Introduction
Pancreatic cancer, principally adenocarcinoma of
ductal origin, is the fourth most common cause of cancer-associated
mortality in the United States (1). Pancreatic cancer has the worst
prognosis among all major human tumor types, with a five-year
survival rate of only 5% when all stages are considered (1). Although surgical resection is
considered to be the sole curative therapeutic approach, only
10–30% of patients have resectable tumors at the time of diagnosis
(2). Previous studies reported
that the five-year survival rate following curative resection is
10–17% (3–7). Given the limited beneficial effect of
conventional chemotherapy in the treatment of pancreatic cancer,
novel targets for the treatment of this disease are required.
Angiogenesis is considered to be one of the most
important factors for tumor development and progression. Vascular
endothelial growth factor (VEGF) plays an important role in tumor
angiogenesis and is significantly correlated with tumor invasion
and metastasis (8–10). In addition, VEGF has a central role
in the emergence of reactive stroma (11). VEGF can be directly released from
cancer cells; however, fibroblasts and inflammatory cells are the
predominant source of host-derived VEGF (12). Furthermore, VEGF induces
microvascular permeability, leading to extravasation of plasma
proteins, including fibrin, which initiates an influx of
fibroblasts, and inflammatory and endothelial cells (11,13,14).
These cells produce extracellular matrix (ECM) that predominantly
consists of fibronectin and type I collagen, which are associated
with the initiation of tumor angiogenesis (11,15,16).
Pancreatic tumors are not considered to be grossly vascularized and
are characterized by a dense stromal reaction that can subsequently
promote tumor invasion (17).
Notably, the majority of pancreatic cancers display overexpression
of angiogenic molecules, including VEGF, which is a key mediator of
tumor angiogenesis (18–20).
A characteristic feature of pancreatic cancer is the
presence of an extensive fibrotic or desmoplastic reaction
surrounding the primary tumor (17). Fibroblast activation protein (FAP)
is a selective protein for tumor stromal fibroblasts. FAP is a 95
kDa cell surface glycoprotein expressed by tumor stromal
fibroblasts in >90% of human epithelial carcinomas, including
breast, lung, colorectal and ovary carcinomas (21,22).
Previous studies have demonstrated that FAP exhibits dipeptidyl
peptidase and collagenase activity (21–23).
A considerable number of studies have presented evidence that
support the importance of stroma and fibroblasts in cancer invasion
and metastasis (17,24). Cancer cells produce enzymes that
degrade basement membranes and the ECM, inducing characteristic
cellular and molecular alterations to the supporting stroma
(24). Carcinoma-associated
fibroblasts (CAFs) that express FAP have been shown to directly
stimulate tumor cell proliferation, angiogenesis and metastasis by
producing various growth factors, hormones and cytokines (22,23,26–28).
Computer-based image analysis is a valuable tool in
pathology for measuring structural parameters concerning all
aspects of neoplasia, including proliferation, apoptosis,
angiogenesis and carcinogenesis, in an objective and reproducible
manner. Confocal laser scanning microscopy (CLSM) is an
immunohistochemical detection technique that is able to recognize
specific cellular proteins. This technique combines the extreme
immunofluorescence sensitivity with improved image resolution by
capturing images that are almost free of out-of-focus signals (NEW
1 and 2). CLSM has been successfully applied in quantitative
pathology to measure the expression of various cellular components,
even in ordinary paraffin-embedded sections (31). However, whether formalin-fixed
tissues are prone to non-specific autofluorescence remains unclear
(NEW 3). Nevertheless, the use of formalin-fixed tissue in the
present study did not appear to raise any technical problems.
The present study used a computer-assisted CLSM
method to quantify FAP expression, while standard
immunohistochemical analysis was used to assess VEGF expression in
a series of human pancreatic adenocarcinomas. The aim of the
current study was to investigate the correlation of the results in
an attempt to elucidate the role of fibroblasts in the progression
of pancreatic cancer. In addition, the impact of FAP expression on
the overall survival of the patients was investigated, following a
standard chemotherapy regimen with gemcitabine after surgery.
Materials and methods
Patients and specimens
A total of 46 patients (male, 26; female, 20) with a
mean age of 66 years (range, 53–80 years) were selected. The
participants of this study were pancreatic adenocarcinoma patients
who had undergone curative pancreatic resection during a period of
six years (2003–2006) at the ‘Laiko’ General University Hospital
(Athens University Medical School, Athens, Greece).
Paraffin-embedded samples of pancreatic adenocarcinoma and normal
adjacent pancreatic tissue were researched retrospectively and
retrieved from the archives of the Pathology Department of ‘Laiko’
General University Hospital (Athens University Medical School,
Athens, Greece) where they were stored from the time of the
operation under appropriate conditions. A pathologist blindly
reviewed the slides to ensure that the cases were consistent with
pancreatic ductal adenocarcinoma, according to the World Health
Organization classification (33).
The surgical procedure included standard pancreatoduodenectomy and
distal pancreatectomy. Patients with distant metastases detected
pre-operatively or receiving any type of cancer treatment prior to
the surgery were excluded from the study. All the patients received
the same chemotherapy regimen following the surgery. The
chemotherapy treatment schedule was: 1,000 mg/m2
gemcitabine intrvenously administered in 30 min on days 1, 8, 15
every 28 days for 6 cycles. Each cycle lasted 28 days and the total
duration of treatment was six months. Outcome data were recorded
from follow-up consultations with the patients, which were arranged
according to each patient’s oncologist’s recommendations. Contact
was maintained with the patients and their families, as well as
with the physicians involved in the patients’ treatment. The
patients surviving until the final follow-up visit or contact were
included in the survival analysis. At the time the present study
was completed, 16 out of 46 patients were alive. A modified Kloppel
grading system (34) was used to
establish the differentiation grade of the tumor samples as
follows: G1, well-differentiated; G2, moderately-differentiated;
G3, poorly-differentiated. Tumor node metastasis staging (35) was performed, according to the
criteria of the National Comprehensive Cancer Network. The present
study was approved by the Ethics Committee of the National and
Kapodistrian University of Athens and the Institutional Review
Board (Athens, Greece). Written informed consent was obtained from
all patients or their families.
Immunohistochemistry and
immunofluorescence
All the biopsy specimens were fixed in formalin
solution and processed, according to the routine protocol of the
Pathology Laboratory of the Athens University Medical School, from
the blocks of tissue retrieved. Paraffin-embedded sections (4
μm) were stained with hematoxylin and eosin for histological
or immunohistochemical analyses. The tissue sections were then
treated with a 1:50 dilution of monoclonal mouse anti-human VEGF
antibody (M7273; Dako, Glostrup, Denmark). Detection was performed
using the Dako Envision Detection system, Peroxidase/DAB+ (K4065;
Dako). The levels of immunostaining were evaluated by examining 10
random high-power fields under x400 magnification. Specimens
expressing >20% VEGF were arbitrarily considered to ‘highly’
express the marker. Each specimen was assessed by two pathologists,
who had no access to the clinical information, with the use of a
two-headed microscope (E400 Eclipse dual viewing microscope; Nikon
Corp., Tokyo, Japan). In the case of disagreement between the
observers the specimens were re-evaluated, in order to reach a
consensus.
Immunofluorescent analysis was also performed on
4-μm sections of neoplastic and adjacent normal tissue from
each patient. The following antibodies were used: Mouse monoclonal
anti-human FAP (F11-24) antibody (sc-65398; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), at a dilution of 1:50; and
fluorescein isothiocyanate (FITC)-labeled goat anti-mouse
polyclonal antibody (sc-2010; Santa Cruz Biotechnology, Inc.), at a
dilution of 1:100. The tissue sections were incubated with anti-FAP
at room temperature for 60 min and with anti-FITC for 60 min at
room temperature in the dark. Normal goat serum was used to block
non-specific antigenic sites (sc-2043; Santa Cruz Biotechnology,
Inc.). All the samples were maintained at 4°C in the dark and
observed within 24 h after staining in order to avoid fluorescence
fading.
CLSM
Fluorescent images were obtained using a Bio-Rad
MRC-600 confocal laser scanning imaging system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) supplied with an argon ion
laser source with a maximum emitting power of 25 mW. The imaging
system was coupled to a Nikon Optiphot microscope (Nikon
Corporation, Tokyo, Japan) under x40 magnification. Integrated
excitation and emission filters at 488 and 515 nm, respectively,
were used to detect FITC (excitation peak, 490 nm; emission
maximum, 525 nm). All the images were acquired using the same
system settings and quantified using a previously described
standard methodology (31). A
unique value of 0–255 units, corresponding to the FAP staining
intensity, was attributed to each case.
Statistical analyses
Statistical analyses were performed using SPSS
version 13.0 software for Windows (SPSS Inc., Chicago, IL, USA).
McNemar’s test was used to assess the difference among VEGF
expression in the various disease stages. A χ2 test of
independence was used to determine whether there was an association
between disease stage and VEGF expression. Independent samples
t-test was used to compare the differences in FAP expression in the
neoplastic tissue, associated with the disease stage and VEGF
expression. The correlation between FAP expression in the
neoplastic and adjacent normal tissue was assessed by calculating
the partial correlation coefficient, controlling the disease stage
and VEGF expression. The effect of VEGF on the survival rate was
assessed using the Kaplan-Meier method, while the effect of FAP
expression and covariates on the survival rate were investigated
using Cox regression. P<0.05 was considered to indicate a
statistically significant difference.
Results
A total of 46 patients (male, 26; female, 20) with a
mean age of 66 years (range, 53–80 years) participated in this
study. All the tumor samples investigated were found to be
moderately or poorly differentiated, with 22 patients presenting
stage IIA (T3N0M0) disease and 24
patients presenting stage IIB (T1, T2 or
T3N1M0) disease. The median
survival time was 11 months (range, 4–32 months). VEGF expression
was detected in the neoplastic cells and in a few stroma cells, as
determined by cytoplasmic staining. VEGF was found to be highly
expressed in 11 out of the 22 patients with stage IIA disease and
in 19 out of 24 patients with stage IIB (P=0.038). FAP expression
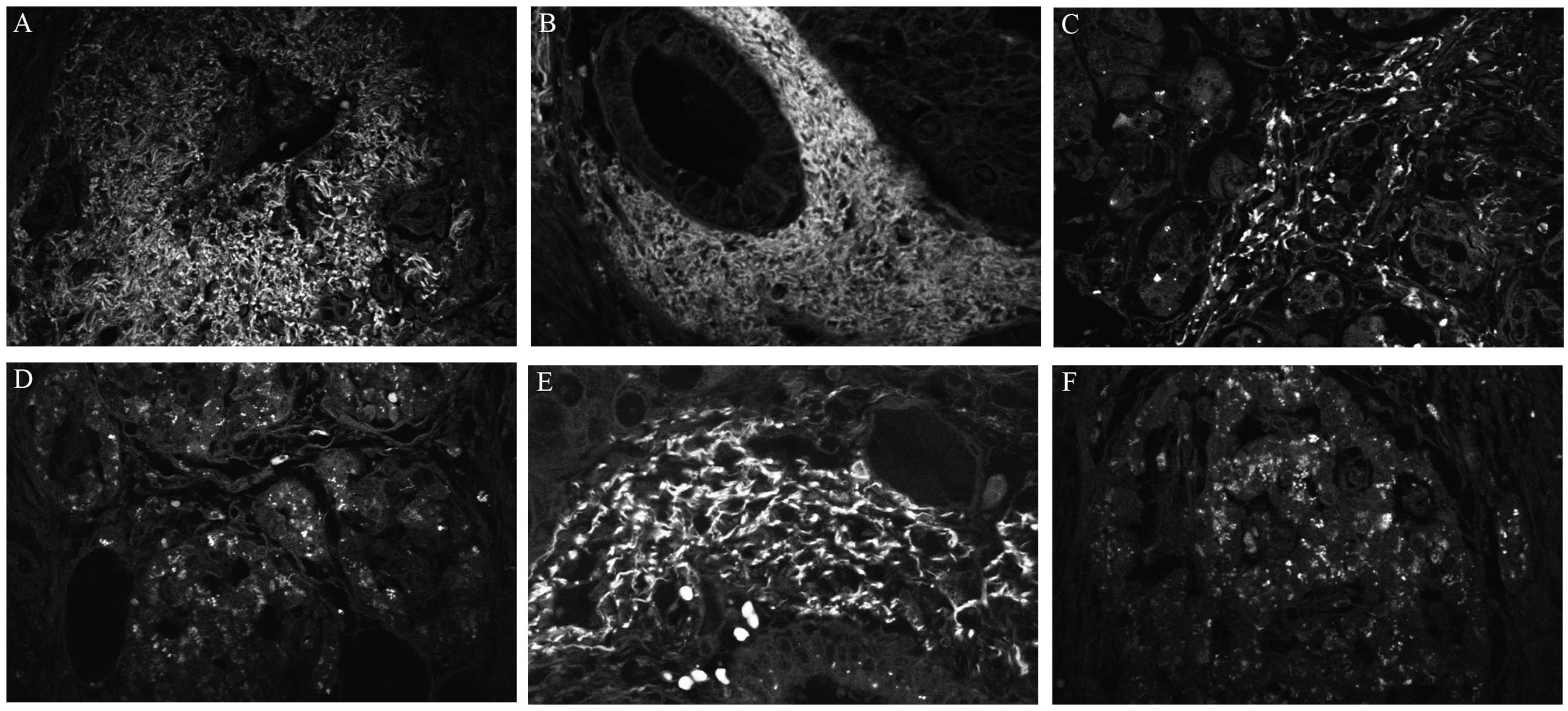
was predominantly expressed in tumor myofibroblasts and, at a
lesser extent, in cancer cells (Fig.
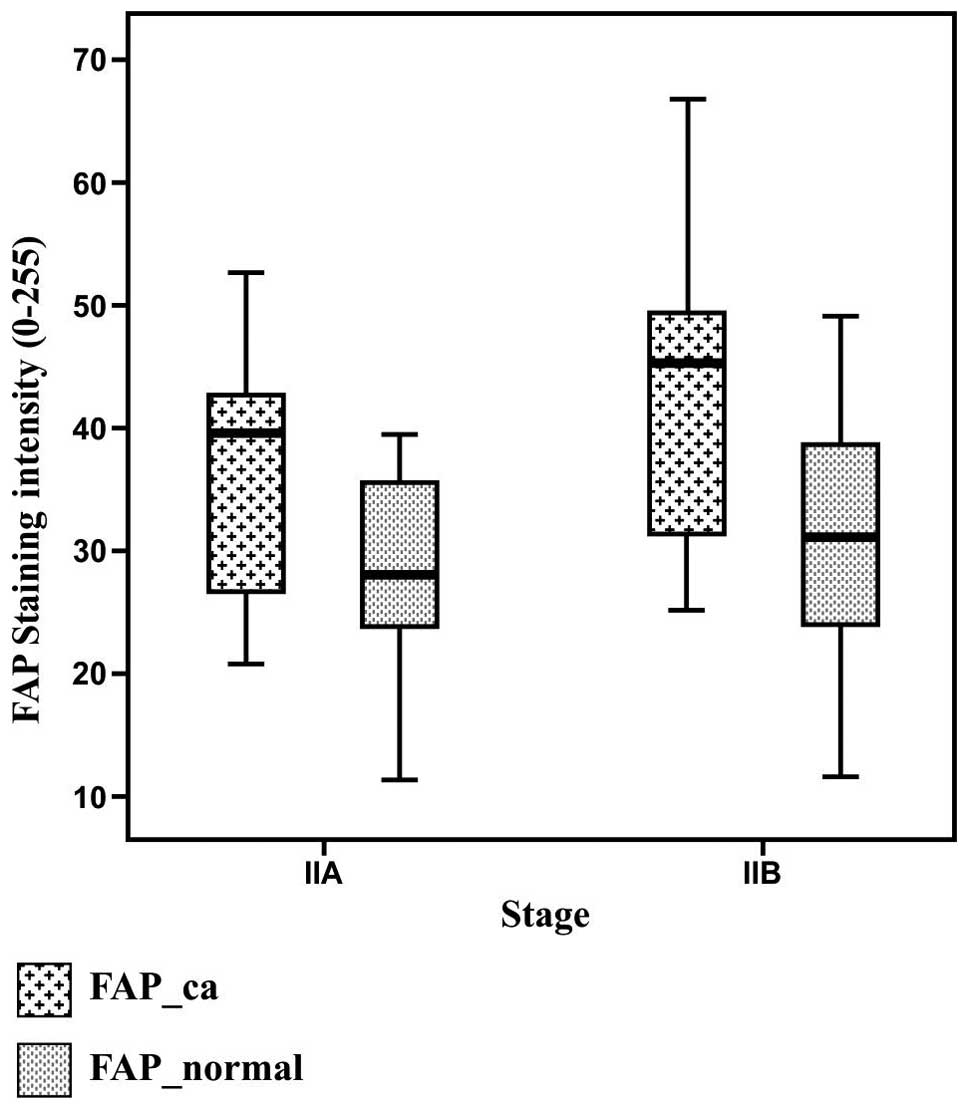
1). FAP expression [mean (standard deviation)] was found to be
36.4 (9) arbitrary units in stage
IIA disease with negative lymph nodes and 42.7 (11.1) arbitrary
units in stage IIB disease with positive lymph nodes (P=0.04). In
patients presenting high expression levels of VEGF, FAP expression
was 42.6 (10.7) arbitrary units, while in the patients with low
expression of VEGF, FAP expression was 34.3 (8) (P=0.03). By contrast, the expression
of FAP in the adjacent normal tissue was 28 (7.9) in the patients
with stage IIA disease and 30.5 (10.3) in the patients with stage
IIB disease (P>0.05; Fig. 2).
Therefore, FAP expression in the neoplastic and adjacent normal
tissue was found to be significantly correlated (partial
correlation coefficient, 0.39; P= 0.007). This correlation was
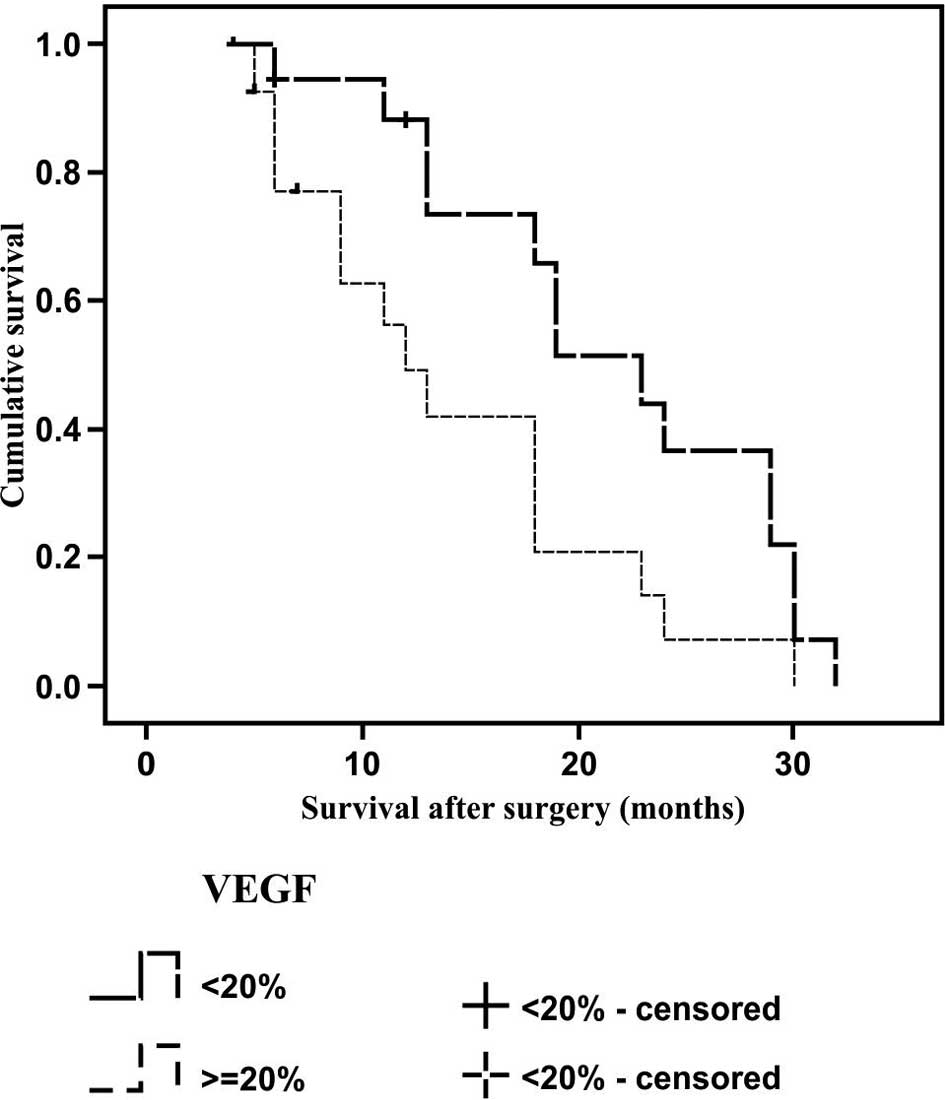
independent of disease stage and VEGF expression (Fig. 2). The median (standard deviation)
survival time for the tumors with low VEGF expression was 23 (2.3)
months, as compared with 11 (1.9) months in the tumors with high
VEGF expression; therefore, a statistically significant difference
was observed between the survival rates (P=0.001; Fig. 3). In the Cox regression model for
survival analysis, FAP expression in the neoplastic tissue was
shown to significantly affect the survival rate (P=0.003), while
this effect was independent of VEGF expression (P=0.065), disease
stage (P=0.930), age (P=0.157) and gender (P=0.076; Table I).
 | Table ICox regression analysis of survival
assessing the impact of multiple covariates in the model. FAP
expression in the neoplastic tissue had a significant effect on
survival (P=0.003), whereas other variables did not affect survival
(P>0.05). |
Table I
Cox regression analysis of survival
assessing the impact of multiple covariates in the model. FAP
expression in the neoplastic tissue had a significant effect on
survival (P=0.003), whereas other variables did not affect survival
(P>0.05).
A, Dependent
variable: Survival
|
|---|
| | | Overall (score)
|
|---|
| −2 Log
likelihood |
χ2-value | Df | P-value | |
|---|
|
|---|
| 142.134 | 15.458 | 7 | 0.009 | |
|---|
|
|---|
B, Variables in the
equation
|
|---|
| | | | | | | 95.0% CI for Exp
(B)
|
|---|
| Variable | B | SE | Wald | DF | P-value | Exp (B) | Lower | Upper |
|---|
| Age | 0.041 | 0.029 | 2.003 | 1 | 0.157 | 1.041 | 0.984 | 1.102 |
| Gender | 1.129 | 0.637 | 3.141 | 1 | 0.076 | 3.091 | 0.887 | 10.768 |
| Stage | −0.043 | 0.493 | 0.008 | 1 | 0.930 | 0.958 | 0.364 | 2.517 |
| VEGF | −0.915 | 0.495 | 3.414 | 1 | 0.065 | 0.401 | 0.152 | 1.057 |
| FAP_ca | 0.042 | 0.014 | 8.542 | 1 | 0.003 | 1.043 | 1.014 | 1.073 |
Discussion
The results of the present study were concordant
with the findings from previous reports, which indicated that FAP
may play a significant role in pancreatic adenocarcinoma. FAP has
been previously shown to be predominantly expressed in the tumor
stroma of epithelial malignancies, while its presence has been
associated with epithelial cancer invasion, tumor angiogenesis, and
subsequent growth and metastasis (22, 23,26–28,36).
The present study demonstrated that FAP expression in the
neoplastic and adjacent normal tissue was significantly higher in
stage IIB patients, when compared with stage IIA patients. In
addition, FAP expression was found to be correlated with positive
lymph nodes and lymphatic vessel invasion, and was therefore
associated with poor prognosis in patients with stage IIB disease.
Cohen et al (26)
demonstrated that high expression of FAP in the surrounding tumor
myofibroblasts was associated with positive lymph nodes, reduced
time period to recurrence and reduced survival rate. Furthermore,
Shi et al (37)
demonstrated that FAP was highly expressed in carcinoma cells, as
well as in the fibroblasts of pancreatic adenocarcinoma tissues.
The authors also identified that patients with a high FAP
expression in carcinoma cells had a significantly reduced overall
survival rate compared with the patients with low FAP expression,
while a multivariate analysis indicated that high expression of FAP
was an independent predictor of overall survival. In the present
study, a survival analysis was conducted using the Cox regression
model, and the expression of FAP in the neoplastic tissues was
found to significantly affect survival; however, this effect was
independent from VEGF expression, disease stage, age and
gender.
In the present study, the survival rate was
considerably reduced in patients with higher expression levels of
VEGF. This result was concordant with a recent meta-analysis, which
suggested that VEGF represents the most consistently reproducible
immunohistochemical marker with prognostic value in resected
pancreatic adenocarcinoma (38).
Furthermore, the results of the present study demonstrated that a
higher VEGF expression in pancreatic cancer is correlated with a
higher expression of FAP in the same tissue. To the best of our
knowledge, this is the first study to demonstrate a correlation
between FAP and VEGF expression levels and evaluate their
association with clinical outcomes in pancreatic
adenocarcinoma.
Currently, anti-VEGF therapy is not recommended for
the treatment of pancreatic cancer, and certain studies have failed
to demonstrate the beneficial effect of combining axitinib (an oral
inhibitor of VEGF receptors 1, 2 and 3) with gemcitabine (39,40).
Due to the essential contribution of stroma in cancer progression,
CAFs have recently gained interest as a novel therapeutic target.
CAFs are genetically stable, as compared with cancer cells, and
thus are more likely to maintain sensitivity to drugs (41). In addition, CAFs are responsible
for the structure of tumor ECM, which interferes with the diffusion
of anti-cancer agents into tumors (41). Furthermore, communication between
CAFs and tumor cells promotes the survival, proliferation and
invasiveness of cancer cells. Tumor stroma-directed therapies can
target the growth factors and cytokines that initiate the
communication within the tumor microenvironment, therefore
promoting an anti-cancer effect (41). FAP has previously been suggested as
a potential candidate for specifically targeting CAFs (42). Notably, CAF-directed therapy may
also be used to overcome the loss of VEGF inhibitor effects. A
previous study has revealed that platelet derived growth factor-C
(PDGF-C) produced by CAFs is able to initiate VEGF production in
tumor cells, thereby sustaining the angiogenic shift. Therefore,
antibodies targeting PDGF-C may be used to inhibit angiogenesis in
tumors, refractory to anti-VEGF treatments (43).
In conclusion, the present study used CLSM to
quantify the expression levels of FAP in a series of human
pancreatic adenocarcinomas. An immumohistochemical investigation
was also used to determine VEGF expression. A significant
correlation was observed between the two markers, and they were
both shown to possess prognostic significance. A multivariate
analysis demonstrated the prognostic superiority of FAP over VEGF,
which is currently considered to be the most consistently
reproducible molecular marker with prognostic value in resected
pancreatic adenocarcinoma. Since the available systemic therapies
for pancreatic adenocarcinoma that target tumor cells have limited
clinical benefit, targeting FAP may be a potential novel
therapeutic strategy against cancer. However, this is concept that
requires further investigation.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clinic. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Crist DW and Cameron JL: The current
status of the Whipple operation for periampullary carcinoma. Adv
Surg. 25:21–49. 1992.PubMed/NCBI
|
|
3
|
Sohn TA, Yeo CJ, Cameron JL, et al:
Resected adenocarcinoma of the pancreas-616 patients: results,
outcomes and prognostic indicators. J Gastrointest Surg. 4:567–579.
2000. View Article : Google Scholar
|
|
4
|
Cleary SP, Gryfe R, Guindi M, et al:
Prognostic factors in resected pancreatic adenocarcinoma: analysis
of actual 5-year survivors. J Am Coll Surg. 198:722–731. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han SS, Jang JY, Kim SW, Kim WH, Lee KU
and Park YH: Analysis of long-term survivors after surgical
resection for pancreatic cancer. Pancreas. 32:271–275. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moon HJ, An JY, Heo JS, Choi SH, Joh JW
and Kim YI: Predicting survival after surgical resection for
pancreatic ductal adenocarcinoma. Pancreas. 32:37–43. 2006.
View Article : Google Scholar
|
|
7
|
Matsuno S, Egawa S, Fukuyama S, et al:
Pancreatic cancer registry in Japan: 20 years of experience.
Pancreas. 28:219–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Whitehurst B, Flister MJ, Bagaitkar J, et
al: Anti-VEGF-A therapy reduces lymphatic vessel density and
expression of VEGFR-3 in an orthotopic breast tumor model. Int J
Cancer. 121:2181–2191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Christiansen A and Detmar M:
Lymphangiogenesis and cancer. Genes Cancer. 2:1146–1158. 2011.
View Article : Google Scholar
|
|
10
|
Shinkaruk S, Bayle M, Laïn G and Déléris
G: Vascular endo-thelial cell growth factor (VEGF), an emerging
target for cancer chemotherapy. Curr Med Chem Anticancer Agents.
3:95–117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brown LF, Guidi AJ, Schnitt SJ, et al:
Vascular stroma formation in carcinoma in situ, invasive carcinoma
and metastatic carcinoma of the breast. Clin Cancer Res.
5:1041–1056. 1999.PubMed/NCBI
|
|
12
|
Fukumura D, Xavier R, Sugiura T, et al:
Tumor induction of VEGF promoter activity in stromal cells. Cell.
94:715–725. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Senger DR, Galli SJ, Dvorak AM, Perruzzi
CA, Harvey VS and Dvorak HF: Tumor cells secrete a vascular
permeability factor that promotes accumulation of ascites fluid.
Science. 219:983–985. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dvorak HF, Sioussat TM, Brown LF, et al:
Distribution of vascular permeability factor (vascular endothelial
growth factor) in tumors: concentration in tumor blood vessels. J
Exp Med. 174:1275–1278. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng D, Nagy JA, Brekken RA, et al:
Ultrastructural localization of the vascular permeability
factor/vascular endothelial growth factor (VPF/VEGF) receptor-2
(FLK-1, KDR) in normal mouse kidney and in the hyperpermeable
vessels induced by VPF/VEGF-expressing tumors and adenoviral
vectors. J Histochem Cytochem. 48:545–556. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leung DW, Cachianes G, Kuang WJ, Goeddel
DV and Ferrara N: Vascular endothelial growth factor is a secreted
angiogenic mitogen. Science. 246:1306–1309. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mahadevan D and Von Hoff DD: Tumor-stroma
interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther.
6:1186–1197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ikeda N, Adachi M, Taki T, et al:
Prognostic significance of angiogenesis in human pancreatic cancer.
Br J Cancer. 79:1553–1563. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Itakura J, Ishiwata T, Shen B, Kornmann M
and Korc M: Concomitant over-expression of vascular endothelial
growth factor and its receptors in pancreatic cancer. Int J Cancer.
85:27–34. 2000. View Article : Google Scholar
|
|
20
|
Fujimoto K, Hosotani R, Wada M, et al:
Expression of two angiogenic factors, vascular endothelial growth
factor and platelet-derived endothelial cell growth factor in human
pancreatic cancer and its relationship to angiogenesis. Eur J
Cancer. 34:1439–1447. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JE, Lenter MC, Zimmermann RN,
Garin-Chesa P, Old LJ and Rettig WJ: Fibroblast activation protein,
a dual specificity serine protease expressed in reactive human
tumor stromal fibroblasts. J Biol Chem. 274:36505–36512. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garin-Chesa P, Old LJ and Rettig WJ: Cell
surface glycoprotein of reactive stromal fibroblasts as a potential
antibody target in human epithelial cancers. Proc Nat Acad Sci USA.
87:7235–7239. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scanlan MJ, Raj BK, Calvo B, et al:
Molecular cloning of fibroblast activation protein alpha, a member
of the serine protease family selectively expressed in stromal
fibroblasts of epithelial cancers. Proc Nat Acad Sci USA.
91:5657–5661. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mueller MM and Fusenig NE: Tumor-stroma
interactions directing phenotype and progression of epithelial skin
tumor cells. Differentiation. 70:486–497. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: an imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cohen SJ, Alpaugh RK, Palazzo I, et al:
Fibroblast activation protein and its relationship to clinical
outcome in pancreatic adenocarcinoma. Pancreas. 37:154–158. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goscinski MA, Suo Z, Florenes VA,
Vlatkovic L, Nesland JM and Giercksky KE: FAP-alpha and uPA show
different expression patterns in premalignant and malignant
esophageal lesions. Ultrastruct Pathol. 32:89–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Henry LR, Lee HO, Lee JS, et al: Clinical
implications of fibroblast activation protein in patients with
colon cancer. Clin Cancer Res. 13:1736–1741. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Földes-Papp Z, Demel U and Tilz GP: Laser
scanning confocal fluorescence microscopy: an overview. Int
Immunopharmacol. 3:1715–1729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amos WB and White JG: How the confocal
laser scanning microscope entered biological research. Biol Cell.
95:335–342. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Papaxoinis K, Patsouris E, Kittas C and
Nicolopoulou-Stamati P: Insulinlike growth factor I receptor and
estrogen receptor beta expressions are inversely correlated in
colorectal neoplasms and affected by the insulin resistance
syndrome. Hum Pathol. 38:1037–1046. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mason DY, Micklem K and Jones M: Double
immunofluorescence labelling of routinely processed paraffin
sections. J Pathol. 191:452–461. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hamilton SR and Aaltonen LA: Pathology and
Genetics of Tumours of the Digestive System. World Health
Organization Classification of Tumours. Kleihues P and Sobin LH:
IARC Press; Lyon: pp. 1–250. 2000
|
|
34
|
Giulianotti PC, Boggi U, Fornaciari G, et
al: Prognostic value of histological grading in ductal
adenocarcinoma of the pancreas. Klöppel vs TNM grading. Int J
Pancreatol. 17:279–289. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Edge SB, Byrd DR, Compton CC, et al: AJCC
Cancer Staging Manual. 7th. Springer; New York, NY: pp. 241–249.
2010
|
|
36
|
Kraman M, Bambrough PJ, Arnold JN, et al:
Suppression of antitumor immunity by stromal cells expressing
fibroblast activation protein-alpha. Science. 330:827–830. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi M, Yu DH, Chen Y, et al: Expression of
fibroblast activation protein in human pancreatic adenocarcinoma
and its clinicopathological significance. World J Gastroenterol.
18:840–846. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Smith RA, Tang J, Tudur-Smith C,
Neoptolemos JP and Ghaneh P: Meta-analysis of immunohistochemical
prognostic markers in resected pancreatic cancer. Br J Cancer.
104:1440–1451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kindler HL, Ioka T, Richel DJ, et al:
Axitinib plus gemcitabine versus placebo plus gemcitabine in
patients with advanced pancreatic adenocarcinoma: a double-blind
randomised phase 3 study. Lancet Oncol. 12:256–262. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Spano JP, Chodkiewicz C, Maurel J, et al:
Efficacy of gemcitabine plus axitinib compared with gemcitabine
alone in patients with advanced pancreatic cancer: an open-label
randomised phase II study. Lancet. 371:2101–2108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cirri P and Chiarugi P: Cancer associated
fibroblasts: the dark side of the coin. Am J Cancer Res. 1:482–497.
2011.PubMed/NCBI
|
|
42
|
Rettig WJ, Garin-Chesa P, Healey JH, et
al: Regulation and heteromeric structure of the fibroblast
activation protein in normal and transformed cells of mesenchymal
and neuroectodermal origin. Cancer Res. 53:3327–3335.
1993.PubMed/NCBI
|
|
43
|
Crawford Y, Kasman I, Yu L, et al: PDGF-C
mediates the angiogenic and tumorigenic properties of fibroblasts
associated with tumors refractory to anti-VEGF treatment. Cancer
Cell. 15:21–34. 2009. View Article : Google Scholar
|