1. Introduction
The metallothioneins (MTs) are a group of low
molecular weight, cysteine-rich intracellular proteins, which are
involved in maintaining intracellular metal homeostasis by binding
metals, including zinc and copper. There are 10 functional isoforms
of MTs, which are divided into four classes, designated MT-1 to -4,
on the basis of small differences in protein sequence, expression
and characteristics (1,2). They maintain transition metal ion
homeostasis and redox balance, serve as anti-oxidants and protect
against DNA damage and apop-tosis (3). Reduced expression of MT has
been observed in liver (4), colon
(5) and prostate (6) cancer. It was suggested that during
the transformation of normal colorectal tissue to adenomatous
polyps and adenocarcinoma, a progressive decrease in the expression
of MT occurs (7,8). The role of MT in these types
of cancer remains to be elucidated, however, considering its
anti-oxidant activity and its protective potential against DNA
damage, this reduction may increase susceptibility to toxin-induced
damage. Indeed, an MT knockout in mice has been reported to
induce a higher rate of induced carcinogenesis (9). Conversely, aberrant overexpression of
MT has been observed in various types of human cancer,
including breast cancer, gallbladder cancer, melanoma and lymphoma
(10–13). It has been suggested that the
overexpression of MT may protect cells from free
radical-induced DNA damage and lipid peroxidation (14). Overexpression of MT has been
demonstrated to be important in drug resistance, since nuclear
expression of MT protects DNA in ovarian cancer cells from
the toxic effect of treatment with cisplatin (15). This indicates that aberrant
under/over-expression of MT are important in various types
of cancer.
A study revealed that the hematopoietic master
transcription factor, PU.1, directly suppresses the MT-1A
and MT-1G promoter through DNA methylation and histone
deacetylase (HDAC) activity (16).
Additionally, it was revealed that MT-1A is suppressed,
while the expression of PU.1 is induced, during
12-O-tetradecanoylphorbol-13-acetate (TPA)-induced monocytic
differentiation of THP-1 cells (17). Notably, the suppression of
MT-1s by PU.1 is required for the proper differentiation of
myeloid cells.
Although there are several reviews regarding the
regulation of the MT gene (18–20),
reviews regarding the suppressive regulation of MT genes are
relatively scarce. Therefore, this review summarized the regulation
of MT genes and particularly focused on PU.1 and other
suppressive regulators of the MT genes.
2. Positive regulators of MT
genes
The basal activity of MT is regulated by
several general transcription factors, including the TFIID complex
comprising TATA-binding protein (TBP), TBP associated factors and
Sp1 (18–20). In addition, MT can be
activated by a variety of stimuli, including metal ions, cytokines
and growth factors (1). Several
inducible expression regulators of the MT genes have been
identified, including metal-responsive element (MRE)-binding
transcription factor (MTF)-1 (21,22),
upstream stimulatory factor (USF)-1 (23) and nuclear factor (NF)1 (24). Since a number of reviews summarize
the details of the positive regulation of MT genes (18–20),
the present review describes the above essential factors.
The MTF-1 gene is a central regulator of the
metal-inducible expression levels of MT-1 and MT-2.
In addition to zinc, other heavy metals (e.g. cadmium), hypoxia,
oxidative stress, stress hormones (glucocorticoids), nitric oxide
and high temperature induce the transcriptional activity of MTF-1
(25–28). Andrews et al (23) reported that MTF-1 is essential for
the upregulation of the gene expression of MT-1 in visceral
endoderm cells and that optimal expression is dependent upon the
interactions of the basic helix-loop-helix transcription factor,
USF -1, with an E-box-1 containing sequence at −223 bp in the
MT-1 promoter (23).
NF1 is a protein expressed ubiquitously in higher
eukaryotes, and distinct highly conserved genes encode four
isoforms of the NF1 protein (NF1-A, NF1-B, NF1-C and NF1-X)
(29–31). NF1 binding sites were identified in
various MT promoters, with the exception of MT-IB
(19). LaRochelle et al
(24) previously demonstrated that
NF1 binds to the mouse MT-1 promoter in vivo and this
binding is zinc inducible and MTF-1 dependent. It was revealed by
transient transfection assays into HepG2 cells, that NF1 activates
the mouse MT-1 promoter. The authors demonstrated that NF1
and MTF-1 synergistically activate the mouse MT-1 gene in
response to metal ions (24).
However, Majumder et al (32,33)
previously demonstrated that NF1 isoforms inhibit the activity of
the MT-1 promoter in HepG2 cells. This is contradictory to
the earlier study (24), however,
this result may be due to the experimental condition in which
Majumder et al have used extremely high expression levels of
the NF1 vector, ~30- to 1000-fold more vector compared with the
earlier study (24). LaRochelle
et al demonstrated that the expression levels of the
transcriptionally active mutant of NF1 reduced the zinc-induced
MT-1 promoter by up to 50%, in a dose-dependent manner and
may also indicate that NF1 is a positive regulator of the gene
expression of MT-1 (24).
3. Negative regulators of MT
genes
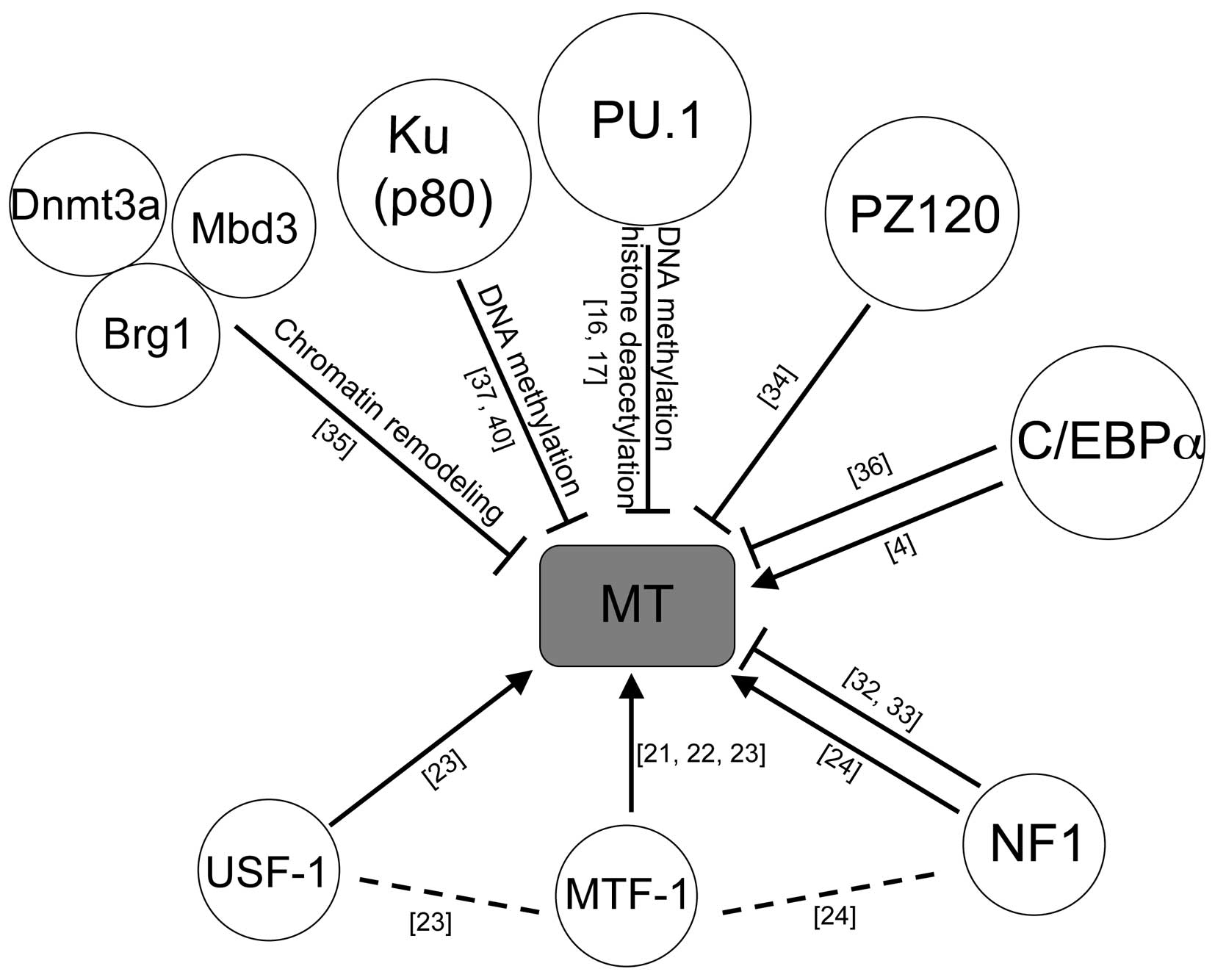
To date, several factors are reported to regulate
the suppression of MT genes, including PZ120 (34), DNA methyltransferase (Dnmt) 3a with
Mbd3 and Brg1 complex (35), C/EBP
α (36), Ku protein (37) and PU.1 (16,17).
Tang et al (34), reported the cloning of a novel zinc
finger protein with a molecular mass of 120 kDa (PZ120), through
Southwestern cloning, which interacts specifically with the human
gene transcription initiation site of MT-2A. PZ120 is a
ubiquitously expressed protein and possesses a conserved poxvirus
and zinc finger (POZ) motif, which is a structure existing in
several transcriptional repressors. This protein has been revealed
to repress the transcription of the MT-2A promoter (34).
Datta et al (35) purified DNA methyltransferase (Dnmt)
3a from mouse lymphosarcoma cells and revealed that Dnmt
3a-associated polypeptides identified the methyl CpG binding
protein, Mbd3, histone deacetylase 1 and components of the Brg1
complex (35). A chromatin
immunoprecipitation assay reveled that Dnmt 3a, Mbd3 and Brg1 are
associated with a transcriptionally silent methylated MT-1
promoter in the mouse lymphosarcoma cells. The authors further
clarified that the catalytic activity of Dnmt3a was not important
for the repression of the MT-1 gene; however, ATP-dependent
chromatin remodeling of Brg1 was (35). It was also revealed that methylated
and unmethylated MT-1 promoters are differentially regulated
by several methyl CpG binding proteins, including methyl CpG
binding protein (MeCP) 2 and Mbd1, 2 and 4 (38).
CCAAT enhancer binding protein (C/EBP) is important
in the terminal differentiation of cells, particularly in myeloid
cells and adipose cells (39). Yin
et al (36) demonstrated
that forced expression of C/EBPα decreased the expression levels of
the MT isoforms 1A, B, F and H, and 2A and 3 in prostate
cancer cells, and that this suppression is mediated through its
promoter activity. Furthermore, it was revealed that the forced
expression of C/EBPα led to an increased cytotoxicity of zinc in
prostate cancer cells (36).
However, in human hepatocellular carcinoma cells, the inactivation
of C/EBPα through the activation of phosphatidylinositol 3-kinase
led to the downregulation of the expression of MT (4). Therefore, the role of C/EBPα in the
gene regulation of MT may differ among tissues.
It was previously reported that the large subunit
(p80) of the Ku protein contained repressor activity for the
MT-1 promoter (37).
Additionally, it was revealed that this repression is due to the
hypermethylation of a CpG island in the MT-1 promoter
(40).
Rodent and human MT genes contain CpG islands
in their promoter (19,41). It was first reported in 1981 that
DNA methylation controls the inducibility of the mouse MT-1
gene (42). Since then, >100
studies have been published demonstrating that the MT
promoter is regulated by DNA methylation in its promoter region.
Arriaga et al (43)
demonstrated from the analysis of colorectal cancer, that the mRNA
expression levels of five isoforms (MT-1G, 1E,
1F, 1H and 1M) were lost during the transition
from normal mucosa to tumor, whereas MT-1X and MT-2
were less downregulated and their expression was correlated with
overall protein positivity. It was also demonstrated that
hypermethylation of the MT-1G gene occurred in cell lines
and in 29% of tumor samples. Faller et al (44) analyzed specimens from patients with
melanoma and demonstrated that in 1/17 (6%) of the benign naevi,
16/43 (37%) primary tumors and 6/13 (46%) of metastases exhibited
MT-1E gene methylation. Peng et al (45) revealed using quantitative
pyrosequencing, unique DNA methylation profiles in the MT-3
promoter region in esophageal adenocarcinomas (EACs). This previous
study concluded that EACs are characterized by frequent epigenetic
silencing of the MT-3 gene. In addition, in colon cancer,
not only DNA methylation (41,43),
but the loss of heterozygosity (5)
is also important in the downregulation of the MT genes
(MT-1F, MT-1G, MT-1X and MT-2A).
4. PU.1-a master hematopoietic transcription
factor previously identified as a novel negative regulator of
MT-1s
A previous study revealed that MT-1s genes
are epigenetically suppressed by the activity of PU.1 (16). PU.1 is a hematopoietic master
transcription factor, predominantly expressed in immature myeloid
cells and B cells, and downregulation of this factor is important
in various hematological malignancies (46,47).
To identify downstream target genes of PU.1, the authors generated
cell lines expressing reduced levels of PU.1 by stable transfection
of PU.1 short inhibitory RNAs into K562 human myeloid leukemia
cells (K562PU.1KD cells) and PU.1-overexpressing K562 cells
(K562PU.1OE cells). Dual microarray analyses were performed using
these cell lines. Notably, the expression levels of all the
functional MT isoforms expressed in humans
(MT-1A, -B, -E, -F, -G,
-H and -X and MT-2) were increased by varying
degrees in the K562PU.1KD cells. Furthermore, there were negative
correlations between the mRNA expression of PU.1 and the mRNA
expression of the MT-1s in 43 primary specimens from
patients with acute myeloid leukemia (AML). Additionally, it was
revealed that PU.1 directly binds and epigenetically suppresses the
MT-1s promoter, in concert with MeCP2, through the
suppression of the enzymatic activities of HDAC and Dnmt. The
proportion of the methylated CpG sites is tightly associated with
the expression levels in MT-1s promoters (16). Next, the authors examined whether
the expression levels of PU.1 and MT-1A are indeed
correlated with each other, and whether the expression of
MT-1A is regulated by PU.1 during TPA-induced THP-1 monocyte
differentiation. As a result, it was revealed that the expression
of MT-1s is suppressed during monocytic differentiation in
the THP-1 cells (17). Chromatin
immunoprecipitation analysis demonstrated that PU.1 and MeCP2 bind
to the same region in the MT-1A promoter, and the binding of
these proteins to this promoter was increased during
differentiation. Consistently, the proportion of methylated CpG
sites was markedly increased during differentiation (17). These results suggest that
MT-1s are repressed through the epigenetic activity of PU.1
in hematopoietic cells.
5. Conclusion
The positive and negative regulators described in
this review are summarized in Fig.
1. The consequences of these MT gene regulations have
been reported to be through the normal physiological aspects to
disease, including inflammation, aging and malignancies (1,3,48,49).
It was recently demonstrated that the overexpression of
MT-1G potently inhibited the retinoic acid induced myeloid
differentiation of NB4 acute promyelocytic leukemia cells (50). This is consistent with the
literature, suggesting that the downregulation of PU.1 is the cause
of AML (46) and results in the
overexpression of MT, leading to the inhibition of
differentiation, which is important in leukemogenesis.
MTs are multifunctional proteins and exhibit
different biological behavior in different tissues. Therefore,
further clarifying the underlying mechanisms and the roles of
MT, may lead to an improved understanding of the biology of
normal physiology and malignancies from another aspects.
Acknowledgments
The author would like to thank everyone who helped
the research during this decade, regarding the analysis of the PU.1
transcription factor, and the regulation and functions of the
MT gene. This study was supported in part by Grants-in-Aid
for Scientific Research (grant no. 26460685) from the Ministry of
Education, Culture, Sports, Science and Technology, Japan, the
Takeda Science Foundation, and a foundation from Kitasato
University School of Allied Health Sciences (Grant-in-Aid for
Research Project, grant no. 2014-1003).
References
|
1
|
Takahashi S: Molecular functions of
metallothionein and its role in hematological malignancies. J
Hematol Oncol. 5:412012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hamer DH: Metallothionein. Annu Rev
Biochem. 55:913–951. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cherian MG, Jayasurya A and Bay BH:
Metallothioneins in human tumors and potential roles in
carcinogenesis. Mutat Res. 533:201–209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Datta J, Majumder S, Kutay H, et al:
Metallothionein expression is suppressed in primary human
hepatocellular carcinomas and is mediated through inactivation of
CCAAT/enhancer binding protein alpha by phosphatidylinositol
3-kinase signaling cascade. Cancer Res. 67:2736–2746. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan DW, Fan JW, Yu ZH, et al:
Downregulation of metallothionein 1F, a putative oncosuppressor, by
loss of heterozygosity in colon cancer tissue. Biochim Biophys
Acta. 1822:918–926. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei H, Desouki MM, Lin S, Xiao D, Franklin
RB and Feng P: Differential expression of metallothioneins (MTs) 1,
2, and 3 in response to zinc treatment in human prostate normal and
malignant cells and tissues. Mol Cancer. 7:72008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Theocharis SE, Margeli AP, Klijanienko JT
and Kouraklis GP: Metallothionein expression in human neoplasia.
Histopathology. 45:103–118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ofner D, Maier H, Riedmann B, et al:
Immunohistochemical metallothionein expression in colorectal
adenocarcinoma: correlation with tumour stage and patient survival.
Virchows Arch. 425:491–497. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang B, Satoh M, Nishimura N, et al:
Metallothionein deficiency promotes mouse skin carcinogenesis
induced by 7,12-dimethylbenz[a]anthracene. Cancer Res.
58:4044–4046. 1998.PubMed/NCBI
|
|
10
|
Poulsen CB, Borup R, Borregaard N, Nielsen
FC, Møller MB and Ralfkiaer E: Prognostic significance of
metallothionein in B-cell lymphomas. Blood. 108:3514–3519. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bay BH, Jin R, Huang J and Tan PH:
Metallothionein as a prognostic biomarker in breast cancer. Exp
Biol Med (Maywood). 231:1516–1521. 2006.
|
|
12
|
Shukla VK, Aryya NC, Pitale A, et al:
Metallothionein expression in carcinoma of the gallbladder.
Histopathology. 33:154–157. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weinlich G, Eisendle K, Hassler E, Baltaci
M, Fritsch PO and Zelger B: Metallothionein-overexpression as a
highly significant prognostic factor in melanoma: a prospective
study on 1270 patients. Br J Cancer. 94:835–841. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
You HJ, Lee KJ and Jeong HG:
Overexpression of human metallothionein-III prevents hydrogen
peroxide-induced oxidative stress in human fibroblasts. FEBS Lett.
521:175–179. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Surowiak P, Materna V, Maciejczyk A, et
al: Nuclear metallothionein expression correlates with cisplatin
resistance of ovarian cancer cells and poor clinical outcome.
Virchows Arch. 450:279–285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Imoto A, Okada M, Okazaki T, Kitasato H,
Harigae H and Takahashi S: Metallothionein-1 isoforms and vimentin
are direct PU.1 downstream target genes in leukemia cells. J Biol
Chem. 285:10300–10309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suzuki S, Nakano H and Takahashi S:
Epigenetic regulation of the metallothionein-1A promoter by PU.1
during differentiation of THP-1 cells. Biochem Biophys Res Commun.
433:349–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghoshal K and Jacob ST: Regulation of
metallothionein gene expression. Prog Nucleic Acid Res Mol Biol.
66:357–384. 2001.
|
|
19
|
Laukens D, Waeytens A, De Bleser P,
Cuvelier C and De Vos M: Human metallothionein expression under
normal and pathological conditions: mechanisms of gene regulation
based on in silico promoter analysis. Crit Rev Eukaryot Gene Expr.
19:301–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haq F, Mahoney M and Koropatnick J:
Signaling events for metallothionein induction. Mutat Res.
533:211–226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Radtke F, Heuchel R, Georgiev O, et al:
Cloned transcription factor MTF-1 activates the mouse
metallothionein I promoter. EMBO J. 12:1355–1362. 1993.PubMed/NCBI
|
|
22
|
Palmiter RD: Regulation of metallothionein
genes by heavy metals appears to be mediated by a zinc-sensitive
inhibitor that interacts with a constitutively active transcription
factor, MTF-1. Proc Natl Acad Sci USA. 91:1219–1223. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Andrews GK, Lee DK, Ravindra R, et al: The
transcription factors MTF-1 and USF1 cooperate to regulate mouse
metallothionein-I expression in response to the essential metal
zinc in visceral endoderm cells during early development. EMBO J.
20:1114–1122. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
LaRochelle O, Labbe S, Harrisson JF, et
al: Nuclear factor-1 and metal transcription factor-1
synergistically activate the mouse metallothionein-1 gene in
response to metal ions. J Biol Chem. 283:8190–8201. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Westin G and Schaffner W: A
zinc-responsive factor interacts with a metal-regulated enhancer
element (MRE) of the mouse metallothionein-I gene. EMBO J.
7:3763–3770. 1988.PubMed/NCBI
|
|
26
|
Dalton T, Palmiter RD and Andrews GK:
Transcriptional induction of the mouse metallothionein-I gene in
hydrogen peroxide-treated Hepa cells involves a composite major
late transcription factor/antioxidant response element and metal
response promoter elements. Nucleic Acids Res. 22:5016–5023. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Murphy BJ, Andrews GK, Bittel D, et al:
Activation of metallothionein gene expression by hypoxia involves
metal response elements and metal transcription factor-1. Cancer
Res. 59:1315–1322. 1999.PubMed/NCBI
|
|
28
|
Günther V, Lindert U and Schaffner W: The
taste of heavy metals: gene regulation by MTF-1. Biochim Biophys
Acta. 1823:1416–1425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Paonessa G, Gounari F, Frank R and Cortese
R: Purification of a NF1-like DNA-binding protein from rat liver
and cloning of the corresponding cDNA. EMBO J. 7:3115–3123.
1988.PubMed/NCBI
|
|
30
|
Rupp RA, Kruse U, Multhaup G, Göbel U,
Beyreuther K and Sippel AE: Chicken NFI/TGGCA proteins are encoded
by at least three independent genes: NFI-A, NFI-B and NFI-C with
homologues in mammalian genomes. Nucleic Acids Res. 18:2607–2616.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meisterernst M, Rogge L, Foeckler R,
Karaghiosoff M and Winnacker EL: Structural and functional
organization of a porcine gene coding for nuclear factor I.
Biochemistry. 28:8191–8200. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Majumder S, Ghoshal K, Gronostajski RM and
Jacob ST: Downregulation of constitutive and heavy metal-induced
metallothionein-I expression by nuclear factor I. Gene Expr.
9:203–215. 2001.PubMed/NCBI
|
|
33
|
Jacob ST, Majumder S and Ghoshal K:
Suppression of metallothionein-I/II expression and its probable
molecular mechanisms. Environ Health Perspect. 110(Suppl 5):
827–830. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang CM, Westling J and Seto E: trans
repression of the human metallothionein IIA gene promoter by PZ120,
a novel 120-kilo-dalton zinc finger protein. Mol Cell Biol.
19:680–689. 1999.
|
|
35
|
Datta J, Majumder S, Bai S, et al:
Physical and functional interaction of DNA methyltransferase 3A
with Mbd3 and Brg1 in mouse lymphosarcoma cells. Cancer Res.
65:10891–10900. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yin H, Smith M and Glass J: Stable
expression of C/EBPalpha in prostate cancer cells down-regulates
metallothionein and increases zinc-induced toxicity. Prostate.
62:209–216. 2005. View Article : Google Scholar
|
|
37
|
Ghoshal K, Li Z and Jacob ST:
Overexpression of the large subunit of the protein Ku suppresses
metallothionein-I induction by heavy metals. Proc Natl Acad Sci
USA. 95:10390–10395. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Majumder S, Kutay H, Datta J, Summers D,
Jacob ST and Ghoshal K: Epigenetic regulation of metallothionein-i
gene expression: differential regulation of methylated and
unmethylated promoters by DNA methyltransferases and methyl CpG
binding proteins. J Cell Biochem. 97:1300–1316. 2006. View Article : Google Scholar
|
|
39
|
Koschmieder S, Halmos B, Levantini E and
Tenen DG: Dysregulation of the C/EBPalpha differentiation pathway
in human cancer. J Clin Oncol. 27:619–628. 2009. View Article : Google Scholar :
|
|
40
|
Majumder S, Ghoshal K, Li Z and Jacob ST:
Hypermethylation of metallothionein-I promoter and suppression of
its induction in cell lines overexpressing the large subunit of Ku
protein. J Biol Chem. 274:28584–28589. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mao J, Yu H, Wang C, et al:
Metallothionein MT1M is a tumor suppressor of human hepatocellular
carcinomas. Carcinogenesis. 33:2568–2577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Compere SJ and Palmiter RD: DNA
methylation controls the inducibility of the mouse
metallothionein-I gene lymphoid cells. Cell. 25:233–240. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Arriaga JM, Levy EM, Bravo AI, et al:
Metallothionein expression in colorectal cancer: relevance of
different isoforms for tumor progression and patient survival. Hum
Pathol. 43:197–208. 2012. View Article : Google Scholar
|
|
44
|
Faller WJ, Rafferty M, Hegarty S, et al:
Metallothionein 1E is methylated in malignant melanoma and
increases sensitivity to cisplatin-induced apoptosis. Melanoma Res.
20:392–400. 2010.PubMed/NCBI
|
|
45
|
Peng D, Hu TL, Jiang A, et al:
Location-specific epigenetic regulation of the metallothionein 3
gene in esophageal adenocarcinomas. PLoS One. 6:e220092011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rosenbauer F, Wagner K, Kutok JL, et al:
Acute myeloid leukemia induced by graded reduction of a
lineage-specific transcription factor, PU.1. Nat Genet. 36:624–630,
Epub 2004 May 2016. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pettersson M, Sundström C, Nilsson K and
Larsson LG: The hematopoietic transcription factor PU.1 is
downregulated in human multiple myeloma cell lines. Blood.
86:2747–2753. 1995.PubMed/NCBI
|
|
48
|
Inoue K and Takano H: Metallothionein as a
negative regulator of pulmonary inflammation. Curr Pharm
Biotechnol. 14:414–419. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mocchegiani E, Costarelli L, Basso A,
Giacconi R, Piacenza F and Malavolta M: Metallothioneins, ageing
and cellular senescence: a future therapeutic target. Curr Pharm
Des. 19:1753–1764. 2013.
|
|
50
|
Hirako N, Nakano H and Takahashi S: A PU.1
suppressive target gene, metallothionein 1G, inhibits retinoic
acid-induced NB4 cell differentiation. PLoS One. 9:e1032822014.
View Article : Google Scholar : PubMed/NCBI
|