Introduction
Chinese medicine has played an invaluable role in
the prevention and treatment of diseases for thousands of years in
China. Although Chinese medicine was considered to have less
toxicity and fewer side effects than traditional medicine,
increasing evidence has shown this to be untrue (1–3).
Given that many pregnant women use Chinese herbs for
pregnancy-related sickness (4,5), the
potential developmental toxicity of these herbs needs to be
studied. In the present study, we investigated the safety and
possible embryonic toxicity of four common herbs: Rhizoma
Atractylodes macrocephala, Radix Isatidis, Coptis
chinensis and Flos Genkwa.
Atractylodes macrocephala has been used in
traditional Chinese medicine (TCM) for approximately 2,000 years.
It exhibits multipharmacological effects and is used to treat
various complaints, including excessive vaginal bleeding (6). It is also used to invigorate the
spleen (7). As a typical Chinese
herbal medicine for replenishing qi, Rhizoma Atractylodes
macrocephala has long been used in the treatment of threatened
preterm labor.
Radix Isatidis is the dried root of crucifer
Isatis tinctoria L, which is widely distributed in northern
and central China. According to a book on herbal medicine written
in 110 B.C. by Shennong, a notable ancient Chinese medicinal
specialist, it has been used as a medicinal plant for more than
2,000 years. It is used to dissipate heat (cold compress), detoxify
the immune system and cool the blood. It is widely used for
preventing and treating infectious diseases caused by viruses,
including influenza, viral pneumonia, mumps and hepatitis (8).
The use of Coptis chinensis as a herbal
remedy was also first recorded in Shennong’s book. This herb is
used to dissipate heat and promote diuresis. It is used as a common
herbal medicine for diarrhea, dysentery, acute febrile and
suppurative infections and vomiting, as well as to protect the
gastric mucosa (9). Coptis
chinensis has been used for thousands of years in China.
However, it has been banned in Singapore since 1978 due to the
belief that taking this herb during pregnancy or lactation causes
serious jaundice in infants.
Flos Genkwa, the dried flower buds of
Daphne genkwa, is a medicinal plant distributed mainly in
mainland China. It is commonly used as an abortifacient (10), with purgative, diuretic and
anti-inflammatory actions (11).
Since the enactment of China’s one-child family planning policy,
studies of Flos Genkwa have focused on its abortion efficacy
and mechanisms (12). One previous
study has investigated its anticancer actions (13).
A systematic study of the use of Chinese herbal
medicines during pregnancy has not been conducted. Currently,
teratogenicity tests in vivo or genetic toxicity tests are
the main methods employed to study the reproductive and
developmental toxicity of TCM (14,15).
Whole embryo cultures or micromass embryo cell cultures have also
been used to reveal the developmental toxicity of TCM (16). Comparatively speaking, in
vivo tests, based on maternal or embryonic exposure of
laboratory animals, are more time-consuming and expensive. In
vitro assays, including the whole-embryo culture assay and the
micromass assay, offer an alternative to in vivo assessment,
although both still rely on embryos (17). Spielmann and Liebsch developed the
embryonic stem cell test (EST), an in vitro assay system to
determine the teratogenic potential of test chemicals (18). It is the only test not requiring
pregnant animals (19). The EST is
based on murine-derived embryonic stem cells (ESCs) from the
blastocyst stage. ESCs are pluripotent cells. They differentiate
in vitro into a wide variety of cell types, representing all
three germ layers (ectoderm, mesoderm and endoderm). Under
appropriate culture conditions, certain ESCs differentiate
spontaneously into beating myocard cells. Three different endpoints
are evaluated in the EST: The inhibition of growth (cytotoxicity)
of 3T3 cells and ESCs after 10 days of treatment, determined by the
Cell Counting Kit-8 (CCK8) cell proliferation assay, and the
inhibition of the differentiation of ESCs into myoblasts following
10 days of treatment. The concentration ± response correlations are
recorded, and 50% inhibition concentrations are determined for the
three endpoints. In the EST, the mutagenic potential of the test
substances are classified into three different classes of in
vivo embryotoxic potencies: strongly embryotoxic, weakly
embryotoxic and non-embryotoxic (20). In a previous study, the EST was
used to verify the embryotoxicity of 20 reference compounds with
the different embryotoxic potencies mentioned above. The accuracy
of the EST assay was 78%. Notably, a predictivity of 100% was
attained for strong embryo-toxicants (21). As a result, the validated EST has
been accepted for assessing the embryotoxicity of test compounds at
an early stage of development (22). However, there are few reports on
the application of the EST to the study of the reproductive and
developmental toxicity of TCM (23,24).
The aim of the present study was to evaluate the
embryonic developmental toxicity of extracts of four TCMs (Rhizoma
Atractylodes macrocephala, Radix Isatidis, Coptis
chinensis and Flos Genkwa). For this purpose, a modified
EST was used. Three endpoints (IC503T3,
IC50ES and ID50ES) were used for each
extract, and each test compound was classified as strongly, weakly
or non-embryotoxic.
Materials and methods
Preparation of crude drug extracts
Roots of Atractylodes macrocephala, Coptis
chinensis, Radix Isatidis and Flos Genkwa were
purchased from Caizhiling, a reputable Chinese medicinal herb store
in Guangzhou, China. Their authenticity was confirmed by Professor
Ma Zhiguo of the Pharmacy College of Jinan University. The aqueous
extract was prepared by a general method. After cutting the herbs
into small pieces, 100 g dried plant material was boiled in 1,000
ml distilled water for 1 h. The decoction was collected and the
residue was boiled another two times. The decoction obtained from
the three separated extractions was mixed, filtered and lyophilized
by freeze drying. The powdered forms of the extracts were stored at
−20°C.
Preparation of extracts and pure compound
solutions
The dried extracts were dissolved in
double-distilled water until the initial concentration was 1 g/ml
and centrifuged at 14,000 g for 5 min before filtration
sterilization to obtain a clear, sterile supernatant for testing.
The chemicals were dissolved in appropriate solvents. The chemical
5-fluorouracil (5-FU; Sigma-Aldrich, St. Louis, MO, USA) was
dissolved in dimethylsulphoxide. Phenytoinum natricum (DPH;
Sigma-Aldrich) and saccharin (SAC; Sigma-Aldrich) were dissolved in
phosphate-buffered saline (PBS). The final solvent concentrations
applied in the differentiation and cytotoxicity assays demonstrated
no undesired background effects.
Cell culture
Undifferentiated mouse ESCs of the OG2
cell line were purchased from the Chinese Academy of Sciences.
Continuous cultures of the cell line were grown on mitomycin C
inactivated mouse embryonic fibroblast (MEF) feeders in a standard
culture medium consisting of 80% high-glucose (4.5 g glucose/l)
Dulbecco’s modified Eagle’s medium (DMEM; Gibco Life Technologies,
Karlsruhe, Germany), 15% fetal calf serum (Hyclone,
Erembodegem-Aalst, Belgium), as well as 2 mM glutamine, 2 mM sodium
pyruvate, antibiotics (50 U/ml penicillin and 50 µg/ml
streptomycin), 1% non-essential amino acids, 0.1 mM
β-mercaptoethanol and 1,000 U/ml murine leukemia inhibitory factor,
which were all purchased from Gibco Life Technologies. BALB/c 3T3
fibroblasts, purchased from the cell bank of Sun Yat-sen University
of Medical Sciences, were cultured in DMEM supplemented with 10%
fetal calf serum, 50 U/ml penicillin and 50 µg/ml
streptomycin. The cells were maintained under 5% CO2 and
95% humidity at 37°C.
Alkaline phosphatase staining
The ESCs were subjected to alkaline phosphatase
(AKP) staining on day 5 of passage. The original medium was
discarded from the culture plates, and the cells were washed with
PBS and fixed in 4% paraformaldehyde for 10 min. They were then
washed again with PBS and stained with 75 mg/ml nitrotetrazolium
blue chloride (Inogent, Hyderabad, India) and 50 mg/ml
5-bromo-4-chloro-3-indolylphosphate (Innogent) for 1 h at room
temperature.
Cytotoxicity assay
The cytotoxic effects on the mESCs and 3T3 cells
were detected by performing a CCK8 (Mbchem, Mumbai, India) cell
proliferation assay on day 10. In brief, on day 1, 500 cells were
seeded into each well of a 96-well tissue plate in a routine cell
culture medium without leukemia inhibitory factor and incubated for
2 h. Following incubation, 200 µl culture medium containing
the appropriate dilution of extracts from the four medicines were
added to each well. On days 3 and 5, the medium was renewed. On day
10, CCK8 assay was carried out. Cytotoxicity was expressed as the
concentration of the compound that reduced the viability of cells
to 50% of the control level (IC503T3 and
IC50ES), determined from a concentration-response
curve.
Differentiation assay
Differentiation assays were performed to detect
compound-induced changes in the differentiation of the mESCs into
contracting cardiomyocytes. Briefly, on day 0, 20 µl stem
cell suspension containing 750 cells was placed as hanging drops on
the inner side of the lid of a petri dish filled with 6 ml PBS,
then incubated for three days at 37°C, with 5% CO2 and
95% humidity, in the presence of the test chemicals at various
concentrations. During this period, the cells formed aggregates
referred to as embryonic bodies (EBs). After three days, the
aggregates that formed were transferred to bacterial petri dishes
and exposed to the appropriate concentration of the test chemical
for another two days. On day 5, the EBs were placed individually
into six wells of a Falcon tissue culture plate. On day 10, the EBs
were collected for quantitative polymerase chain reaction (qPCR)
detection to observe the expression of related genes.
qPCR
The cell samples for analysis were collected on day
10 of the differentiation assay, and total RNA was extracted using
an EZNA™ Total RNA kit II (Omega Bio-Tek, Norcross, GA, USA)
according to the instructions of the manufacturer. cDNA was
synthesized using 1 µg RNA and PrimeScript™ RT Master mix
(Takara, Otsu, Japan) as per the instructions of the manufacturer.
The reaction mixture was incubated at 37°C for 30 min, followed by
5 sec at 85°C. To verify the undifferentiated marker genes Sox2 and
Oct4 and the myocardial-specific marker β-myosin heavy chain
(β-MHC), qPCR was performed using SsoAdvanced™ SYBR-Green (Bio-Rad
Laboratories, Hercules, CA, USA). qPCR reactions were conducted in
a 20-µl mixture that included 10 µl 2X qPCR Master
mix, 8 µl nuclease-free water with mouse-specific primers
for glyceral-dehyde-3-phosphate dehydrogenase (GAPDH; 5′-GCCTTC
TCCATGGTGGTGAA-3′ and 5′-GCACAGTCAAGGCCGA GAAT-3′) and β-MHC
(5′-TCCGCAACCGAGAGAATCAG-3′ and 5′-TGTCGCCAGAAATTGTGCCTT-3′), and 2
µl cDNA template. The thermal cycle profile consisted of an
initial 30-min step at 95°C, followed by 40 cycles of 95°C for 5
sec, and 60°C for 20 min. The CFX Connect Real-Time system and CFX
Manager Software (version 2.0; Bio-Rad) were used to collect the
PCR data. The RNA levels of each gene were normalized to GAPDH.
Classification of the embryotoxic
potential of the four extracts
Two permanent mouse cell lines of ESCs and 3T3 cells
were used to predict the cytotoxicity of the test compounds using
the EST. The concentrations of the compounds that inhibited the
proliferation of 50% of the ESCs and 50% of the 3T3 cells
(IC50ES and IC503T3) were determined with
CCK8 assay. In addition, the concentration that inhibited the
differentiation of 50% of the ESCs (ID50ES) was obtained
from the results of qPCR in a differentiation assay. The three
endpoints obtained in each experiment were used to calculate linear
discriminant functions (I, II, III) for each extract.
5.9157 lg (IC503T3) + 3.500 lg
(IC50ES) − 5.307 (IC503T3-ID50ES)
/ IC503T3-15.72;
3.6511 lg (IC503T3) + 2.3941 lg
(IC50ES) − 2.033 (IC503T3-ID50ES)
/ IC503T3-6.85;
−0.125 lg (IC503T3) + 1.917 lg
(IC50ES) + 1.500 (IC503T3-ID50ES)
/ IC503T3-2.67.
Depending on the variables of the three functions I,
II and III, the embryotoxicity of a test compound can be classed as
non-embryotoxic (Class 1), weakly embryotoxic (Class 2) or strongly
embryotoxic (Class 3).
The classification criteria were as follows: Class
1: if I>II and I>III; Class 2: if II>I and II>III; and
Class 3: if III>I and III>II.
We first used a modified EST to determine the effect
of 5-FU, DPH and SAC on differentiation and to classify the
embryotoxic potential of each compound, which was revealed to be
strong, weak and non-existent, respectively, to verify the
predictive validity of the EST we established. Subsequently, the
embryotoxic classification of the four herbal extracts was
determined with the modified EST.
Statistical analysis
The statistical analysis was performed using
GraphPad Prism 5 (GraphPad Prism Software, Inc., San Diego, CA,
USA). Each data point represented three independent experiments.
Data are given as the mean ± SEM. A one-way ANOVA was used to
assess the statistical significance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Verification of the undifferentiated
ESCs
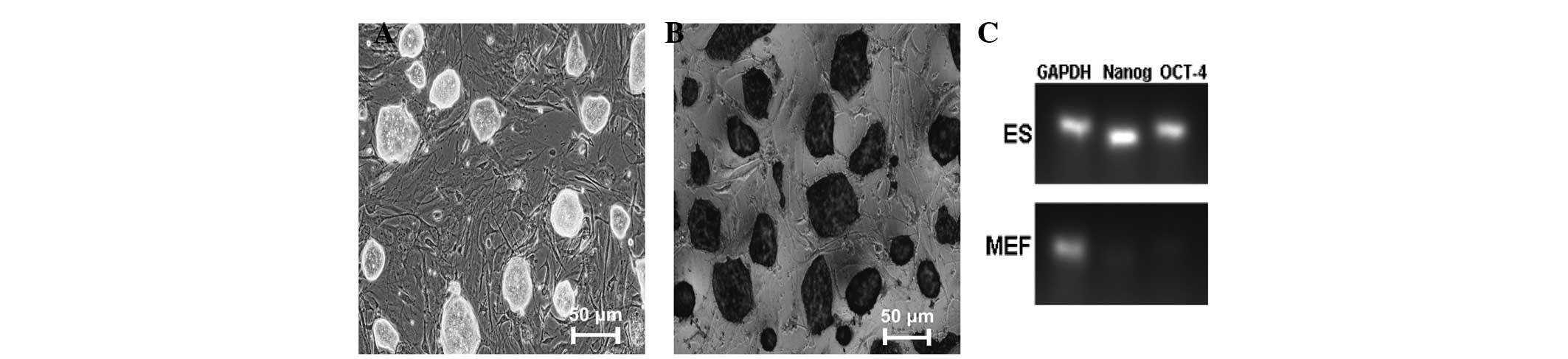
We verified the undifferentiated cells through
morphology, AKP staining and qPCR to determine the expression
levels of undifferentiated markers in undifferentiated cells.
Observed under the microscope, the ESCs were nest-like, compact
clones, with clear boundaries (Fig.
1A). They were AKP-positive and deep brown in color (Fig. 1B). The expression levels of the
undifferentiated markers Sox2, Oct4 and Nanog were much higher than
in the negative control, MEF (Fig.
1C). These results indicated that the ESCs were
undifferentiated, and they were used in subsequent experiments.
Validity check of EST
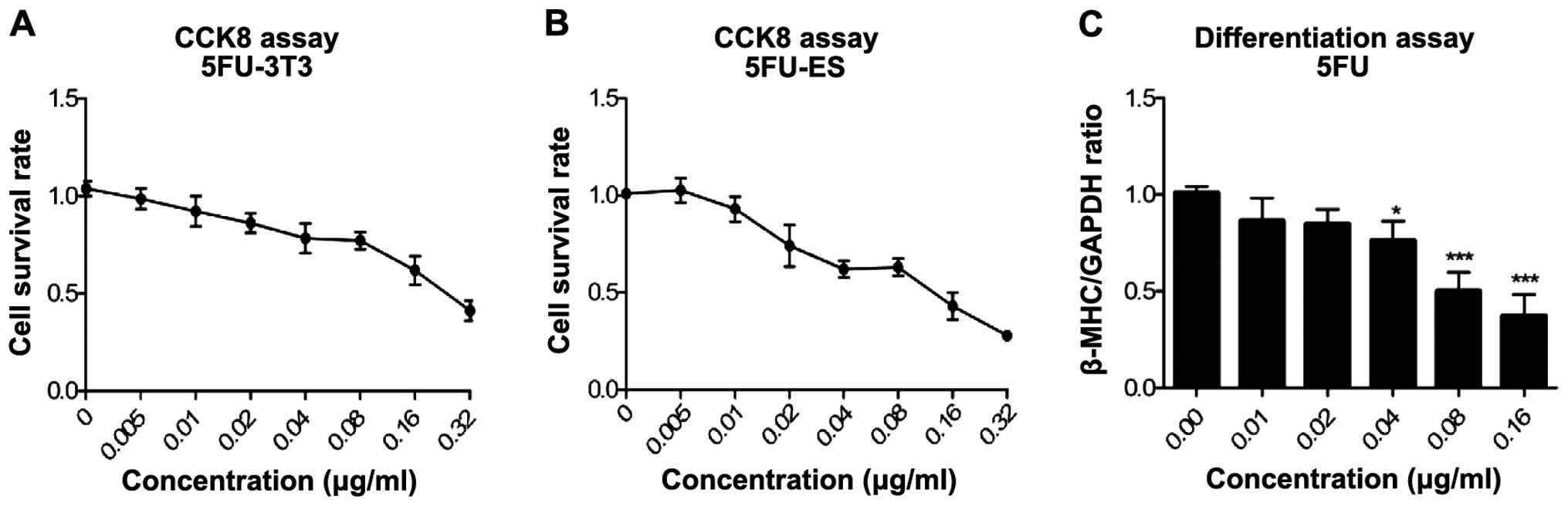
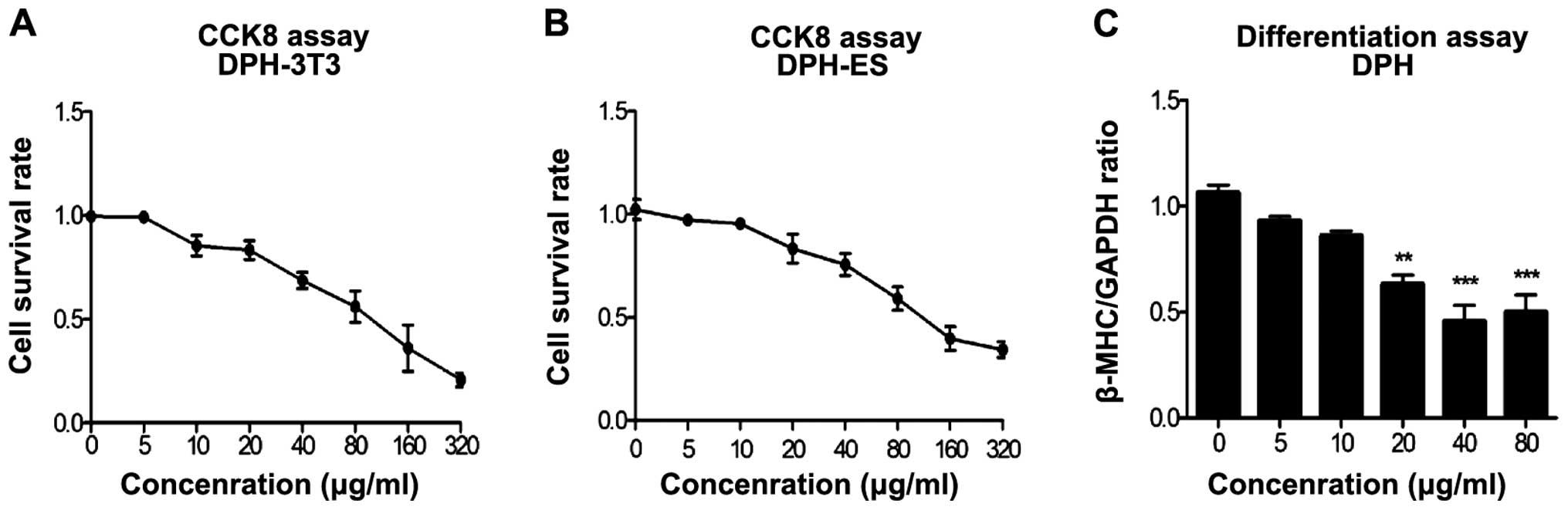
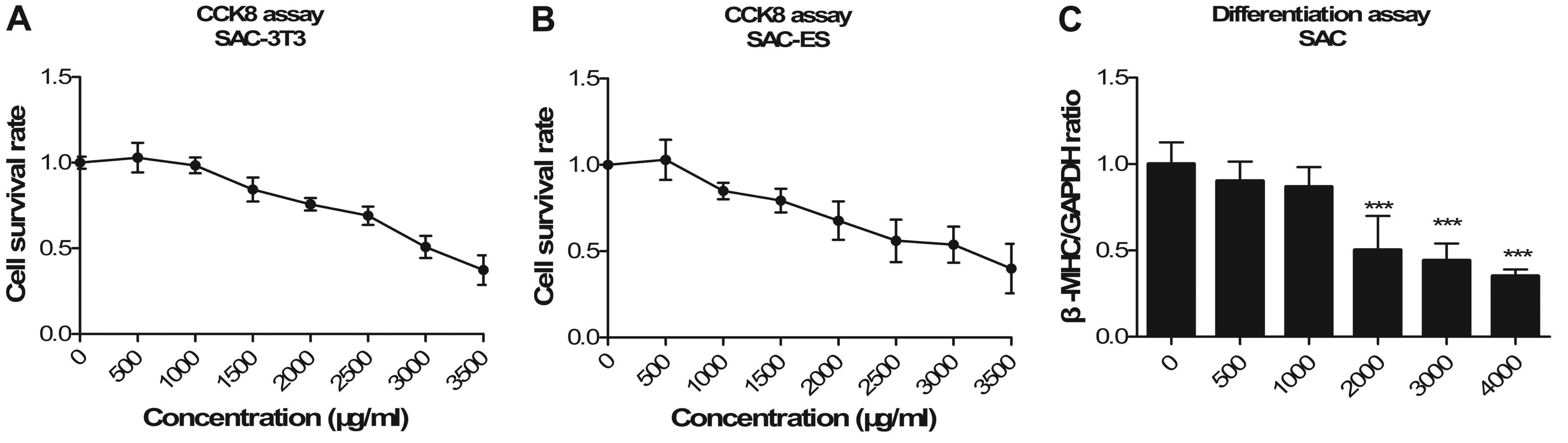
The modified EST model was verified by determining
the embryotoxicity of 5-FU (Fig.
2), DPH (Fig. 3) and SAC
(Fig. 4). Three endpoint values
were obtained for each compound. Detailed data on the endpoint
values are shown in Table I. We
confirmed that 5-FU had strong embryotoxicity, DPH was weakly
embryotoxic and SAC was non-embryotoxic.
 | Table IThree endpoint values of test
compounds in the embryonic stem cell test. |
Table I
Three endpoint values of test
compounds in the embryonic stem cell test.
| Test |
IC503T3 |
IC50ES |
ID50ES | I | II | III | Criteria | Classification |
|---|
| SAC | 2790.00 | 3009.00 | 2156.00 | 15.63 | 13.60 | −9.43 | I>II and
I>III | 1 |
| DPH | 89.76 | 67.87 | 14.13 | −2.23 | 2.95 | −5.16 | II>I and
II>III | 2 |
| 5-FU | 0.25 | 0.05 | 0.07 | −27.79 | −13.72 | 1.05 | III>I and
III> II | 3 |
|
Atractylodes | 4.82 | 42.42 | 37.67 | 30.19 | 13.40 | −16.10 | I>II and
I>III | 1 |
| R.
Isatidis | 1.76 | 27.68 | 15.00 | 30.00 | 12.79 | −16.74 | I>II and
I>III | 1 |
| C.
chinensis | 0.40 | 2.73 | 1.06 | −7.73 | −3.89 | −5.95 | II>I and
II>III | 2 |
| Flos
Genkwa | 0.51 | 0.75 | 1.15 | −11.18 | −5.65 | −4.77 | III>I and
III> II | 3 |
Embryotoxicity of four TCM extracts
The four TCMs (Rhizoma Atractylodes
macrocephala, Radix Isatidis, Coptis chinensis
and Flos Genkwa) were decocted with water, and the EST was
performed to determine the embryotoxic potential of the extracts.
Three endpoints were obtained for each extract from three
independent experiments. The cytotoxicity of the extracts on the
OG2 cells and the 3T3 cells (IC503T3 and
IC50ES) was determined with a CCK8 assay, and the
inhibition of cardiomyocyte differentiation of the ESCs
(ID50ES; based on the expression levels of the β-MHC
gene) was determined by qPCR. The three parameters of each extract
were substituted into three linear discriminant functions (I, II
and III). Detailed data on the classification of the embryotoxic
potential of each extract using the criteria for embryotoxicity are
provided in Table I.
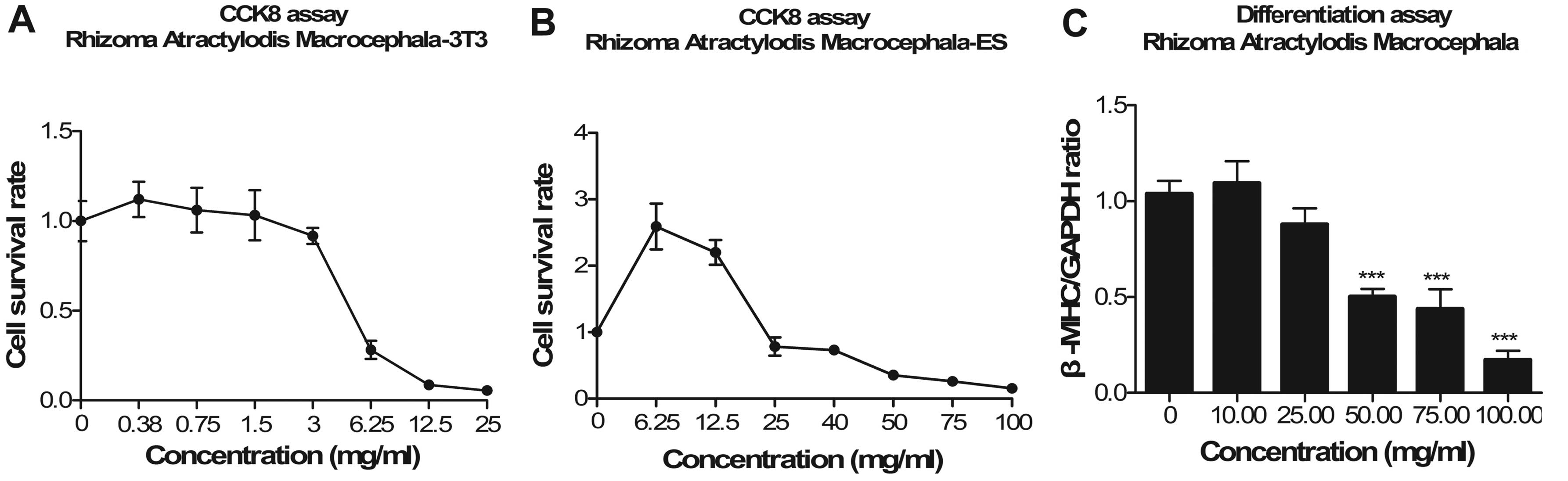
Rhizoma Atractylodes macrocephala
In the cytotoxicity assay, the IC503T3
value was 4.82 mg/ml, which was ~1/10 that of the IC50ES
value (42.42 mg/ml; Fig. 5A and
B). The ID50 value for Rhizoma Atractylodes
macrocephala was 37.67 mg/ml (Fig.
5C). It promoted the survival of ESCs and led to cardiac
differentiation of ESCs at a low concentration. Thus, it was
defined as a non-embryotoxic compound.
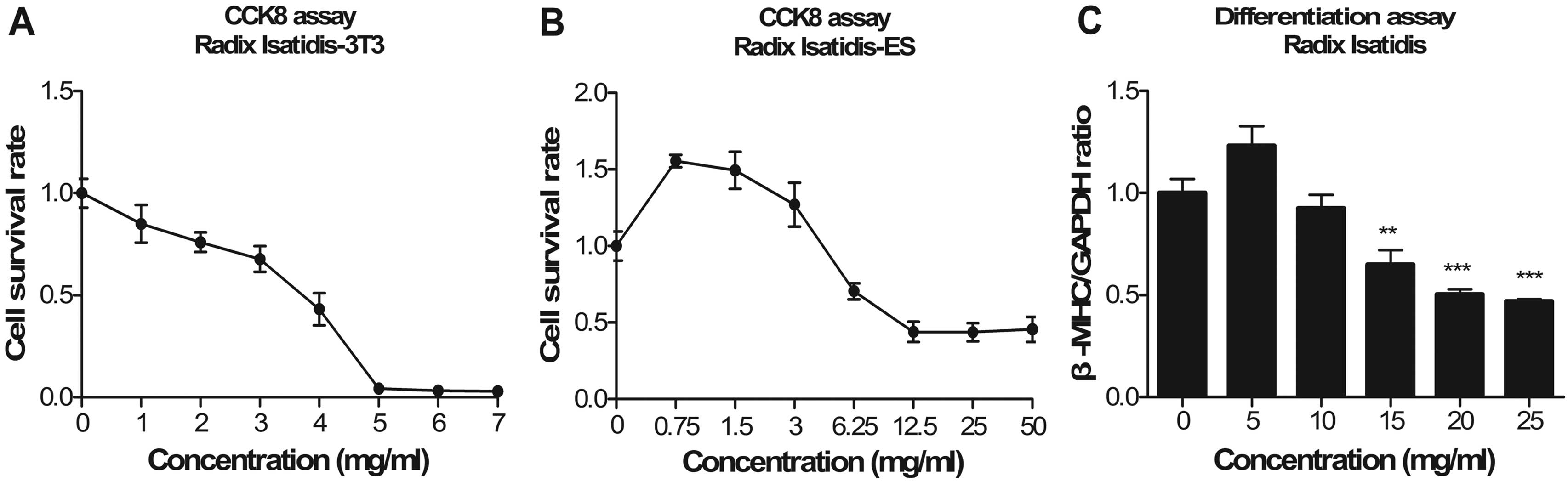
Radix Isatidis
ESCs increased at a low Radix Isatidis
concentration. As the concentration increased, the survival rate of
the ESCs declined. The proliferation of the 3T3 cells was
suppressed at a low concentration, whereas the proliferation of the
ESCs was promoted (Fig. 6A and B).
The IC50 values were 1.76 and 27.68 mg/ml for the 3T3
fibroblasts and the ESCs, respectively. The expression levels of
β-MHC decreased in a concentration-dependent manner following
exposure to Radix Isatidis extract (Fig. 6C). The ID50 values were
15 mg/ml. Radix Isatidis extract was classified as
non-embryotoxic.
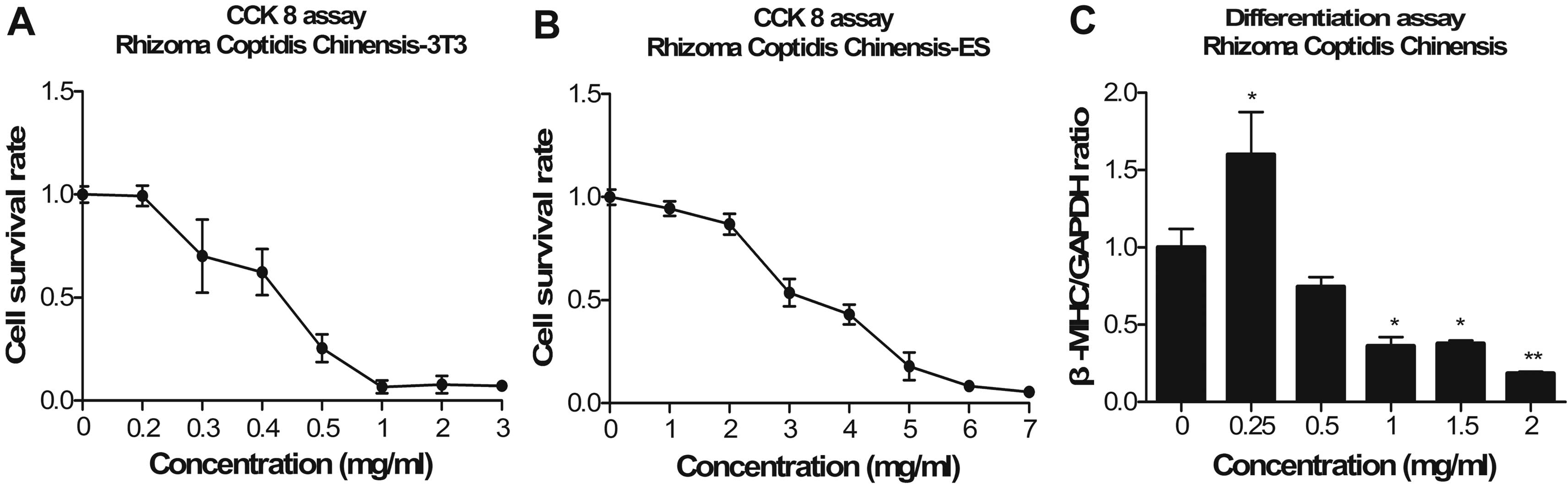
Coptis chinensis
In both cell lines, the Coptis chinensis
extract inhibited the survival of cells in a dose-dependent manner
(Fig. 7A and B). The cytotoxic
sensitivity of the 3T3 fibroblasts was much higher than that of the
ESCs exposed to Coptis chinensis extract. The
IC50 values were 0.398 and 2.73 mg/ml for the 3T3
fibroblasts and the ESCs, respectively. The ID50 value
was 1.06 mg/ml. The Coptis chinensis extract was defined as
weakly embryotoxic.
Flos Genkwa
The 3T3 cells and ESCs were both sensitive to
Flos Genkwa. The IC50 values for the 3T3 cells
(0.51 mg/ml) and those for the ESCs (0.75 mg/ml) were below 1 mg/ml
(Fig. 7A and B). The ESCs were
slightly more sensitive than the 3T3 cells when the concentration
was under 0.25 mg/ml. However, as the concentration was increased,
the opposite trend was observed. The value of ID50 was
1.15 mg/ml. Flos Genkwa was classified as strongly
embryotoxic.
Discussion
The EST is an in vitro tool to assess the
developmental toxic potency of test compounds in early development.
Its coincidence rate is 78% compared with in vivo test
results (21). The EST has been
successfully used to classify the embryo-toxicity of a large number
of chemical compounds (25). It is
now starting to be used to estimate the embryonic toxicity of
natural compounds of Chinese medicinal herbs. Using the EST,
bisphenol A and genistein were classified as weakly embryo-toxic
and strongly embryotoxic, respectively (23). Baicalin, an active constituent of
Radix Scutellariae, demonstrated weak embryotoxicity based
on the EST (24).
In the current study, we attempted to apply the EST
to aqueous extracts of Chinese herbs. A mouse embryonic stem cell
line, OG2, was used as a substitute for the D3 cell line
in the EST, and the embryotoxicity of 5-FU, DPH and SAC was
evaluated to verify the reliability of our findings. Our results
revealed that 5-FU, DPH and SAC were strongly embryotoxic, weakly
embryotoxic and non-embryotoxic, respectively, which is in
accordance with the results of the EST validation test. This
indicated that the findings were reliable when the D3
cell line was replaced with the OG2 cell line in the
EST. We then used the EST to detect the embryotoxicity of the four
selected tested Chinese herbal medicine aqueous extracts.
Rhizoma Atractylodes macrocephala is commonly
used in TCM for pregnant women to treat abnormal fetal movement. In
safety evaluation research, water-soluble extract of Rhizoma
Atractylodes macrocephala revealed no genotoxicity based on
four genotoxicity tests (26). Our
in vitro results revealed that it exhibited no
embryotoxicity.
Radix Isatidis is a valuable Chinese medicine
with antibacterial and antiviral activities as well as an excellent
safety profile (27). However, it
was demonstrated that water boiled juice of Isatic tinctoria
L. caused micronuclei and sperm abnormalities in mice, and it
was deemed a potential mutagen (28). Our results indicated that Radix
Isatidis has no embryotoxicity.
A survey of Chinese herbal medicines used during
pregnancy previously revealed that Coptis chinensis was one
of the five most commonly used Chinese herbal medicines (4). It exhibited acute toxicity at
LD50 under 10 g/kg in mice (29). Berberine, one of the major
constituents of Coptis chinensis, was also reported to
exhibit genotoxicity (30). The
present study suggests that Coptis chinensis has weak
embryotoxic potential.
Since China’s one-child family planning policy was
enacted, Flos Genkwa has been widely used in abortion. No
studies have investigated the embryonic toxicity of Flos
Genkwa, although it is considered a toxic Chinese medicine.
According to our results, Flos Genkwa was strongly
embryotoxic.
In conclusion, the results of the present study
suggest that extracts of Rhizoma Atractylodes macrocephala
and Radix Isatidis are non-embryotoxic, whereas those of
Coptis chinensis and Flos Genkwa are weakly
embryotoxic and strongly embryotoxic, respectively. Our study
potentially offers valuable information that may be used to expand
the application of EST to the field of TCM. Moreover, as ESCs are
pluripotent, herbal remedies with an ability to induce cell
differentiation may be discovered. Such a finding would be of great
significance in the field of TCM.
Acknowledgments
The present study was funded by the Guangdong
Province Natural Science Foundation of China (grant no.
S2011010001898) and Guangdong Province Plan of Scientific and
Technological Development (grant no. 2011B031700009). The authors
would like to thank Professor Ma Zhiguo for expert assistance.
References
|
1
|
Lu F, Cao M, Wu B, et al: Urinary
metabonomics study on toxicity biomarker discovery in rats treated
with Xanthii Fructus. J Ethnopharmacol. 149:311–320. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu F, Gu Q, Wu R and Xu J: A
structure-similarity-based software for the cardiovascular toxicity
prediction of traditional Chinese medicine. Bioinformation.
8:110–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang ZH, Zhao YY, Cheng XL, et al:
General toxicity of Pinellia ternata (Thunb) Berit in rat: a
metabonomic method for profiling of serum metabolic changes. J
Ethnopharmacol. 149:303–310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chuang CH, Hsieh WS, Guo YL, et al:
Chinese herbal medicines used in pregnancy: a population-based
survey in Taiwan. Pharmacoepidemiol Drug Saf. 16:464–468. 2007.
View Article : Google Scholar
|
|
5
|
Nordeng H and Havnen GC: Use of herbal
drugs in pregnancy: a survey among 400 Norwegian women.
Pharmacoepidemiol Drug Saf. 13:371–380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu CL, Zhao YF, Shang XY and Niu WN: The
effects of supplementing diets with Atractylodes macrocephala Koidz
rhizomes on growth performance and immune function in piglets. J
Animal Feed Sci. 21:302–312. 2012.
|
|
7
|
Jin C, Zhang PJ, Bao CQ, et al: Protective
effects of Atractylodes macrocephala polysaccharide on liver
ischemia-reperfusion injury and its possible mechanism in rats. Am
J Chin Med. 39:489–502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng HZ, Dong ZH and Yu J: Modern Study
of Traditional. Chin Med J. 328–334. 1997.
|
|
9
|
Xiao PG and Lian WY: The Illustrated
Medicinal Plants of China. Shanghai Education Press; Shanghai:
1998
|
|
10
|
Zhou BN: Some progress on the chemistry of
natural bioactive terpenoids from Chinese medicinal plants. Mem
Inst Oswaldo Cruz. 86(Suppl 2): 219–226. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee MY, Park BY, Kwon OK, et al:
Anti-inflammatory activity of (−)-aptosimon isolated from Daphne
genkwa in RAW264.7 cells. Int Immunopharmacol. 9:878–885. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao Y, Yuan S, Li A, Zhang B and Wang Z:
Effects of processing on toxicity and pharmacological action of
Flos Genkwa. Zhongguo Zhong Yao Za Zhi. 23:344–347. 382–343.
1998.In Chinese.
|
|
13
|
Hong JY, Chung HJ, Lee HJ, Park HJ and Lee
SK: Growth inhibition of human lung cancer cells via
down-regulation of epidermal growth factor receptor signaling by
yuanhuadine, a daphnane diterpene from Daphne genkwa. J Nat Prod.
74:2102–2108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goel RK, Prabha T, Kumar MM, Dorababu M,
Prakash and Singh G: Teratogenicity of Asparagus racemosus Willd
root, a herbal medicine. Indian J Exp Biol. 44:570–573.
2006.PubMed/NCBI
|
|
15
|
Moallem SA, Ahmadi A, Moshafi M and
Taghavi MM: Teratogenic effects of HESA-A, a natural anticancer
product from Iran, in mice. Hum Exp Toxicol. 30:851–859. 2011.
View Article : Google Scholar
|
|
16
|
Chen CC and Chan WH: Impact effects of
puerarin on mouse embryonic development. Reprod Toxicol.
28:530–535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Jong E, Barenys M, Hermsen SA, et al:
Comparison of the mouse Embryonic Stem cell Test, the rat Whole
Embryo Culture and the Zebrafish Embryotoxicity Test as alternative
methods for developmental toxicity testing of six 1,2,4-triazoles.
Toxicol Appl Pharmacol. 253:103–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spielmann H and Liebsch M: Lessons learned
from validation of in vitro toxicity test: from failure to
acceptance into regulatory practice. Toxicol In Vitro. 15:585–590.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bremer S, Worth AP, Paparella M, et al:
Establishment of an in vitro reporter gene assay for developmental
cardiac toxicity. Toxicology In Vitro. 15:215–223. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Genschow E, Scholz G, Brown N, et al:
Development of prediction models for three in vitro embryotoxicity
tests in an ECVAM validation study. In Vitr Mol Toxicol. 13:51–66.
2000.PubMed/NCBI
|
|
21
|
Whitlow S, Bürgin H and Clemann N: The
embryonic stem cell test for the early selection of pharmaceutical
compounds. ALTEX. 24:3–7. 2007.PubMed/NCBI
|
|
22
|
Paquette JA, Kumpf SW, Streck RD, Thomson
JJ, Chapin RE and Stedman DB: Assessment of the Embryonic Stem Cell
Test and application and use in the pharmaceutical industry. Birth
Defects Res B Dev Reprod Toxicol. 83:104–111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kong D, Xing L, Liu R, et al: Individual
and combined developmental toxicity assessment of bisphenol A and
genistein using the embryonic stem cell test in vitro. Food Chem
Toxicol. 60:497–505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang W, Song DR, Wang YN and Zhu Z:
Evaluation of embryotoxicity of baicalin based on embryonic stem
cell test system. Chin J Pharmacol Toxicol. 26:864–869. 2012.
|
|
25
|
Spielmann H and Liebsch M: Validation
successes: chemicals. Altern Lab Anim. 30(Suppl 2): 33–40.
2002.
|
|
26
|
Zhao AS, Sun YL and Zhang LS: Safety
assessment of Baizhu. Chin J Public Health. 1:43–45. 2006.
|
|
27
|
Wang HY, Ma YF and Zhou WY: On IRPS
toxicity test and effects on immune system. Journal of Mianyang
Normal University. 31:52012.
|
|
28
|
Pang Z, Tang J, Zhu W and Lu Y: Genetic
toxicity of water boiled juice of Isatic Tinctoria L. in mice.
Academic Journal of Guangzhou Medical College. 28:41–42. 2000.
|
|
29
|
Qiu SH, Tang WB, Li FY, Xiao JR and Chen
BY: Experimental study of the acute toxicity on common-used bitter
and cold medicines. Central South Pharmacy. 2:37–38. 2004.
|
|
30
|
Pasqual M, Lauer C, Moyna P and Henriques
JA: Genotoxicity of isoquinoline alkaloid berberine in prokaryotic
and eukaryotic organisms. Mutat Res. 286:243–152. 1993. View Article : Google Scholar : PubMed/NCBI
|