Introduction
Mechanical strain is pivotal in bone remodeling as
physiological dynamic loading promotes bone formation, whereas the
absence of mechanical forces results in bone loss (1,2). In
bone tissue, osteoblasts are important mechanical receptors, which
can transform mechanical stimuli into biochemical signals for bone
matrix formation and mineralization (3). Previous studies have demonstrated
that mechanical forces are crucial regulators of osteoblastic
proliferation, differentiation and apoptosis (4,5).
However, the mechanism underlying the response of osteoblasts to
mechanical strain remains to be fully elucidated, particularly the
role of microRNAs (miRNAs; miRs) in the mecchano-response.
miRNAs are a class of small non-coding RNAs,
typically 18–22 nucleotides in length, which repress gene
expression at the post-transcriptional level by degrading their
target mRNAs or through translational repression (6,7).
miRNAs regulate cell proliferation, differentiation and apoptosis,
and control physiological changes, including growth and development
(6–8). Several miRNAs, which regulate bone
formation or osteoblastic differentiation, have been found
(9,10).
In previous years, certain mechanoresponsive or
mechanosensitive miRNAs have been detected and identified in
endothelial cells, chondrocytes and smooth muscle cells (11–13).
For example, in mechanical strained chondrocytes, miR-365 is
expressed at higher levels compared with unstrained cells, and
regulates chondrocyte differentiation (12). Osteoblasts are a type of
mechanoresponsive cell, therefore, the present study hypothesized
that they contain mechanoresponsive miRNAs.
In the present study, mouse pre-osteoblastic
MC3T3-E1 cells were stimulated with mechanical tensile strain,
which was performed to stimulate osteoblastic differentiation.
Subsequently, miRNA microarray and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analyses were performed to identify the presence of
mechanoresponsive miRNAs.
Materials and methods
Application of mechanical strain to
cultured cells
The MC3T3-E1 cells (provided by the Institute of
Basic Medicine of Peking Union Medical College, Beijing, China), a
mouse pre-osteoblastic cell line, at the third passage, were seeded
into mechanical loading dishes, which were reformed from cell
culture dishes (Nunc International, Roskilde, Denmark) in α-minimal
essential medium (Invitrogen Life Technologies, Carslbad, CA, USA),
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin (Invitrogen Life Technologies).
At confluence, the medium was replaced with FBS-free
medium, and the MC3T3-E1 cells were subjected to mechanical tensile
strain of 2,500 με at 0.5 Hz for different durations (0, 2,
4, 8, 12 and 24 h). The mechanical strain was generated by a
specially designed four-point bending device (Institute of Medical
Equipment, Academy of Military Medical Sciences, Tianjin, China),
as previously described (14).
ALP activity assay
Following the induction of mechanical strain, the
MC3T3-E1 cells were lysed by brief sonication on ice in
radioimmunoprecipitation lysis buffer (Cw Biotech, Beijing, China),
and the protein concentration of the cell lysates were measured
using the Bichinchoninic Acid Protein Assay kit (Cw Biotech). The
activity of ALP in the lysates was measured using a fluorometric
detection kit (Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing,
China) using a p-nitrophenyl phosphate (Sigma-Aldrich, St. Louis,
MO, USA) method, according to manufacturer’s instructions. A single
unit of ALP activity represented 1 μmol p-nitrophenyl
phosphate hydrolyzed to p-nitrophenol/min, therefore the ALP
activity in the proteins was expressed in U/g protein.
RT-qPCR
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen Life Technologies), following which cDNA was
synthesized using a Quant Script RT kit (Tiangen Biotechnology Co.,
Ltd., Beijing, China). qPCR was performed to detect the mRNA levels
of ALP, OCN, Col I and glyceraldehyde3-phosphate dehydrogenase
(GAPDH), as an internal reference, using SYBR Green I PCR mix
(Cowin Biotech Co, Ltd., Beijing, China) on a Real-Time PCR system
(7900; Applied Biosystems Life Technologies, Foster City, CA, USA),
according to the manufacturer’s instructions. The sequences of the
primers are listed in Table I. The
amplification reaction included a denaturation step at 94°C for 180
sec, followed by 40 cycles of 94°C for 15 sec, and annealing and
extension at each annealing temperature for 30 sec at 60°C. The
relative quantitative 2−ΔΔCt method (15) was used to determine the mRNA levels
of the PCR products relative to the control group.
 | Table IPrimers used for qPCR analysis of
mRNA. |
Table I
Primers used for qPCR analysis of
mRNA.
| Gene | Primer sequence
(5′-3′) | Product size
(bp) | Annealing temperature
(°C) |
|---|
| ALP | F:
CGGGACTGGTACTCGGATAA
R: ATTCCACGTCGGTTCTGTTC | 156 | 58 |
| OCN | F:
AGTCTGACAAAGCCTTCA | 134 | 56 |
| R:
AAGCAGGGTTAAGCTCACA | | |
| Col I | F:
GGTATGCTTGATCTGTATCTG
R: TCTTCTGAGTTTGGTGATACG | 130 | 58 |
| GAPDH | F:
ACCCATCACCATCTTCCAGGAG
R: GAAGGGGCGGAGATGATGAC | 159 | 58 |
Enzyme-linked immunosorbent assay (ELISA)
of the protein levels of bone morphogenetic proteins (BMPs)
Following mechanical strain, the cell culture medium
was collected, and the protein levels of BMP-2 and BMP-4 in the
culture medium were detected using an ELISA kit (Wuhan Boster
Bioengineering Co., Ltd., Wuhan China), according to the
manufacturer’s instructions. The absorbance was measured at 450 nm
on a Multiskan FC ELISA reader (Thermo Fisher Scientific, Rockford,
IL, USA), with the results presented as the percentage of activity
change, compared with the unstrained control.
Microarray and RT-qPCR validation of
miRNA
The Agilent Mouse miRNA microarray (Agilent
Technologies, Santa Clara, CA, USA) was used to detect the miRNA
expression levels in the MC3T3-E1 cells. The miRNA expression
profiles of the mechanically strained cells were compared with the
unstrained cells.
In brief, the total RNA extraction and miRNA
enrichment procedures were performed using an mirVana™ miRNA
Isolation kit (Ambion Life Technologies, Carlsbad, CA, USA),
according to the manufacturer’s instructions. Target labeling,
hybridization, imaging and data processing were performed,
according to the manufacturer’s instructions, at CapitalBio
Corporation (Beijing, China) using Agilent Mouse miRNA (Agilent
Technologies), 8×60 K and Sanger miRBase V18.0 software (http://www.mirbase.org/). Data were acquired using
Agilent Feature Extraction software version 10.7 (Agilent
Technologies). Further data analyses were performed using
GeneSpring GX 10.0 software (Agilent Technologies).
The expression levels of miRNA were confirmed using
RT-qPCR at CapitalBio Corporation. The primers for RT-qPCR were
synthesized by Invitrogen Life Technologies, and the sequences are
shown in Table II. Following cDNA
synthesis using Megaplex™ RNA RT mix, qPCR was performed using
Power SYBR & Green PCR Master mix (ABI 4367659; Thermo Fisher
Scientific). The reactions were incubated in a 96-well optical
plate at 95°C for 10 min, followed by 40 cycles of 15 sec at 95°C,
1 min at 60°C (annealing and extension). Expression analysis was
performed in triplicate for each sample. Mus musculus
(mmu)-Actin was used as the normalization control. The miRNA
expression levels were quantified using an ABI Prism 7300 Sequence
Detection system (Applied Biosystems Life Technologies).
 | Table IIForward and reverse primers used for
reverse transcription-quantitative polymerase chain reaction of
microRNA. |
Table II
Forward and reverse primers used for
reverse transcription-quantitative polymerase chain reaction of
microRNA.
| MicroRNA | Oligonucleotide
sequence (5′-3′) |
|---|
| mmu-let-7e* | F:
ATCCTATACGGCCTCCTAGCTT
R: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGGAAAG |
| mmu-miR-191 | F:
CAACGGAATCCCAAAAGCAG
R: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCAGCTG |
| mmu-miR-32 | F:
TGCCGTATTGCACATTACTAAGTT
R: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGCAAC |
| mmu-miR-218 | F:
AGCCTTGTGCTTGATCTAACCA
R: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACATGG |
| mmu-miR-210 | F:
CTGTGCGTGTGACAGCGG
R: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAGCC |
| mmu-miR-33 | F:
TGCGTGCATTGTAGTTGCATT
R: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGCAAT |
| mmu-miR-3070a | F:
TCGTAGTGCTACCGTCAGGGG
R: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCTACC |
| UPL_U6 | F:
TTCCTCCGCAAGGATGACACGC
R: GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACAAAAATAT |
| Universal reverse
primer |
GTGCAGGGTCCGAGGT |
Prediction of miRNA target genes
The TargetScan (http://wwwargetscan.org) and PicTar (http://www.pictar.org) software programs were used to
predict the miRNA target genes. The target genes associated with
osteoblastic differentiation or the response of the cell to
mechanical strain, were selected.
Statistical analysis
To determine miRNAs, which were differentially
expressed among the groups, Student’s t-test was performed using
SPSS version 12.0 (SPSS, Inc., Chicago, IL, USA). The experiments
were repeated in triplicate. Statistical significance between the
groups was measured using Student’s t-test; P<0.05 was
considered to indicate a statistically significant difference.
Results
Mechanical strain promotes osteoblastic
differentiation
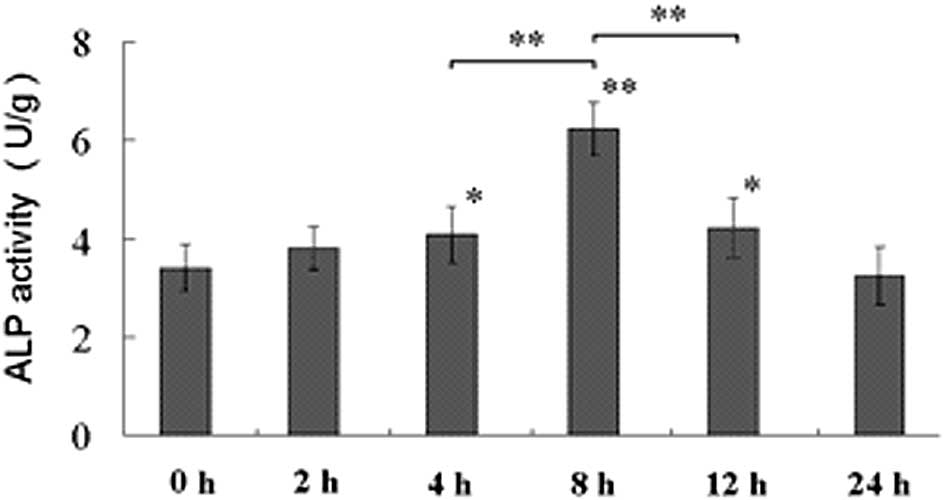
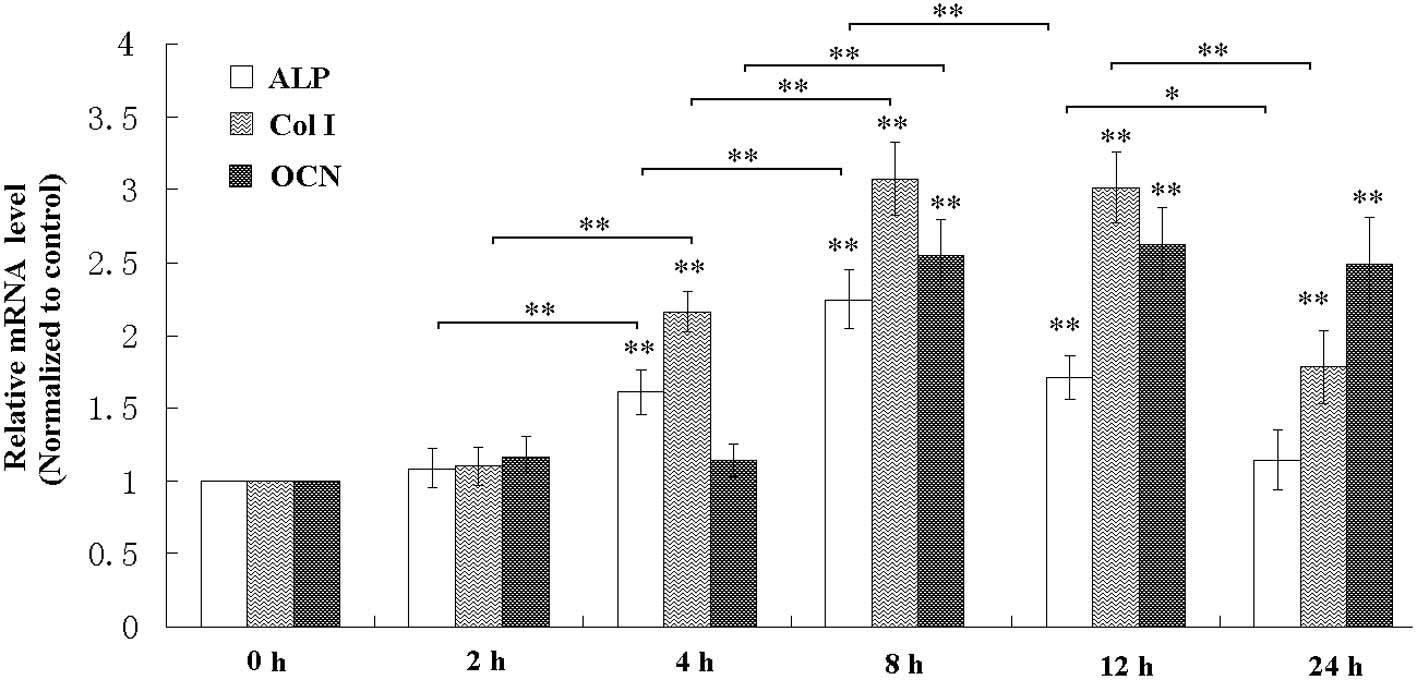
Following exposure of the MC3T3-E1 osteoblastic
cells to a mechanical tensile strain of 2,500 με at 0.5 Hz,
the activity and mRNA level of ALP were elevated (Figs. 1 and 2), the mRNA expression levels of Col I
and OCN were also increased (Fig.
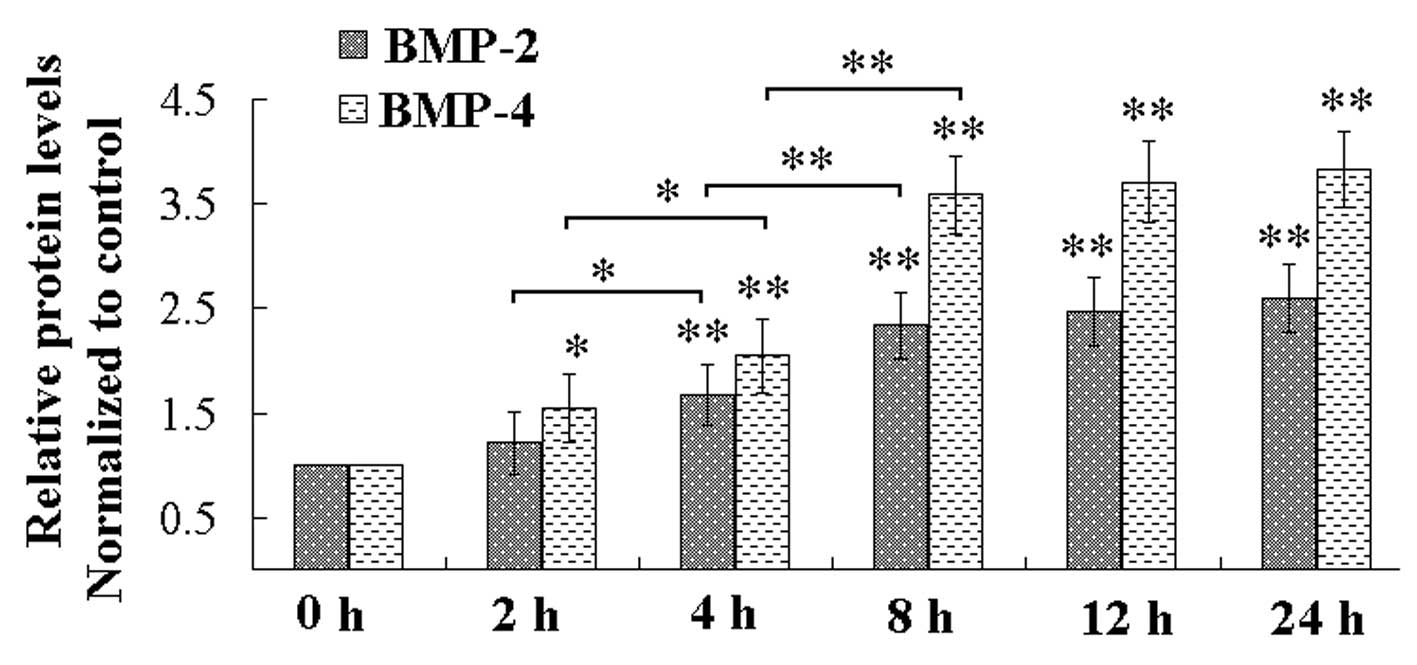
2). In addition, the ELISA indicated that the mechanical strain
increased the levels of BMP-2 and BMP-4 in the culture medium
(Fig. 3). ALP, Col I, OCN, and
BMP-2/4 are all markers of osteoblastic differentiation (16–18),
therefore, the mechanical strain promoted osteoblastic
differentiation of the MC3T3-E1 cells. These results demonstrated
that mechanical strain for a duration of 8 h had the most marked
effect on the enhancement of osteoblastic differentiation (Figs. 1Figure 23).
Identification of four miRNAs responsive
to mechanical strain applied to MC3T3-E1 cells
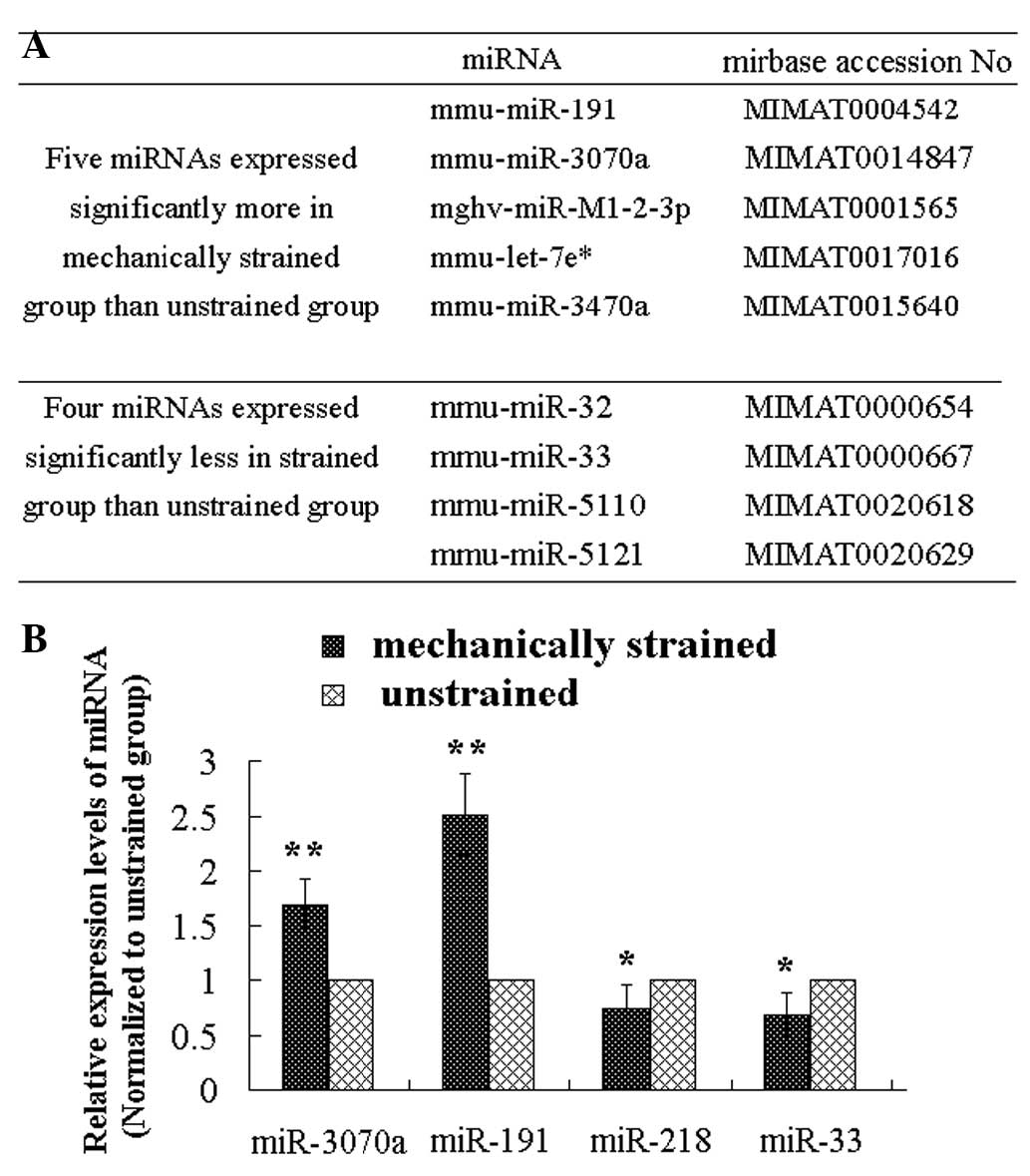
The microarray images are shown in Fig. 4. The results of the miRNA
micro-array indicated that the expression levels of five miRNAs
(mmu-miR-191*, mmu-miR-3070a, mmu-miR-M1-2-3p,
mmu-miR-let-7e*, mmu-miR-3470a and mmu-miR-) were higher
in the mechanically strained group, compared with the unstrained
control group, and the expression levels of four miRNAs
(mmu-miR-32, mmu-miR-33, mmu-miR-5110 and mmu-miR-5121) were lower
in the mechanically strained group, compared with the unstrained
control group (Fig. 5A). The
results of the RT-qPCR confirmed that the expression levels of
miR-218, miR-191*, miR-3070a and miR-33 differed between
the strained group and the unstrained group (Fig. 5B). Therefore, these four miRNAs
were considered to be responsive to the mechanical strain, which
was applied to the MC3T3-E1 cells.
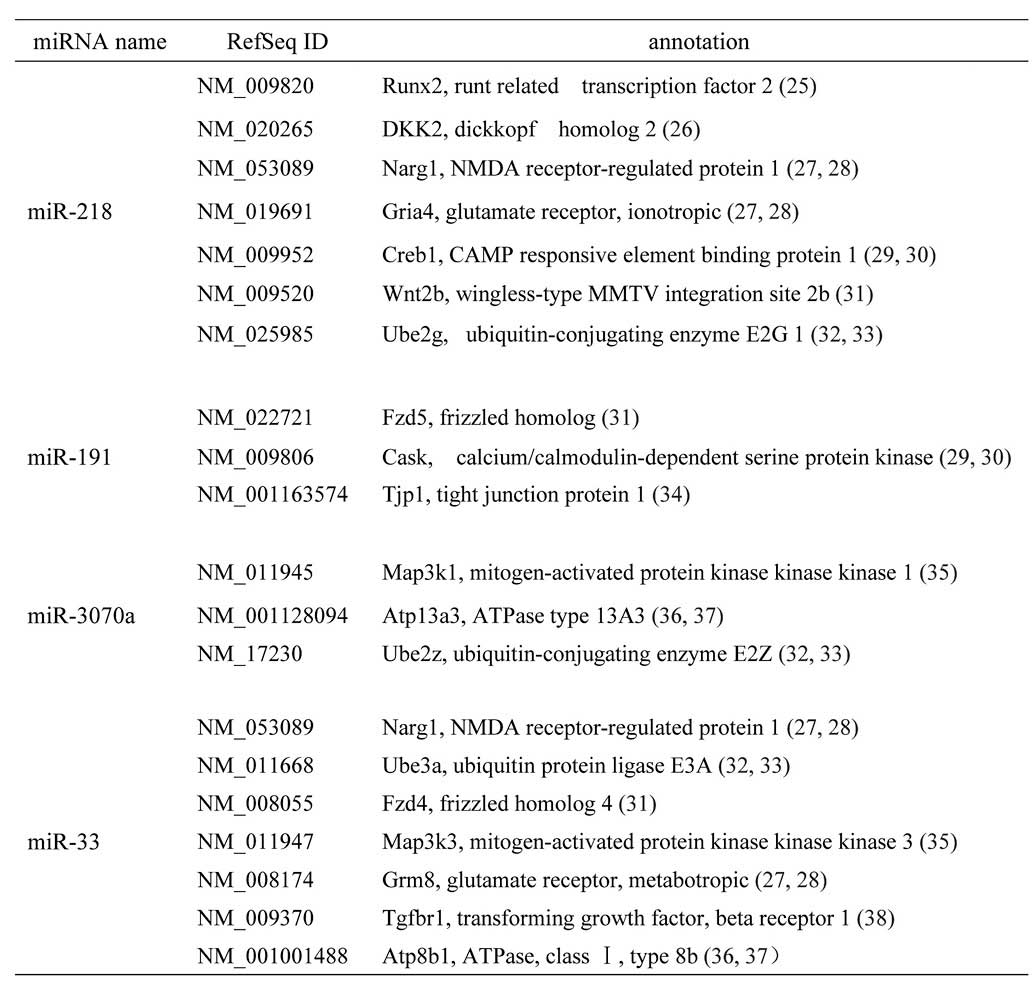
Target genes of miR-218,
miR-191*, miR-3070a and miR-33 may be involved in
osteoblast differentiation
Using the TargetScan and PicTar databases, the
predicted target genes of the differentially expressed,
mechanoresponsive, miRNAs, were determined. The target genes, which
were identified aso being involved in osteoblast differentiation
are shown in Fig. 6.
Discussion
Bones and the skeleton are responsive to dynamic
mechanical loading, in which a suitable dynamic mechanical loading
promotes bone formation and removal of mechanical loading reduces
bone mass (19–21). The ability of bone tissues to
respond to mechanical strain depends on the bone cells (22). Osteoblasts are located on the
surface of bone and are bone-forming cells, which can be stimulated
by dynamic mechanical strain in vivo.
Several studies have indicated that osteoblasts are
responsive to mechanical strain (4,5,23,24).
In the present study, the activity of ALP and the mRNA levels of
ALP, Col I, OCN and BMP-2/4 in the cell culture were all increased,
which confirmed that osteoblasts are also sensitive to mechanical
strain in vitro. Additionally, the results of the present
study indicated that mechanical strain for a duration of 8 h had
the most marked effect on the enhancement of osteoblastic
differentiation and was, therefore, selected for the following
experiments.
Differentially expressed miRNAs in mechanical
stimulated tissues or cells are regarded as mechanoresponsive, or
mechanosensitive, miRNAs. In endothelial cells, chondrocytes and
smooth muscle cells, the mechanoresponsive miRNAs have been
identified, and are reported to be involved in cell differentiation
(11–13). However, the mechanoresponsive
miRNAs of osteoblasts remain to be fully elucidated.
In the present study, miRNA microarray and RT-qPCR
analyses were performed, and the four mechanoresponsive miRNAs,
miR-218, miR-191*, miR-3070a and miR-33 were identified. Using
bioinformatics analysis, the target genes of the miRNAs were
predicted and, of all the putative target genes, 19 genes were
involved in osteoblast differentiation. As mechanical strain was
observed to promote osteoblastic differentiation, these
mechanoresponsive miRNAs may be regulators of osteoblastic
differentiation. These target genes require further verification,
and the mechanism underlying the involvement of mechanoresponsive
miRNAs in the mechanical response of osteoblasts requires further
investigation.
In conclusion, the present study demonstrated that a
mechanical strain of 2,500 με, particularly for a period of
8 h, promoted osteoblastic differentiation, and four miRNAs were
identified as mechanoresponsive, which are potential regulators of
osteoblastic differentiation and the response of osteoblasts to
mechanical strain.
Acknowledgments
This study was supported by grants from the National
Nature Science Foundation of China (nos. 11372351 and 31370942),
and was supported by funding from Shandong Provincial Key
Laboratory of Functional Macromolecular Biophysics (no. 11172062).
The authors would like to thank their colleagues at the Institute
of Medical Equipment (Tianjin, China), for their support.
References
|
1
|
Schriefer JL, Warden SJ, Saxon LK, et al:
Cellular accommodation and the response of bone to mechanical
loading. J Biomech. 38:1838–1845. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thompson WR, Rubin CT and Rubin J:
Mechanical regulation of signaling pathways in bone. Gene.
503:179–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wozniak M, Fausto A, Carron CP, Meyer DM
and Hruska KA: Mechanically strained cells of the osteoblast
lineageorganize their extracellular matrix through unique sites of
alphavbeta3-integrin expression. J Bone Miner Res. 15:1731–1745.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaneuji T, Nogami S, Ariyoshi W, et al:
Regulatory effect on osteoclastogenesis of mechanical strain-loaded
osteoblasts. Int J Oral Maxillofac Surg. 40:12152011. View Article : Google Scholar
|
|
5
|
Rumney RM, Sunters A, Reilly GC and
Gartland A: Application of multiple forms of mechanical loading to
human osteoblasts reveals increased ATP release in response to
fluid flow in 3D cultures and differential regulation of immediate
early genes. J Biomech. 45:549–554. 2012. View Article : Google Scholar :
|
|
6
|
Zhao Y and Srivastava D: A developmental
view of microRNA function. Trends Biochem Sci. 32:189–197. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stefani G and Slack FJ: Small non-coding
RNAs in animal development. Nat Rev Mol Cell Biol. 9:219–230. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Taipaleenmäki H, Bjerre Hokland L, Chen L,
Kauppinen S and Kassem M: Mechanisms in endocrinology: micro-RNAs:
targets for enhancing osteoblast differentiation and bone
formation. Eur J Endocrinol. 166:359–371. 2012. View Article : Google Scholar
|
|
10
|
Vimalraj S and Selvamurugan N: MicroRNAs:
synthesis, gene regulation and osteoblast differentiation. Curr
Issues Mol Biol. 15:7–18. 2012.PubMed/NCBI
|
|
11
|
Ni CW, Qiu H and Jo H: MicroRNA-663
upregulated by oscillatory shear stress plays a role in
inflammatory response of endothelial cells. Am J Physiol Heart Circ
Physiol. 300:H1762–H1769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guan YJ, Yang X, Wei L and Chen Q:
MiR-365: a mechanosen-sitive microRNA stimulates chondrocyte
differentiation through targeting histone deacetylase 4. FASEB J.
25:4457–4466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song JT, Hu B, Qu HY, et al: Mechanical
stretch modulates microRNA-21 expression, participating in
proliferation and apoptosis in cultured human aortic smooth muscle
cells. PLoS One. 7:e476572012. View Article : Google Scholar
|
|
14
|
Tang LL, Wang YL, Pan J and Cai SX: The
effect of step-wise increased stretching on rat calvarial
osteoblast collagen production. J Biomech. 37:157–161. 2004.
View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Jang WG, Kim EJ and Koh JT: Tunicamycin
negatively regulates BMP2-induced osteoblast differentiation
through CREBH expression in MC3T3E1 cells. BMB Rep. 44:735–740.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wan M and Cao X: BMP signaling in skeletal
development. Biochen Biophys Res Commun. 328:651–657. 2005.
View Article : Google Scholar
|
|
18
|
Guo Y, Zhang CQ, Zeng QC, et al:
Mechanical strain promotes osteoblast ECM formation and improves
its osteoinductive potential. Biomed Eng Online. 11:802012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rubin CT and Lanyon LE: Regulation of bone
formation by applied dynamic loads. J Bone Joint Surg Am.
66:397–402. 1984.PubMed/NCBI
|
|
20
|
Lanyon LE and Rubin CT: Static versus
dynamic loads as an influence on bone remodelling. J Biomech.
17:897–905. 1984. View Article : Google Scholar
|
|
21
|
Hillam RA and Skerry TM: Inhibition of
bone resorption and stimulation offormulation by mechanical loading
of the modeling rat ulna in vivo. J Bone Miner Res. 10:683–689.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Turner CH and Pavalko FM:
Mechanotransduction and functional responseof the skeleton to
physical stress: the mechanisms and mechanics of bone adaptation. J
Orthop Sci. 3:346–355. 1998. View Article : Google Scholar
|
|
23
|
Wozniak M, Fausto A, Carron CP, Meyer DM
and Hruska KA: Mechanically strained cells of the osteoblast
lineage organize their extracellular matrix through unique sites of
alphavbeta3-integrin expression. J Bone Miner Res. 15:1731–1745.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bhatt KA, Chang EI, Warren SM, et al:
Uniaxial mechanical strain: an in vitro correlate to distraction
osteogenesis. J Surg Res. 143:329–336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ducy P, Zhang R, Geoffroy V, Ridall AL and
Karsenty G: Osf2/Cbfa1: a transcriptional activator of osteoblast
differentiation. Cell. 89:747–754. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olivares-Navarrete R, Hyzy S, Wieland M,
Boyan BD and Schwartz Z: The roles of Wnt signaling modulators
Dickkopf-1 (Dkk1) and Dickkopf-2 (Dkk2) and cell maturation state
in osteogenesis on microstructured titanium surfaces. Biomaterials.
31:2015–2024. 2010. View Article : Google Scholar
|
|
27
|
Lin TH, Yang RS, Tang CH, Wu MY and Fu WM:
Regulation of the maturation of osteoblasts and osteoclastogenesis
by glutamate. Eur J Pharmacol. 589:37–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Szczesniak AM, Gilbert RW, Mukhida M and
Anderson GI: Mechanical loading modulates glutamate receptor
subunit expression in bone. Bone. 37:63–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Danciu TE, Adam RM, Naruse K, Freeman MR
and Hauschka PV: Calcium regulates the PI3K-Akt pathway in
stretched osteoblasts. FEBS Lett. 536:193–197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Futatsugi A, Nakamura T, Yamada MK, et al:
IP3 receptor types 2 and 3 mediate exocrine secretion underlying
energy metabolism. Science. 309:2232–2234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin GL and Hankenson KD: Integration of
BMP, Wnt and notch signaling pathways in osteoblast
differentiation. J Cell Biochem. 112:3491–501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ito Y, Inoue D, Kido S and Matsumoto T:
c-Fos degradation by the ubiquitin-proteasome proteolytic pathway
in osteoclast progenitors. Bone. 37:842–849. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xing L, Zhang M and Chen D: Smurf control
in bone cells. J Cell Biochem. 110:554–563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hatakeyama N, Kojima T, Iba K, et al:
IGF-I regulates tight-junction protein claudin-1 during
differentiation of osteoblast-like MC3T3-E1 cells via a MAP-kinase
pathway. Cell Tissue Res. 334:243–254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Greenblatt MB, Shim JH, Zou W, et al: The
p38 MAPK pathway is essential for skeletogenesis and bone
homeostasis in mice. J Clin Invest. 120:2457–2473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakano Y, Forsprecher J and Kaartinen MT:
Regulation of ATPase activity of transglutaminase 2 by MT1-MMP:
implications for mineralization of MC3T3-E1 osteoblast cultures. J
Cell Physiol. 223:260–269. 2010.PubMed/NCBI
|
|
37
|
Sun D, Junger WG, Yuan C, et al:
Shockwaves induce osteogenic differentiation of human mesenchymal
stem cells through ATP release and activation of P2×7 receptors.
Stem Cells. 31:1170–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen G, Deng C and Li YP: TGF-β and BMP
signaling in osteoblast differentiation and bone formation. Int J
Biol Sci. 8:272–288. 2012. View Article : Google Scholar
|