1. Introduction
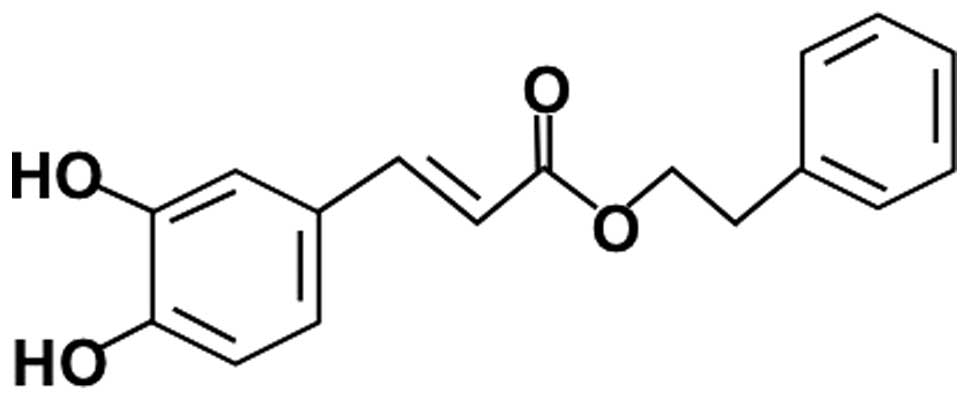
Caffeic acid phenethyl ester (CAPE; Fig. 1), a well known component of the
natural honeybee product, propolis, has been used for centuries in
medicine, due to its anti-inflammatory, antioxidant and
antineoplastic properties (1–4).
CAPE is a naturally occurring phenolic compound, and is an ester
derived from caffeic acid and phenethyl alcohol. It downregulates a
number of pro-inflammatory cytokines and inflammatory mediators, by
inhibiting the transcription of nuclear factor
κ-light-chain-enhancer of activated B cells (NF-κB) (5). CAPE exhibits antimitogenic,
anticarcinogenic, anti-inflammatory and immunomodulatory properties
in vitro (6). There are a
number of articles that have reviewed the positive effects of CAPE
in models of neoplasm (7),
prostate, lung and melanoma cancers (8), chemotherapy and radiotherapy-induced
toxicity (9), and in heart disease
(10). However, to the best of our
knowledge, there has been no review discussing the protective role
of CAPE on diseases of the ear, such as ototoxicity.
Reactive oxygen species (ROS) are associated with
ototoxicity and presbycusis (11).
Not all cell types found in the cochlea share the same
vulnerability to ROS injury. Outer hair cells on the base of the
cochlea are susceptible to ROS, while supporting cells show
significantly greater survival capacity compared with hair cells,
following exposure to ROS (12).
Glutathione levels were found to be higher in apical outer hair
cells, compared with outer hairs at the base (12). The literature demonstrates that
oxidative stress, regardless of its origin, is associated with
ototoxicity (13).
Since a number of oxidative pathways may affect the
structures in the ear (Fig. 2),
CAPE may be considered as a promising agent with which to prevent
ROS-induced ototoxicity caused by certain medicines,
myringosclerosis, otitis media and inflammation of the ear.
Therefore, the present review aims to summarize and critically
evaluate the evidence for the protective role of CAPE in
ototoxicity.
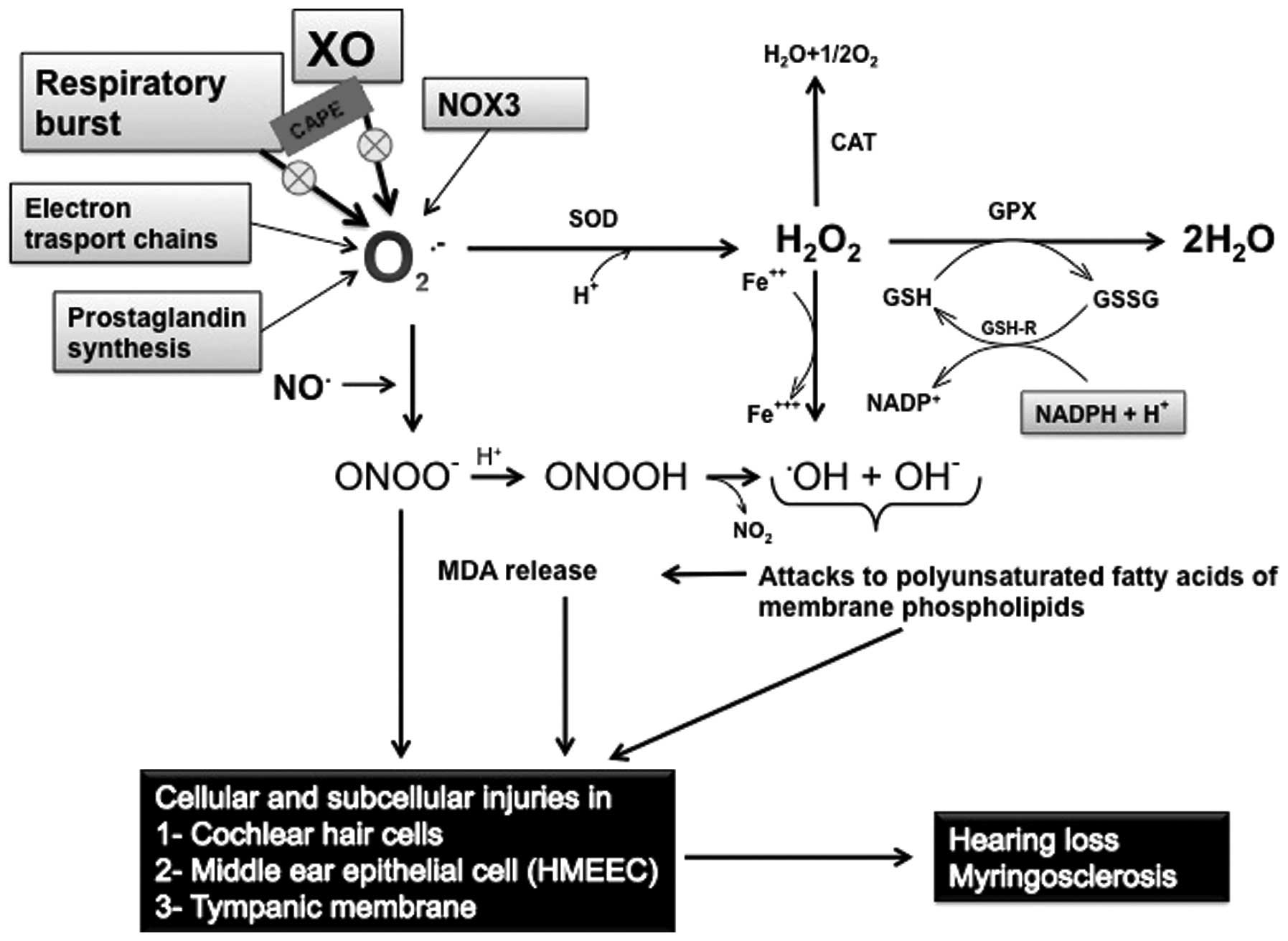 | Figure 2Proposed mechanism for the induction
of oxidative stress, and its effects on hearing pathways and cells,
as well as the use of CAPE for mitigating the damaging effects of
ROS. CAT, catalase; Fe++, ferrous iron; GPx, glutathione
peroxidase; GSH, reduced glutathione; GSH-Red, glutathione
reductase; GSSG, oxidized glutathione; H+, hydrogen ion
proton; H2O, water; H2O2, hydrogen
peroxide; MDA, malondialdehyde; NADP+, oxidized
nicotinamide adenine dinucleotide phosphate; NADPH+H+,
reduced nicotinamide adenine dinucleotide phosphate;
NO•, nitric oxide radical; NO2, nitrite;
NOX3, NADPH oxidase 3; O2, molecular oxygen;
O2−, superoxide anion radical;
OH−, hydroxyl ion; •OH, hydroxyl radical;
ONOO−, peroxynitrite; PUFA, polyunsaturated fatty acid;
SOD, superoxide dismutase; tNOS, total nitric oxide synthases; XO,
xanthine oxidase; ROS, reactive oxygen species; CAPE, caffeic acid
phenethyl ester. |
2. Use of CAPE in antibiotic-induced
ototoxicity
Aminoglycoside antibiotics, which are polycationic
compounds, are widely utilized in clinical practice. However, they
induce adverse ototoxic effects in 2–5% of patients (14). The common cochleotoxic or
vestibulotoxic side effects thus limit the use of aminoglycoside
antibiotics. A number of the oxidation products of polyunsaturated
fatty acids (PUFA) in phospholipids act as mediators of apoptosis.
ROS activate apoptotic or necrotic intracellular pathways,
including the c-Jun N-terminal kinase (JNK) pathway (15). The inhibition of the JNK pathway
has been demonstrated to help hair cells injured by aminoglycosides
(15). Due to the fact that the
trigger mechanism starts with ROS activation, CAPE may be a
promising agent to block this cascade at two different points
(Fig. 3). A number of studies have
investigated underlying mechanisms of action of numerous
antibiotics and cytotoxic medications, which result in ototoxicity
(16–18). Once inside the negatively charged
apex of the hair cell, aminoglycosides induce the generation of
ROS, which are involved in molecular pathways leading to
ototoxicity (19). Bakir et
al (20) conducted a study in
order to assess the antioxidant properties of CAPE in the
prevention or attenuation of ototoxicity caused by long-term use of
aminoglycosides in a rat model. Specifically, the authors
investigated the toxic effects of intramuscular injections of
streptomycin (20 mg/kg/day for 45 days). On day 45, a marked
decrease in cochlear activity was observed in the streptomycin
group compared with that in the control group, according to
distortion product otoacoustic emissions (DPOAEs). However, no
significant differences were observed between the control and CAPE
groups. The distortion product-gram, DP-gram (a measurement for
DPOAEs) of the streptomycin group had significantly deteriorated
compared with the control and streptomycin plus CAPE-treated rats.
The number of cochlear hair cells was shown to be reduced in rats
treated with streptomycin. Immunohistochemical examination revealed
caspase-3 immunoreactivity in rats treated with streptomycin, while
this was not observed in streptomycin plus CAPE-treated rats, which
indicates a protective effect of CAPE on hair cells, supporting
cells, and the basilar membrane. CAPE, when applied alone,
exhibited no obvious harmful effects on hair cells.
Histopathological examination with hematoxylin and eosin, and
immunohistochemistry, in addition to hearing test measurements, did
not reveal deterioration in the cochlear hair cells of rats in the
CAPE-treated group. These results demonstrate that CAPE exhibits
protective effects against ROS generated by oxidative pathways in
the ear (Fig. 2).
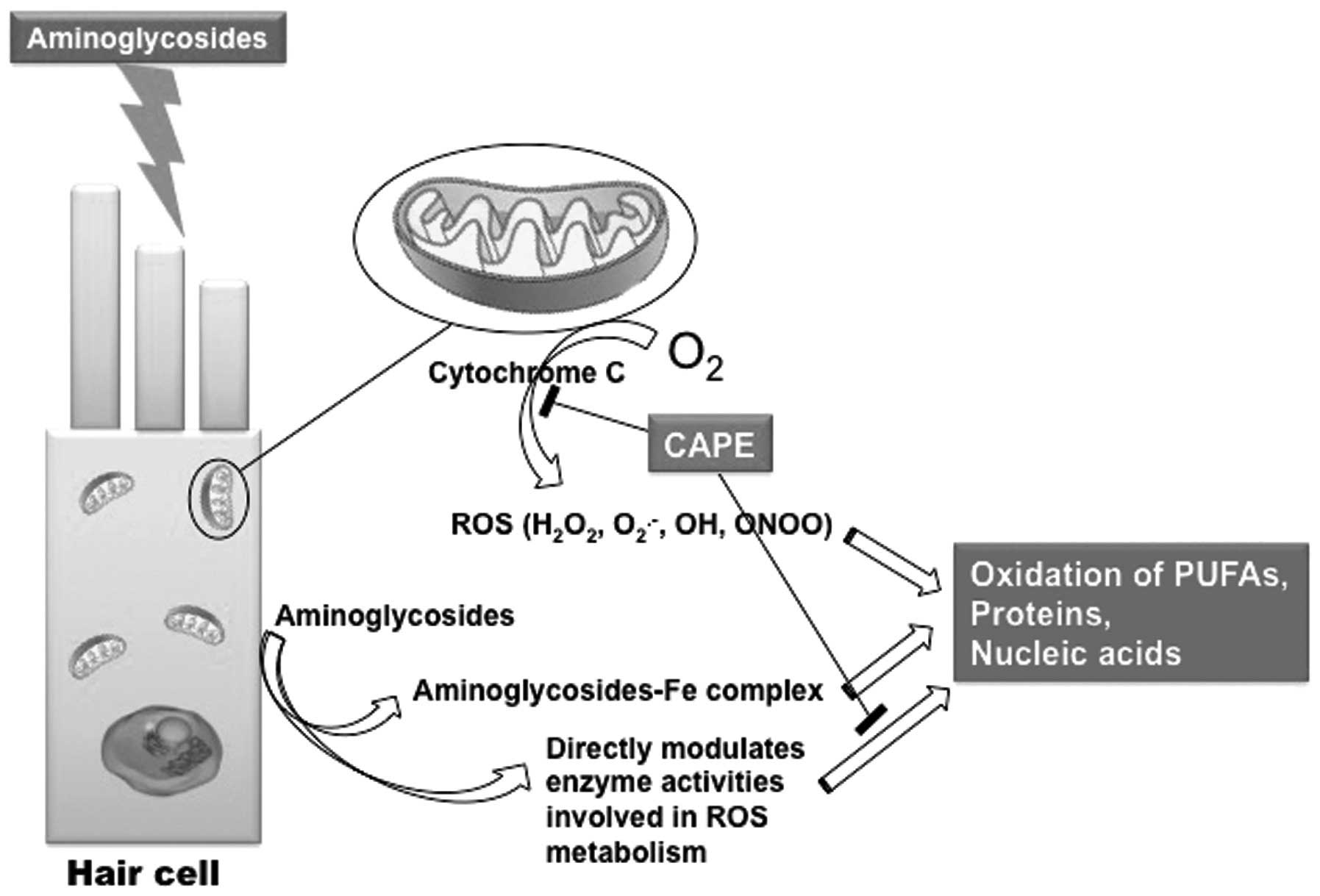 | Figure 3Schematic representation of the
ototoxic effects of aminoglycosides. A number of the oxidation
products of PUFA in phospholipids, which are located particularly
in membranous structures, act as mediators of apoptosis.
H2O2, hydrogen peroxide; O2,
molecular oxygen; O2−, superoxide radical;
OH, hydroxyl radical; ONOO, peroxynitrite; ROS, reactive oxygen
species; PUFA, polyunsaturated fatty acids; ROS, reactive oxygen
species; CAPE, caffeic acid phenethyl ester; JNK, c-Jun N-terminal
kinase. |
More recently, Sakallioglu et al (21) examined the effects of exogenous
glucocorticoid exposure during the prenatal effect on hearing, in
addition to the protection of this inner ear injury by CAPE.
Dexamethasone, which is capable of causing an excessive production
of ROS, was administered to pregnant Sprague-Dawley rats (one group
received only dexamethasone, and the other received dexamethasone
plus CAPE) prior to exposure to 110 dB of noise for 4 h. Exogenous
dexamethasone administration did not alter hearing thresholds prior
to the noise exposure. Following noise exposure, dexamethasone
treatment elevated the hearing thresholds. Therefore, prenatal
exposure to dexamethasone may cause inner ear susceptibility to
noise. In this study, CAPE treatment did not prevent the damage to
hearing caused by dexamethasone. This finding supports the
hypothesis that the protective effects of CAPE are due to its
antioxidant and anti-inflammatory properties. In conclusion, CAPE
does not appear protect the inner ear against dexamethasone-induced
oxidative stress.
3. Use of CAPE in otitis media
Otitis media (OM) is the leading cause of visits to
physicians and consequent antibiotic prescriptions, and is a common
cause of hearing impairment in children (22). Pro-inflammatory mediators have been
reported to be responsible for the inflammation observed in OM.
Several cytokines, including fibroblast growth factor, tumor
necrosis factor α (TNF-α), platelet activating factor, interleukin
(IL) 2, 6, 8 and 1β, and interferon γ, have been isolated in middle
ear effusions, and are responsible for increases in vascular
permeability, chemotaxis, secretion, and consequently, middle ear
inflammation (Fig. 4) (23). Additionally, lipopolysaccharide
(LPS) is a pro-inflammatory mediator that is associated with middle
ear inflammation. Furthermore, a number of other ROS products may
directly damage the epithelium of the middle ear. For example,
nitric oxide (NO) has been reported to contribute to
vasodilatation, increased vascular permeability and mucoid
effusions (24). Furthermore, NO
produced by activated inflammatory cells, regulates cells and
induces inflammation. Therefore, NO may be a secondary inflammatory
mediator, produced by the middle ear epithelium in response to
primary cytokines, such as IL-1β, TNF-α (25).
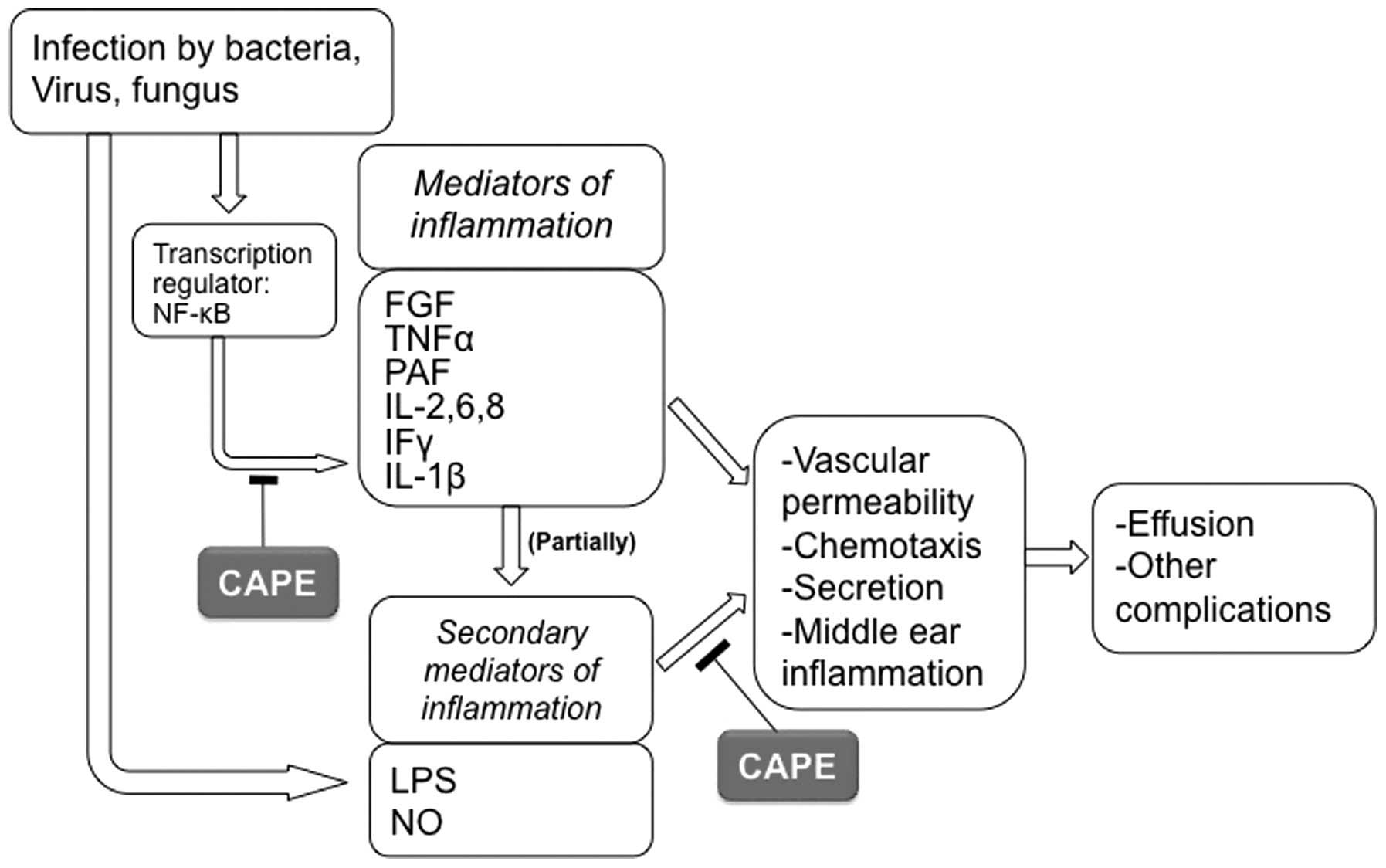 | Figure 4Proposed mechanism by which CAPE
diminishes or blocks otitis media via different pathways. The
transcription regulator, NF-κB has been shown to be involved in
regulating the expression of large numbers of genes, such as
cytokines, chemokines and other mediators involved in inflammatory
responses. CAPE is a potent and specific inhibitor of NF-κB. FGF,
fibroblast growth factor; PAF, platelet activating factor; LPS,
lipopolysaccharide; NO, nitric oxide; CAPE, caffeic acid phenethyl
ester; IL, interleukin; NF-κB, nuclear factor-κB; TNF-α, tumor
necrosis factor α. |
Previously, in vivo and in vitro
studies have shown that cytokines increase inducible nitric oxide
synthase (iNOS) production in middle ear epithelial cells (26). Intracellular NO production
inhibition prevents the hypersecretion of mucin that is stimulated
by ROS and other inflammatory mediators (27). A number of studies have suggested
that CAPE is involved in the suppression of LPS-induced
inflammatory responses in the HMEEC human middle ear epithelial
cell line, via the inhibition pro-inflammatory mediators (Fig. 4) (28). Thus, the effects of CAPE on TNF-α,
may underlie the LPS-stimulation of HMEECs. In these studies, the
bacterial endotoxin, LPS (10 µg/ml), which is isolated from
Pseudomonas aeruginosa, was used in order to induce an
inflammatory response in HMEEC cells. CAPE was administered at
doses of 0, 10, 50, 100 and 200 µM for 1 h. LPS treatment
led to an increase in TNF-α gene expression in HMEEC cells. CAPE
treatment suppressed LPS-induced TNF-α expression, IL-8 production
and NF-κB activity in HMEEC cells, according to the results of
quantitative polymerase chain reaction (qPCR), enzyme-linked
immunosorbent assay (ELISA) and western blot analyses.
Previous reports have demonstrated that CAPE is an
inhibitor of NF-κB, which is a regulatory molecule that modifies
the expression of genes associated with cell proliferation,
inflammatory responses and cell adhesion. Downregulation of several
pro-inflammatory cytokines is caused by the inhibition of NF-κB
(29). Ordinarily, NF-κB is
inactive in the cytoplasm, as it is bound to the IκB-α protein,
which prevents NF-κB activity. When inflammation occurs, IκB-α is
phosphorylated and degraded, which results in the translocation of
NF-κB into the nucleus, where it activates the expression of a
number of genes associated with inflammatory responses. Therefore,
the inhibition of IκB-α phosphorylation may prevent NF-κB
translocation (30). LPS treatment
induces the degradation of IκB-α. CAPE treatment results in the
inhibition of LPS-induced IκB-α degradation in a dose-dependent
manner, which prevents inflammation. Overall, the literature
provides an insight into the molecular pathways underlying the
anti-inflammatory effects of CAPE in association with OM and other
inflammatory conditions.
4. Use of CAPE in hydrogen peroxide
(H2O2)-induced oxidative stress in HMEEC
cells
Recently, Song et al examined the inhibitory
effects of CAPE on H2O2-induced oxidative
stress in HMEEC cells (31).
H2O2 was added at different concentrations
for up to 6 h. The H2O2-induced inflammatory
response was subsequently analyzed by quantifying TNF-α and COX-2
mRNA expression, using reverse transcription-quantitative PCR. CAPE
was applied at different concentrations for 30 and 60 min,
following which, ROS production, including superoxide dismutase
(SOD) expression, was determined. H2O2 caused
an increase in SOD protein expression. However, treatment of the
cells with CAPE inhibited the expression of the SOD protein. The
authors observed that CAPE treatment decreased
H2O2-induced ROS production in HMEEC cells.
Additionally, CAPE treatment inhibited COX-2 protein and TNF-α mRNA
expression, which were upregulated following treatment with
H2O2 alone. Therefore, CAPE may inhibit
H2O2-induced oxidative injury and reduce the
expression of inflammatory mediators, which are involved in the
pathophysiology of OM.
5. Effect of CAPE in myringosclerosis
(MS)
MS is described as hyalinization and calcification
in the collagen layer of the tympanic membrane. Histopathological
examination indicates an increase in collagen fibers, as well as in
extracellular calcium deposition and hyaline degeneration in the
lamina propria (32). Although the
exact mechanisms underlying the development of MS are unknown, ROS
and related oxidative stress are among the possible causes of this
condition (33). Following
placement of tubes into the tympanic membranes, hyperoxia occurs,
which results in the excessive production of ROS (34). The preventive effects of CAPE on
the development of MS in tympanic membranes of myringotomized rats
have been investigated, using otomicroscopy and histopathology
(35). Following myringotomy, rats
were treated with CAPE for two weeks. Otomicroscopic evaluation was
performed and extensive myringosclerotic plaques were observed.
However, the plaques observed in myringotomized rats treated with
CAPE were less extensive, compared with those treated with saline
solution. Similarly, histopathological evaluation revealed
extensive sclerotic membranes in the tympanic membranes of
non-treated blank control as well as in saline-treated groups.
Sclerotic deposits and fibroblast infiltration/production were
observed in the lamina propria. Thickening of the tympanic
membranes was also observed. The tympanic membranes of the
myringotomized rats that had been subjected to CAPE treatment were
thinner compared with those in the group without CAPE treatment.
Furthermore, the degree of fibroblastic activity in the lamina
propria was lower in CAPE-treated myringotomized rats compared with
those that were not treated with CAPE. In conclusion, the
preventive effects of CAPE on the development of MS in
myringotomized rats appears to be associated with a number of
mechanisms, including the scavenging activity of free radicals.
Conclusions
This review discusses only a small number of in
vivo studies on ear disease, and the use of CAPE exceeds the
scope of this article. In addition to its use in a number of in
vivo studies of ear pathology, as an antioxidant and
anti-inflammatory agent, beneficial effects of CAPE have been
reported in a number of types of cancer, arthritis, allergy, heart
disease, diabetes, kidney disease, liver disease and neurological
disease. CAPE modulates a number of biochemical pathways and
several targets involved in ear diseases, such as ototoxicity. CAPE
treatment has demonstrated promising effects in oxidative
stress-mediated and inflammation-mediated diseases (Fig. 2). CAPE may be a useful treatment
for patients with ear disease. Further investigation of the effects
of different doses of CAPE, as well as appropriate administration
regimens and the bioavailability of this agent in humans is
required.
References
|
1
|
Koltuksuz U, Ozen S, Uz E, et al: Caffeic
acid phenethyl ester prevents intestinal reperfusion injury in
rats. J Pediatr Surg. 34:1458–1462. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ilhan A, Koltuksuz U, Ozen S, Uz E,
Ciralik H and Akyol O: The effects of caffeic acid phenethyl ester
(CAPE) on spinal cord ischemia/reperfusion injury in rabbits. Eur J
Cardiothorac Surg. 16:458–463. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ozyurt H, Söğüt S, Yildirim Z, et al:
Inhibitory effect of caffeic acid phenethyl ester on
bleomycine-induced lung fibrosis in rats. Clin Chim Acta.
339:65–75. 2004. View Article : Google Scholar
|
|
4
|
Iraz M, Ozerol E, Gulec M, et al:
Protective effect of caffeic acid phenethyl ester (CAPE)
administration on cisplatin-induced oxidative damage to liver in
rat. Cell Biochem Funct. 24:357–361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Orban Z, Mitsiades N, Burke TR Jr, Tsokos
M and Chrousos GP: Caffeic acid phenethyl ester induces leukocyte
apoptosis, modulates nuclear factor-kappa B and suppresses acute
inflammation. Neuroimmunomodulation. 7:99–105. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Natarajan K, Singh S, Burke TR Jr,
Grunberger D and Aggarwal BB: Caffeic acid phenethyl ester is a
potent and specific inhibitor of activation of nuclear
transcription factor NF-kappaB. Proc Natl Acad Sci USA.
93:9090–9095. 1996. View Article : Google Scholar
|
|
7
|
Huang Q and Tang J: Age-related hearing
loss or presbycusis. Eur Arch Otorhinolaryngol. 267:1179–1191.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sha SH, Taylor R, Forge A and Schacht J:
Differential vulnerability of basal and apical hair cells is based
on intrinsic susceptibility to free radicals. Hear Res. 155:1–8.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akyol S, Ozturk G, Ginis Z, Armutcu F,
Yigitoglu MR and Akyol O: In vivo and in vitro antineoplastic
actions of caffeic acid phenethyl ester (CAPE): Therapeutic
perspectives. Nutr Cancer. 65:515–526. 2013. View Article : Google Scholar
|
|
10
|
Ozturk G, Ginis Z, Akyol S, Erden G, Gurel
A and Akyol O: The anticancer mechanism of caffeic acid phenethyl
ester (CAPE): Review of melanomas, lung and prostate cancers. Eur
Rev Med Pharmacol Sci. 16:2064–2068. 2012.
|
|
11
|
Akyol S, Ginis Z, Armutcu F, Ozturk G,
Yigitoglu MR and Akyol O: The potential usage of caffeic acid
phenethyl ester (CAPE) against chemotherapy-induced and
radiotherapy-induced toxicity. Cell Biochem Funct. 30:438–443.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khalil MI and Sulaiman SA: The potential
role of honey and its polyphenols in preventing heart diseases: a
review. Afr J Tradit Complement Altern Med. 7:315–321.
2010.PubMed/NCBI
|
|
13
|
Kizilay A, Kalcioglu MT, Ozerol E, et al:
Caffeic acid phenethyl ester ameliorated ototoxicity induced by
cisplatin in rats. J Chemother. 16:381–387. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rizzi MD and Hirose K: Aminoglycoside
ototoxicity. Curr Opin Otolaryngol Head Neck Surg. 15:352–357.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Poirrier AL, Pincemail J, Van Den
Ackerveken P, Lefebvre PP and Malgrange B: Oxidative stress in the
cochlea: An update. Curr Med Chem. 17:3591–3604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sanli A, Aydin S, Sarisoy ZA, Paksoy M,
Ayduran E and Erdivanli OC: The protective effect of dexamethasone
and lactate against cisplatin-induced ototoxicity. Turk J Med Sci.
41:467–474. 2011.
|
|
17
|
van den Berg JH, Beijnen JH, Balm AJ and
Schellens JH: Future opportunities in preventing cisplatin induced
ototoxicity. Cancer Treat Rev. 32:390–397. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yorgason JG, Luxford W and Kalinec F: In
vitro and in vivo models of drug ototoxicity: Studying the
mechanisms of a clinical problem. Expert Opin Drug Metab Toxicol.
7:1521–1534. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rybak LP and Whitworth CA: Ototoxicity:
Therapeutic opportunities. Drug Discov Today. 10:1313–1321. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bakir S, Ozbay M, Gun R, et al: The
protective role of caffeic acid phenethyl ester against
streptomycin ototoxicity. Am J Otolaryngol. 34:16–21. 2013.
View Article : Google Scholar
|
|
21
|
Sakallioglu O, Yalcin S, Ozel HB,
Colakoglu N and Alpay HC: Prenatally exposure to exogenous
glucocorticoids and stress may affect the inner ear. Kulak Burun
Bogaz Ihtis Derg. 23:104–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barrett TQ, Kristiansen LH and Ovesen T:
NF-kappaB in cultivated middle ear epithelium. Int J Pediatr
Otorhinolaryngol. 67:895–903. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yellon RF, Leonard G, Marucha PT, et al:
Characterization of cytokines present in middle ear effusions.
Laryngoscope. 101:165–169. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rose AS, Prazma J, Randell SH, Baggett HC,
Lane AP and Pillsbury HC: Nitric oxide mediates mucin secretion in
endotoxin-induced otitis media with effusion. Otolaryngol Head Neck
Surg. 116:308–316. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li W, Lin J, Adams GL and Juhn SK:
Expression of inducible nitric oxide synthase (iNOS) in middle ear
epithelial cells by IL-1beta and TNF-alpha. Int J Pediatr
Otorhinolaryngol. 55:91–98. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Watanabe K, Tomiyama S, Jinnouchi K,
Pawankar R and Yagi T: Expression of inducible nitric oxide
synthase in the cochlea following immune response in the
endolymphatic sac of guinea pigs. ORL J Otorhinolaryngol Relat
Spec. 63:155–159. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wright DT, Fischer BM, Li C, Rochelle LG,
Akley NJ and Adler KB: Oxidant stress stimulates mucin secretion
and PLC in airway epithelium via a nitric oxide-dependent
mechanism. Am J Physiol. 271:L854–L861. 1996.PubMed/NCBI
|
|
28
|
Song JJ, Cho JG, Hwang SJ, Cho CG, Park SW
and Chae SW: Inhibitory effect of caffeic acid phenethyl ester
(CAPE) on LPS-induced inflammation of human middle ear epithelial
cells. Acta Otolaryngol. 128:1303–1307. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shin KM, Kim IT, Park YM, et al:
Anti-inflammatory effect of caffeic acid methyl ester and its mode
of action through the inhibition of prostaglandin E2, nitric oxide
and tumor necrosis factor-alpha production. Biochem Pharmacol.
68:2327–2336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kopp EB and Ghosh S: NF-kappaB and rel
proteins in innate immunity. Adv Immunol. 58:1–27. 1995. View Article : Google Scholar
|
|
31
|
Song JJ, Lim HW, Kim K, Kim KM, Cho S and
Chae SW: Effect of caffeic acid phenethyl ester (CAPE) on
H2O2 induced oxidative and inflammatory
responses in human middle ear epithelial cells. Int J Pediatr
Otorhinolaryngol. 76:675–679. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mattsson C, Marklund SL and Hellstrom S:
Application of oxygen free radical scavengers to diminish the
occurrence of myringosclerosis. Ann Otol Rhinol Laryngol.
106:513–518. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mattsson C and Hellstrom S: Inhibition of
the development of myringosclerosis by local administration of
fenspiride, an anti-inflammatory drug. Eur Arch Otorhinolaryngol.
254:425–429. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Felding JU, Rasmussen JB and Lildholdt T:
Gas composition of the normal and the ventilated middle ear cavity.
Scand J Clin Lab Invest Suppl. 186:31–41. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song JJ, Kwon SK, Cho CG and Park SW: The
effect of caffeic acid phenethyl ester on the prevention of
experimentally induced myringosclerosis. Int J Pediatr
Otorhinolaryngol. 71:1287–1291. 2007. View Article : Google Scholar : PubMed/NCBI
|