Introduction
Ovarian cancer is a common malignant tumor of the
female reproductive system and has one of the highest mortality
rates out of all gynecological malignancies, presenting a serious
threat to women's health and lives (1). Previous studies have observed that
the transformation of epithelial cells to mesenchymal cells
(epithelial-mesenchymal transition; EMT) is important in various
stages of development of various types of cancer, including colon,
skin, breast, ovarian and pancreatic cancer, hepatocellular and
lung adenocarcinoma. In particular, EMT has been reported to
promote invasion and metastasis (2). EMT occurs in specific physiological
and pathological conditions, including the basic process of
biological embryonic development, organ formation and cell
migration, and the early stages of the tumor metastasis cascade
reaction as a key factor in the process of invasion and metastasis
(2).
EMT involves multiple signaling pathways and a
variety of complex molecular mechanisms. Previous studies have
demonstrated that the Snail transcription factor functions as an
inducer of the EMT during embryonic development, and Snail is able
to directly inhibit the expression of E-cadherin to regulate EMT
(3,4). E-cadherin is a calcium-dependent
glycoprotein distributed in the epithelial tissue, which mediates
homogeneous adhesion between cells. As a typical epithelial
phenotypic marker, it is an important molecule in the maintenance
of the epithelial phenotype; the reduction and loss of E-cadherin
expression have been reported to result in a decline in cell
adhesion (5,6). E-cadherin is an inhibitory factor for
the malignant transformation of tumors, invasion and metastasis;
the functional loss of adhesion in cell-cell contacts mediated by
E-cadherin is a rate-limiting step of cell dedifferentiation and
invasion (7,8). Previous studies have observed that in
a number of malignancies, including ovarian cancer, certain factors
are able to regulate the expression of E-cadherin by regulating
Snail, thus affecting the occurrence of tumor cell invasion and
metastasis (7,8).
Hypoxia is a common characteristic of the vast
majority of malignant tumors, particularly solid tumors (9). However, the association between the
hypoxic microenvironment and Snail genes in tumors remains to be
elucidated. Hypoxia-inducible factor lα (HIF-1α) serves a key role
in the regulation of the intracellular oxygen metabolism and its
overexpression is closely associated with malignant behaviors,
including tumor cell proliferation, invasion and metastasis
(9). Luo et al (10) identified a hypoxia-response element
(HRE) at the Snail gene promoter sequence using luciferase reporter
gene and ChIP assays. Through a combination of HRE sites, HIF-lα
directly regulates Snail transcription and expression, indicating
that the Snail gene contains the structural basis of hypoxia
regulation.
Therefore, the present study aimed to investigate
the expression and clinical significance of HIF-1α, Snail and
E-cadherin in the human mucinous ovarian cancer cell line 3AO, the
serous ovarian cancer cell line SKOV3, the human clear cell ovarian
cancer cell line ES-2 and the undifferentiated human ovarian cancer
cell line TYK, as well as in ovarian cancer tissues.
Materials and methods
Cell lines
The serous ovarian cancer cell line SKOV3 was
purchased from the Cell Center of the Institute of Basic Medical
Sciences, Chinese Academy of Medical Sciences (Beijing, China), the
mucinous ovarian carcinoma cell line 3AO was purchased from the
Central Laboratory of Tianjin Medical University (Tianjin, China),
the human clear cell ovarian cancer cell line ES-2 was purchased
from Shanghai Aiyan Research Biotechnology Co., Ltd. (Shanghai,
China) and the undifferentiated ovarian cancer cell line TYK was
provided by the Cancer Center of Qilu Hospital at Shandong
University (Jinan, China).
Patient samples
The ovarian cancer tissues were obtained via
surgical resection from 182 patients with ovarian cancer at Renmin
Hospital of Wuhan University between February 2010 and 2012 and the
tissue was divided into histological grades I, II and III (11). The patients were 32–57 years of age
and surgical resection was performed 1–6 months post-diagnosis. The
inclusion criteria for the present study were as follows: Female,
≥18 years, confirmed histologically as Stage I–III epithelial ovary
primary cancer, white blood cell content ≥3,000/µl, absolute
neutrophil count ≥1,500/µl, hemoglobin content ≥9 g/ml and
platelet count ≥100,000/mm3. Patients with other medical
conditions, which precluded the study were excluded depending on
the opinion of the authors. The present study was approved by the
ethics committee of Renmin Hospital of Wuhan University (Wuhan,
China) and written consent was obtained from all patients.
Main reagents
RPMI-1640 culture media and trypsin were purchased
from Gibco-BRL (Invitrogen Life Technologies, Carlsbad, CA, USA).
Fetal calf serum was purchased from Hangzhou Evergreen Biological
Engineering Materials Co., Ltd. (Hangzhou, China), MTT was
purchased from Amresco LLC (Solon, OH, USA) and dimethyl sulfoxide
was purchased from Sigma-Aldrich (St. Louis, MO, USA). The
Transwell chamber was purchased from Corning-Costar (Corning, NY,
USA), the artificial basement membrane (Matrige1) was purchased
from BD Biosciences (Franklin Lakes, NJ, USA), the reverse
transcription polymerase chain reaction (RT-PCR) kit (Access Quick
RT-PCR system) was purchased from Promega Corporation (Madison, WI,
USA); anti-human Snail and anti-human E-cadherin mouse monoclonal
antibodies were purchased from Santa Cruz Biotechnology, Inc.,
(Dallas, TX, USA) and the prestained protein marker (cat. no.
BRP-125) was purchased from SBS Genetech (Beijing, China).
Cell culture
The cells were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum, 2 mmol/l glutamine, 100
U/ml penicillin and 100 U/m1 streptomycin, all purchased from
Invitrogen Life Technologies. Cells were cultured in incubators at
37°C in humidified air containing 5% CO2.
Cell invasion assay
The Transwell chamber assay was applied to detect
cancer cell invasion (12). The
number of cells transgressed through a polycarbonate membrane was
counted under the light microscope (CKX41-A32RC; Olympus, Tokyo,
Japan; magnification, x400), and the relative number of invasive
cells represented the tumor cell invasion. The number of cells was
counted within six random fields of view, the average was deduced
and three samples were counted for each group.
Total RNA extraction and
identification
Total RNA extraction was conducted using TRIzol
reagent (Invitrogen Life Technologies) in accordance with the
manufacturer's instructions for the kit used (Huamei Company).
Subsequent to extraction of the RNA, the concentration and purity
of the RNA sample was determined by measuring the optical density
within 6 h.
RT-PCR using the two-step method
Total RNA extraction was performed in accordance
with the manufacturer's instructions for TRIzol. Synthesis of
first-strand cDNA was performed in accordance with the kit.
Briefly, l µl oligo (dT) were added to 2 µg total RNA
and heated at 70°C for 5 min. A total of 5 µl 5X avian
myeloblastosis virus (AMV) buffer, 2.5 µl
desoxyribonucleotide triphosphate (10 mmol/l), 1 µl AMV-RT
(10 U/µl), l µl RNasin (40 U/µl; Promega
Corp.) and diethylpyrocarbonate-treated water were then added to a
final volume of 25 µl, mixed and reacted at 42°C for 60
min.
The PCR primers were synthesized by Sangon Biotech
Co., Ltd. (Shanghai, China; Table
I). PCR cycling conditions were as follows: 94°C for 2 min, 35
cycles of 94°C for l min and 58°C for 1 min, followed by extension
at 72°C for l min, on a GeneAmp PCR system 9600 (Applied
Biosystems). Expression levels were calculated relative to GAPDH
levels, which acted as the endogenous control. The PCR products
then underwent 1% agarose gel electrophoresis and were observed
under an ultraviolet lamp and images were captured on a Bio-Rad
VersaDoc 3000 Imaging system (Bio-Rad). Image J software (NIH,
Bethesda, MD, USA) was used for grey value analysis.
 | Table IPolymerase chain reaction primers. |
Table I
Polymerase chain reaction primers.
| Protein | Primer sequences | Gene length (bp) |
|---|
| Snail | F:
5′-ATGCATGACCGGACACACCCATTAC-3′ | 2048 |
| R:
5′-AGATTTATTCTGCATCTGAATGCTC-3′ | |
| E-cadherin | F:
5′-AAGTGCTGCCACCAAAGACAGA-3′ | 1581 |
| R:
5′-AGGTCAAGGCACCTCAATCATCCTC-3′ | |
| HIF-1α | F:
5′-TGATACCAAAACCAATACACCTC-3′ | 2472 |
| R:
5′-TGCTGAATTCACACAGTCACAAC-3′ | |
| GAPDH | F:
5′-ATTCCATGGCACCGTCAAGGCT-3′ | 527 |
| R:
5′-TCAGGTCCACCACTGACACGTT-3′ | |
Western blot analysis
The total protein was extracted from the cells and
quantified using a bicinchoninic acid assay (Pierce Biotechnology,
Inc., Rockford, IL, USA). Equal quantities (40 µg) of
protein sample were loaded, separated by electrophoresis at 120 V
for 1.5 h and transferred onto polyvinylidene difluoride (PVDF)
membranes (Bio-Rad) at 90 mA for l2–16 h at 4°C. The film was then
stained with 0.1% Ponceau S (Bio-Rad) to determine the location of
the transfer efficiency and protein marker. The PVDF membranes were
blocked by freshly prepared blocking buffer (1X Tris-buffered
saline, 5% skimmed milk and 0.05% Tween-20; Sigma-Aldrich, St.
Louis, MO, USA) prior to incubation with the primary antibodies,
mouse anti-human HIF-1α (cat. no. sc-13515), anti-human Snail (cat.
no. sc-393172), anti-human E-cadherin (cat. no. sc-21791),
anti-human GAPDH (cat. no. sc-365062) monoclonal antibodies (Santa
Cruz Biotechnology) and rabbit anti-mouse IgG horseradish
peroxidase conjugated secondary antibody (sc-358914) (Santa Cruz
Biotechnology), according to the manufacturer's instructions. This
was followed by incubation with the secondary antibody and the
protein was visualized using an enhanced chemiluminescent (ECL)
detection system, following the manufacturer's instructions (ECL;
GE Healthcare Life Sciences, Little Chalfont, UK). Image J software
(NIH) was used for grey value analysis.
Immunohistochemistry
Streptvidin-peroxidase two-step immunohistochemical
staining was performed in paraffin block of filed case; the
experimental procedure was conducted in accordance with the
manufacturer's instructions. Instead of the primary antibody,
phosphate-buffered saline (Invitrogen Life Technologies) was used
for the negative control while a known positive slide was used as
the positive control.
Evaluation of immunohistochemical
staining
HIF-1α and Snail proteins are predominantly
localized in the nucleus, occasionally accompanied by cytoplasmic
staining. Endometrial glandular epithelial cells was predominantly
localized to the nucleus and clear staining were selected as
positive samples. Staining was graded as follows (13): i) Percentage of positively stained
cells ≤5%, 0 points; 6–25%, 1 point; 26–50%, 2 points; 51–75%, 3
points; and 75%, 4 points. ii) Staining intensity was graded as:
Colorless, 0 points; pale yellow, 1 point; brown, 2 points; and
tan, 3 points. Samples in which the multiplication of the scores
for the percentage of positively stained cells and the staining
intensity resulted in values <2 were classified as negative
expression and those ≥2 were classified as positive expression.
E-cadherin protein was predominantly localized in the cell
membrane, and E-cadherin expression in normal endometrial tissue
was used as a positive control. In cancer cells, E-cadherin
staining was graded as follows (11): No staining or positive staining
rate ≤l0%, 0 points; low staining and positive staining rate ≥10, 1
point; moderate staining and positive staining rate ≥10, 2 points;
staining as in normal tissue, classified as a high degree of
staining, with a positive staining rate ≥10, 3 points. In order to
simplify the statistical analysis, 0, 1 and 2 points were
classified as reduced E-cadherin expression (reduced expression)
and 3 points was classified as normal E-cadherin expression
(preserved expression).
Statistical analysis
SPSS software, version 13.0 (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. The data are expressed
as the mean ± standard deviation of 'n' experiments/samples. The
comparison of numerical data was conducted using the χ2
test or Fisher's exact test and correlation analysis was performed
using Spearman's rank correlation analysis. A two-tailed
P<0.05 was considered to indicate a statistically significant
difference.
Results
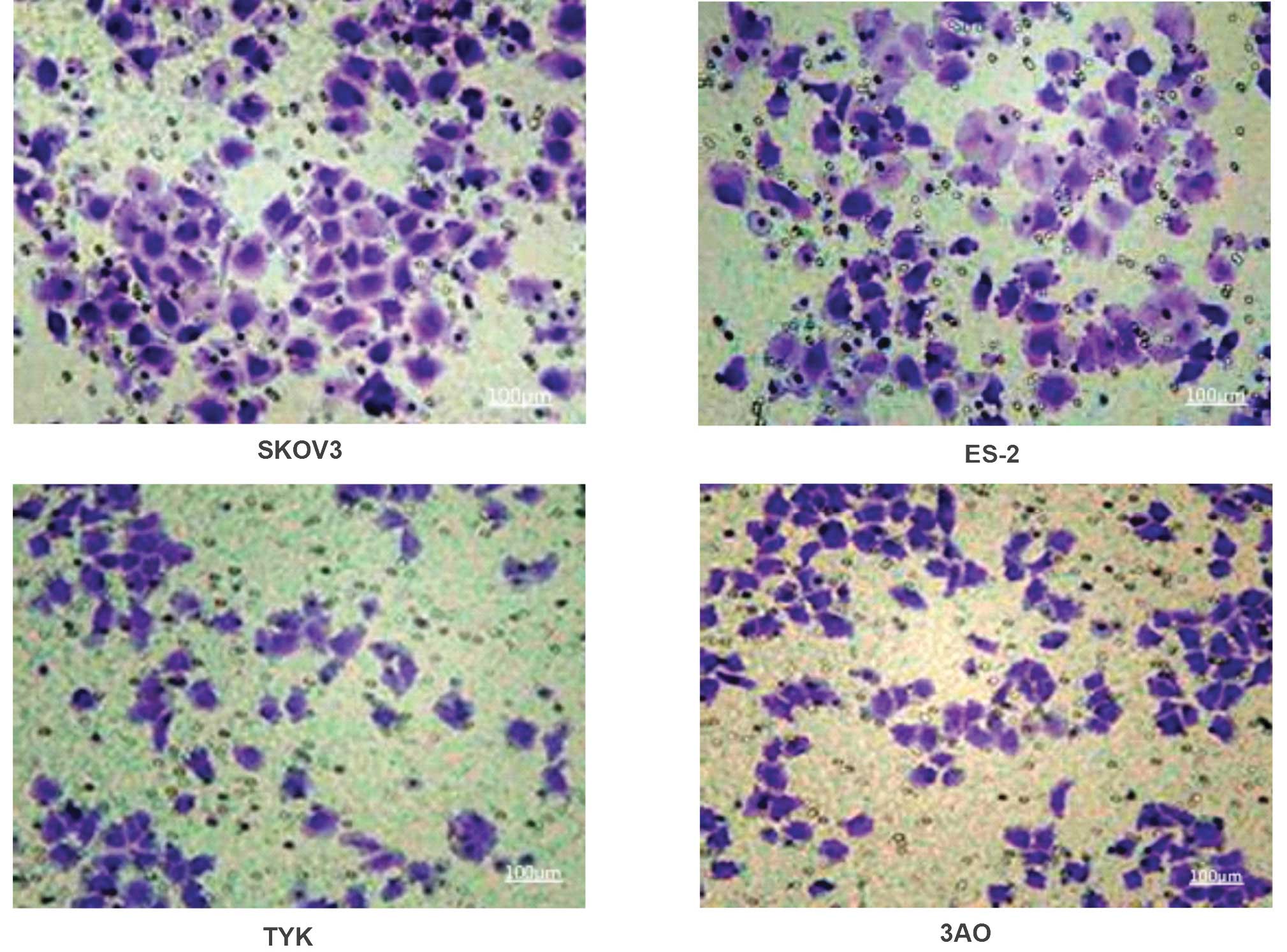
Invasiveness varies among ovarian cancer
cell lines
The number of invasive cells in the human serous
ovarian cancer cell line SKOV3 (132.6±6.4) and the human clear cell
ovarian cancer cell line ES-2 (130.4±5.6) was significantly higher
than that in the human undifferentiated ovarian cancer cell line
TYK (102.3±4.7) and the human mucinous ovarian cancer cell line 3AO
(104.1±5.2) (P<0.01).
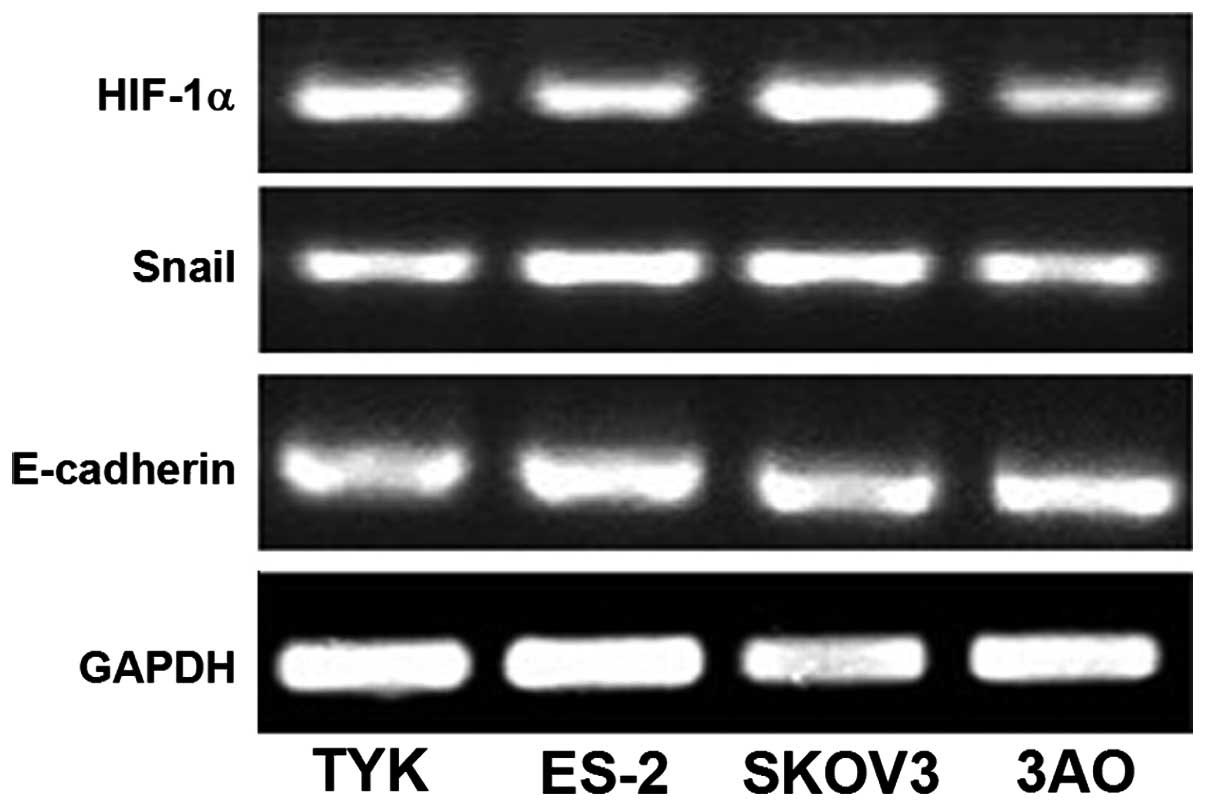
mRNA expression of HIF-1α and Snail is
high and that of E-cadherin is low in invasive ovarian cancer cell
lines
The mRNA expression levels of HIF-1α and Snail were
greater in the highly invasive ovarian cancer cell lines SKOV3 and
ES-2 as compared with those in the TYK and 3AO cell lines
(104.1±5.2; P<0.01; Fig. 1). By
contrast, the mRNA expression levels of E-cadherin were
significantly lower in the SKOV3 and ES-2 cell lines than those in
TYK and 3AO (P<0.05), as presented in Table II and Fig. 2.
 | Table IImRNA expression levels of the HIF-1α,
Snail and E-cadherin genes in ovarian cancer cells. |
Table II
mRNA expression levels of the HIF-1α,
Snail and E-cadherin genes in ovarian cancer cells.
| Ovarian cancer cell
line | HIF-1α/GAPDH | Snail/GAPDH | E-cadherin/GAPDH |
|---|
| 3AO | 0.64±0.12 | 0.56±0.08 | 1.11±0.15 |
| SKOV3 | 1.22±0.25 | 1.17±0.20 | 0.48±0.08 |
| ES-2 | 0.98±0.18 | 1.14±0.18 | 0.45±0.06 |
| TYK | 0.47±0.08 | 0.92±0.17 | 1.37±0.26 |
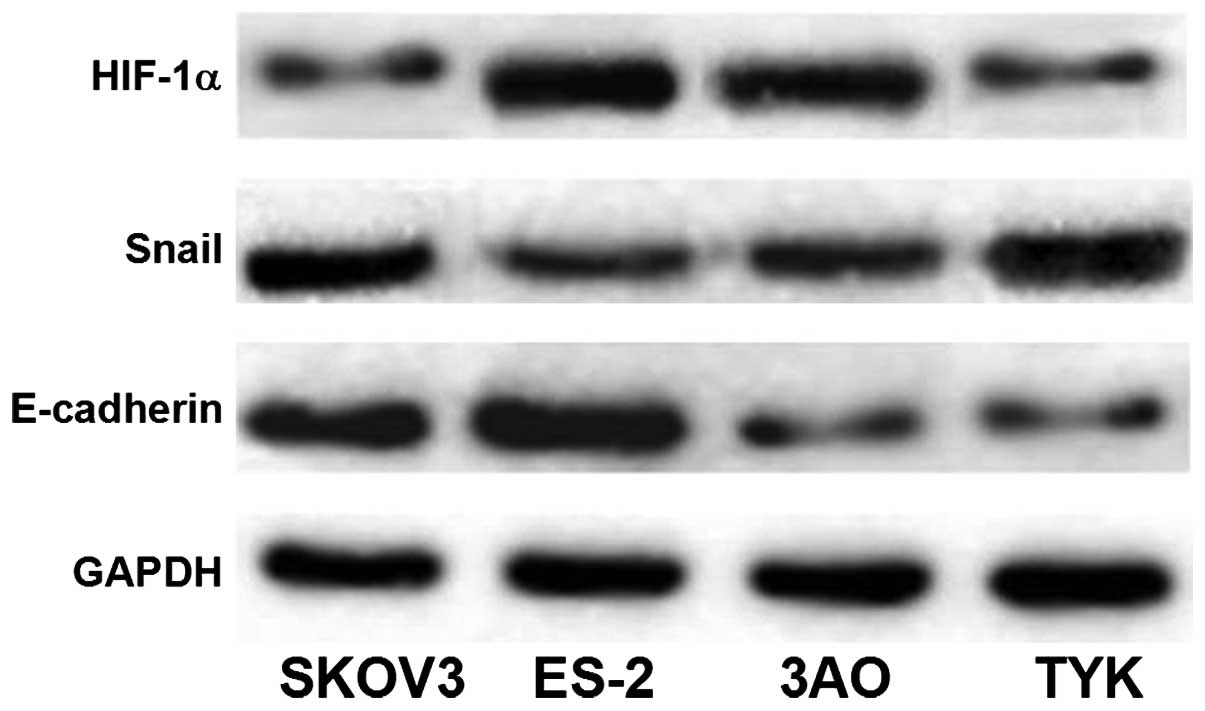
Protein expression of HIF-1α and Snail is
high, while that of E-cadherin is low in invasive ovarian cancer
cell lines
The protein expression levels of HIF-1α and Snail
were greater in the highly invasive ovarian cancer cell lines SKOV3
and ES-2 than those in TYK and 3AO. By contrast, the expression
levels of E-cadherin protein in SKOV3 and ES-2 were significantly
lower than those in TYK and 3AO (P<0.05), as presented in
Table III and Fig. 3. The results regarding protein
expression were consistent with those of gene expression.
 | Table IIIProtein expression levels of HIF-1α,
Snail and E-cadherin in ovarian cancer cells. |
Table III
Protein expression levels of HIF-1α,
Snail and E-cadherin in ovarian cancer cells.
| Types of ovarian
cancer | HIF-1α/GAPDH | Snail/GAPDH | E-cadherin/GAPDH |
|---|
| 3AO | 0.87±0.13 | 0.65±0.10 | 1.12±0.21 |
| SKOV3 | 1.25±0.22 | 1.34±0.27 | 0.71±0.17 |
| ES-2 | 1.79±0.45 | 1.87±0.51 | 0.88±0.11 |
| TYK | 0.36±0.05 | 0.45±0.09 | 1.16±0.23 |
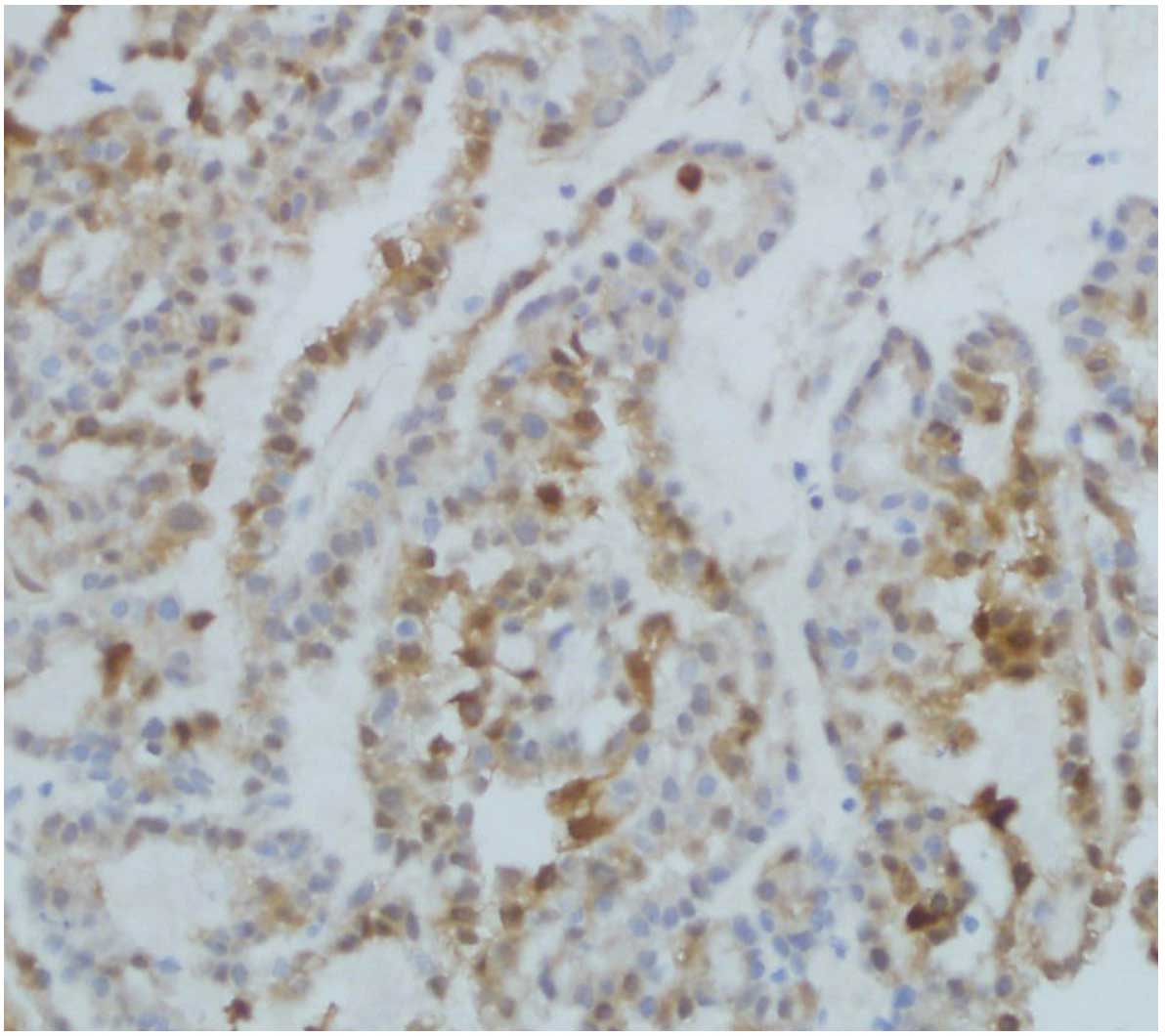
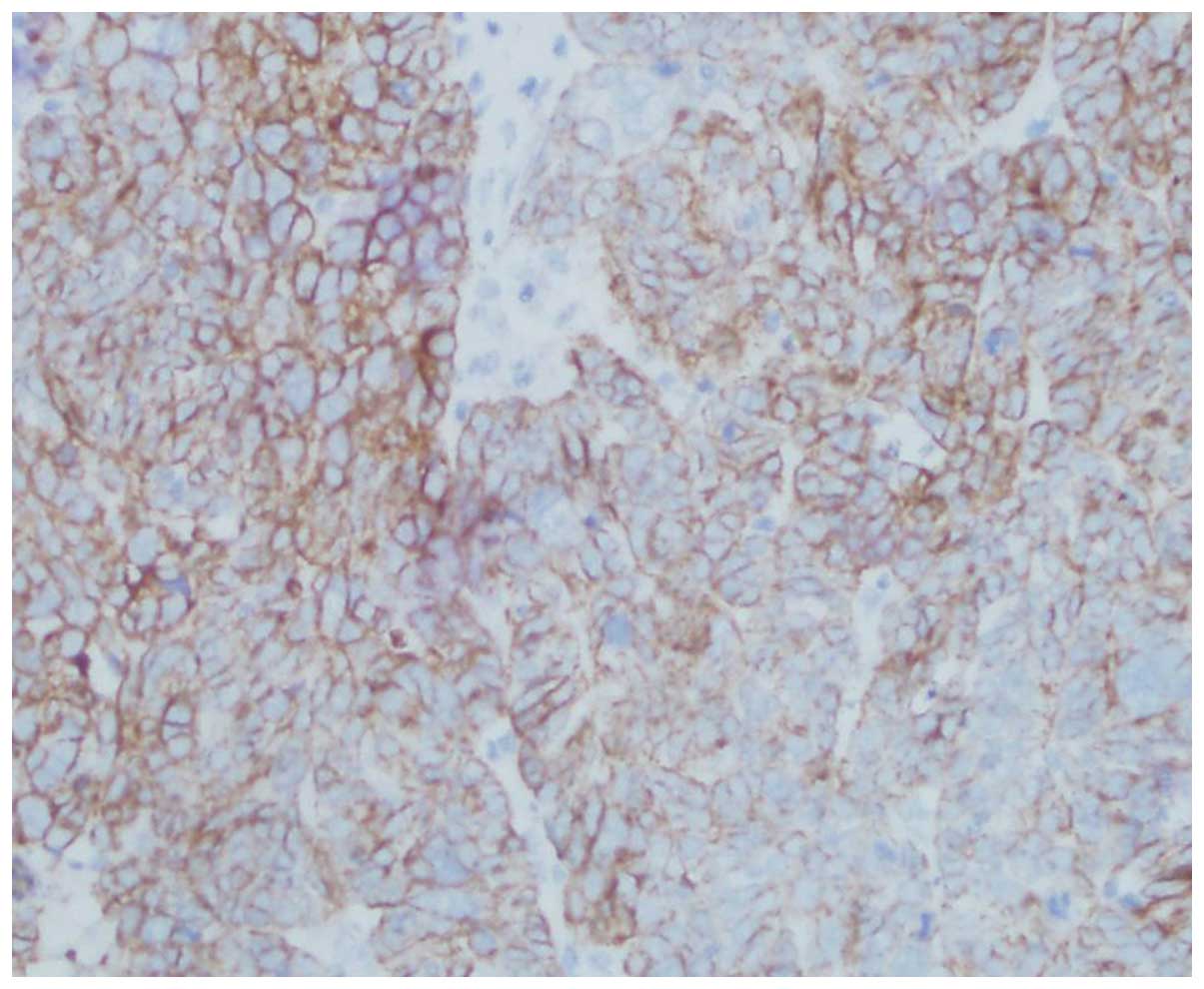
HIF-1α, Snail and E-cadherin expression
are correlated with clinicopathological factors of ovarian
cancer
HIF-1α expression was significantly correlated with
histological grading, tumor myometrial invasion, type of tissue and
lymph node metastasis (P<0.05). Positive expression of Snail was
closely correlated with surgical pathology stage, type of tissue
and lymph node metastasis (P<0.05). Reduced expression of
E-cadherin was significantly correlated with the histological type
of tissue, tumor myometrial invasion and lymph node metastasis
(P<0.01), as presented in Table
IV and Figs. 4Figure 5–6.
 | Table IVAssociations between expression of
HIF-1α, Snail and E-cadherin in ovarian cancer and clinical
pathological factors. |
Table IV
Associations between expression of
HIF-1α, Snail and E-cadherin in ovarian cancer and clinical
pathological factors.
| Clinical factors | Cases (n) | HIF-1α
| Snail
| E-cadherin
|
|---|
| (−) | (+) | P | (−) | (+) | P | (−) | (+) | P |
|---|
| Age (years) | | | | >0.05 | | | >0.05 | | | >0.05 |
| <50 | 47 | 16 | 31 | | 27 | 20 | | 30 | 17 | |
| ≥50 | 135 | 57 | 78 | | 71 | 64 | | 89 | 46 | |
| Histological
grade | | | | <0.05 | | | >0.05 | | | >0.05 |
| I | 80 | 42 | 38 | | 48 | 32 | | 53 | 27 | |
| II | 57 | 20 | 37 | | 26 | 31 | | 37 | 20 | |
| III | 45 | 11 | 34 | | 24 | 21 | | 29 | 16 | |
| FIGO stage | | | | >0.05 | | | <0.05 | | | >0.05 |
| I+II | 148 | 67 | 81 | | 92 | 56 | | 96 | 52 | |
| III+IV | 34 | 6 | 28 | | 6 | 28 | | 23 | 11 | |
| Type of tissue | | | | <0.05 | | | <0.05 | | | <0.01 |
| Mucinous | 53 | 29 | 24 | | 43 | 10 | | 30 | 23 | |
| Serous | 78 | 24 | 54 | | 28 | 50 | | 64 | 14 | |
| Clear cell | 21 | 6 | 15 | | 7 | 14 | | 15 | 6 | |
|
Undifferentiated | 30 | 14 | 16 | <0.05 | 20 | 10 | | 10 | 20 | |
| Myometrial
invasion | | | | | | | >0.05 | | | <0.01 |
| <1/2 | 125 | 59 | 66 | | 75 | 50 | | 62 | 53 | |
| ≥1/2 | 57 | 14 | 43 | | 23 | 34 | | 47 | 10 | |
| Lymph node
metastasis | | | | <0.05 | | | <0.05 | | | <0.01 |
| No | 136 | 63 | 73 | | 87 | 49 | | 81 | 55 | |
| Yes | 46 | 10 | 36 | | 11 | 35 | | 38 | 8 | |
Significant correlation between HIF-1α,
Snail and E-cadherin expression in ovarian cancer tissues
The expression levels of HIF-1α and Snail in ovarian
cancer exhibited a positive correlation (r=0.231; P=0.021), whereas
the expression levels of Snail and E-cadherin were negatively
correlated (r=−0.225; P=0.028), as presented in Table V, and there was a negative
correlation between HIF-1α and E-Cadherin (r=−0.306;
P<0.05).
 | Table VCorrelation between Snail and
HIF-1α/E-cadherin in ovarian cancer tissue. |
Table V
Correlation between Snail and
HIF-1α/E-cadherin in ovarian cancer tissue.
| Snail | Cases (n) | HIF-1α
| Spearman
| E-cadherin
| Spearman
|
|---|
| (−) | (+) | r | P | (−) | (+) | r | P |
|---|
| Negative | 98 | 49 | 47 | | | 57 | 42 | | |
| Positive | 84 | 24 | 62 | 0.231 | 0.021 | 62 | 21 | −0.225 | 0.028 |
Discussion
Cancer metastasis requires the occurrence of a
primary tumor, local invasion and the formation of metastasis,
involving a variety of molecular mechanisms which have remained to
be fully elucidated to date. Thus, the clarification of these
molecular mechanisms is an important challenge in cancer research
(14).
A previous study identified that HIF-1α serves a
critical role in the process of tumor adaptation to hypoxia
(15), and HIF-1α expression was
shown to be closely associated with tumor proliferation, invasion,
metastasis, patient prognosis and resistance to treatment (16). In a study on ovarian cancer, Imai
et al (7) demonstrated that
the HIF-lα content of SKOV3 and OVCAR3 cells was upregulated
following culturing under hypoxic conditions (5% oxygen) for 72 h.
In addition, the invasiveness and metastatic potential were
increased, accompanied by a significant increase in Snail gene
expression and a significant reduction in E-cadherin gene
expression, while normal ovarian epithelial OSE cells exhibited no
such alterations. Immunohistochemical analysis demonstrated that
high protein expression of HIF-lα in ovarian cancer tissue was
associated with the lack of expression of E-cadherin (17,18).
However, studies have also demonstrated that mRNA expression of
HIF-lα in ovarian cancer and breast cancer exhibited no significant
correlation with tumor grade, clinical stage and the survival rate
(17,18). Yang et al (19) identified that overexpression of
HIF-1α inhibits E-cadherin expression to promote the occurrence of
EMT, which is mediated by Twist. The present study demonstrated
that the protein and gene expression levels of HIF-1α in ovarian
cancer cells were as follows: SKOV3 > ES-2 > 3AO > TYK
(P<0.01), with the positive expression being significantly
increased in specimens with lymph node metastasis. The expression
levels of the Snail gene in ovarian cancer cells were as follows:
SKOV3 = ES-2 > 3AO > TYK and the expression levels of Snail
protein in ovarian cancer cells were as follows: ES-2 > SKOV3
> 3AO > TYK. The expression of Snail was in parallel with
increases in HIF-lα, exhibiting a significant positive correlation
in ovarian cancer (r=0.231; P=0.021). In addition, the present
study revealed that HIF-1α protein expression was significantly
correlated with histological grading, tumor myometrial invasion,
tissue type and lymph node metastasis (P<0.05), which indicated
that HIF-1α is involved in malignant processes of ovarian cancer
development and progression and is closely correlated with invasion
and metastasis.
Kurrey et al (20) demonstrated that ovarian cancer
cells transfected with exogenous Snail were able to undergo EMT,
following which the cell invasiveness and motility were observed to
be increased. This suggested that the transcription factor Snail
has an important role in the process of metastasis in ovarian
cancer. Snail expression has been demonstrated to be significantly
associated with clinicopathological tumor staging, with a previous
study identifying that Snail was associated with lymph node
metastasis (20). An additional
study observed that high Snail expression was closely associated
with tumor recurrence and poor prognosis (21,22).
Blechschmidt et al (13)
demonstrated, through immunohistochemical analysis of Snail and
E-cadherin in 48 cases of primary ovarian cancer and metastatic
cancer, that the expression of Snail was significantly associated
with E-cadherin expression in the primary tumor and metastasis of
ovarian cancer. E-cadherin expression was significantly reduced in
primary cancer tissues, while Snail expression was significantly
increased. Their expression levels were observed to be
significantly correlated with clinicopathological staging and a
poor prognosis of ovarian cancer, and the expression trend was more
marked in the metastatic cancer. In a study on 95 patients with
epithelial ovarian cancers, Yoshida et al (23) observed that, as benign ovarian
epithelial cells progressed into borderline tumors and then to
ovarian cancer, Snail protein expression was gradually increased,
whereas the expression levels of E-cadherin continued to decrease.
In analogy with these results, the present study identified an
inverse correlation between the expression of Snail and E-cadherin
(r=−0.225; P=0.028), positive expression of Snail was significantly
correlated with surgical stage, histological type and lymph node
metastasis (P<0.05), while negative expression of E-cadherin was
significantly associated with histological type, tumor myometrial
invasion and lymph node metastasis (P<0.01). Based on previous
studies and the results of the present study, E-cadherin is likely
to be a direct target of Snail, serving an important role in
invasion and metastasis in ovarian cancer.
HIF-1α may inhibit the expression levels of
E-cadherin by upregulating the expression of Snail, serving an
important role in invasion and metastasis in ovarian cancer. The
results of the present study lead to the hypothesis that as a
transcription factor, Snail is at the center of a signaling pathway
cascade (HIF-1α regulates Snail, which in turn regulates
E-cadherin), regulating upstream signal transduction pathways and
downstream Snail transcriptional regulation of gene expression. It
was suggested that the degree of malignancy of ovarian cancer can
be reduced by blocking the Snail signaling pathway, reducing the
invasive and metastatic potential of the tumor cells, thereby
improving the prognosis of the patients. In addition, Snail is a
potential tumor marker for metastatic potential, which may aid in
the development of novel tumor treatment strategies and improve
survival rates of patients with ovarian cancer.
Acknowledgments
The authors would like to thank Professor Yang Jing
for the helpful advice with the experiments and careful reading of
this manuscript. This study was supported partially by the National
Natural Science Foundation of China (no. 30973196).
References
|
1
|
Berkenblit A and Cannistra SA: Advances in
the management of epithelial ovarian cancer. J Reprod Med.
50:426–438. 2005.PubMed/NCBI
|
|
2
|
Radisky DC: Epithelial-mesenchymal
transition. Cell Sci. 118:4325–4326. 2005. View Article : Google Scholar
|
|
3
|
Batlle E, Sancho E, Francí C, et al: The
transcription factor snall is a repressor of E-cadherin gene
expression in epithelial tumour cells. Nat Cell Biol. 2:84–89.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cano A, Pérez-Moreno MA, Rodrigo I, et al:
The transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thiery JP: Epithelial mesenchymal
transition in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Imai T, Horiuchi A, Wang C, et al: Hypoxia
attenuates the expression of E-cadherin via up-regulation of SNAIL
in ovarian carcinoma cells. Am J Pathol. 163:1437–1447. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dohadwala M, Yang SC, Luo J, et al:
Cyclooxygenase-2-dependent regulation of E-cadherin: Prostaglandin
E (2) induces transcriptional repressors ZEB1 and snail in
non-small cell lung cancer. Cancer Res. 66:5338–5345. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Semenza GL: Defining the role of
hypoxia-inducible factor 1 in cancer biology and therapeutics.
Oncogene. 29:625–634. 2010. View Article : Google Scholar :
|
|
10
|
Luo D, Wang J, Li J and Post M: Mouse
snail is a target gene for HIF. Mol Cancer Res. 9:234–245. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blechschmidt K, Sassen S, Schmalfeldt B,
et al: The E-cadherin repressor Snail is associated with lower
overall survival of ovarian cancer patients. Br J Cancer.
98:489–495. 2008. View Article : Google Scholar
|
|
12
|
Siegal GP, Wang MH, Rinehart CA Jr, et al:
Development of a novel human extracellular matrix for quantitation
of the invasiveness of human cells. Cancer Lett. 69:123–132. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan H, Ye K, Wang Z and Tang H:
Clinicopathologic evaluation of immunohistochemical CD147 and MMP-2
expression in differentiated thyroid carcinoma. Jpn J Clin Oncol.
38:528–533. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katoh M and Katoh M: Pharmacogenomics on
gastric cancer. Cancer Biol Ther. 3:566–567. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bachtiary B, Schindl M, Pötter R, et al:
Overexpression of hypoxia-inducible factor 1 alpha indicates
diminished response to radiotherapy and unfavorable prognosis in
patients receiving radical radiotherapy for cervical cancer. Clin
Cancer Res. 9:2234–2240. 2003.PubMed/NCBI
|
|
16
|
Jiang YA, Fan LF, Jiang CQ, et al:
Expression and significance of PTEN, hypoxia-inducible factor-1
alpha in colorectal adenoma and adenocarcinoma. World J
Gastroenterol. 9:491–494. 2003.PubMed/NCBI
|
|
17
|
Nakayama K, Kanzaki A, Hata K, et al:
Hypoxia-inducible factor 1 alpha (HIF-1 alpha) gene expression in
human ovarian carcinoma. Cancer Lett. 176:215–223. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang BH, Agani F, Passaniti A and
Semenza: V-SRC induces expression of hypoxia-inducible factor 1
(HIF-1) and transcription of genes encoding vascular endothelial
growth factor and enolase l: Involvement of HIF-1 in tumor
progression. Cancer Res. 57:5328–5335. 1997.PubMed/NCBI
|
|
19
|
Yang MH, Wu MZ, Chiou SH, et al: Direct
regulation of TWIST by HIF-1 alpha promotes metastasis. Nat Cell
Biol. 10:295–305. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kurrey NK, K A and Bapat SA: Snail and
Slug are major determinants of ovarian cancer invasiveness at the
transcription level. Gynecol Oncol. 97:155–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Craene B and Berx G: Snail in the frame
of malignant tumor recurrence. Breast Cancer Res. 8:1052006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Olmeda D, Moreno-Bueno G, Flores JM, et
al: SNAI1 is required for tumor growth and lymph node metastasis of
human breast carcinoma MDA-MB-231 cells. Cancer Res.
67:11721–11731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoshida J, Horiuchi A, Kikuchi N, et al:
Changes in the expression of E-cadherin repressors, Snail, Slug,
SIP1 and Twist, in the development and progression of ovarian
carcinoma: The important role of Snail in ovarian tumorigenesis and
progression. Med Mol Morphol. 42:82–91. 2009. View Article : Google Scholar : PubMed/NCBI
|