Introduction
Prostate cancer is the most common type of cancer
among males in Western countries (1). Although the initial cause of the
onset of prostate cancer remains to be elucidated, previous studies
have demonstrated potential links to dietary habits and fat intake.
For example, a controlled case study provides evidence of a
positive correlation between dietary fat and mortality from
prostate cancer (2–5). The dietary intake of essential fatty
acids, including ω-3 and ω-6 polyunsaturated fatty acids (PUFAs),
is crucial for several cellular processes, including cell
proliferation and differentiation (6). A number of previous studies have
demonstrated that PUFAs are important in promoting or inhibiting
several types of tumor, including hormone-responsive prostate
tumors (7–9).
The contribution of ω-3 and ω-6 PUFAs to prostate
carcinogenesis has gained considerable importance in previous
years. It has been reported by previous in vitro and in
vivo studies that ω-3 PUFAs, including docosahexaenoic acid
(DHA) and eicosapentaenoic acid (EPA), can repress the development
and progression of prostate cancer, whereas ω-6 PUFAs promote the
growth of prostate cancer (9–12).
In addition, epidemiological studies demonstrated that males who
consumed large quantities of fish have a lower risk of prostate
cancer and those who eat low quantities of seafood were associated
with an increased prostate cancer risk, suggesting that there is an
inverse correlation between diets rich in ω-3 PUFAs and the
incidence of prostate cancer (13–15).
Therefore, the ω-3 PUFAs contained in fish oil and other dietary
factors may be beneficial for prostate cancer chemoprevention.
However, the association between ω-3 PUFAs and the progression from
hormone dependency to hormone independency, and the mechanisms by
which they may be involved in mediating their effects on androgen
dependence remain to be elucidated.
The tumor-suppressive effects of ω-3 PUFAs are
hypothesized to be partly due to the modulation of signal
transduction pathways (16–18).
Androgens are important in the development and progression of
prostate cancer (19). Androgens
function via binding to the androgen receptor (AR), which is a
ligand-dependent transcription factor of the nuclear hormone
receptor superfamily. Therefore, AR is critical in the development
of prostate cancer (19). Several
previous studies have reported that the overexpression of AR is
characteristic of prostate cancer that progresses to hormone
independency (20–23). For instance, LNCaP clones, which
progressed to hormone independency demonstrated increased protein
expression levels of the AR, compared with their hormone-dependent
syngenic clones. Exposure to ω-3 PUFAs caused a significant effect
on suppressing the androgen deprivation-induced expression of the
AR (24).
The LNCaP cell line is an androgen-responsive
prostate cancer cell line expressing the AR and a number of
androgen-inducible genes, including prostate-specific antigen
(PSA). The present study aimed to investigate whether treatment
with DHA impedes the growth of hormone-responsive LNCaP cells, and
whether the effect of DHA is associated with changes in the
androgen receptor and androgen-regulated genes.
Materials and methods
Cell lines
All cells types used in the present study were
obtained from the American Type Culture Collection (Rockville, MD,
USA) and maintained in RPMI-1640 medium (Life Technologies, Grand
Island, NY, USA), supplemented with 10% fetal bovine serum (FBS;
Life Technologies), at 37°C and 5% CO2, until reaching
70% confluence. The cells were subsequently treated with DHA
(Sigma-Aldrich, St. Louis, MO, USA) dissolved in ethanol at the
designated concentrations and for the indicated duration. For the
AR stability experiment, the cells were treated with either 50
µg/ml of the protein synthesis inhibitor, cycloheximide
(CHX; Sigma-Aldrich) for the indicated duration or 25 µM
proteasome inhibitor, MG132 (Sigma-Aldrich) for 24 h prior to
harvesting.
Cell proliferation assay and PSA
quantification
Cell growth was assessed by
3,(4,5-dimethylthiazol-2-yl)2,5-diphenyltetra-zoliumbromide
(Sigma-Aldrich) dye conversion, according to the manufacturer's
instructions. Briefly, the cells were seeded
(5×103/well) into a 96-well flat bottom plate and were
treated with 0.4% trypan blue staining (Sigma-Aldrich). The cells
were grown in different treatment conditions and cell growth was
subsequently assessed following the indicated duration of
continuous treatment. The number of viable cells was counted using
a hemocytometer (XBK25; Qiujing Instrument, Shanghai, China) under
a light microscope (×20 magnification; CKX31; Olympus, Tokyo,
Japan).
The LNCaP cells were seeded at 3×104
cells/well in 24-well plates. Following culturing for 48 h, the
cells were treated with serum-free medium for 24 h and subsequently
incubated in medium, containing 10% charcoal-stripped serum (Life
Technologies) with indicated concentrations of DHA, in the absence
or presence of 1 nM R1881 (Perkin Elmer Life Sciences, Waltham, MA,
USA). Following treatment for 5 days, the culture medium was
collected for measuring the total protein expression levels of PSA,
using the PSA Human kit (Abcam, Cambridge, MA, USA). The expression
levels of PSA in the culture medium were normalized to the cell
number.
Immunoblot and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The cells were harvested and analyzed by
immunoblotting, as previously described (25). The AR (#3202) and GAPDH (D16H11;
#5174) antibodies were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). For RT-qPCR analysis, the cells were
suspended in 1 ml TRIzol reagent (Life Technologies) and the total
RNA was extracted, followed by cDNA synthesis as described
previously (25). The RNA was
amplified by RT-qPCR, performed with an SYBR Green Master Mix
(Takara Biotechnology, Inc., Dalian, China) on a
LightCycler® 96 Real-Time PCR System (Roche, Mannheim,
Germany). The cycling conditions were as follows: Initial
denaturation at 95°C for 15 min, followed by 40 cycles of 95°C for
5 sec and 60°C for 30 sec. β-actin was used as the reference gene
and the relative quantification comparative CT method was used. The
primer sequences used are as follows: Forward
5′-GATGCTGTGAAGGTCATGGA-3′ and reverse 5′-TGGAGGTCCACACACTGAAG-3′
for PSA; forward 5′-TTGACTGCCACTTCCTCG-3′ and reverse
5′-CATCCTTCGCCGACATGG-3′ for ODC1; forward
5′-CTGGTGGCTGATAGGGGAT-3′ and reverse 5′-GTCTGCCCTCATTTGTCGAT-3′
for TMPRSS2; forward 5′-TCCCTCGAATGCAACTCTCT-3′ and reverse
5′-GCCACATCTCTGCAGTCAAA-3′ for FKBP51; and forward
5′-GCCAAGAACCTCAAGCTCAC-3′ and reverse 5′-AGAAGGCCTCCTCTTTCAGG-3′
for NKX3-1.
Statistical analysis
All data are presented as the mean ± standard
deviation. Data that followed a normal distribution were analyzed
using Student's t-test or the one-way analysis of variance test for
comparisons between two groups. Dunn's method was used for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference. All statistical values were calculated
using SPSS software, version 19.0 (IBM SPSS, Armonk, NY, USA).
Results
DHA inhibits the growth of LNCaP
cells
DHA has been demonstrated to suppress the growth of
AR-positive, hormone-dependent LNCaP cells. The present study
examined the efficacy of DHA on LNCaP cells, under conditions of
hormone presence (in the presence of FBS), similar to the
conditions in patients undergoing androgen-dependent carcinogenesis
of prostate cancer. Firstly, increasing concentrations up to 100
µM DHA were selected to treat the LNCaP cells for 6 h, to
assess whether DHA has a toxic effect. Trypan blue staining
revealed no difference in the cells treated with DHA compared with
the control cells (Fig. 1A),
indicating that the concentrations of DHA used in the present study
caused no toxic effect on LNCaP cells. As shown in Fig. 1B, when LNCaP cells growing in
complete FBS were treated with DHA, there was decreased cell growth
in a dose-dependent manner. In addition, treatment with 50
µM DHA for varying durations on the LNCaP cells demonstrated
a time-dependent suppression of cell growth (Fig. 1C). However, DHA-treated AR-negative
PC3 and DU145 prostate cancer cells exhibited no response (Fig. 1D and E). This data suggested that
AR potentiates the inhibitory effect of DHA on the growth of LNCaP
cells when compared with those without the AR.
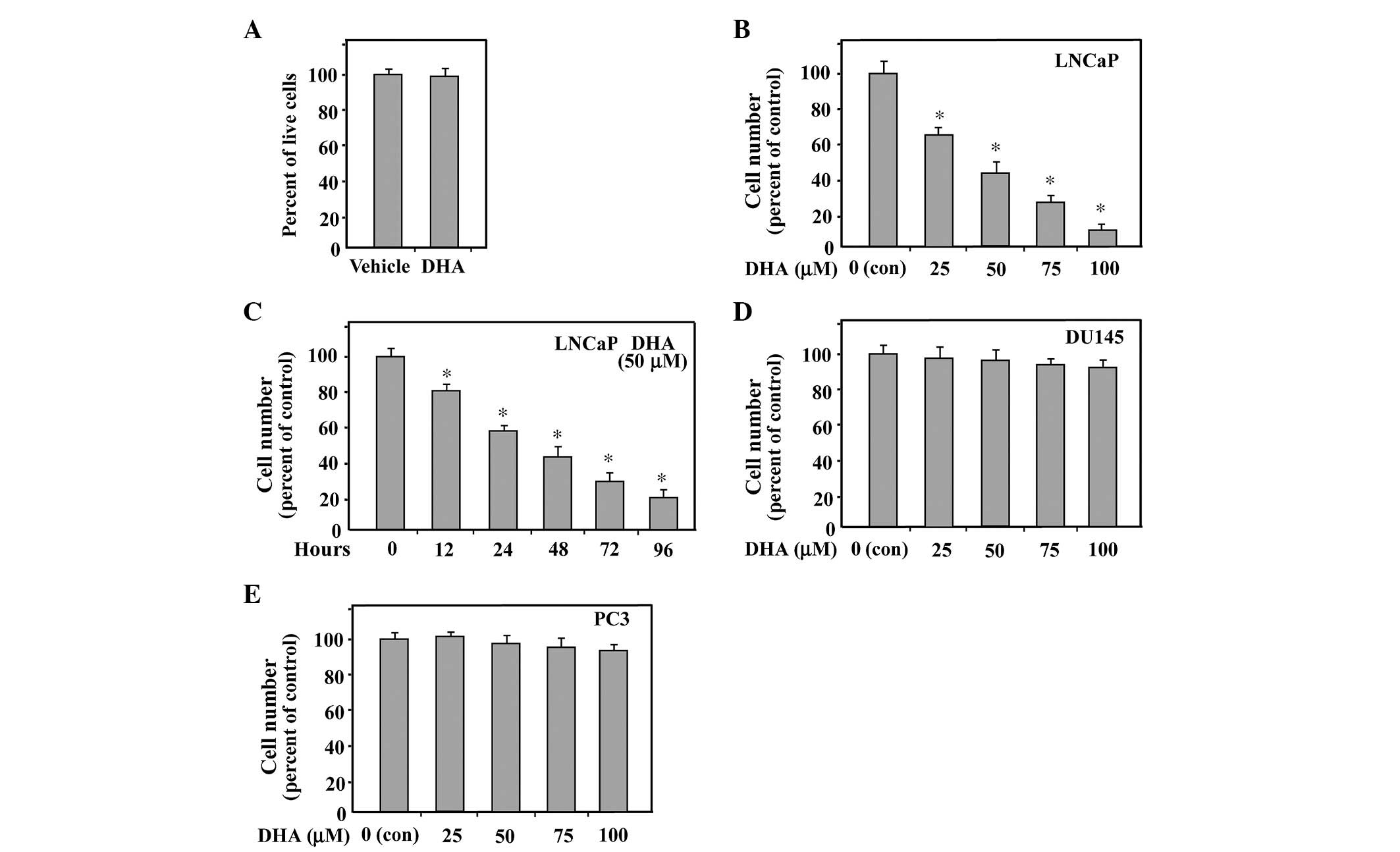 | Figure 1DHA exhibits no cytotoxic effect and
inhibits hormone-dependent growth of LNCaP cells. (A) LNCaP cells
cultured in medium containing 10% FBS were treated with 100
µM DHA for 6 h and subsequently trypan blue staining was
performed. The cell numbers were counted to measure the viability.
Ethanol treatment was used as a vehicle control. (B) LNCaP cells
were assessed by an MTT assay for viability following exposure for
48 h to media containing 10% FBS and varying concentrations of DHA.
Equal quantities of ethanol were used as a vehicle control. (C) An
MTT assay was performed on LNCaP cells following treatment with 50
µM DHA for the indicated duration. (D and E) PC3 and DU145
cells growing in media containing 10% FBS were treated with varying
concentrations of DHA for 48 h and cell viability was measured
using an MTT assay. The data are expressed as the mean ± standard
deviation for triplicate experiments. P-values were determined with
Student's t-test. *P<0.01, compared with control.
DHA, docosahexaenoic acid; MTT, 3, (4,5-dimethylthiazol-2-yl)
2,5-diphenyltetrazoliumbromide; con, control; FBS, fetal bovine
serum 3, (4,5-dimethylthiazol-2-yl)
2,5-diphenyltetrazoliumbromide. |
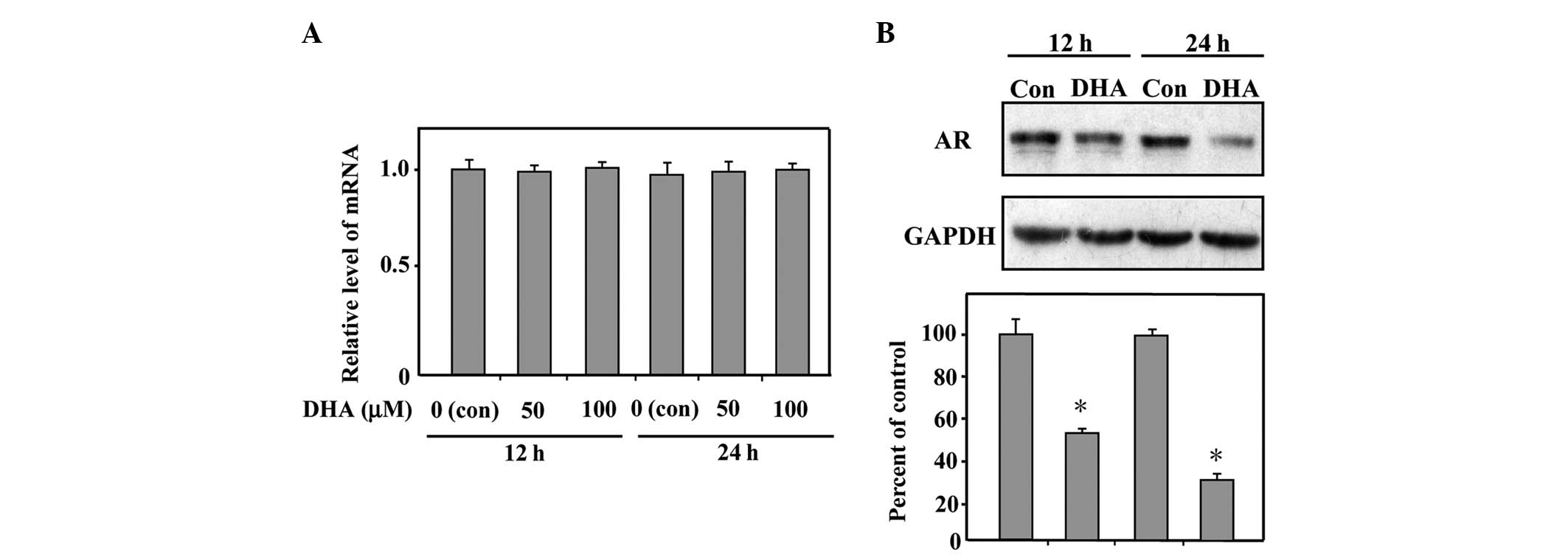
DHA reduces the protein expression level
of AR
To ascertain that DHA indeed affects the AR in LNCaP
cells, the present study examined the effect of DHA on the
expression levels of the AR. RT-qPCR analysis was performed to
confirm whether the transcribed mRNA expression levels of the AR
were affected by treatment with DHA. As shown in Fig. 2A, the mRNA expression levels of the
AR in the DHA-treated LNCaP cells were unaltered compared with
those from the control cells. The protein expression level of the
AR was further assessed and the result of the immunoblotting
revealed that treatment with 50 µM DHA for 12 or 24 h
downregulated the protein expression levels of the AR by 50 and
65%, respectively (Fig. 2B). These
data demonstrated that DHA exhibits no effect on the transcription
of the AR gene, however, significantly reduces the protein
expression level of the AR in LNCaP cells.
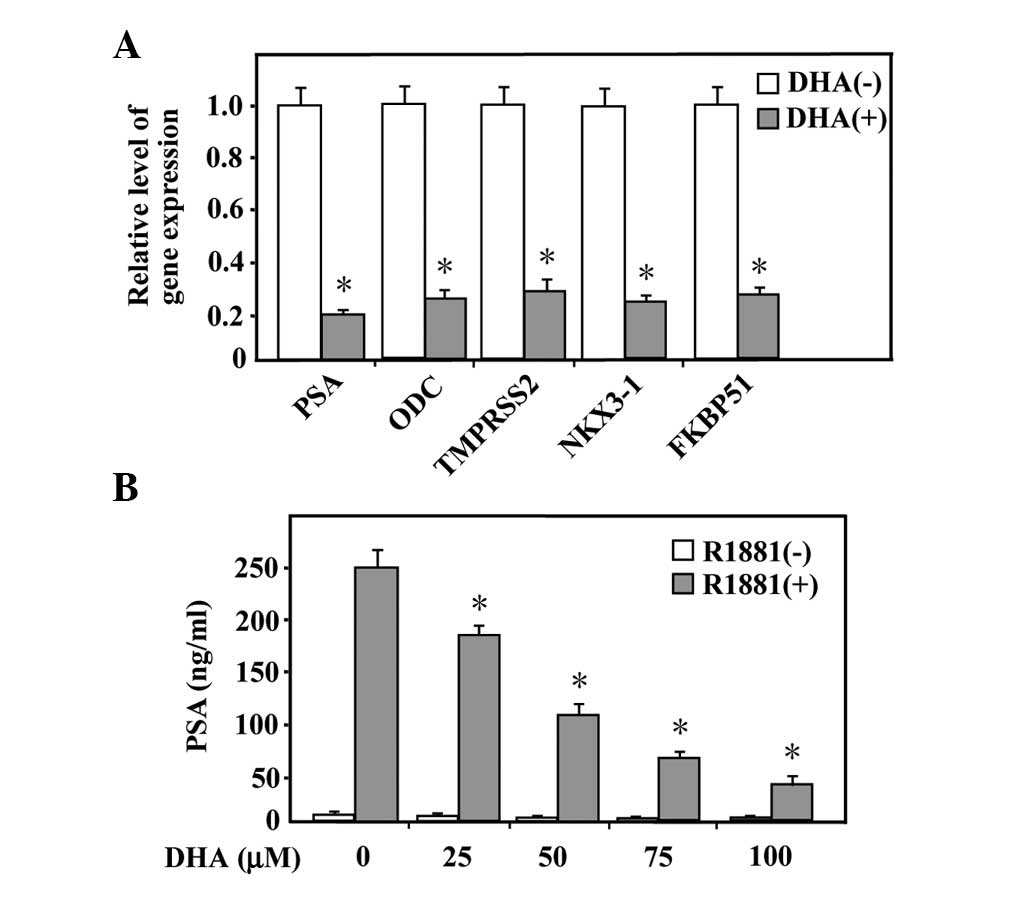
DHA represses androgen-regulated gene
expression
Since androgen functions via the androgen receptor,
which has been demonstrated to be reduced by DHA in LNCaP cells,
the present study further investigated whether the androgen action
was affected by DHA. RT-qPCR was performed to assess whether the
mRNA expression level of androgen-responsive genes, including PSA,
ODC, TMPRSS2, NKX3-1 and FKBP51, were affected by treatment with
DHA. As shown in Fig. 3A, the mRNA
expression levels of the selected genes were upregulated by
androgen and treatment with 100 µM DHA for 24 h
significantly repressed the induced response. In addition, the
quantity of secreted PSA was measured. The LNCaP cells cultured in
serum-free media or exposed to R1881 were treated with different
concentrations of DHA prior to the collection of the culture medium
for measurement of the total protein expression levels of secreted
PSA. As shown in Fig. 3B, androgen
stimulated the expression of PSA and treatment with DHA decreased
the androgen-induced expression of PSA in a dose-dependent manner.
These data indicated that the actions of androgens can be inhibited
in LNCaP cells by DHA.
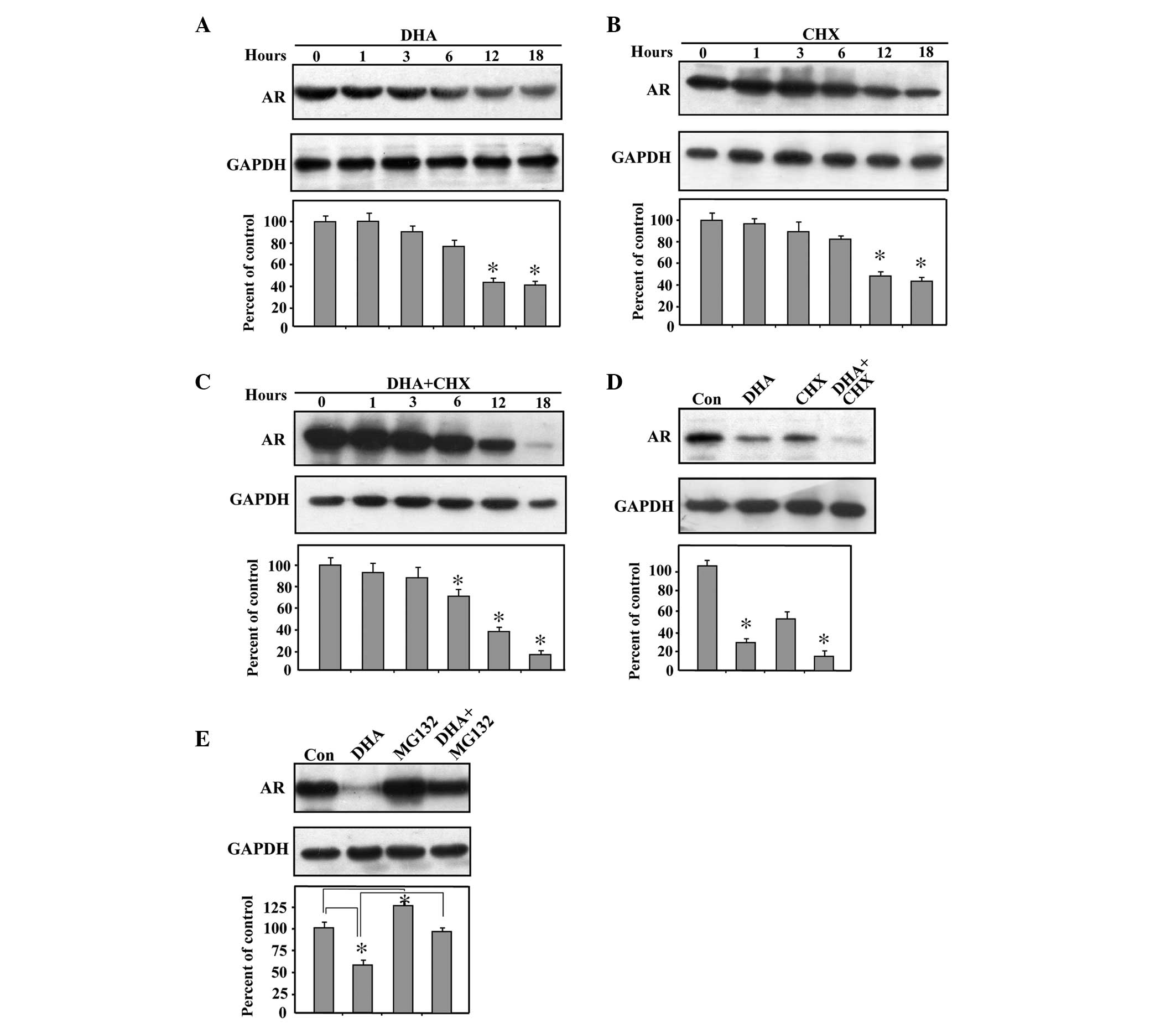
DHA promotes the proteasome-mediated
degradation of AR
To further elucidate the discrepant effects of DHA
on the mRNA and protein expression levels of the AR, the present
study examined the effects of DHA on the protein expression of the
AR at a range of durations. Treatment of the LNCaP cells with DHA
revealed a time-dependent decrease in the protein expression level
of the AR over the interval of 6–18 h (Fig. 4A). To ascertain whether this
decline in AR protein level reflects a reduced protein synthesis or
increased degradation by DHA, the protein translation inhibitor,
CHX, was used. Under conditions of CHX treatment and therefore, no
protein translation, it was observed that the AR protein declined
in a time-dependent manner, demonstrating a half-life of 12 h
(Fig. 4B). Furthermore, the
reduction in the AR protein was more pronounced with DHA in the
presence of CHX (Fig. 4C). In
addition, at the 18 h time point, there was a significant additive
effect between DHA and CHX when LNCaP cells were treated with each
drug (Fig. 4D). The additive
reduction in the protein expression levels of the AR by addition of
DHA beyond that already elicited by CHX indicated that the
DHA-induced decrease in AR protein levels was not mediated by an
inhibition of protein translation.
Since the evidence suggested that DHA has no effect
on protein translation, however, reduces the protein level of AR,
the present study next examined whether DHA acts as a regulator of
AR stability. The LNCaP cells were treated with MG132, a proteasome
inhibitor, to avoid proteasome-mediated degradation. As shown in
Fig. 4E, treatment with MG132
increased the protein expression levels of the AR compared with the
control, suggesting that AR is degraded by the proteasome. Notably,
the combined treatment of MG132 and DHA significantly increased the
protein expression level of AR compared with treatment with DHA
alone. The above data indicated that DHA promoted
proteasome-mediated degradation of the AR.
Discussion
The incidence and mortality rate of prostate cancer
differs among countries and regions. For instance, American males
have a higher incidence and mortality of prostate cancer compared
with Asian males (1). Several
previous studies have indicated that dietary factors may be
important in the incidence, progression and clinical outcome of
prostate cancer (26–28). In addition, dietary fat has been
demonstrated to promote or inhibit the growth of prostate cancer
(29). Epidemiological and
laboratory investigations have suggested that ω-3 fatty acids
inhibit the growth of prostate cancer cells and ω-6 fatty acids
promote the disease (11,14,30,31).
Based on this evidence, it has been speculated that the ω-3 fatty
acids may reduce the risk of prostate cancer and also inhibit the
growth of developing prostate tumors.
It has been revealed that ω-3 PUFAs repress the
growth of prostate cancer cells in vitro and reduce the
protein expression levels of the AR in LNCaP cells (24). However, the mechanism underlying
the reduced protein expression level of the AR remains to be
elucidated. The present study demonstrated for the first time, to
the best of our knowledge, that DHA, a ω-3 PUFA, promoted the
degradation of the AR in LNCaP cells. Furthermore, androgenic
induction of several androgen-regulated genes were significantly
inhibited by DHA at steady-state mRNA expression levels. The above
data indicated that DHA treatment repressed androgen action,
including the cell growth response.
The present study also used EPA, another ω-3 PUFA,
to treat LNCaP cells, however, EPA has been demonstrated to have no
significant repressive effect on LNCaP cell growth and revealed no
reduction in the protein expression levels of the AR at
concentrations <100 µM. Although DHA and EPA are each
long chain ω-3 PUFAs, EPA contains less unsaturated bonds, which
may result in a reduced inhibitory effect compared with DHA. It is
also possible that the concentrations of EPA used were lower than
required to exhibit its effect, since high concentrations of EPA
have an inhibitory effect on the growth of LNCaP cells.
The present study demonstrated that DHA exhibits an
inhibitory effect on the androgenic induction of gene expression.
DHA inhibited the expression of the prostate-specific gene, PSA,
and the ODC gene, which is ubiquitously expressed, which are
well-known direct target genes of the AR. In addition, TMPRSS2,
NKX3-1 and FKBP51, which are all upregulated by androgens, were
also repressed by treatment with DHA. These results indicated that
DHA can impair the transactivation ability of the AR. ODC is a
rate-limiting enzyme in the polyamine biosynthesis pathway, which
is known to be involved in the proliferation and differentiation of
normal and neoplastic cells (32).
Overexpression of ODC may be involved in the oncogenic process.
Therefore, the repressed expression of ODC by DHA may partially
explain the decrease in cell growth. The function of nuclear
receptors, including the AR, can be affected by expression level.
Androgens can stabi-lize the AR and therefore, increase the
expression level of the AR. Immunoblot analysis of the AR
demonstrated that DHA affected the androgen-mediated stabilizing
effect by reducing the level of the AR.
It has been elucidated that one of the mechanisms by
which prostate cancer cells become hormone-independent is by
increasing the levels of the AR, thereby sensitizing the receptor
to low levels of circulating androgens (33). Previous studies revealed that the
hormone-independent LNCaP clones demonstrated a significant
increase in the expression levels of the AR, as compared with their
hormone dependent clones (24).
Therefore, it may be helpful to reduce the levels of the AR during
the progression to hormone-independency, to prevent the growth of
LNCaP cells. The results from the present study demonstrated that
treatment with DHA inhibited the upregulation of the AR, indicating
that DHA may possibly be involved in modulating and regulating the
AR pathway.
Acknowledgments
This study was supported by the Tianjin Municipal
Science and Technology Commission (grant no. 12JCZDJC21600 to Dr
Wei Hong), the National Natural Science Foundation of China (grant
no. 81271203 to Dr Wei Hong), the German Academic Exchange Service
DAAD (to Mr. Mohsen Esmaeili) and the German Cancer Aid (to
Professor Aria Baniahmad) The authors would like to thank Dr David
Fisher for critically reading the manuscript.
Abbreviations:
|
PUFAs
|
polyunsaturated fatty acids
|
|
DHA
|
docosahexaenoic acid
|
|
AR
|
androgen receptor
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
MTT
|
3, (4,5-dimethylthiazol-2-yl)
2,5-diphenyltetrazoliumbromide
|
|
CHX
|
cycloheximide
|
References
|
1
|
Saman DM, Lemieux AM, Nawal Lutfiyya M and
Lipsky MS: A review of the current epidemiology and treatment
options for prostate cancer. Dis Mon. 60:150–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gathirua-Mwangi WG and Zhang J: Dietary
factors and risk for advanced prostate cancer. Eur J Cancer Prev.
23:96–109. 2014. View Article : Google Scholar :
|
|
3
|
Masko EM, Allott EH and Freedland SJ: The
relationship between nutrition and prostate cancer: Is more always
better? Eur Urol. 63:810–820. 2013. View Article : Google Scholar :
|
|
4
|
Pelser C, Mondul AM, Hollenbeck AR and
Park Y: Dietary fat, fatty acids and risk of prostate cancer in the
NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev.
22:697–707. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wright JL, Plymate S, D'Oria-Cameron A, et
al: A study of caloric restriction versus standard diet in
overweight men with newly diagnosed prostate cancer: A randomized
controlled trial. Prostate. 73:1345–1351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang JX, Wan JB and He C: Concise review:
Regulation of stem cell proliferation and differentiation by
essential fatty acids and their metabolites. Stem Cells.
32:1092–1098. 2014. View Article : Google Scholar
|
|
7
|
Olivo SE and Hilakivi-Clarke L: Opposing
effects of prepubertal low- and high-fat n-3 polyunsaturated fatty
acid diets on rat mammary tumorigenesis. Carcinogenesis.
26:1563–1572. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Z, Zhang Y, Jia C, et al: mTORC1/2
targeted by n-3 polyunsaturated fatty acids in the prevention of
mammary tumorigenesis and tumor progression. Oncogene.
33:4548–4557. 2014. View Article : Google Scholar
|
|
9
|
Apte SA, Cavazos DA, Whelan KA and
Degraffenried LA: A low dietary ratio of omega-6 to omega-3 Fatty
acids may delay progression of prostate cancer. Nutr Cancer.
65:556–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chua ME, Sio MC, Sorongon MC and Dy JS:
Relationship of dietary intake of omega-3 and omega-6 Fatty acids
with risk of prostate cancer development: A meta-analysis of
prospective studies and review of literature. Prostate Cancer.
2012:8262542012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akinsete JA, Ion G, Witte TR and Hardman
WE: Consumption of high ω-3 fatty acid diet suppressed prostate
tumorigenesis in C3 (1) Tag mice. Carcinogenesis. 33:140–148. 2012.
View Article : Google Scholar :
|
|
12
|
Berquin IM, Edwards IJ and Chen YQ:
Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer
Lett. 269:363–377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Virtanen JK, Mozaffarian D, Chiuve SE and
Rimm EB: Fish consumption and risk of major chronic disease in men.
Am J Clin Nutr. 88:1618–1625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Williams CD, Whitley BM, Hoyo C, et al: A
high ratio of dietary n-6/n-3 polyunsaturated fatty acids is
associated with increased risk of prostate cancer. Nutr Res.
31:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brasky TM, Till C, White E, et al: Serum
phospholipid fatty acids and prostate cancer risk: Results from the
prostate cancer prevention trial. Am J Epidemiol. 173:1429–1439.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Comba A, Lin YH, Eynard AR, Valentich MA,
Fernandez-Zapico ME and Pasqualini ME: Basic aspects of tumor cell
fatty acid-regulated signaling and transcription factors. Cancer
Metastasis Rev. 30:325–342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stillwell W, Shaikh SR, Zerouga M,
Siddiqui R and Wassall SR: Docosahexaenoic acid affects cell
signaling by altering lipid rafts. Reprod Nutr Dev. 45:559–579.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bazan NG: Omega-3 fatty acids,
pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr
Metab Care. 10:136–141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Balk SP: Androgen receptor functions in
prostate cancer development and progression. Asian J Androl.
16:561–564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taplin ME and Balk SP: Androgen receptor:
A key molecule in the progression of prostate cancer to hormone
independence. J Cell Biochem. 91:483–490. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang LG, Ossowski L and Ferrari AC:
Androgen receptor level controlled by a suppressor complex lost in
an androgen-independent prostate cancer cell line. Oncogene.
23:5175–5184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen CD, Welsbie DS, Tran C, Baek SH, Chen
R, Vessella R, Rosenfeld MG and Sawyers CL: Molecular determinants
of resistance to antiandrogen therapy. Nat Med. 10:33–39. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Linja MJ, Savinainen KJ, Saramäki OR,
Tammela TL, Vessella RL and Visakorpi T: Amplification and
overexpression of androgen receptor gene in hormone–refractory
prostate cancer. Cancer Res. 61:3550–3555. 2001.PubMed/NCBI
|
|
24
|
Friedrichs W, Ruparel SB, Marciniak RA and
deGraffenried L: Omega-3 fatty acid inhibition of prostate cancer
progression to hormone independence is associated with suppression
of mTOR signaling and androgen receptor expression. Nutr Cancer.
63:771–777. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hong W, Li J, Wang B, et al: Epigenetic
involvement of Alien/ESET complex in thyroid hormone-mediated
repression of E2F1 gene expression and cell proliferation. Biochem
Biophys Res Commun. 415:650–655. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Joshi AD, John EM, Koo J, Ingles SA and
Stern MC: Fish intake, cooking practices, and risk of prostate
cancer: Results from a multi-ethnic case-control study. Cancer
Causes Control. 23:405–420. 2012. View Article : Google Scholar
|
|
27
|
Huang M, Narita S, Numakura K, Tsuruta H,
Saito M, Inoue T, Horikawa Y, Tsuchiya N and Habuchi T: A high-fat
diet enhances proliferation of prostate cancer cells and activates
MCP-1/CCR2 signaling. Prostate. 72:1779–1788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang SN, Han J, Abdelkader TS, Kim TH,
Lee JM, Song J, Kim KS and Park JH and Park JH: High animal fat
intake enhances prostate cancer progression and reduces glutathione
peroxidase 3 expression in early stages of TRAMP mice. Prostate.
74:1266–1277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Berkow SE, Barnard ND, Saxe GA and
Ankerberg-Nobis T: Diet and survival after prostate cancer
diagnosis. Nutr Rev. 65:391–403. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Astorg P: Dietary N-6 and N-3
polyunsaturated fatty acids and prostate cancer risk: a review of
epidemiological and experimental evidence. Cancer Causes Control.
15:367–386. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McEntee MF, Ziegler C, Reel D, et al:
Dietary n-3 polyunsaturated fatty acids enhance hormone ablation
therapy in androgen-dependentprostate cancer. Am J Pathol.
173:229–241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Symes AJ, Eilertsen M, Millar M, et al:
Quantitative analysis of BTF3, HINT1, NDRG1 and ODC1 protein
over-expression in human prostate cancer tissue. PLoS One.
8:e842952013. View Article : Google Scholar
|
|
33
|
Chen Y, Sawyers CL and Scher HI: Targeting
the androgen receptor pathway in prostate cancer. Curr Opin
Pharmacol. 8:440–448. 2008. View Article : Google Scholar : PubMed/NCBI
|