1. Introduction
An oncogene is defined as a mutated gene, whose
product contributes to the initiation or progression of cancer
(1). Activation of oncogenes are
critical in the molecular pathogenesis of human neoplasms (2). Known oncogene, including Ras, BRAF,
β-catenin, and Myc, are predominantly protein-coding genes, the
functions of which are mediated by their gene products-proteins
(3–6). Genome sequencing projects have
revealed that the human genome contains <2% protein coding
genes, while>90% of the genome is transcripted into non-coding
(nc)RNAs (7,8). Based on nucleotide size, ncRNAs can
be classified into two major classes: Small ncRNAs and long
non-coding RNAs (lincRNAs) (9–11).
Small ncRNAs include the well-documented microRNAs. Several
microRNAs are increased in multiple types of cancer which exhibit
pro-oncogenic activity through the induction of oncogenes or the
inhibition of multiple genes with tumor suppressor-like activity
(12–14). LincRNAs are mRNA-like transcripts
consisting of >200 nucleotides. Increasing evidence has reported
the deregulation of lincRNAs across numerous types of cancer,
indicating that lincRNA may be involved in tumorigenesis (15). Several lincRNAs act as scaffolds,
which regulate molecular (protein, RNA and DNA) interactions that
are required for various signaling networks, and is accomplished
partly through association with chromatin-modifying c o mpl exes
(15–17).
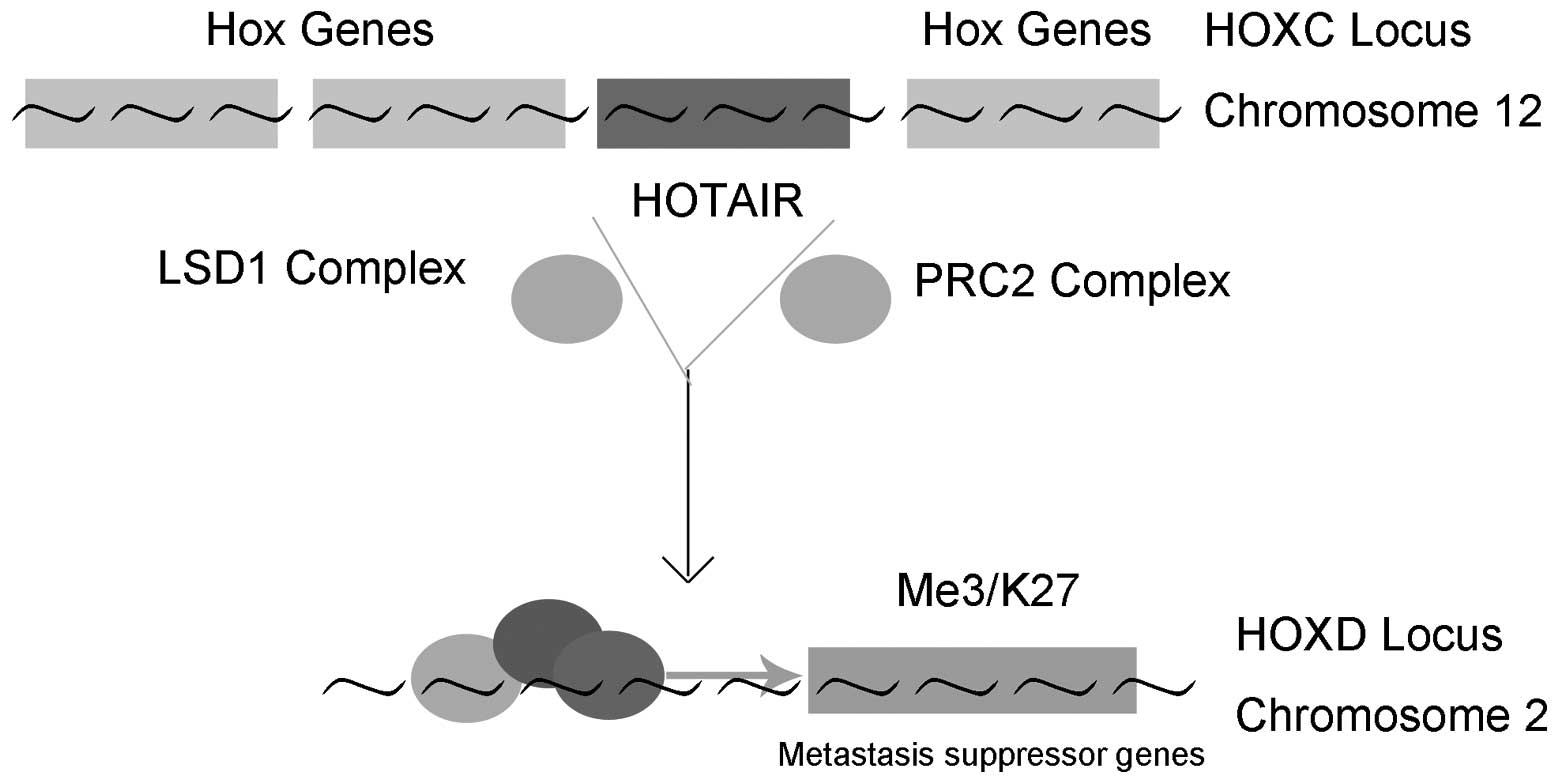
Hox transcript antisense intergenic RNA (HOTAIR) was
one of the first lincRNAs reported to be involved in the
development of cancer (18).
HOTAIR is transcribed from the HOXC locus but represses expression
in the more distal HOXD locus and genes on other chromosomes,
leading to the decreased expression of multiple genes, particularly
the metastasis suppressing genes (18–20).
HOTAIR is the first lincRNA identified to regulate genes at a
distance (18). HOTAIR is
overexpressed in several types of malignancy, including breast
cancer, gastric cancer, liver cancer and sarcoma (21–24).
Furthermore, in vitro analysis has demonstrated that HOTAIR
can promote cancer cells proliferation, invasion and metastasis,
while inhibit apoptosis (25).
Notably, in murine xenograft models, HOTAIR-knockout can reduce
tumor growth in vivo (26,27).
As a result, HOTAIR has been suggested as a potential oncogene.
The present review aims to discuss the current
knowledge of the properties of HOTAIR, its status in human tumors,
the mechanism in tumorigenesis and their potential implications in
cancer management.
2. Structure and biological function of
HOTAIR
HOTAIR, first identified from a custom-tilling array
of the HOXC locus, is encoded from the HOXC locus on chromosome
12q13.13 (18,28). It belongs to the long non-coding
RNAs, with 2,158 nucleotides. HOTAIR RNA does not encode any
proteins, however it is important in gene regulation by modifying
chromatin structure (16,18,20).
Polycomb proteins are a group of proteins involved
in the repression of transcription of thousands of genes, which are
critical in differentiation, maintenance of cell identity and
cancer development (20,29,30).
Polycomb proteins act in several protein complexes, polycomb
repressive complex (PRC) 1 and PRC2. PRC2 is a histone H3 lysine 27
(H3K27) methylase and, by modulating H3K27 methylation, PRC2 is
involved in developmental gene silencing and cancer progression
(28,31). The association between lincRNA and
plycomb proteins to induce silencing is considered a common
mechanism in the epigenetic regulation of lncRNAs, including
HOTAIR. The core PRC2 cannot target and silence genomic regions
alone. Instead, HOTAR binding is required to guide PRC2 to the
specific regions of the genome, where PRC2 associates and
epigenetically silences the gene expression. HOTAR binding results
in genome-wide retargeting of PRC2. HOTAIR contains two
independently binding domains, 5′ and 3′ domains, which bind the
PRC2 and lysine-specific demethylase 1 (LSD1) complexes,
respectively. The HOTAIR-PRC2-LSD1 complex then targets the HOXD
locus on chromosome 2, silencing the genes involved in the
suppression of metastasis (32),
as shown in Fig. 1. It is clear
that HOTAIR reprograms the chromatin to promote cancer metastasis.
However, the precise mechanism of the activities of HOTAIR remains
to be elucidated.
3. HOTAIR status in human malignancies
Since its identification in breast cancer, the
aberrant expression of HOTAIR has been reported in various types of
human cancer (22–24). lncRNA HOTAIR is an independent
prognostic marker of metastasis in estrogen receptor-positive
primary breast cancer. HOTAIR is overexpressed in multiple types of
cancers, and its overexpression is associated with metastasis and
poor survival rates. The regulation and function of HOTAIR in
cancer is summarized in Table
I.
 | Table IChanges in the expression of HOTAIR
in different types of human cancer. |
Table I
Changes in the expression of HOTAIR
in different types of human cancer.
| Type of cancer | Expression | Effect on
invasion/metastasis | Reference |
|---|
| Breast cancer | Increased | Promote | 20,33 |
| Esophageal
cancer | Increased | Promote | 35,36,37 |
| Lung cancer | Increased | Promote | 38,39,40 |
| Gastric cancer | Increased | Promote | 22,41 |
| Liver cancer | Increased | Promote | 23,42 |
| Endometrial
cancer | Increased | Unknown | 43 |
| Prostate
cancer | Increased | Promote | 44 |
| Nasopharyngeal
cancer | Increased | Promote | 45 |
| Laryngeal
cancer | Increased | Promote | 26, |
| Pancreatic
cancer | Increased | Promote | 27,46 |
| Colorectal
cancer | Increased | Unknown | 47 |
| Melanoma | Increased | Promote | 48 |
| Glioma | Increased | Promote | 49 |
| Sarcoma | Increased | Promote | 24 |
| Pituitary
adenoma | Increased | Unknown | 50 |
HOTAIR and breast cancer
A significantly higher level of HOTAIR expression is
observed in primary and metastatic breast cancer, as compared with
normal breast epithelium, and HOTAIR is an independent biomarker
for predicting the risk of metastasis and mortality in breast
cancer (20). Overexpression of
HOTAIR increases the invasive ability of breast cancer cells in
vitro and in vivo (20). High expression levels of HOTAIR
correlate positively with DNA methylation in primary breast cancer
(33). Methylation has been
associated with poor disease prognosis, however, no significant
associations have been identified between the expression of HOTAIR
and clinical outcomes, indicating that HOTAIR may not be an
independent prognostic biomarker in breast cancer (33). Sorensen et al demonstrated
that high expression levels of HOTAIR correlated with decreased
prognosis, serving as an independent predictor of metastasis in
patients with estrogen receptor (ER)-positive breast cancer, but
not those with ER-negative breast cancer (34).
HOTAIR and esophageal cancer
HOTAIR is upregulated in esophageal squamous cell
cancer (ESCC). A positive correlation exists between high levels of
HOTAIR and clinical stage, serving as an independent prognostic
factor in ESCC patients and cell lines (35,36).
Higher expression levels of HOTAIR are associated with advanced
disease stage, a poorer prognosis and reduced survival rate
(36). Depletion of HOTAIR in ESCC
cells reduces proliferation, foci formation, migration and invasion
of the extracellular matrix, alters cell cycle progression and
increases the sensitivity of cells to apoptosis in vitro
(35, 37). I n addition, microarray analysis
has revealed that HOTAIR reprograms the gene expression profile in
ESCC cells, leading to an increase in genes involved in
tumorigenesis, including genes regulating migration and cell cycle
(37). HOTAIR can also decrease
the expression of Wnt inhibitory factor 1 (WIF-1), which is
important in cell proliferation, migration and tumor progression
(36). The HOTAIR/WIF-1 axis
provides a potential molecular mechanism of HOTAIR in the
pathogenesis of ESCC (36).
HOTAIR and lung cancer
Using reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) analysis in 42 non-small cell lung cancer
(NSCLC) samples, HOTAIR levels were found to be significantly
higher in the cancerous tissues, compared with normal lung cells,
correlating positively with the pathological stage and lymph node
metastasis (38,39). HOTAIR is a prognostic parameter for
survival rates, and higher expression levels of HOTAIR are
associated with a poorer prognosis in patients with NSCLC (39,40).
In addition, subsequently functional analysis in vitro has
revealed its mediation in the migration and invasion of NSCLC cell
lines. HOTAIR knockdown was observed to affect the level of HOXA5,
which inhibited the migration and invasion of NSCLC cells,
indicating that HOTAIR may partially exert its effects through the
regulation of HOXA5 (38,39). HOTAIR is induced by type I collagen
(Col-1), which was a type of interstitial extracellular matrix
(ECM), aberrantly enriched in the tumor microenvironment (39).
HOTAIR and gastric cancer
The expression level of HOTAIR are significantly
increased in gastric cancer tissues, significantly correlating with
lymph node metastasis and tumor-node-metastasis stage (22,41).
High expression levels of HOTAIR predict poorer overall survival
rates in patients with gastric cancer (41). In addition, gastric cancer cells
expressing HOTAIR exhibit higher proliferation ability in
vitro and a higher rate of liver metastasis (22). Loss of functional analysis has
revealed the importance of HOTAIR in gastric cancer cell invasion
in vitro. The expression of MMP1 and MMP3, which can favor
metastasis by remodeling the ECM and degrading the basement
membrane, are also inhibited by HOTAIR knockdown (41).
HOTAIR and liver cancer
HOTAIR is significantly upregulated in
hepatocellular carcinoma (HCC) tissues compared with adjacent
normal tissues (23). The
expression of HOTAIR is an independent prognostic factor for HCC
recurrence following liver transplantation (23). Patients with higher expression
levels of HOTAIR have been observed to have increased size and
reduced prognosis, compared with those with lower expression levels
of HOTAIR (42). Depletion of
HOTAIR in HepG2 cells decreases cell viability and invasiveness,
and increases the sensitivity to tumor necrosis factor-α-induced
apoptosis and chemotherapy (23).
In addition, the introduction of HOTAIR into liver cancer cells
increased the proliferation rate in vitro (42). These results indicate the
functional role of HOTAIR in the progression of liver cancer.
HOTAIR and endometrial cancer
The levels of HOTAIR are significantly higher in
endometrial cancer (EC) tissues than the normal tissues (43). A positive correlation has been
observed between the expression of HOTAIR and the EC grade, depth
of the myometrium, lymphovascular invasion and lymph node
metastasis (43). Similar to the
types of cancer mentioned above, the expression of HOTAIR is also a
predictor of poor prognosis in patients with EC (43).
HOTAIR and prostate cancer
The expression of HOTAIR is upregulated in
castration-resistant prostate cancer (PCa) cell lines, compared
with normal prostate cells. Knockdown of the expression of HOTAIR
by small interfering RNA leads to a reduction in PCa cell
proliferation, migration and invasion, and increased apoptosis and
cell cycle arrest (44).
HOTAIR and nasopharyngeal cancer
The expression of HOTAIR is higher in nasopharyngeal
cancer, compared with non-cancerous tissue, which is positively
associated with tumor size, clinical stage and lymph node burden.
Furthermore, HOTAIR overexpression has been associated with
significantly decreased survival rates (45). In addition, in vitro
analysis has demonstrated that HOTAIR is important in the
proliferation, migration and invasion of nasopharyngeal cancer
cells (45).
HOTAIR and laryngeal cancer
According to qPCR analysis, HOTAIR is significantly
overexpressed in laryngeal squamous cell carcinoma, compared with
adjacent non-neoplastic tissue (26), and its expression level is higher
in high-grade carcinoma than in low-grade carcinoma. Furthermore,
patients with higher expression levels of HOTAIR have been observed
to have a relatively poorer prognosis. In vitro analysis
demonstrated that knockdown of HOTAIR in the Hep-2 cells inhibited
the invasion and promoted apoptosis (26). In addition, HOTAIR knockout murine
xenograft models reduce the growth of laryngeal squamous cell
carcinoma tumors in vivo. Knockdown of HOTAIR in Hep-2 cells
resulted in a significant decrease in the methylation level of
PTEN, which is a tumor suppressor gene involved in various types of
human malignancy. Therefore, the epigenetic modification of PTEN
may be the underlying molecular mechanism for tumorigenesis by
HOTAIR (26).
HOTAIR and pancreatic cancer
The expression of HOTAIR is high in pancreatic
cancerous tissue, compared with non-cancerous tissue (27,46).
Its expression level is also higher in high-grade carcinoma than in
low-grade carcinoma. In addition, the expression of HOTAIR is
significantly increased in primary pancreatic tumors, which have
metastasized to lymph nodes, compared with those that are localized
in the pancreas only (27).
Consistent with the findings in other cancer cell lines, HOTAIR
depletion in Panc1 and L3.6pL pancreatic cancer cells inhibits cell
proliferation, alters cell cycle progression and induces apoptosis,
indicating its functional role in the progression of pancreatic
cancer (27). Of note, in murine
xenograft models, HOTAIR knockout in the pancreatic cancer cells
inhibited tumor growth in vivo, further demonstrating the
pro-oncogenic function of HOTAIR in pancreatic cancer (27). HOTAIR knockdown resulted in changes
in the expression of 1,006 genes, which may contribute to the
functional pro-oncogenic activity of HOTAIR in Panc1 cells
(27).
HOTAIR and colorectal cancer
qPCR analysis has revealed that the expression
levels of HOTAIR are higher in colorectal cancer compared with
non-tumor tissues (47).
Furthermore, HOTAIR has also been revealed as a negative prognostic
factor for patients with colorectal cancer (47). The expression levels of HOTAIR were
found to be higher in tumors, which metastasized to the liver,
compared with those that did not metastasize; indicating that the
upregulation of HOTAIR may be a critical element in the metastatic
progression of colorectal cancer (47).
HOTAIR and melanoma
Melanoma is a type of skin cancer with high
potential to metastasize to the vital organs. HOTAIR has been
demonstrated to be involved in the metastasis of melanoma.
Expression levels of HOTAIR are higher in metastatic lymph nodes
than in corresponding primary melanoma (48). Furthermore, in vitro
analysis has revealed that HOTAIR depletion decreases the motility
and invasion of human melanoma cells and inhibits degradation of
the gelatin matrix (48).
HOTAIR and glioma
Glioma is the most common primary tumor in the brain
(49). Expression levels of HOTAIR
are higher in high-grade glioma, compared with low-grade glioma.
Higher levels of HOTAIR were identified as an indicator for reduced
survival rates, serving as an independent overall survival factor
(49). Gene set enrichment
analysis has revealed that the expression of HOTAIR modulates gene
sets involved in cell cycle progression, which may be the mechanism
underlying the pro-oncogenic activity of HOTAIR (49).
HOTAIR and sarcoma
Sarcomas consist of a heterogenous group of soft
tissue and bone malignant tumors (24). Expression of HOTAIR has been
detected in the primary and metastatic sarcoma tissues using
western blotting and RT-qPCR analysis. In addition, high expression
levels of HOTAIR have been correlated with a high probability of
metastasis in primary sarcoma. Of note, the inhibition of HOTAIR
has been observed to result in a good response to treatment, in
terms of necrosis (24).
HOTAIR and pituitary adenomas
Pituitary adenomas belong to primary intracranial
tumors, the majority of which secrete pituitary hormones that
result in increased levels of blood hormones and clinical
syndromes. A non-functioning pituitary adenoma (NFPA) is defined as
a pituitary adenomas, which does not cause clinical hormone
hypersecretion. The majority of pituitary adenomas are
histologically benign; however, they have a marked ability to
invade surrounding structures. The expression of HOTAIR has been
reported to increase between normal anterior pituitaries,
non-invasive NFPAs and invasive NFPAs, indicating a potential role
of HOTAIR in NFPA invasion (50).
In conclusion, a substantial number of studies have
revealed that HOTAIR is upregulated in multiple types of human
cancer, and its overexpression is correlated with metastasis and
poor prognosis. HOTAIR may serve as a potential tumor marker and
therapeutic target in the future. Further investigations are
required to clarify its expression in other types of cancer,
particularly of the molecular mechanisms associated with the
suppression and activation of genes by HOTAIR in cancer.
4. Mechanisms of HOTAIR in
tumorigenesis
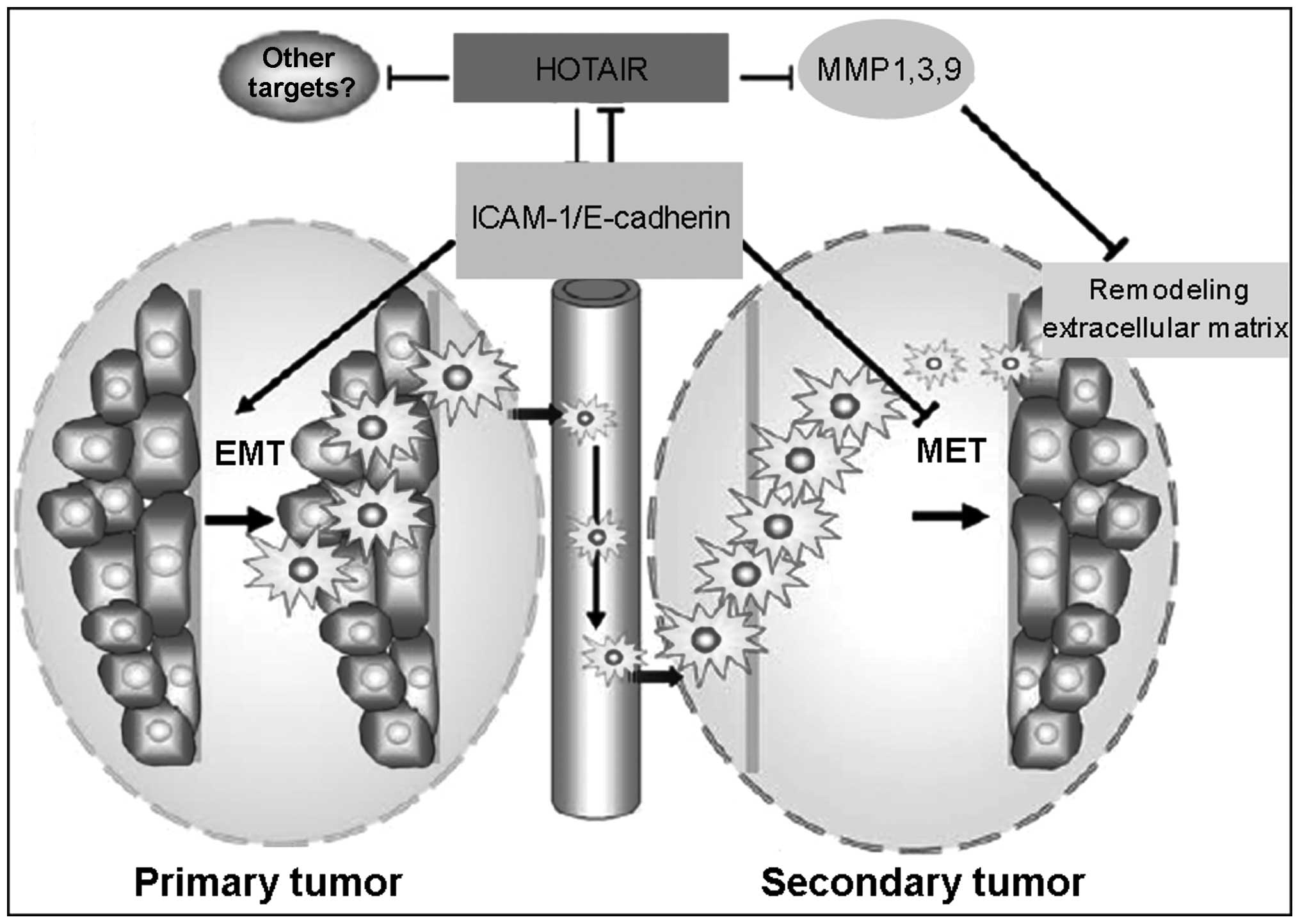
Epithelial-to-mesenchymal transition
(EMT) and maintenance of cancer stem cells (CSCs)
The EMT is a multistep process by which epithelial
cells lose epithelial characteristics and gain mesenchymal
characteristics, including motility and invasive properties
(51). The metastatic process
involves several steps. EMT is considered to be the first step of
metastatic spread. Dissociation of cells from the epithelial layer
results in the deregulation of intercellular contacts and the
acquisition of migratory abilities.
CSCs are pluripotent tumor-initiating cells, which
have the ability to self-renew. Due to their unique
characteristics, CSCs are considered the basis for tumor
initiation, development, metastasis and recurrence (52).
The overexpression of HOTAIR has been reported to
induce EMT and support CSCs, with HOTAIR silencing leading to the
failure of EMT and CSC establishment (53). In addition, in vitro
analysis has demonstrated that suppression of HOTAIR can reverse
EMT process in gastric cancer cells (41). The molecular mechanism by which
HOTAIR induces EMT remains to be elucidated. The overexpresion of
HOTAIR increases the expression of snail, which is a potent EMT
inducer (53). HOTAIR can also
accelerate the degradation of Ataxin-1, which can activate the
promoter of E-cadherin. E-cadherin is the key regulator of the EMT,
as it regulates epithelial cell-to-cell interactions (54).
It has been suggested that HOTAIR may be involved in
EMT/CSC establishment by regulating certain critical molecular
signaling pathways. A potential explanation may be found in the
HOTAIR targeting of intercellular adhesion molecule (ICAM)-1 and
members of the matrix metalloproteinase (MMP) family, including
MMP1, MMP3 and MMP9 in gastric cancer (41). MMP1 and MMP3 have been reported to
be suppressed by HOTAIR knockdown (41), and MMPs can favor metastasis by
remodeling the ECM and degrading the basement membrane. In
addition, HOTAIR facilitates the ubiquitination and, thus,
accelerates the degradation of Ataxin-1. Ataxin-1 has been
demonstrated to activate the promoter of E-cadherin, which is a key
tumor suppressor that suppresses the invasiveness of cancer cells
(55–57). These findings indicate that HOATIR
may promote metastasis through the regulation of the
metastasis-associated genes, including MMPs and Ataxin-1 (Fig. 2).
HOTAIR and the control of gene
transcription
Several genes are induced or repressed by HOTAIR,
including WIF-1, HOX D10, M M P1/3, PTEN and snail (20,36,41).
In esophageal cancer, HOTAIR decreases the expression of WIF-1
(36), which is critical in cell
proliferation, migration and tumor progression. HOTAIR directly
decreases the expression of WIF-1 by promoting its histone, H3K27,
methylation in the promoter region, activating Wnt/b-catenin
signaling pathway. The Wnt/b-catenin signaling pathway is important
in mediating cell proliferation and migration, and in controlling
tumor progression, and ha been found to be aberrantly activated in
multiple types of cancer, including ESCC (5,58).
In breast cancer, the overexpression of HOTAIR leads to epigenetic
silencing of multiple genes, including HOXD10 and other metastasis
suppressor genes (20). In gastric
cancer, as mentioned above, overexpression of HOTAIR is associated
with the increase of ICAM-1 and certain members of the MMPs family
including MMP1, MMP3, and MMP9 (41), indicating that HOATIR may promote
metastasis through the regulation of MMPs.
A significant role of lncRNAs is in the regulation
of gene expression via a mechanism involving interaction with the
epigenetic silencing complex, polycomb repressive complex 2 (PRC2),
with ~20% of all lncRNA transcripts estimated to bind PRC2
(20,47). Modulation of HOTAIR alters the
levels of various genes in several cancer cells, the majority of
which are PRC2-dependent (20,47).
HOTAIR-induced PRC2 target genes include JAM2, PCDH10 and PCDHB5,
and the majority are positive regulators of cancer metastasis
(20). In addition, the modulation
of the expression of these genes and the metastatic effect of
HOTAIR can be reversed by simultaneous PRC2 depletion, revealing a
HOTAIR-polycomb pathway in cancer invasion (46). HOTAR binding is required to target
PRC2 to the specific regions of the genome. As a H3K27 methylase,
PRC2 can modulate H3K27 methylation. Methylation of H3K27 leads to
transcriptional repression and is, therefore, involved in
controlling gene expression patterns (59). Deregulation of H3K27 methylation
patterns are also commonly observed in multiple types of cancer
(60).
Ubiquitination is a process commonly leading to the
proteasome-mediated degradation of the target protein (61). Ubiquitination is one of the most
important post-translational modifications, with a pivotal role in
tumor development, and can regulate tumor suppressors and
oncogenes. HOTAIR can control protein levels by promoting
ubiquitin-mediated proteolysis (62). HOTAIR facilitates the
ubiquitination of Ataxin-1 and Snurportin-1, in cells and in
vitro, by associating with E3 ubiquitin ligases bearing
RNA-binding domains, Dzip3 and Mex3b, as well as with their
respective ubiquitination substrates, Ataxin-1 and Snurportin-1. In
this manner, HOTAIR can accelerate the degradation of Ataxin-1 and
Snurportin-1. Ataxin-1 has been demonstrated to activate the
promoter of E-cadherin, a key tumor suppressor that suppresses the
invasiveness of cancer cells (55–57).
In conclusion, by binding PRC2, HOTAIR can regulate
the expression of genes that are conducive to cell motility, matrix
invasion and remodeling the chromatin state in cancer cells.
5. Regulation of HOTAIR expression
As mentioned above, HOTAIR is frequently increased
in a variety of human malignances. However, the mechanism leading
to its deregulation in human cancer remains to be elucidated. In
lung cancer cells, HOTAIR is induced by type I collagen (Col-1), a
type of interstitial ECM that is aberrantly enriched in the tumor
microenvironment, indicating that the expression of HOTAIR in
cancer cells may result from the response of the cancer cells to
Col-1 (63). In breast cancer,
bisphenol-A (BPA) and diethylstilbestrol (DES) induce HOTAIR
expression in cultured human breast cancer cells and in vivo
in the mammary glands of rats (64). BPA and DES exposure induce HOTAIR
expression by altering the epigenetic programming of the HOTAIR
promoters. In the presence of BPA and DES, HOTAIR promoter
estrogen-response-elements (EREs) are bound by ERs and ER
co-regulators, their chromatin is modified via histone methylation
and acetylation, and they are activated by HOTAIR.
6. Implications in cancer management
As the overexpression of HOTAIR has been documented
in a number of human malignancies, it provides a promising approach
for anti-cancer therapies. Firstly, HOTAIR can be used as a
biomarker for cancer diagnosis. High expression levels of HOTAIR
are correlated with enhanced metastasis and is presents a negative
prognostic factor for patient survival rates. By monitoring the
levels of HOTAIR in certain types of tumor, including alterations
in gene or protein levels, the risk of tumor development and
progression, and the prognosis of the tumor, can be predicted. The
observation in murine xenograft models that HOTAIR knockout can
reduce tumor growth in vivo (26,27),
knockout of the HOTAIR gene or decreasing the protein level of
HOTAIR may provide a promising target for cancer therapy.
7. Conclusions and future directions
Since its first observation, high expression levels
of HOTAIR in various types of human tumor have been well
documented. High expression levels of HOTAIR correlate with
enhanced metastasis in cancer, and overexpression of HOTAIR is
associated with poorer prognosis in cancer patients, most notably
due to the increased metastasis.
To further clarify its role, the expression of
HOTAIR in other types of cancer requires investigation. Although
the expression of HOTAIR has been extensively investigated in
various types of cancer, the molecular mechanism underlying the
effects of HOTAIR in tumorigenesis remain to be elucidated. It
appears that HOTAIR is involved in modulating the cancer epigenome
and reprogramming the chromatin state, which is accomplished
through the interaction with PRC2. HOTAIR binding can induce PRC2
to specific regions of the genome, which can repress or promote the
transcription of genes, including WIF-1, HOXD10, MMP1/3, PTEN and
snail. The majority of these genes regulate cancer cell
proliferation and invasion, and are associated with tumor
progression. By binding PRC2, HOTAIR can regulate gene expression
and remodel chromatin states, which is conducive to cell motility
and matrix invasion in cancer cells.
The molecular mechanism underlying the upregulation
of HOTAIR in cancer also requires further investigation, to
determine which transcription factors or signaling pathways mediate
HOTAIR induction in cancer, In lung cancer cells, HOTAIR is induced
by Col-1, which is a type of ECM aberrantly enriched in the tumor
microenvironment. Col-1 has been demonstrated to promote the
development of cancer, indicating that the expression of HOTAIR in
cancer cells may result from the response of cancer cells to Col-1
(63). However, further
investigations are required to fully elucidated the function of
this highly expressed molecule in cancer.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81401847).
References
|
1
|
Kim H, Huang W, Jiang X, Pennicooke B,
Park PJ and Johnson MD: Integrative genome analysis reveals an
oncomir/oncogene cluster regulating glioblastoma survivorship. Proc
Natl Acad Sci USA. 107:2183–2188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
3
|
Young A, Lou D and McCormick F: Oncogenic
and wild-type Ras play divergent roles in the regulation of
mitogen-activated protein kinase signaling. Cancer Discov.
3:112–123. 2013. View Article : Google Scholar
|
|
4
|
Thiel A and Ristimaki A: Toward a
molecular classification of colorectal cancer: the role of BRAF.
Front Oncol. 3:2812013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: components, mechanisms and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimizu T, Ishikawa T, Sugihara E, et al:
c-MYC overexpression with loss of Ink4a/Arf transforms bone marrow
stromal cells into osteosarcoma accompanied by loss of
adipogenesis. Oncogene. 29:5687–5699. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stein LD: Human genome: end of the
beginning. Nature. 431:915–916. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ponting CP and Belgard TG: Transcribed
dark matter: meaning or myth? Hum Mol Genet. 19:R162–R168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brosnan CA and Voinnet O: The long and the
short of noncoding RNAs. Curr Opin Cell Biol. 21:416–425. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mattick JS: Non-coding RNAs: the
architects of eukaryotic complexity. EMBO Rep. 2:986–991. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Costa FF: Non-coding RNAs: new players in
eukaryotic biology. Gene. 357:83–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu J, Getz G, Miska EA, et al: MicroRNA
expression profiles classify human cancers. Nature. 435:834–838.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nelson KM and Weiss GJ: MicroRNAs and
cancer: past, present and potential future. Mol Cancer Ther.
7:3655–3660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Farazi TA, Spitzer JI, Morozov P and
Tuschl T: miRNAs in human cancer. J Pathol. 223:102–115. 2011.
View Article : Google Scholar :
|
|
15
|
Huarte M and Rinn JL: Large non-coding
RNAs: missing links in cancer? Hum Mol Genet. 19:R152–R161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khalil AM, Guttman M, Huarte M, et al:
Many human large intergenic noncoding RNAs associate with
chromatin-modifying complexes and affect gene expression. Proc Natl
Acad Sci USA. 106:11667–11672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spitale RC, Tsai MC and Chang HY: RNA
templating the epigenome: long noncoding RNAs as molecular
scaffolds. Epigenetics. 6:539–543. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rinn JL, Kertesz M, Wang JK, et al:
Functional demarcation of active and silent chromatin domains in
human HOX loci by noncoding RNAs. Cell. 129:1311–1323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Woo CJ and Kingston RE: HOTAIR lifts
noncoding RNAs to new levels. Cell. 129:1257–1259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta RA, Shah N, Wang KC, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chisholm KM, Wan Y, Li R, Montgomery KD,
Chang HY and West RB: Detection of long non-coding RNA in archival
tissue: correlation with polycomb protein expression in primary and
metastatic breast carcinoma. PloS one. 7:e479982012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Endo H, Shiroki T, Nakagawa T, et al:
Enhanced expression of long non-coding RNA HOTAIR is associated
with the development of gastric cancer. PloS One. 8:e770702013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Z, Zhou L, Wu LM, et al:
Overexpression of long non-coding RNA HOTAIR predicts tumor
recurrence in hepatocellular carcinoma patients following liver
transplantation. Ann Surg Oncol. 18:1243–1250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Milhem MM, Knutson T, Yang S, et al:
Correlation of MTDH/AEG-1 and HOTAIR expression with metastasis and
response to treatment in Sarcoma patients. J Cancer Sci Ther.
S5:pii0042011.
|
|
25
|
Ono H, Motoi N, Nagano H, et al: Long
noncoding RNA HOTAIR is relevant to cellular proliferation,
invasiveness and clinical relapse in small-cell lung cancer. Cancer
Med. 3:632–642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li D, Feng J, Wu T, et al: Long intergenic
noncoding RNA HOTAIR is overexpressed and regulates PTEN
methylation in laryngeal squamous cell carcinoma. Am J Pathol.
182:64–70. 2013. View Article : Google Scholar
|
|
27
|
Kim K, Jutooru I, Chadalapaka G, et al:
HOTAIR is a negative prognostic factor and exhibits pro-oncogenic
activity in pancreatic cancer. Oncogene. 32:1616–1625. 2013.
View Article : Google Scholar
|
|
28
|
Sparmann A and van Lohuizen M: Polycomb
silencers control cell fate, development and cancer. Nature reviews
Cancer. 6:846–856. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Orlando V: Polycomb, epigenomes and
control of cell identity. Cell. 112:599–606. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mohammad HP, Cai Y, McGarvey KM, et al:
Polycomb CBX7 promotes initiation of heritable repression of genes
frequently silenced with cancer-specific DNA hypermethylation.
Cancer Res. 69:6322–6330. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kleer CG, Cao Q, Varambally S, et al: EZH2
is a marker of aggressive breast cancer and promotes neoplastic
transformation of breast epithelial cells. Proc Natl Acad Sci USA.
100:11606–11611. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsai MC, Manor O, Wan Y, et al: Long
noncoding RNA as modular scaffold of histone modification
complexes. Science. 329:689–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu L, Zhu G, Zhang C, et al: Association
of large noncoding RNA HOTAIR expression and its downstream
intergenic CpG island methylation with survival in breast cancer.
Breast Cancer Res Treat. 136:875–883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sorensen K P, Thomassen M, Tan Q, et al:
Long non-coding RNA HOTAIR is an independent prognostic marker of
metastasis in estrogen receptor-positive primary breast cancer.
Breast Cancer Res Treat. 142:529–536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lv XB, Lian GY, Wang HR, Song E, Yao H and
Wang MH: Long noncoding RNA HOTAIR is a prognostic marker for
esophageal squamous cell carcinoma progression and survival. PloS
One. 8:e635162013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ge XS, Ma HJ, Zheng XH, et al: HOTAIR, a
prognostic factor in esophageal squamous cell carcinoma, inhibits
WIF-1 expression and activates Wnt pathway. Cancer Sci.
104:1675–1682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Wu Z, Mei Q, Guo M, Fu X and Han W:
Long non-coding RNA HOTAIR, a driver of malignancy, predicts
negative prognosis and exhibits oncogenic activity in oesophageal
squamous cell carcinoma. Br J Cancer. 109:2266–2278. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu XH, Liu ZL, Sun M, Liu J, Wang ZX and
De W: The long non-coding RNA HOTAIR indicates a poor prognosis and
promotes metastasis in non-small cell lung cancer. BMC Cancer.
13:4642013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Egeblad M, Rasch MG and Weaver VM: Dynamic
interplay between the collagen scaffold and tumor evolution. Curr
Opin Cell Biol. 22:697–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakagawa T, Endo H, Yokoyama M, et al:
Large noncoding RNA HOTAIR enhances aggressive biological behavior
and is associated with short disease-free survival in human
non-small cell lung cancer. Biochem Biophys Res Commun.
436:319–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu ZY, Yu QM, Du YA, et al: Knockdown of
long non-coding RNA HOTAIR suppresses tumor invasion and reverses
epithelial-mesenchymal transition in gastric cancer. Int J Biol
Sci. 9:587–597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ishibashi M, Kogo R, Shibata K, et al:
Clinical significance of the expression of long non-coding RNA
HOTAIR in primary hepatocellular carcinoma. Oncol Rep. 29:946–950.
2013.PubMed/NCBI
|
|
43
|
He X, Bao W, Li X, et al: The long
non-coding RNA HOTAIR is upregulated in endometrial carcinoma and
correlates with poor prognosis. Int J Mol Med. 33:325–332.
2014.
|
|
44
|
Chiyomaru T, Yamamura S, Fukuhara S, et
al: Genistein inhibits prostate cancer cell growth by targeting
miR-34a and oncogenic HOTAIR. PloS One. 8:e703722013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nie Y, Liu X, Qu S, Song E, Zou H and Gong
C: Long non-coding RNA HOTAIR is an independent prognostic marker
for nasopharyngeal carcinoma progression and survival. Cancer Sci.
104:458–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stratford JK, Bentrem DJ, Anderson JM, et
al: A six-gene signature predicts survival of patients with
localized pancreatic ductal adenocarcinoma. PLoS Med.
7:e10003072010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kogo R, Shimamura T, Mimori K, et al: Long
noncoding RNA HOTAIR regulates polycomb-dependent chromatin
modification and is associated with poor prognosis in colorectal
cancers. Cancer Res. 71:6320–6326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tang L, Zhang W, Su B and Yu B: Long
noncoding RNA HOTAIR is associated with motility, invasion and
metastatic potential of metastatic melanoma. Biomed Res Int.
2013:2510982013. View Article : Google Scholar
|
|
49
|
Zhang JX, Han L, Bao ZS, et al: HOTAIR, a
cell cycle-associated long noncoding RNA and a strong predictor of
survival, is preferentially expressed in classical and mesenchymal
glioma. Neuro Oncol. 15:1595–1603. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li Z, Li C, Liu C, Yu S and Zhang Y:
Expression of the long non-coding RNAs MEG3, HOTAIR and MALAT-1 in
non-functioning pituitary adenomas and their relationship to tumor
behavior. Pituitary. 18:42–47. 2015. View Article : Google Scholar
|
|
51
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen K, Huang YH and Chen JL:
Understanding and targeting cancer stem cells: therapeutic
implications and challenges. Acta Pharmacol Sin. 34:732–740. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Padua Alves C, Fonseca AS, Muys BR, et al:
Brief report: The lincRNA HOTAIR is required for
epithelial-to-mesenchymal transition and stemness maintenance of
cancer cell lines. Stem Cells. 31:2827–2832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rangel MC, Karasawa H, Castro NP, Nagaoka
T, Salomon DS and Bianco C: Role of Cripto-1 during
epithelial-to-mesenchymal transition in development and cancer. Am
J Pathol. 180:2188–2200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee S, Hong S, Kim S and Kang S: Ataxin-1
occupies the promoter region of E-cadherin in vivo and activates
CtBP2-repressed promoter. Biochim Biophys Acta. 1813:713–722. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jeanes A, Gottardi CJ and Yap AS:
Cadherins and cancer: how does cadherin dysfunction promote tumor
progression? Oncogene. 27:6920–6929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Stemmler MP: Cadherins in development and
cancer. Mol Biosyst. 4:835–850. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nakajima M, Fukuchi M, Miyazaki T, Masuda
N, Kato H and Kuwano H: Reduced expression of Axin correlates with
tumour progression of oesophageal squamous cell carcinoma. Br J
Cancer. 88:1734–1739. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kouzarides T: Chromatin modifications and
their function. Cell. 128:693–705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shi Y, Lan F, Matson C, et al: Histone
demethylation mediated by the nuclear amine oxidase homolog LSD1.
Cell. 119:941–953. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pickart CM: Mechanisms underlying
ubiquitination. Annu Rev Biochem. 70:503–533. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yoon JH, Abdelmohsen K, Kim J, et al:
Scaffold function of long non-coding RNA HOTAIR in protein
ubiquitination. Nat Commun. 4:29392013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hebner C, Weaver VM and Debnath J:
Modeling morphogenesis and oncogenesis in three-dimensional breast
epithelial cultures. Annu Rev Pathol. 3:313–339. 2008. View Article : Google Scholar
|
|
64
|
Bhan A, Hussain I, Ansari KI, Bobzean SA,
Perrotti LI and Mandal SS: Bisphenol-A and diethylstilbestrol
exposure induces the expression of breast cancer associated long
noncoding RNA HOTAIR in vitro and in vivo. J Steroid Biochem Mol
Biol. 141:160–170. 2014. View Article : Google Scholar : PubMed/NCBI
|