Introduction
In dentistry, >20 metallic elements are processed
into various types of dental metal alloy. These alloys are then
cast and processed for use as metal restorations. Previous studies
have demonstrated that various symptoms are associated with
different metals (1,2). Nickel (Ni), chromium (Cr), mercury
(Hg), palladium (Pd) and cobalt (Co) are metals, which are commonly
used in dentistry, and have been known to cause allergies. Allergic
reactions to these materials occur not only in the mucosa of the
oral cavity, but also on the hands, feet and entire body (3,4). A
previous study performed a patch test on 212 patients with
suspicious metal allergies, and demonstrated that Ni exhibited the
highest rate of positivity (25%), followed by Pd (24.4%), Cr
(16.7%) and Co (15.9%) (5). Patch
tests are considered the most reliable method for the diagnosis of
a metal allergy. When the allergic antigen is a metal ion, primary
irritation responses occur readily, and it is often difficult to
distinguish irritation from allergic reactions. In the case of type
IV hypersensitivity, patch tests are usually used to determine the
allergen, and are considered the gold standard in the diagnosis of
type IV hypersensitivity reactions (6). Studies have shown that the levels of
HLA-DR expression allows the identification of patients with
clinical marginal rejection.
Metals used in dentistry can lead to metal
sensitization, and the sensitization rates of metals differ
(7). The present study used a
patch test to comparatively analyze the various metal allergic
reactions of patients, who had undergone repair work or dental
restorations using oral metals, in order to provide guidance for
dentists in terms of the selection of appropriate metals, as well
as to provide a reference for patients with oral mucosa and skin
diseases. In addition, the present study aimed to identify the most
suitable metallic material in order to provide a foundation for
patient treatment.
Materials and methods
Subjects
The present study was performed by recruitment of 92
outpatients of dental clinics in Huashan Hospital (Shanghai, China)
between September 2011 and December 2012. The inclusion criteria
were as follows: (i) All patients (age, 18–65 years; 43 male, 49
female) provided written, informed consent prior to involvement in
the investigation, were able to receive tests in accordance with
the program requirements and attend follow-up sessions; (ii) no
lesions were present on the tested area; (iii) patients had
previously received an alloy restoration in the oral cavity; (iv)
patients had stopped using oral antihistamines at least 3 days, and
systemic corticosteroids and immunosuppressive drugs at least 2
weeks prior to the start of the investigation; (v) topical systemic
corticosteroids and immunosuppressive drugs had not been applied to
the test site at least 2 weeks prior to the start of the
investigation.
The present study was approved by the Ethics
Committee of the Huashan Hospital affiliated to Fudan University
(Shanghai, China).
Patch test evaluation method
Using the Ruimin patch series (Chemotechnique MB
Diagnostics AB, Vellinge, Sweden), 20 allergens were assessed,
including 19 types of metal allergens and a control
(Vaseline®; Unilever, London, UK). The metal allergens
assessed comprised normal metal components contained in dental
restorations (Table I). The patch
test (positioned on the back skin on eithe side of the spine) was
performed using an IQ Test Core Chamber (Nanjing Allergy
Biotechnology Co.,. Ltd., Jiangsu, China). The International
Contact Dermatitis Group's recommended patch test recording method
was adopted, as follows: +++, strong positive reaction (erythema,
significant invasion, papula, blisters, bullous pemphigoid); ++,
positive reaction (erythema, invasion, papula, blisters); +, weak
positive reaction (erythema, invasion, small pimple); ?+,
suspicious reaction (mild erythema); -, negative reaction; IR,
irritation; NT, not tested. +, ++ and +++ were considered a
positive allergic reaction (8).
 | Table IAllergen composition and concentration
in the patch test. |
Table I
Allergen composition and concentration
in the patch test.
| No. | Allergen | Concentration
(%) |
|---|
| 1 |
H12AlCl3O6 | 2.0 |
| 2 |
Na3Au(S2O3)2·2H2O | 2.0 |
| 3 | SnO2 | 1.0 |
| 4 | FeCl3 | 2.0 |
| 5 |
(NH4)2PtCl6 | 0.1 |
| 6 | PdCl2 | 2.0 |
| 7 | InCl3 | 10.0 |
| 8 | IrCl3 | 1.0 |
| 9 | ZnCl2 | 1.0 |
| 10 | MnO2 | 2.0 |
| 11 | AgNO3 | 1.0 |
| 12 |
Cr2K2O7 | 0.5 |
| 13 | CoCl2 | 1.0 |
| 14 | CuSO4 | 2.0 |
| 15 | HgCl2 | 0.1 |
| 16 | NiSO4 | 5.0 |
| 17 | CdCl2 | 1.0 |
| 18 |
H8MoN2O4 | 1.0 |
| 19 |
TiC2O4 | 5.0 |
| 20 |
Vaseline® | 100.0 |
Metal component assessment
An X-ray fluorescence microscope spectrometer (XFMS;
XGT-5000XIISL, HORIBA Trading Co., Ltd., Shanghai, Japan) was used
to detect metal components. The measurements were performed using a
charge-coupled device camera. This method is able to detect
elements in the periodic table from 11Na to
92U, with a resolution ≤150 eV, measurement
range/accuracy of 0–40.96 keV, temperature of 23°C and relative
humidity of 55%.
Silicone OneGloss (Japanese Pine Corp., Kariya,
Japan) was specifically developed for the repair, polishing and
shaping of resin and glass ionomer. Silicon particles were removed,
and XFMS was used to detect the metal components.
Immunohistochemical analysis
Gingival tissues were fixed in 4% paraformaldehyde
(Sangon Biotech Co., Ltd., Shanghai, China) for 24 h and embedded
in paraffin (Sangon Biotech Co., Ltd.). The tissue was cut into 4
mm sections, blocked in 0.5% bovine serum albumin (Sigma-Aldrich,
St. Louis, MO, USA) for 30 min and incubated with rabbit anti-human
leukocyte antigen (HLA)-DR polyclonal antibody (cat. no. ab175085;
Abcam, Cambridge, UK; dilution 1:1,000) overnight at 4°C in a humid
chamber, prior to being washed three times with 0.01 M
phosphate-buffered saline (PBS). The tissue samples were
subsequently incubated with secondary antibody (goat
anti-rabbit/mouse; Dako, Glostrup, Denmark) at 37°C for 1 h, prior
to being washed three times with 0.01 M PBS. The immune complex was
visualized using a Dako REAL™EnVision™ Detection system containing
peroxidase/DAB, according to the manufacturer's protocol. The
nuclei were counterstained with hematoxylin (Sangon Biotech Co.,
Ltd.), and the sections were observed under a Nikon Eclipse 50i
microscope (Nikon Corporation, Tokyo, Japan).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The tissue samples (100 mg) and TRIzol®
reagent (1 ml; Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) were homogenized in a homogenate machine at 120 hz for 5
min, and then centrifuged at 12,000 × g for 15 min at 4°C in order
to obtain the supernatant. Total RNA (1 μg) was isolated
from the gingival tissues (3 cm3) obtained from the
patients who exhibited allergic reactions using TRIzol®
reagent and was converted into cDNA using a cDNA synthesis kit
(cat. no. DRR037A; Takara Bio, Inc., Otsu, Japan), according to the
manufacturer's protocol. RT-qPCR was performed to determine the
expression levels of HLA-DR using SYBR Supermix (Takara Bio Inc.,
Otsu, Japan) and RT-qPCR Supermix (Takara Bio, Inc.) with the
following thermocycling conditions: Denaturation at 95°C for 30
sec; annealing at 95°C for 5 sec; and extension at 60°C for 30 sec
for 40 cycles. The relative expression levels of HLA-DR were
calculated using the 2−Δ(ΔCq) method (9). The expression of the HLA-DR
transcripts were normalized to the expression of glyceraldehyde
3-phosphate dehydrogenase (GAPDH) in the same sample. Primer
sequences (Sangon Biotech Co., Ltd.) were as follows: HLA-DR,
forward 5′-CAG GCG AGT TTA TGT TTG-3′ and reverse 5′-GAT TTC CAG
GTT GGC TTT-3′; GAPDH forward 5′-CCA CTC CTC CAC CTTTG-3′ and
reverse 5′-CAC CAC CCT GTT GCTGT-3′.
Immunoblotting
A total of 100 mg gingival tissue sample was added
to 1 ml radioimmunoprecipitation assay (Beyotime Institute of
Biotechnology, Shanghai, China) and homogenized in a homogenate
apparatus at 120 Hz for 5 min prior to being centrifuged at 10,000
× g at 4°C for 15 min in order to obtain the supernatant. Total
protein (80 ng) was extracted from gingival tissue and quantified
using a Bicinchoninic Acid Protein Assay kit (Pierce Biotechnology,
Inc., Rockford, USA). The cell lysates were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (Beyotime
Institute of Biotechnology), transferred to PVDF membranes (Merck
Millipore Corporation, Darmstadt, Germany), and blocked with 5%
skimmed milk powder for 1 h at room temperature prior to being
washed three times with Tris-buffered saline with 0.1% Tween 20.
The membranes were subsequently incubated with anti-HLA-DR primary
antibodies (Abcam) and appropriate horseradish
peroxidase-conjugated secondary antibody. The blots were visualized
by chemiluminescence (Cell Signaling Technology, Inc., Danvers, MA,
USA). GAPDH (Abcam) was used as a loading control. Proteins
expression levels were quantified using Image J 2× 2.1.4.7 software
(National Institutes of Health, Bethesda, MA, USA).
Statistical analysis
Each experiment was performed at least three times.
Data are presented as the mean ± standard deviation. Statistical
significance between groups was determined using one-way analysis
of variance and a one-sample t-test. SPSS software (version 19.0;
IBM SPSS, Armonk, NY, USA) was used analyze the data. P<0.05 was
considered to indicate a statistically significant difference.
According to the conditions, Pearson's χ2 test,
corrected χ2 test and Fisher's exact test were used.
Results
Comparative analysis of the patch
test
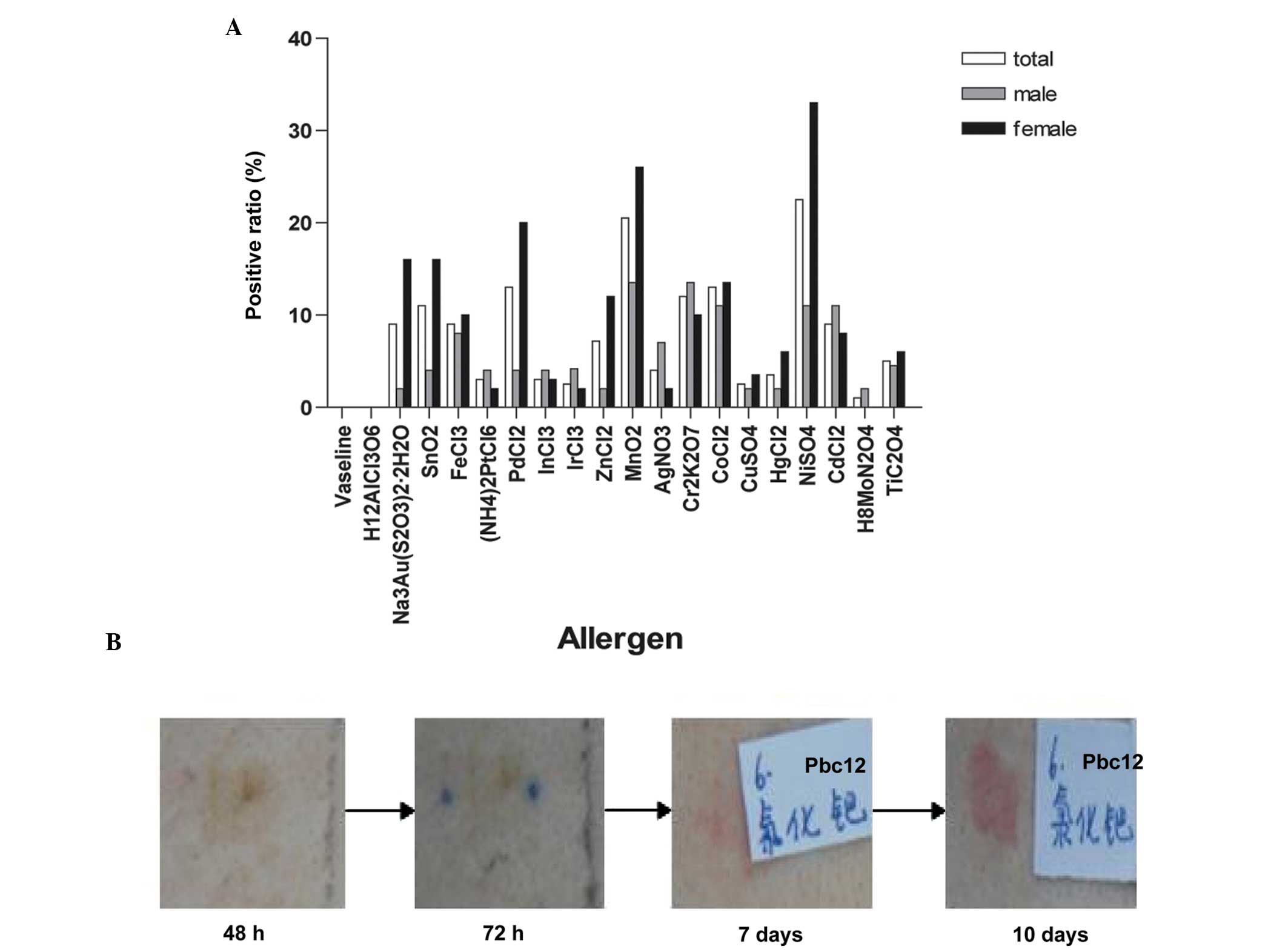
A total of 19 metal allergens and one control were
comparatively analyzed in the patch test (Fig. 1A); the control group
(Vaseline®) resulted in a negative reaction. There were
49 cases of at least one metal allergy, in which males accounted
for 20 cases (46.5%) and females accounted for 29 cases (59.2%).
There were positive reactions in response to at least two metal
allergens in 36 patients: 12 males (27.9%) and 24 females
(49%).
Delayed reaction
A delayed reaction was observed in the patch test.
Usually, results are observed at 72 h; however, in the present
study, results were observed at 96 h, 7 days or longer, in order to
exclude false-negative results. In total, six subjects exhibited a
delayed reaction, including five subjects whose first positive
reaction occurred in 7 days or whose original positive reaction was
more severe. Delayed reactions were observed in response to Ni, Hg
and Cr metal allergens. In one subject, the Pd test sites were
negative at 48 and 72 h; however, after 7 days the Pd test sites
exhibited minor erythema, and after 10 days a significant positive
reaction was detected (Fig.
1B).
Patch test analysis
In patients diagnosed with metal allergy, the
majority of clinical symptoms were relieved in the follow-up,
following removal or replacement of the prosthesis.
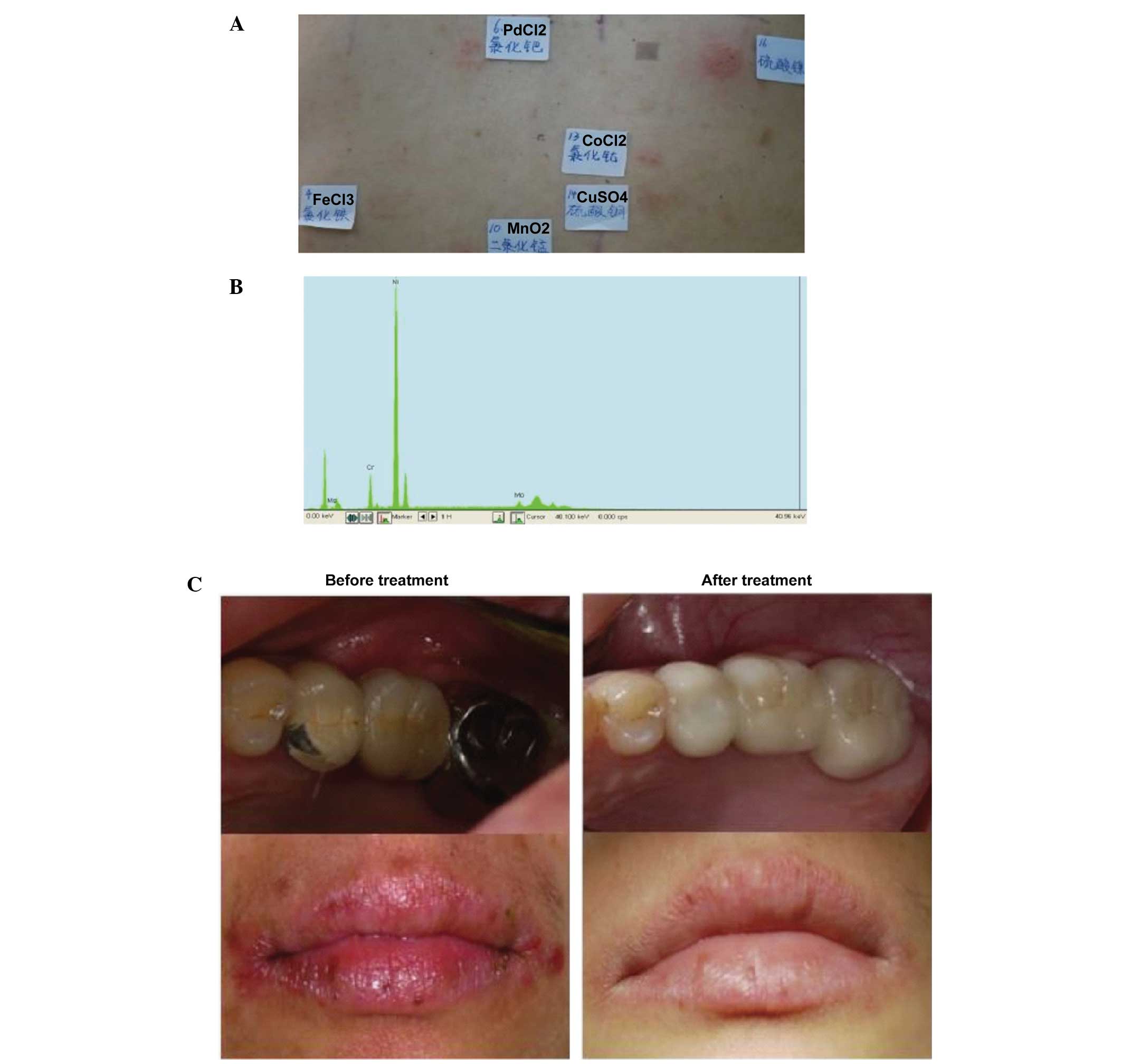
Case 1
Patch test result: NiSO4 (++),
PdCl2 (+), CoCl2 (+), MnO2 (+)
(Fig. 2A). The following metal
components were detected in the restoration: Ni (87.52%), Cr
(9.65%) and molybdenum (Mo; 2.65%) by XFMS (Fig. 2B). The patient had a had a strong
positive reaction to Ni in the patch test and, using XFMS, the
restoration was shown to contain up to 87.52% Ni; clinical symptoms
occurred following dental repair, and the patch test results and
metal prosthesis component test results were consistent. Therefore,
removal of the metal restoration was recommended in this patient,
which was replaced with a ceramic fixed bridge. The patient's
symptoms were relieved after 1 month, detected on follow-up
observation (Fig. 2C).
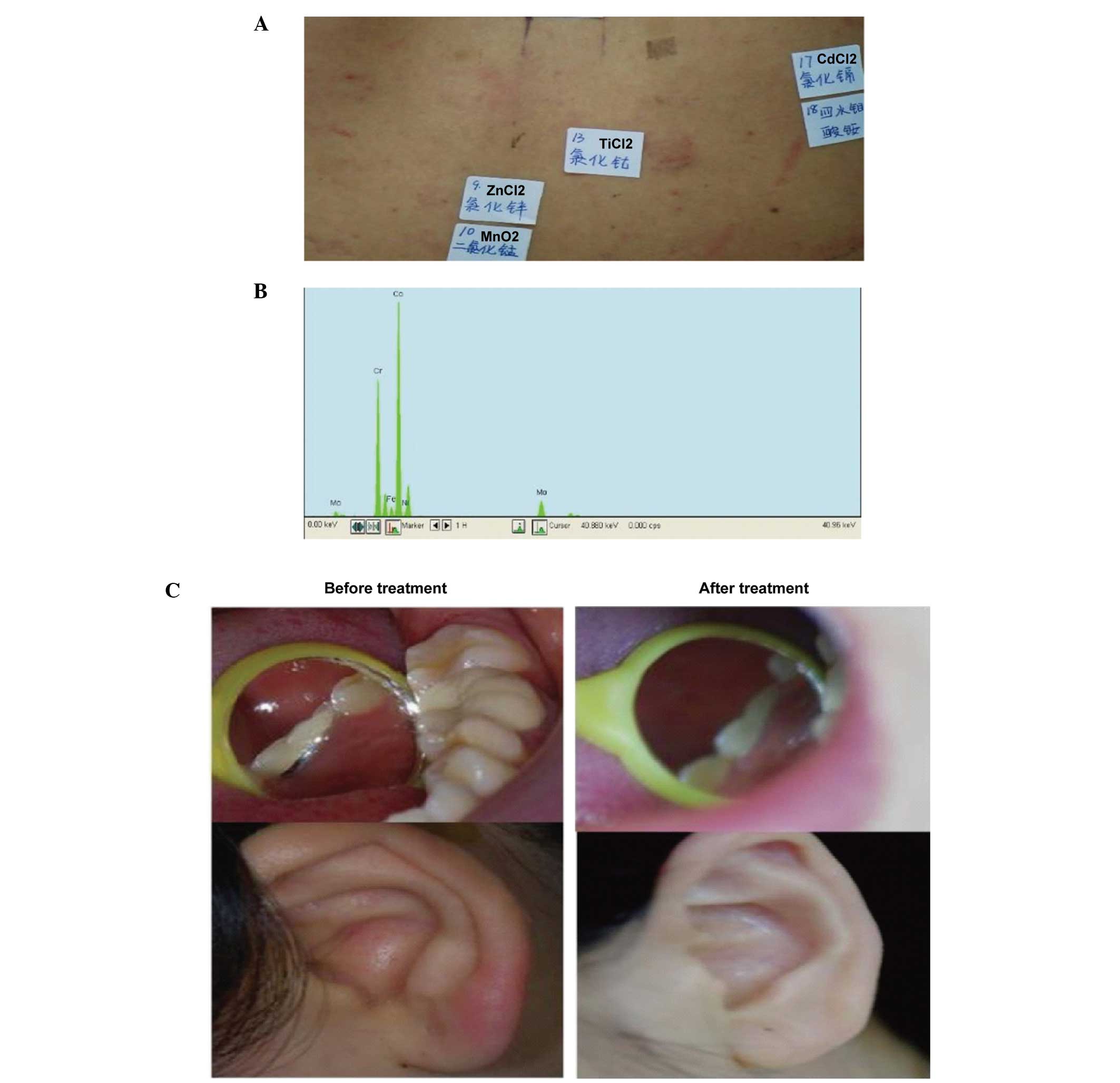
Case 2
Patch test result: ZnCl2 (+),
CoCl2 (+) (Fig. 3A).
The following metal components were detected in the restoration: Co
(73.96%), Cr (17.82%) and iron (Fe; 8.22%) by XFMS (Fig. 3B). The patient had a strong
positive reaction to Co and, using XFMS, the restoration was shown
to contain up to Co 73.96%; clinical symptoms occurred following
dental repair, and the patch test results and metal prosthesis
component test results were consistent. Therefore, it was
determined that the clinical symptoms of patients was associated
with metal prostheses sensitivity and. It was recommended that the
metal restoration be removed in this patient, which was replaced
with a ceramic crown. The patient's symptoms had improved at the 1
month follow-up observation (Fig.
3C).
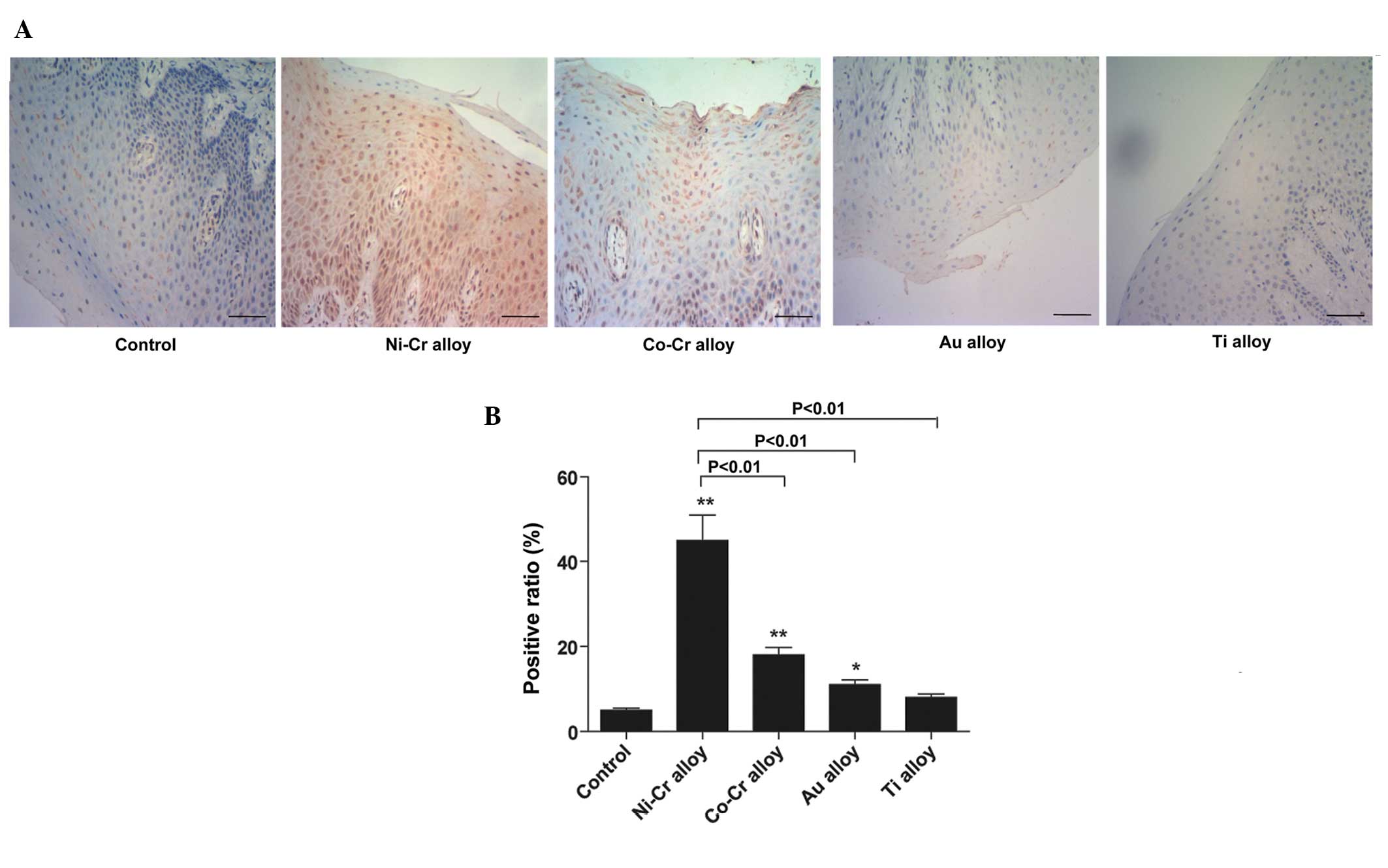
Expression levels of HLA-DR in gingival
tissue of patients with metal restorations
The protein expression levels were of HLA-DR were
significantly higher in the gingival tissues of the patients with
metal restorations, compared with gingival tissues in those
without, as detected using immunohistochemistry (P<0.01). In
normal gingival tissues, HLA-DR was visible only in a small number
of interstitial cells, including lymphocytes and dendritic cells.
In the patients with metal restorations extending submucosaly, the
mucosal epithelium and connective tissue had increased protein
expression levels of HLA-DR (Fig. 4A
and B). A small amount of light yellow staining was observed in
the cytoplasm, indicating strong positive expression.
The protein and gene expression levels of HLA-DR
were significantly lower in the control group, compared with those
in the other groups (P<0.01). The protein and gene expression
levels of HLA-DR were significantly higher in the Ni-Cr prosthesis
group, compared with the other groups (P<0.01); followed by the
Co-Cr alloy, Au alloy and Ti alloy groups, sequentially (Fig. 5A and B).
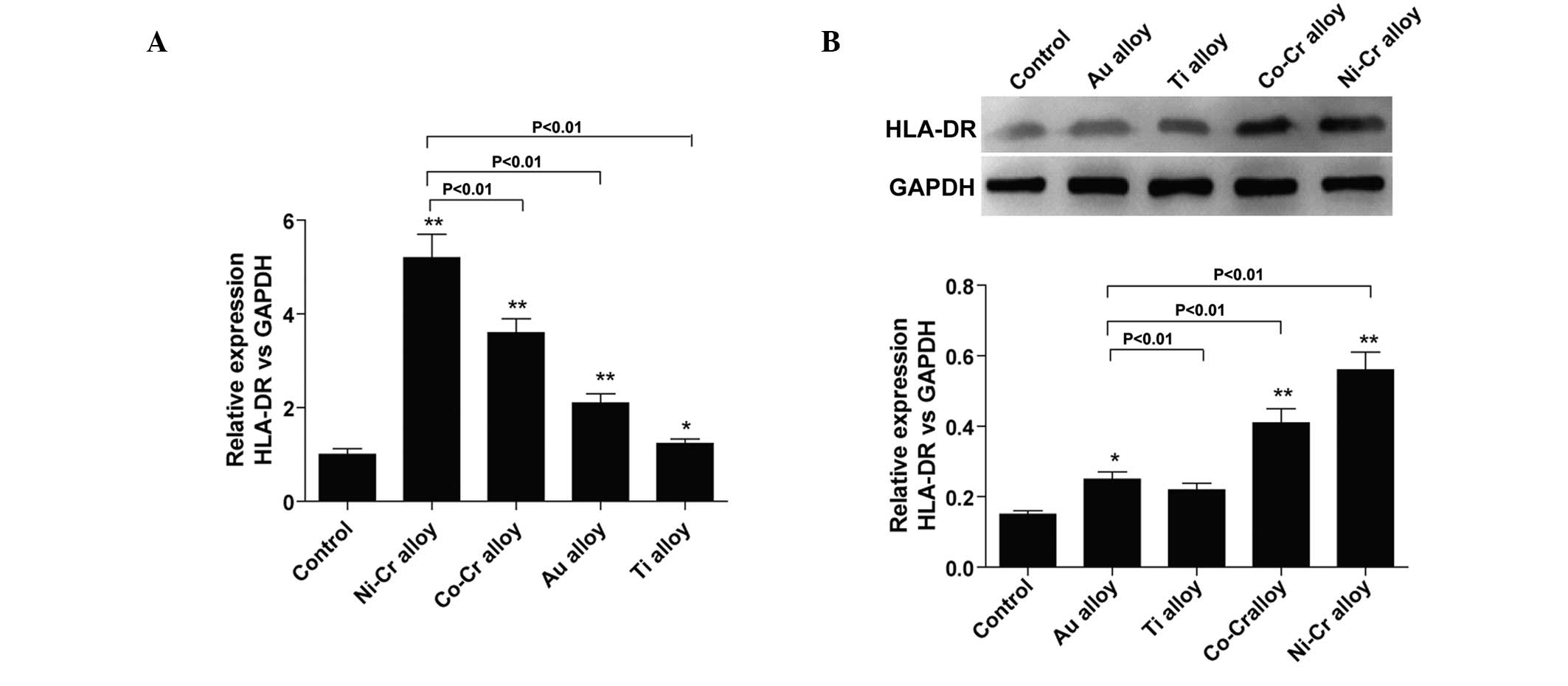 | Figure 5(A) Total RNA was extracted from
gingival tissues and relative expression levels of HLA-DR were
detected by polymerase chain reaction. *P<0.05 and
**P<0.01, vs. control. (B) Following treatment with
alloy restorations for ~1 year, the protein expression levels of
HLA-DR were detected by western blotting. *P<0.05 and
**P<0.01, vs. control. HLA, human leukocyte antigen;
Ni, nickel; Cr, chromium; Co, cobalt; Au, gold; Ti, titanium;
GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
Discussion
Metal allergies have been a concern for domestic and
overseas researchers for several years. Ni, Cr, Hg, Pd and Co are
commonly used components in dental metal prostheses, and readily
cause allergic reactions (10).
Patients who are allergic to Ni and Cr also exhibit skin allergic
reactions, including eczema caused by stainless steel jewelry
(4). Patch tests are considered
the most reliable method for the diagnosis of
delayed-hypersensitivity reactions (type IV hypersensitivity)
(5). Briefly, a patch test
requires the preparation of a solution or ointment containing a
certain concentration of a suspected allergen, which is then
applied to the skin of patients. The response to the preparation,
for example eczema-like skin lesions, are used to identify specific
allergens. The patch test only exposes the skin surface to the
allergen, and the allergen cannot pass through the epidermis into
the dermis to cause bleeding; therefore, it is considered a safe
method. In the present study, the results of patch testing
indicated that the number of allergies induced by Ni (83.3%) were
significantly higher, compared with the other metals. It is
well-known that Ni, Co and Cr can induce allergic reactions in
humans (10–12), and Ni is considered one of the most
common contact allergens. Further evidence of marked sensitization
to Ni and Co was provided by XFMS analysis, in which Ni (87.52%)
and Co (73.96%) had markedly higher sensitivity, compared with
other metal ions, including Mo (2.65%) and Fe (8.22%). Schmidt
et al (13) demonstrated
that Ni ions activate the innate immune response by stimulating
Toll-like receptor 4. However, the underlying mechanism of dental
metal alloy-induced activation of hypersensitivity requires further
investigation.
Allergens enter the human body at different
concentrations and via different routes, resulting in uncertainty
in the sensitization phase duration, which may last between 3 days
and several years (14,15). Furthermore, allergic reactions
differ among individuals, resulting in difficulties in clinical
diagnosis. Nakada et al demonstrated that allergies to
cobalt appeared in patients as palm or foot pustules 1 month
following receipt of a dental Co-Cr alloy crown restoration.
However, following removal of the gold and restoration the patients
no longer exhibited clinical symptoms at follow-up (11). Further evidence of Ni-Cr and
Co-Cr-induced delayed hypersensitivity reactions was provided by
the expression of HLA-DR in the present study. Previous studies
have indicated that metal ions are common allergens, which
sensitize T cells and induce delayed hypersensitivity reactions
through its surface receptor, HLA (16,17).
The significant increase in the expression levels of HLA-DR in the
Ni-Cr and Co-Cr groups reflected the increased delayed
hypersensitivity reaction. However, the expression levels of HLA-DR
in the Ti alloy group showed minimal difference, compared with the
healthy control, which may be due to its biocompatibility and lack
of tissue sensitization (18).
In vitro experiments have demonstrated that
Ni can cause an inflammatory reaction in epidermal cells, increase
the expression levels of interleukin (IL)-1a, IL-8 and
prostaglandin E2, and induce apoptosis (10). Evidence that gold leads to gum
inflammation is suggestive of sensitization. The expression levels
of CD4 and CD8 in the peripheral blood of patients with Ni
allergies is relatively high; therefore, Ni ions may result in
allergic reactions in the oral mucosa or skin (11). Allergies are usually benign;
however, symptoms, including itching, can significantly lower the
quality of life of patients. Therefore, identification of metal
allergies and avoiding contact with specific metal allergens is the
predominant therapeutic strategy. A patch test is necessary in the
diagnosis of contact allergy. Dentists require an understanding of
the corrosion and allergy rates of the alloys used in restorations,
in order to reduce the application of highly allergic alloys. Prior
to restoration, a patch test for hypersensitive patients is
recommended, and the use of different metal alloys in the same
patient requires caution.
In conclusion, sensitization to, and the biological
safety of metals is an important topic in dental investigations.
The present study exhibited clear evidence that sensitization to
certain dental metals, including Ni and Co, can be identified by a
patch test prior to implantation, thus providing guidance for
dental clinicians in the selection of repair materials.
Acknowledgments
This study was supported by the 2010 Shanghai
Committee of Science and Technology, China (grant. no.
10411950900).
References
|
1
|
Lundström IM: Allergy and corrosion of
dental materials in patients with oral lichen planus. Int J Oral
Surg. 13:16–24. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Magnusson B, Bergman M, Bergman B and
Söremark R: Nickel allergy and nickel-containing dental alloys.
Scand J Dent Res. 90:163–167. 1982.PubMed/NCBI
|
|
3
|
Gawkrodger DJ: Investigation of reactions
to dental materials. Br J Dermatol. 153:479–485. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yanagi T, Shimizu T, Abe R and Shimizu H:
Zinc dental fillings and palmoplantar pustulosis. Lancet.
366:10502005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu Z and Zhan DS: Mutagenicity of two
Ni-Cr porcelain alloys. JMutagenicity of two Ni-Cr porcelain
alloys. Journal of Clinical Rehabilitative Tissue Engineering
Research. 14:2683–2685. 2008.
|
|
6
|
Bayindir F, Körkut O and Güngör H:
Potentiodynamic polarization technique for corrosion testing of
Cr-Co and Cr-Ni alloys in artificial saliva and soft drinks. J
Mater Res Innov. 14:280–284. 2010. View Article : Google Scholar
|
|
7
|
Danaei SM, Safavi A, Roeinpeikar SM,
Oshagh M, Iranpour S and Omidkhoda M: Ion release from orthodontic
brackets in 3 mouthwashes: An in-vitro study. Am J Orthod
Dentofacial Orthop. 139:730–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uter W, Rämsch C, Aberer W, Ayala F,
Balato A, Beliauskiene A, Fortina AB, Bircher A, Brasch J,
Chowdhury MM, et al: The European baseline series in 10 European
countries, 2005/2006 - results of the European Surveillance System
on Contact Allergies (ESSCA). Contact Dermatitis. 61:31–38. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeng J, Wang J, Gao W, Mohammadreza A,
Kelbauskas L, Zhang W, Johnson RH and Meldrum DR: Quantitative
single-cell gene expression measurements of multiple genes in
response to hypoxia treatment. Anal Bioanal Chem. 401:3–13. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lidén C and Norberg K: Nickel on the
Swedish market. Follow-up after implementation of the Nickel
Directive. Contact Dermatitis. 52:29–35. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakada T, Iijima M, Nakayama H and Maibach
HI: Role of ear piercing in metal allergic contact dermatitis.
Contact Dermatitis. 36:233–226. 228–233. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Merritt K and Rodrigo JJ: Immune response
to synthetic materials. Sensitization of patients receiving
orthopaedic implants. Clin Orthop Relat Res. 71–79. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmidt M, Raghavan B, Müller V, Vogl T,
Fejer G, Tchaptchet S, Keck S, Kalis C, Nielsen PJ, Galanos C, et
al: Crucial role for human Toll-like receptor 4 in the development
of contact allergy to nickel. Nat Immunol. 11:814–819. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abraham CM, Ownby DR, Peterson EL,
Wegienka G, Zoratti EM, Williams LK, Joseph CL and Johnson CC: The
relationship between seroatopy and symptoms of either allergic
rhinitis or asthma. J Allergy Clin Immunol. 119:1099–1104. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arshad SH, Tariq SM, Matthews S and Hakim
E: Sensitization to common allergens and its association with
allergic disorders at age 4 years: A whole population birth cohort
study. Pediatrics. 108:E332001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thomas P, Maier S and Summer B: Allergic
reactions to metal implants. Materwiss Werksttech. 35:997–1000.
2004. View Article : Google Scholar
|
|
17
|
Forte G, Petrucci F and Bocca B: Metal
allergens of growing significance: Epidemiology, immunotoxicology,
strategies for testing and prevention. Inflamm Allergy Drug
Targets. 7:145–162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harloff T, Hönle W, Holzwarth U, Bader R,
Thomas P and Schuh A: Titanium allergy or not? 'Impurity' of
titanium implant materials. Health. 2:306–310. 2010. View Article : Google Scholar
|