Introduction
Cancer is a life-threatening disease and one of the
leading causes of mortality worldwide. Cancer cells are
characterized by inhibited apoptosis, uncontrolled growth and
proliferation, and metastasis (1).
The development of cancer, often initiated by genetic alterations,
is a complex process that involves interactions between oncogenic
proteins and tumor suppressors forming an intricate signaling
network. For example, the oncogene Myc has been found to be
amplified or overexpressed in various types of cancer and the
Myc-encoded oncoprotein can promote tumorigenesis by driving
cell cycle progression, stimulating cell growth and inducing
angiogenesis (2). However, the
Myc-triggered oncogenic signals can activate the p53 tumor
suppressor pathway via the induction of the tumor suppressor, ARF
that mitigates the oncogenic E3-ligase mouse double minute
(MDM)2-mediated p53 degradation (3). This is an auto-regulated,
self-protective mechanism that prevents cells from malignant
transformation. Notably, p53 can repress Myc activity by
transcriptionally activating, for example, miR-145 which targets
Myc mRNA for translation silencing (4,5),
thus forming a negative feedback regulatory loop.
The most straightforward and effective strategy to
treat cancer is to kill the cancerous cells. The commonly used
anticancer drugs, such as cisplatin (6), actinomycin D (7) and adriamycin (8) are shown to inhibit tumor growth by
promoting apoptosis. Recently, growing evidence has demonstrated
that a number of natural products and derivatives from plants,
particularly from medicinal plants used in traditional Chinese
medicine (TCM), exhibit a tumor suppressive function by inducing
the apoptosis of cancer cells and have potential for clinical
application in cancer therapy (9).
For instance, the natural anthraquinone emodin isolated from the
TCM, Radix rhizome Rhei, can suppress the growth of numerous
types of cancer cells (10,11).
Camptothecin, derived from the Chinese 'happy tree', Camptotheca
acuminate, is a valuable natural product that inhibits the
ligation of DNA following topo I-mediated strand breaks (12). In another retrospective
population-based cohort study of a total of 729 patients with
advanced breast cancer, it was suggested that TCM therapy can
contribute to cancer treatment. Of this cohort, 115 patients were
TCM users while 614 patients did not use TCM. The multivariate
analysis demonstrated that, compared with non-users, the use of TCM
was associated with a significantly decreased risk of all-cause
mortality (13). All the
aforementioned findings indicated that TCM is an important
complementary and alternative medicine that can be used in the
treatment of cancer.
Echinacoside (ECH) is a phenylethanoid isolated from
the stems of Cistanches salsa, a Chinese herbal medicine,
which is an important crude drug used as an antisenium and
antifatigue agent (14). It has
been found that several phenylethanoids possess free radical
scavenging properties and protect against oxidative stress-induced
toxic injuries (15). In addition,
more biological properties of ECH have since been elucidated. For
example, ECH can rescue the increased levels of inflammatory
cytokines and improve lung histopathological abnormalities in mice
with acute lung injury (16,17).
Also, ECH has been shown to have protective effects on nerve tissue
and improve behavioral disorders in murine models of Parkinson's
disease (18). Notably, it has
been found that ECH promotes cell proliferation and inhibits
apoptosis in the mouse intestinal epithelial MODE-K cells (19). Thus far, however, less attention
has been paid to the potential role of ECH in cancer
prevention.
In this study, it was explored whether ECH treatment
affects tumor cell growth and proliferation, and whether ECH
induces apoptosis, elevates the production of reactive oxygen
species (ROS) and reduces mitochondrial membrane potential (MMP),
and consequently suppresses tumor cell growth. Furthermore the
present study aimed to identify the molecular mechanisms
responsible for ECH-mediated cell growth inhibition.
Materials and methods
Cell line, reagent and antibodies
SW1990 pancreatic adenocarcinoma cells (ATCC,
Manassas, VA, USA) were cultured in Dulbecco's modified Eagle's
medium supplemented with 10% fetal bovine serum, 50 U/ml penicillin
and 0.1 mg/ml streptomycin at 37°C in a 5% CO2
humidified atmosphere. ECH was purchased from Jrdun Biotechnology
Corp. (Shanghai, China). Antibodies against AKT, P-AKT, ERK, P-ERK,
JNK, p-JNK, P38, p-P38 and GAPDH were purchased from Cell Signaling
Technology (Danvers, MA, USA); anti-Bax and anti-Bcl-2 were
purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA);
and anti-Caspase-3 was purchased from Abcam (Shanghai, China).
Cell viability assay
To assess the rate of tumor cell growth, Cell
Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Rockville,
MD, USA) was used according to the manufacturer's instructions.
Cell suspensions were seeded at 5,000 cells per well with ECH
treatment for 0, 12, 24, 48 or 72 h in 96-well culture plates. Cell
growth inhibition was determined by adding WST-8 reagent from the
CCK-8 kit at a final concentration of 10% to each well, and the
absorbance of the samples was measured at 450 nm using a microplate
reader (Multiskan MK3; Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Hoechst 33342 staining
Cells (60% confluence) were treated with Hoechst
33342 (Beyotime Institute of Biotechnology, Haimen, China) at a
final concentration of 1 µg/ml, incubated in 37°C incubator
for 15 min, washed with phosphate-buffered saline twice, fixed in
4% paraformaldehyde for 30 min at room temperature, and mounted
onto slides. The morphological changes of the cell nuclei were
observed under a fluorescence microscope (Olympus BX51, Melville,
NY, USA). The normal nuclei were round and stained light blue,
while the apoptotic nuclei were shrunken and stained bright
blue.
Fluorescence-activated cell sorting
(FACS) analyses
For the assessment of apoptosis, the fluorescein
isothiocyanate (FITC)-Annexin V Apoptosis Detection kit (BD
Biosciences, Shanghai, China) was used according to the
manufacturer's instructions. Briefly, 5×104 cells were
washed with ice-cold PBS, resuspended in 0.1 ml binding buffer
(Beyotime Institute of Biotechnology), and stained with 10 ml of
FITC-conjugated Annexin V (10 mg/ml) and 10 ml propidium iodide
(PI) (50 mg/ml). Following incubation for 15 min at room
temperature in the dark and addition of 400 ml binding buffer, the
cells were analyzed by a flow cytometer (C6; BD Biosciences,
Shanghai, China).
Measurement of reactive oxygen species
(ROS)
To evaluate the production of ROS, the Reactive
Oxygen Species Assay kit (Vigorous Biotechnology, Beijing, China)
was used according to the manufacturer's instructions. Briefly,
cells (80% confluence) were harvested and washed with PBS prior to
staining with the dihydroethidium (DHE) solution (Beyotime
Institute of Biotechnology). Cells were then analyzed by flow
cytometric analyses.
Measurement of mitochondrial membrane
potential (MMP)
A tetramethylrhodamine methyl ester (TMRM) Assay kit
(ImmunoChemistry Technologies, Bloomington, MN, USA) was used to
detect the changes in MMP. Briefly, cells (80% confluence) were
harvested, washed with PBS and stained with TMRM for 15–20 min in a
37°C incubator. Cells were then washed once with PBS and subjected
to flow cytometric analyses.
Immunoblotting analyses
The cells (80% confluence) were harvested and lysed
in RIPA buffer (Jrdun Biotechnology) consisting of 50 mM Tris-HCl,
pH 7.4; 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1% sodium deoxycholic
acid and 0.1% SDS and freshly added proteasome inhibitors. Equal
quantities of clear cell lysate were used for immunoblotting
analysis as described previously (20).
Statistical analysis
Quantitative data are expressed as the mean ±
standard deviation. Statistical differences were evaluated by
unpaired Student's t-test using statistical SPSS 15.0 software.
P<0.05 was considered to indicate a statistically significant
difference.
Results
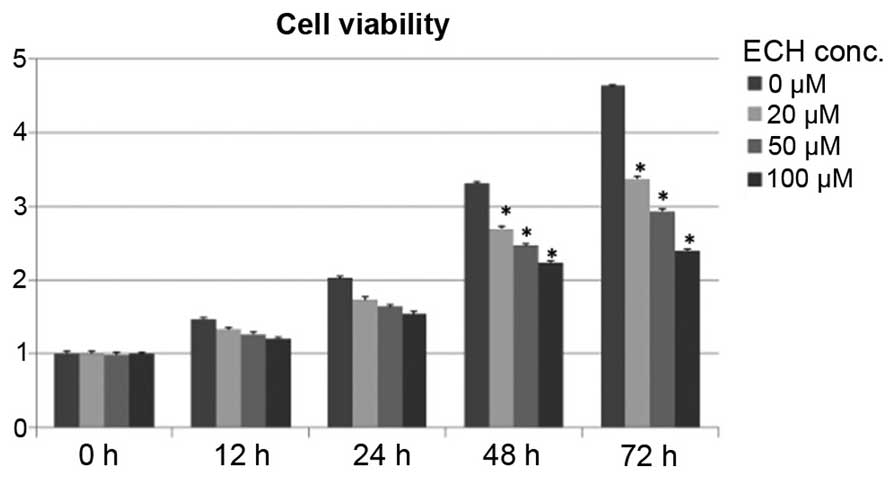
ECH suppresses tumor cell growth
Although it has been reported that ECH exhibits a
protective role by inhibiting apoptosis and inflammatory signals in
somatic cells, such as neuronal and intestinal epithelial cells
(16–19), it remains elusive whether ECH
controls cancerous cell growth and proliferation. To test this, a
cell survival assay was conducted by treating SW1990 cells, derived
from a grade II pancreatic adenocarcinoma, with titrated doses of
ECH as shown in Fig. 1. Notably,
it was demonstrated that ECH significantly retards tumor cell
proliferation in a dose-dependent manner during a 5-day culture
period (Fig. 1).
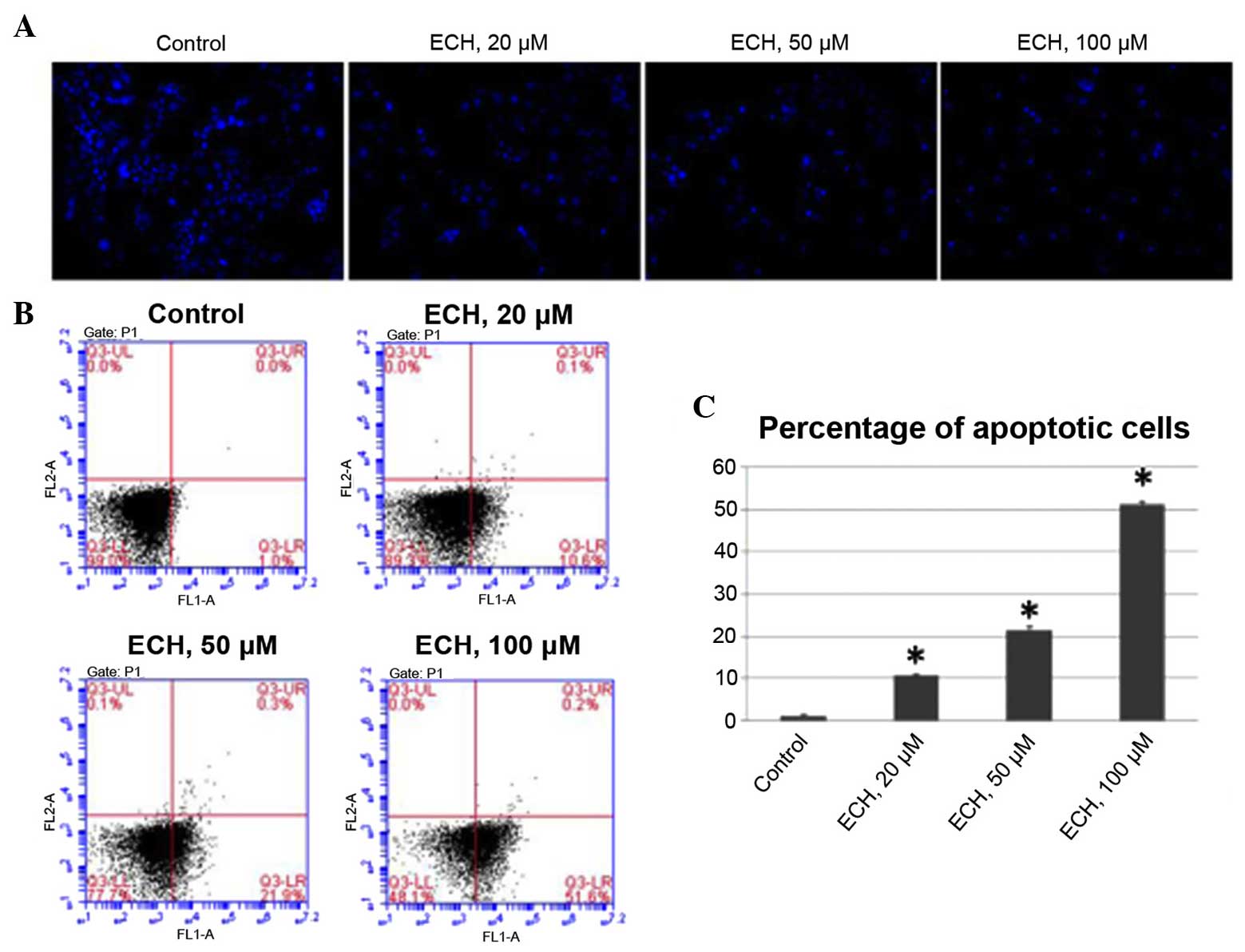
ECH triggers apoptosis
As loss of apoptosis is one of the major causative
mechanisms underlying the uncontrolled proliferation of pancreatic
cancer cells (21), the present
study performed a set of experiments to determine whether ECH
triggers apoptosis. Firstly, by staining the nuclei of tumor cells
with Hoechst 33342, it was demonstrated that ECH results in
apoptosis in a dose-dependent manner (Fig. 2A). Additionally, FACS analyses was
conducted using Annexin V/PI staining to further confirm the
apoptotic effect of ty ECH (Fig.
2B). The average percentage of apoptotic cells was 1.1% under
normal culture conditions, while this percentage significantly
increased to 10.6, 21.4 and 51.3% in response to ECH treatment in a
dose-dependent manner (Fig. 2C).
These results, together with the cell viability assay shown in
Fig. 1, demonstrate that ECH
treatment suppresses the proliferation of tumor cells by triggering
apoptosis.
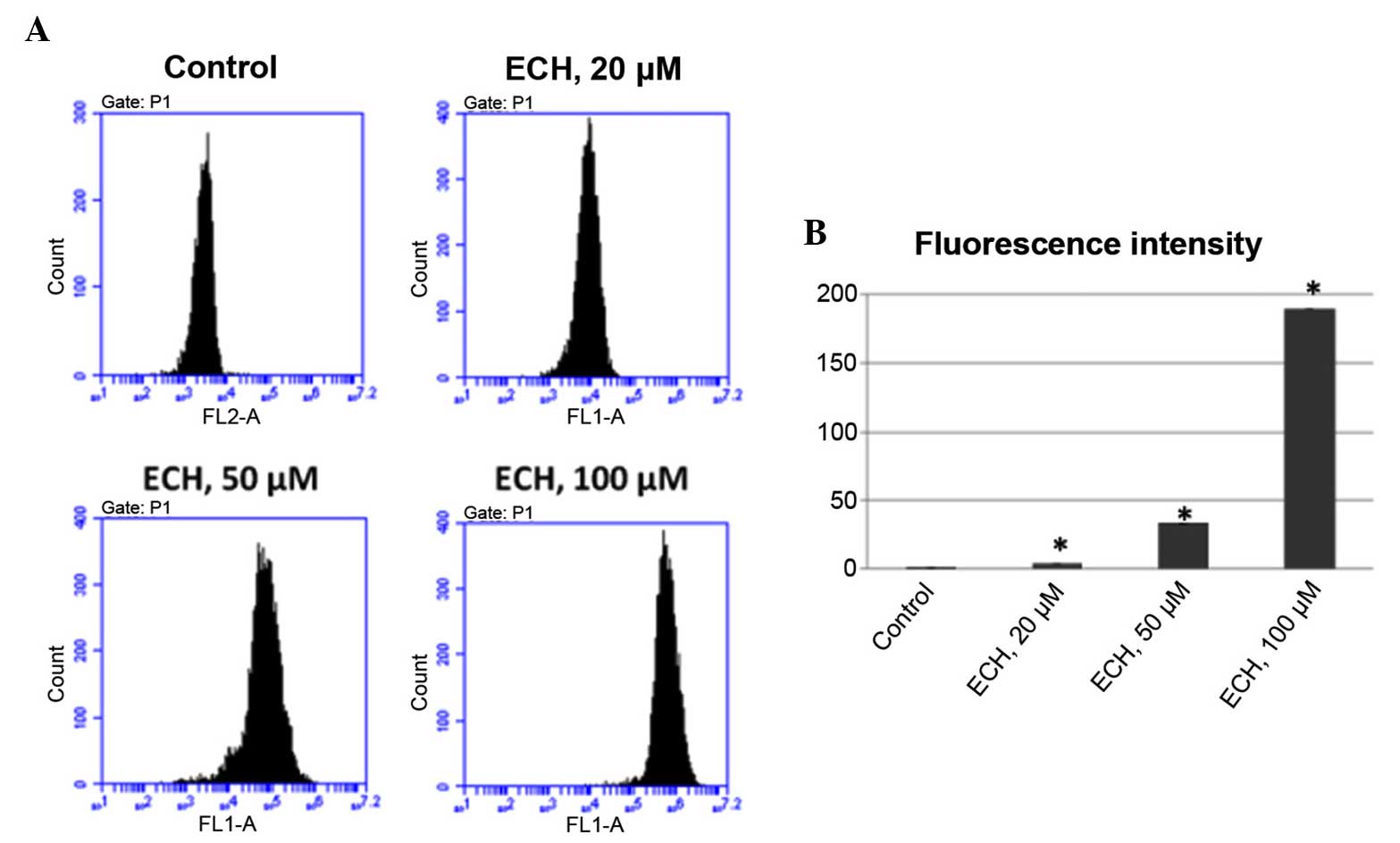
ECH prompts the production of reactive
oxygen species (ROS)
Several cancer chemotherapeutic drugs, such as
doxorubicin and cisplatin, have been shown to be potent generators
of ROS which are crucial role in apoptosis induction under
physiological and pathological conditions (22). Thus, it was investigated whether
ECH treatment could also regulate ROS production. To test this,
DHE, which can be oxidized to generate ethidium that intercalates
with DNA, was used in this study to evaluate ROS production. It was
found that, like other anticancer drugs, ECH also stimulates ROS
production in a dose-dependent manner as shown by the elevated
fluorescence intensity upon ECH treatment (Fig. 3).
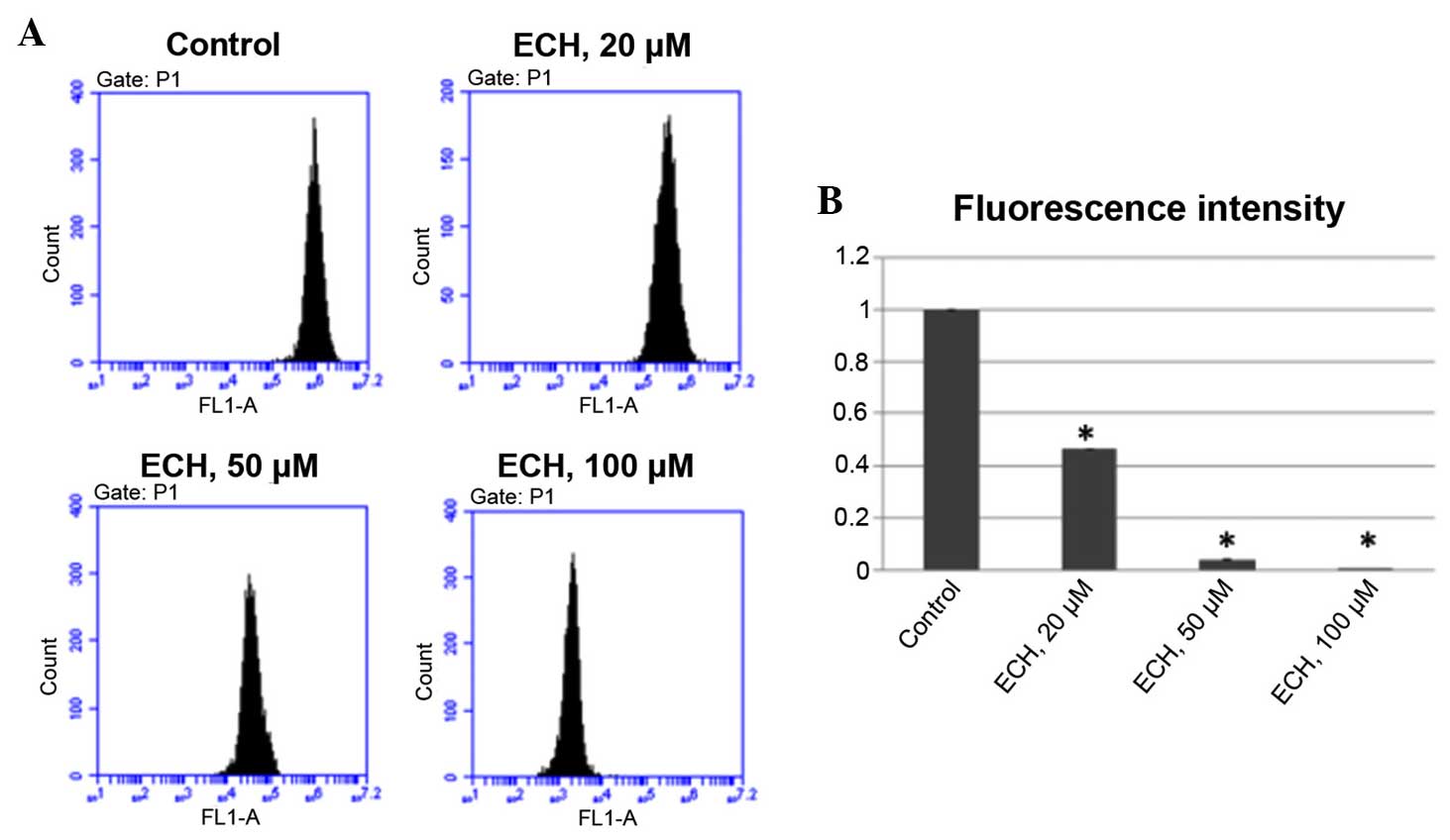
ECH reduces MMP
Mitochondrial dysfunction has been shown to be
implicated in the induction of apoptosis. Opening of the
mitochondrial permeability transition pore has been demonstrated to
induce the depolarization of the transmembrane potential and the
release of pro-apoptotic factors (23). Therefore, it was tested whether ECH
could induce loss of MMP in tumor cells by performing a TMRM assay,
which is a well-established approach, as the intensity of TMRM
fluorescence is proportional to the membrane potential. It was
demonstrated that ECH treatment significantly reduces MMP in a
dose-dependent manner (Fig.
4).
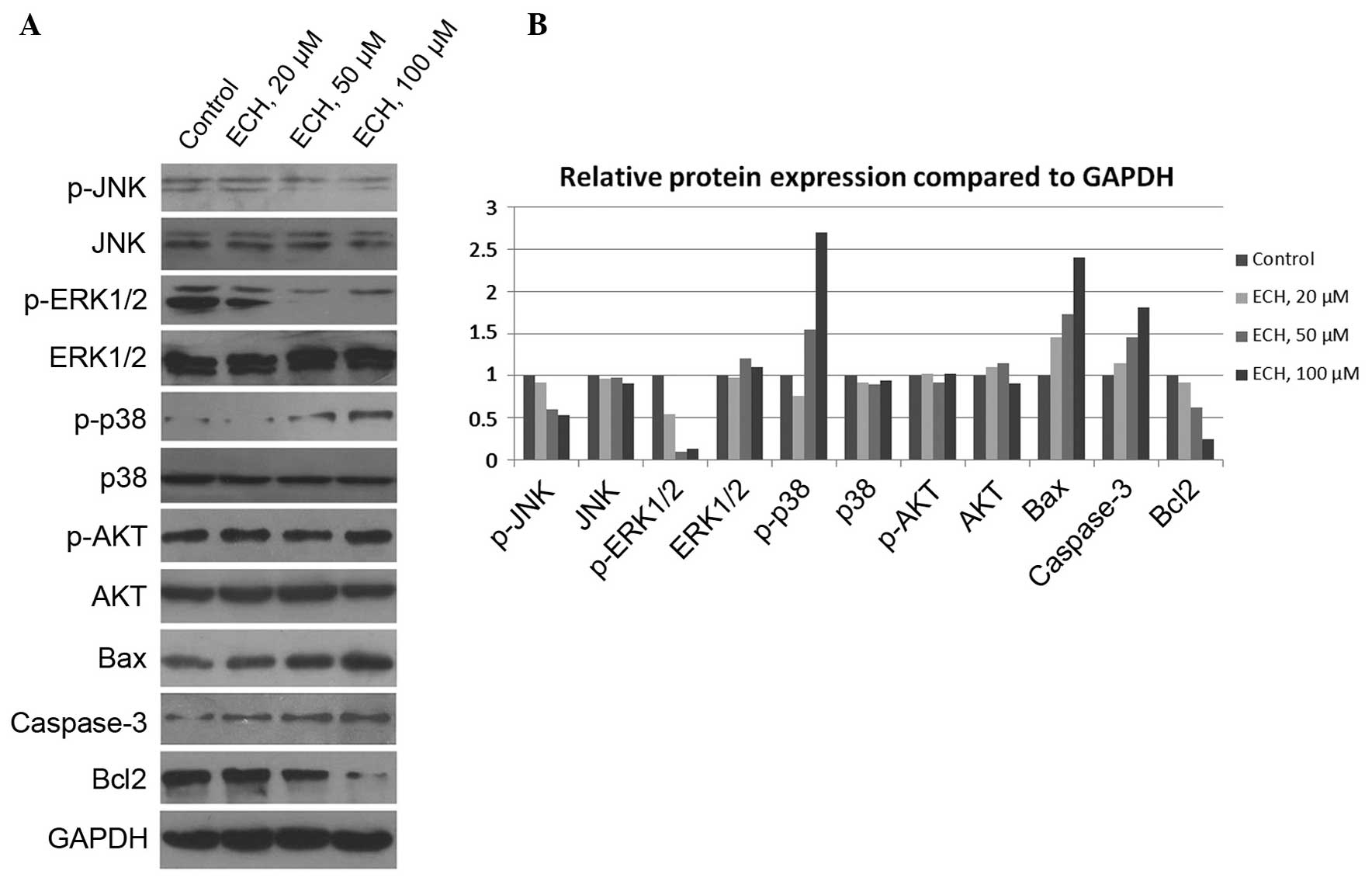
ECH controls tumor cell growth via
mitogen-activated protein kinase (MAPK), but not AKT, signals
To further investigate the molecular basis of
ECH-mediated tumor cell death, the activity of several vital
signaling pathways, such as MAPK and AKT (24,25),
which control cell survival and death was examined. The MAPKs are
evolutionarily conserved, proline-directed Ser/Thr protein kinases,
including extracellular signal-regulated kinases (ERKs), c-Jun
NH2-terminal kinase (JNKs) and the p38 family members
which are activated through three-tier kinase signaling cascades
(26,27). In this study, the expression of
MAPKs and AKT, as well as their activated phosphorylated forms, was
assessed and it was revealed that ECH markedly suppresses JNK and
ERK1/2 activity, but enhances p38 activity (Fig. 5). Notably, it was demonstrated that
AKT activity, which is also critically important for cell
proliferation, is not affected by ECH treatment (Fig. 5). In addition, it was shown that
ECH treatment elevates the expression of Bax and Caspase-3 whilst
reduces Bcl-2 expression (Fig. 5),
which is consistent with Fig. 2.
Thus, the results indicate that ECH triggers tumor cell apoptosis
via the MAPK pathway.
Discussion
To the best of our knowledge, this is the first
study to show that ECH has a tumor suppressive function by
triggering apoptosis (Fig. 2),
promoting ROS production (Fig. 3)
and inducing mitochondrial membrane potential depolarization
(Fig. 4), consequently leading to
tumor cell growth inhibition (Fig.
1). Furthermore, the molecular basis of ECH-mediated tumor cell
death was shown to occur by regulating MAPK signaling pathways
(Fig. 5). These findings
demonstrate a novel function of ECH in preventing tumorigenesis and
thus suggest that it may be a candidate agent for cancer
therapy.
The majority of anticancer drugs can induce tumor
cell apoptosis, senescence and/or cell cycle arrest, which leads to
inhibition of tumor cell growth and proliferation. Cell cycle
arrest is a cellular response to mild stress signals that allow
cells to repair damaged DNA prior to initiating replicative DNA
synthesis or mitosis, whereas apoptosis and senescence (permanent
cell cycle arrest) occur in response to stress signals that
eliminate irreparable or malignant cells (28,29).
Therefore, only the apoptotic effect of ECH on tumor cells was
assessed in this study, as killing cancerous cells is a major
criterion for evaluating the potency of an anticancer agent.
Notably, it was demonstrated that ECH induces the expression of
Bax (Fig. 5), a
pro-apoptotic gene, transcriptionally activated by the tumor
suppressor p53 (30). Thus, it is
worthwhile to test whether ECH can activate the p53 signaling
pathway. If so, ECH may also be able to elicit p53-dependent cell
cycle arrest, senescence, apoptosis or autophagy. In this study,
the tumor suppressive function of ECH in the SW1990 pancreatic
adenocarcinoma cell line was elucidated. However, further studies
observing more pancreatic cancer cell lines, are required. It has
been shown that mutations in the oncogenic protein RAS and the
tumor suppressor p53 are associated with the development of
pancreatic cancer (31); however,
the SW1990 cell line does not harbor any p53 mutation, according to
the IARC p53 database (http://p53.iarc.fr/CellLines.aspx). Hence, it is
important to investigate whether ECH can affect the growth and
proliferation of other pancreatic cancer cell lines with different
p53 mutations. Additionally, it would be interesting to determine
whether ECH is able to promote apoptosis and inhibit the growth of
other types of tumors.
ROS elevation and MMP reduction, which were caused
by ECH treatment, have been shown to be essential in apoptosis
induction (22). In addition, it
has been found that ROS can induce the oxidation of the
mitochondrial pores contributing to the release of cytochrome
c, an intermediate in apoptosis, due to disruption of the
MMP (22). Thus, it remains to be
determined whether ECH disrupts MMP indirectly through induction of
ROS. Moreover, ROS-elicited oxidative stress has also been shown to
be involved in modulating a myriad of cell-growth controlling
signals, including p53, NF-κB, HIFs and PI3K (32). It remains to be determined whether
and, if so, how ECH regulates these important signaling pathways.
Notably, oxidative stress causes various neurodegenerative diseases
due to the high oxygen consumption, weak antioxidative systems and
terminal-differentiation characteristics of the central nervous
system (33). However, several
studies have shown that ECH has protective and anti-apoptotic
effects on nervous tissue. In this regard, it is reasonable to
hypothesize that ECH may reduce ROS production in the
terminal-differentiated neural cells. Therefore, it also remains to
be determined whether the regulation of ROS production by ECH is
dependent on the cell differentiation status. Hence, the present
results together with other studies reveal that ECH, the widely
used TCM, can be an important chemotherapeutic strategy not only
for the treatment of neurodegenerative diseases but also malignant
carcinomas.
Recently, using TCM in cancer therapy has gained
increasingly more attention. The potential of natural products from
medicinal plants used in TCM has been recognized by the scientific
community even in the western world (9). Efforts are required to elucidate the
underlying mechanisms of action of these natural products, which
may eventually lead to the development of efficient and safe
medicines for cancer therapy.
In conclusion, the present study demonstrated the
tumor inhibitory function of ECH and also elaborates the molecular
basis of ECH-mediated tumor suppression, thus suggesting the
potential clinical application of ECH in cancer therapy.
Acknowledgments
This study was funded and supported by the Key
Program of Scientific Research of FMU(grant no. 09ZD014). The
authors would like to thank Biomedworld (Shanghai, China) for help
with editing the manuscript.
References
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alderton GK: Transcription: The
transcriptional effects of MYC. Nat Rev Cancer. 14:5132014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zindy F, Eischen CM, Randle DH, Kamijo T,
Cleveland JL, Sherr CJ and Roussel MF: Myc signaling via the ARF
tumor suppressor regulates p53-dependent apoptosis and
immortalization. Genes Dev. 12:2424–2433. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sachdeva M, Zhu S, Wu F, Wu H, Walia V,
Kumar S, Elble R, Watabe K and Mo YY: p53 represses c-Myc through
induction of the tumor suppressor miR-145. Proc Natl Acad Sci USA.
106:3207–3212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao JM, Cao B, Zhou X and Lu H: New
insights into p53 functions through its target microRNAs. J Mol
Cell Biol. 6:206–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zamble DB and Lippard SJ: Cisplatin and
DNA repair in cancer chemotherapy. Trends Biochem Sci. 20:435–439.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kleeff J, Kornmann M, Sawhney H and Korc
M: Actinomycin D induces apoptosis and inhibits growth of
pancreatic cancer cells. Int J Cancer. 86:399–407. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tacar O, Sriamornsak P and Dass CR:
Doxorubicin: An update on anticancer molecular action, toxicity and
novel drug delivery systems. J Pharm Pharmacol. 65:157–170. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Efferth T, Li PC, Konkimalla VS and Kaina
B: From traditional Chinese medicine to rational cancer therapy.
Trends Mol Med. 13:353–361. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yim H, Lee YH, Lee CH and Lee SK: Emodin,
an anthraquinone derivative isolated from the rhizomes of Rheum
palmatum, selectively inhibits the activity of casein kinase II as
a competitive inhibitor. Planta Med. 65:9–13. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Chang CJ, Bacus SS and Hung MC:
Suppressed transformation and induced differentiation of
HER-2/neu-overexpressing breast cancer cells by emodin. Cancer Res.
55:3890–3896. 1995.PubMed/NCBI
|
|
12
|
Pommier Y: Topoisomerase I inhibitors:
Camptothecins and beyond. Nat Rev Cancer. 6:789–802. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee YW, Chen TL, Shih YR, Tsai CL, Chang
CC, Liang HH, Tseng SH, Chien SC and Wang CC: Adjunctive
traditional Chinese medicine therapy improves survival in patients
with advanced breast cancer: A population-based study. Cancer.
120:1338–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiong Q, Hase K, Tezuka Y, Namba T and
Kadota S: Acteoside inhibits apoptosis in D-galactosamine and
lipopolysaccharide-induced liver injury. Life Sci. 65:421–430.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao JJ, Igalashi K and Nukina M: Radical
scavenging activity of phenylpropanoid glycosides in Caryopteris
incana. Biosci Biotechnol Biochem. 63:983–988. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Xing J, Ai T, Wen T, Guan L and
Zhao J: Protection of echinacoside against acute lung injury caused
by oleic acid in rats. Free Radic Res. 41:798–805. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Gou C, Yang H, Qiu J, Gu T and Wen
T: Echinacoside ameliorates D-galactosamine plus
lipopolysaccharide-induced acute liver injury in mice via
inhibition of apoptosis and inflammation. Scand J Gastroenterol.
49:993–1000. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Q, Gao J, Li W and Cai D:
Neurotrophic and neurorescue effects of Echinacoside in the
subacute MPTP mouse model of Parkinson's disease. Brain Res.
1346:224–236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jia Y, Guan Q, Guo Y and Du C:
Echinacoside stimulates cell proliferation and prevents cell
apoptosis in intestinal epithelial MODE-K cells by up-regulation of
transforming growth factor-β1 expression. J Pharmacol Sci.
118:99–108. 2012. View Article : Google Scholar
|
|
20
|
Liao P, Wang W, Shen M, Pan W, Zhang K,
Wang R, Chen T, Chen Y, Chen H and Wang P: A positive feedback loop
between EBP2 and c-Myc regulates rDNA transcription, cell
proliferation and tumorigenesis. Cell Death Dis. 5:e10322014.
View Article : Google Scholar
|
|
21
|
Westphal S and Kalthoff H: Apoptosis:
Targets in pancreatic cancer. Mol Cancer. 2:62003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar
|
|
23
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Munshi A and Ramesh R: Mitogen-activated
protein kinases and their role in radiation response. Genes Cancer.
4:401–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abraham AG and O'Neill E:
PI3K/Akt-mediated regulation of p53 in cancer. Biochem Soc Trans.
42:798–803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tournier C: The 2 Faces of JNK Signaling
in Cancer. Genes Cancer. 4:397–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koul HK, Pal M and Koul S: Role of p38 MAP
kinase signal transduction in solid tumors. Genes Cancer.
4:342–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brady CA and Attardi LD: p53 at a glance.
J Cell Sci. 123:2527–2532. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carvajal LA and Manfredi JJ: Another fork
in the road-life or death decisions by the tumour suppressor p53.
EMBO Rep. 14:414–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyashita T and Reed JC: Tumor suppressor
p53 is a direct transcriptional activator of the human bax gene.
Cell. 80:293–299. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morton JP, Timpson P, Karim SA, Ridgway
RA, Athineos D, Doyle B, Jamieson NB, Oien KA, Lowy AM, Brunton VG,
et al: Mutant p53 drives metastasis and overcomes growth
arrest/senescence in pancreatic cancer. Proc Natl Acad Sci USA.
107:246–251. 2010. View Article : Google Scholar :
|
|
32
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li J, O W, Li W, Jiang ZG and Ghanbari HA:
Oxidative stress and neurodegenerative disorders. Int J Mol Sci.
14:24438–24475. 2013. View Article : Google Scholar : PubMed/NCBI
|