Introduction
Myopia is a prevalent ocular disorder, which is
accompanied by the potentially blinding complications of high
myopia and the primary structural cause of which is increased axial
length of the eye (1). Eye growth
and refractive development are regulated by local retinal optical
signal in chicks, tree shrews and monkeys (2). A blurred image on the retina
initiates a signaling cascade, which begins in the retinal amacrine
cells (3), and traverses the
choroid (4) to alter extracellular
matrix remodeling in the sclera (5), thus facilitating accelerated eye
growth and, in the longer term, changes in eye size. This signaling
pathway within the eye, comprising a retina to sclera cascade,
affects retinal pigment epithelium (RPE) physiology (6) and choroidal thickness (7), thus contributing to ocular refractive
change in the short-term.
Several studies have focused on identifying the
components of the retinal and choroidal signaling cascade
responsible for the transduction of the blur signal (8), and the transmission of this
information from these tissues to the sclera (9). Several factors have been confirmed to
be involved in this process, including retinoic acid (4), transforming growth factor (TGF)-β and
basic fibroblast growth factor (bFGF) (10,11).
Bone morphogenetic proteins (BMPs) are the largest
subfamily of the TGF-β family (12), and several ocular tissues,
including the cornea, lens, RPE and retina express BMPs (13). BMP-2 belongs to the BMPs family and
is an important factor for early eye morphogenesis, ocular
development and growth (14–16).
The involvement of BMP-2 in retinal and choroidal signaling during
myopia has been previously investigated. In form-deprived myopia of
guinea pigs, the scleral expression of BMP-2 decreased (17). In vitro, BMP-2 promotes
scleral fibroblast proliferation and extracellular matrix synthesis
(18,19). This suggests that BMP-2 may be
involved in scleral remodeling in myopia development. The genetic
analysis of humans has confirmed that BMP-2 is associated with
refractive error and myopia (20,21).
However, whether BMP2 affects ocular growth via the choroid and
retina or by directly acting on the sclera remains unknown.
The aim of the present study was to characterise the
contribution of retinal and choroidal BMP-2 to the retinoscleral
cascade in a mammalian model of myopia development. BMP-2
expression levels changes in the retina and choroid were analyzed
during myopia development and recovery from myopia.
Materials and methods
Lens-induced myopia model
preparation
A total of 24 pigmented guinea pigs, aged 3 weeks
and weighing 180–220 g, were provided by Shandong Traditional
Chinese Medicine University (Shandong, China). Animals were
maintained in an air-conditioned room with an ambient temperature
of 16–26°C, 40–70% relative humidity and 12-h light/dark cycle
(daytime light intensity, 500 lux). Animals had ad libitum
access to water supplemented with vitamin C and food (guinea pig
pellets, hay and fresh vegetables). All the guinea pigs were fed
indoors in a standardized manner. In all guinea pigs defocused
myopia was induced by the animals wearing a concave lens (−4.00 D;
polymethyl methacrylate; King Tak & Jia Run Co., Beijing,
China) in the right eye, with the left eye serving as a control.
The animals were randomly assigned to either the lens-induced
myopia (LIM) group, in which animals wore lenses for 3 weeks, and a
recovery group, in which animals wore lenses for 3 weeks, followed
by 1 week without the lenses. Each group contained 12 guinea pigs.
During the experimental period, the lenses were cleaned once daily
in order to prevent the form-deprival effect (22). Animals were treated three times
with local cycloplegia in both eyes in order to produce mydriasis
and cycloplegia and, 30 mins after completion of the three
treatments, the animals were examined using cycloplegic streak
retinoscopy (66 Vision-Tech Co., Ltd., Suzhou, China). The axial
length was measured by A-ultrasonic scanning with a 10-MHz probe
(KN-1800; Kangning Medical Device Co., Ltd., Wuxi, China) every
week.
All animals were treated according to the
Association for Research in Vision and Ophthalmology statement for
the Use of Animals in Ophthalmic and Vision Research, and the
present study was approved by the Institutional Animal Care and Use
Committee of Zhongshan Ophthalmic Center of Sun Yat-Sen University
(Guangzhou, China).
Immunofluorescent microscopy
Following the final measurements of axial length and
refractive power (LIM group, 3 weeks; recovery group, 4 weeks), the
guinea pigs were sacrificed via an intraperitoneal injection of 10%
chloral hydrate (200 mg/kg; Boster Biological Technology Co., Ltd.,
Wuhan, China). Their eyes were rapidly enucleated and then fixed in
4% neutral-buffered formalin (Boster Biological Technology Co.,
Ltd.) for 24 h at 4°C. The tissue was dehydrated and cut into
frozen sections (5 µm thick), with the posterior eye ball
around the optic nerve selected for analysis. The samples were
blocked with normal serum (Boster Biological Technology Co., Ltd.)
for 30 min at room temperature, followed by incubation with rabbit
anti-BMP-2 polyclonal antibody (1:500; ab82511; Abcam, Cambridge,
MA, USA) at 4°C overnight and incubation with fluorescein
isothiocyanate-conjugated goat anti-rabbit IgG antibodies (1:50;
BA1105; Boster Biological Technology Co., Ltd.) for 1 h at room
temperature. Finally, the nuclei were stained with
4,6-diamidino-2-phenylindole (Roche Diagnostics, Indianapolis, IN,
USA) for 15 min, and the slides were mounted. The samples were
observed under a confocal fluorescent microscope (LSM 510 META;
Carl Zeiss, Inc., Oberkochen, Germany).
Western blot analysis
The eyes of the animals were rapidly enucleated and
hemisected. The posterior neural retina was dissected with minimal
adherent retinal pigment epithelium, and the choroid was then
removed from the underlying sclera. The tissues were collected and
frozen in liquid nitrogen. Each frozen tissue sample was ground,
and the total protein was collected using lysis buffer with 1%
phenylmethanesulfonyl fluoride (Beyotime Institute of
Biotechnology, Shanghai, China). The protein concentration was
assayed using a Bicinchoninic Acid Protein Assay kit (Beyotime
Institute of Biotechnology). Subsequently, 30 µg of the
protein samples were separated by 10% SDS-PAGE (Boster Biological
Technology Co., Ltd.) and electrotransferred onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA) at 200 mA
for 2 h, which were then blocked with 5% bovine serum albumin
(Sigma-Aldrich, St. Louis, MO, USA) for 2 h at room temperature.
The membranes were then incubated with mouse anti-β-actin
monoclonal antibody (1:3,000; ab8226; Abcam) and rabbit anti-BMP-2
polyclonal antibody (1:500; ab82511), respectively, overnight at
4°C. The membranes were then washed three times with Tris-buffered
saline with Tween-20 (TBST) and incubated with horseradish
peroxidase (HRP)-conjugated goat anti-mouse (1:2,000; BA1050) and
goat anti-rabbit (1,2000; BA1054; both Boster Biological
Technology) IgG secondary antibodies for 1 h at room temperature.
Following washing three times with TBST, the proteins were detected
using an enhanced chemiluminescence detection system (EMD
Millipore, Billerica, MA, CA), exposed onto negative film,
developed and fixed. The film was scanned and subsequently analyzed
using Quantity One Imaging Software (version 1; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). β-actin served as an
internal reference, and the experiments were repeated at least
three times.
Statistical analysis
Each experiment was repeated at least three times.
Data are expressed as the mean ± standard error of the mean. The
measurements of refractive power and axial lengths in each group of
guinea pigs were analyzed using a paired sample t-test. The
statistical significance of differences were determined by one-way
analysis of variance followed by Tukey's post-hoc multiple
comparison test, using a Mann-Whitney U test when significance was
detected. All data analysis was performed using SPSS version 19
(SPSS, Inc, Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
Refractive power and axial lengths of
guinea pigs
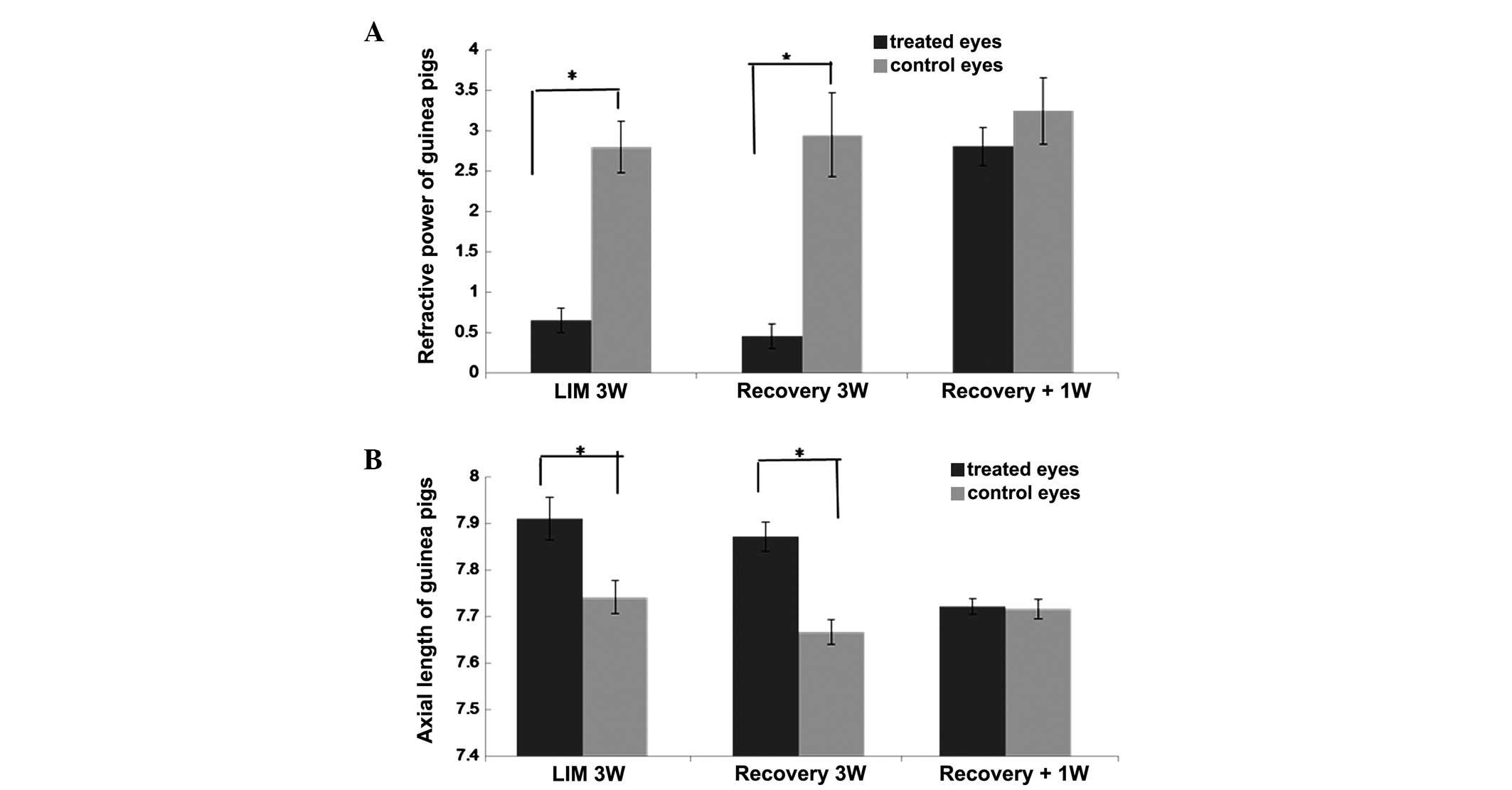
No significant differences in refraction power or
axial length were found between the treated and untreated eyes of
the two groups of guinea pigs prior to myopia induction
(P>0.05). Following treatment to induce myopia using minus
lenses for 3 weeks, the treated eyes in the two groups developed
myopia (P<0.05; Fig. 1A), and
had longer axial lengths (P<0.05; Fig. 1B), compared with the contralateral
eyes. In the recovery group, without lenses for 1 week, the changes
in the refractive power and axial lengths of the treated eyes had
regressed, and there were no differences in refraction (P>0.05)
or axial length (P>0.05) between the treated and untreated
eyes.
Expression of BMP-2 in the eyes of the
LIM and myopia recovery groups of guinea pigs
Immunofluorescence staining was used to examine the
expression levels of BMP-2 in the posterior retina, RPE, choroid
and sclera of the guinea pig eyes (Fig. 2). In the LIM group, the protein
expression of BMP-2 in the myopic retina appeared weaker, compared
with that in the control eyes (Fig. 2A
and B). However, following recovery from myopia, no significant
differences were observed in the expression of BMP-2 in the treated
and untreated control eyes (Fig. 2C
and D).
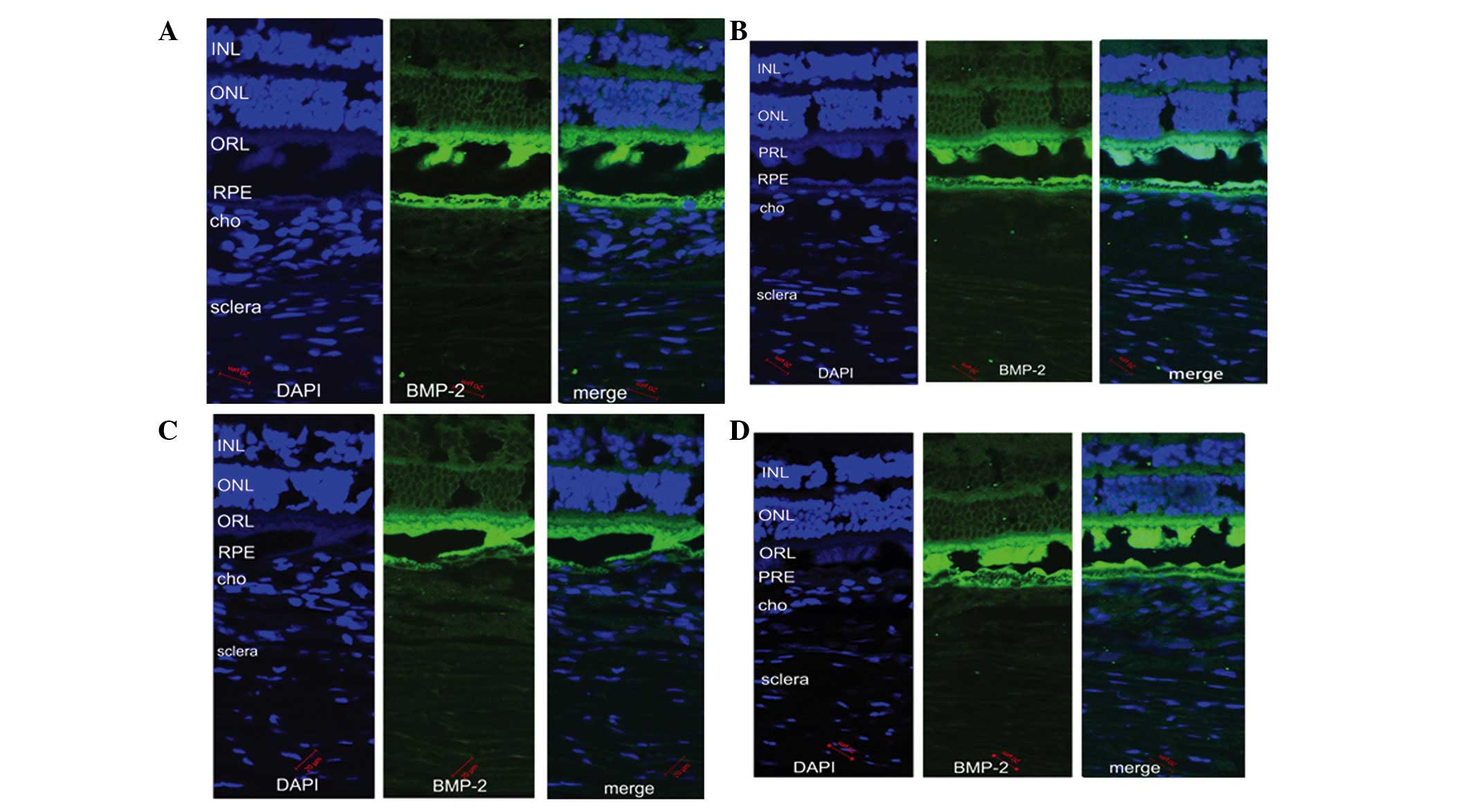 | Figure 2Immunoreactivity for BMP-2 (green)
antibody and DAPI (blue) in the retina, RPE, cho and sclera of
guinea pig eyes. Compared with the (A) untreated eyes, BMP-2
antibody staining in the retina of the (B) myopic eyes was weaker.
In the recovery group, in which myopic eyes were without lenses for
1 week, BMP-2 antibody staining in the retina of the (C) treated
eyes and (D) untreated eyes were similar. Images were captured
using a confocal microscope (magnification, ×200; scale bar, 20
µm). BMP-2, bone morphogenetic protein-2; INL, inner nuclear
layer; ONL, outer nuclear layer; ORL, optical receptor layer; RPE,
retinal pigment epithelium; cho choroid; DAPI,
4,6-diamidino-2-phenylindole. |
Retinal expression of BMP-2 in the LIM
and myopia recovery groups of guinea pigs
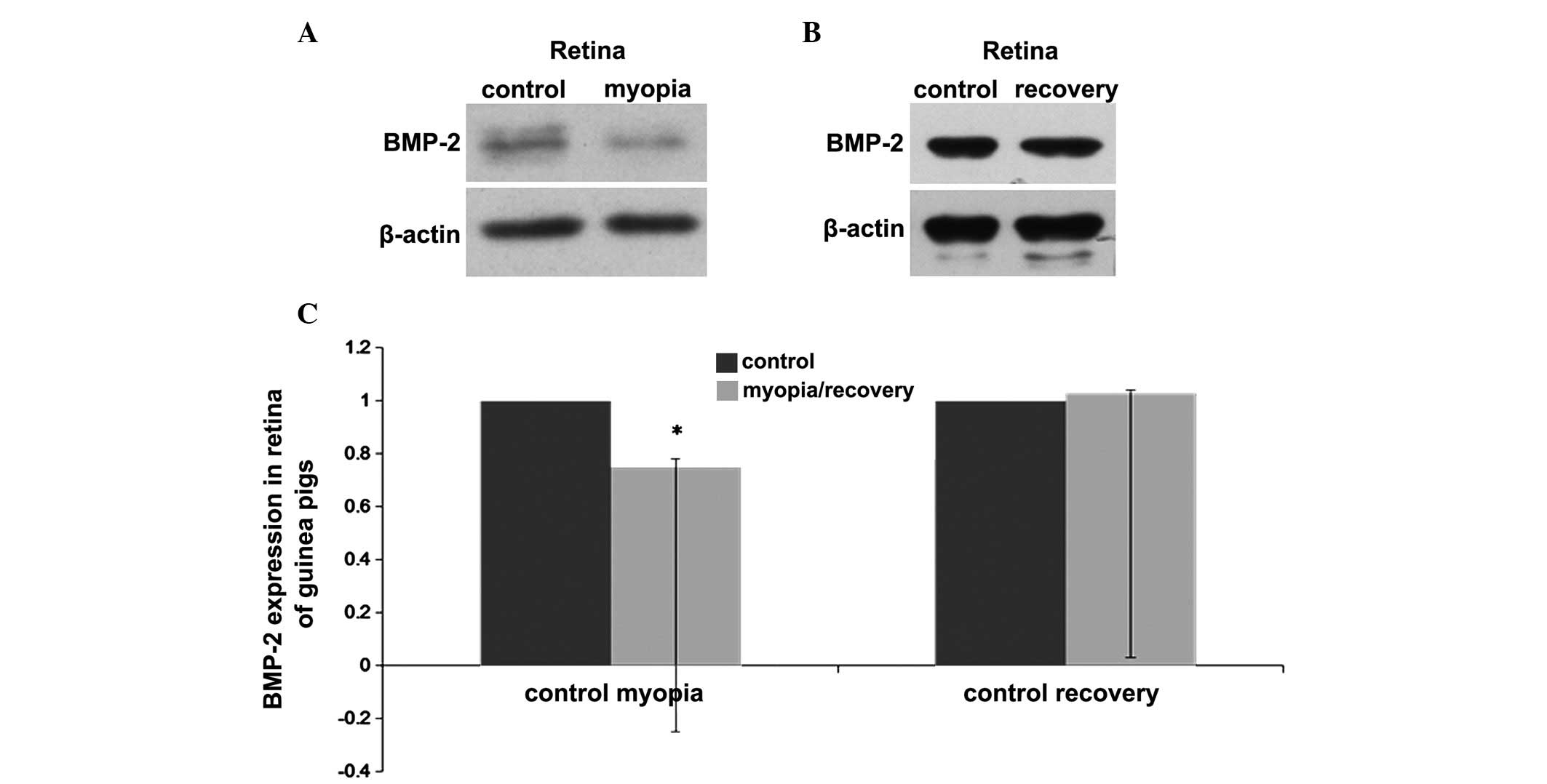
Western blot analysis was used to further verify the
changes in the protein expression levels of BMP-2 observed in the
posterior retina and choroid of the guinea pigs. Following 3 weeks
of lens wearing in the LIM group, the retinal protein levels of
BMP-2 in the myopic eyes were decreased significantly, compared
with levels in the contralateral eyes (P<0.05; Fig. 3A). In the recovery group, following
1 week without lenses subsequent to 3 weeks wearing lenses, no
significant differences were observed in the protein levels of
BMP-2 between the treated eyes and the contralateral untreated eyes
(P>0.05; Fig. 3B).
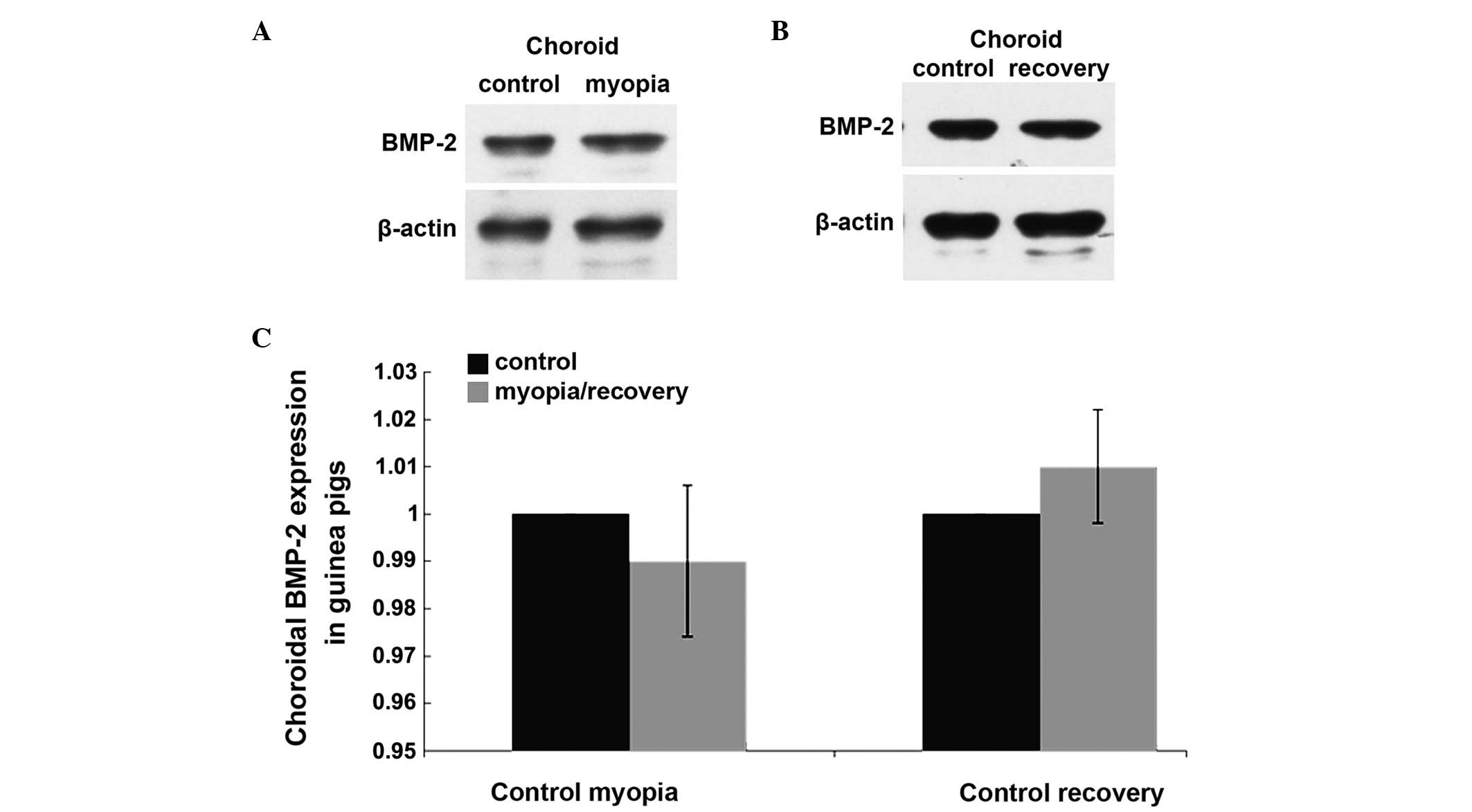
Choroidal expression of BMP-2 in the LIM
and myopia recovery groups of guinea pigs
Following 3 weeks of lens wearing in the LIM group,
no differences were observed between the choroidal protein levels
of BMP-2 in the treated eyes and those in the contralateral
untreated eyes (P>0.05; Fig.
4A). Following a further 1 week without lenses, no differences
in the choroidal protein expression of BMP-2 were observed between
the treated and untreated eyes of the guinea pigs in the recovery
group (P>0.05; Fig. 4B).
Discussion
Alterations in the expression of BMP-2 are
associated with scleral remodeling during myopia (17,23),
however, whether the expression of BMP-2 in the retina and choroid
is involved in the retinoscleral cascade remains to be fully
elucidated. The present study demonstrated that the retinal
expression of BMP-2 decreased in the development of myopia, and
that its level increased on recovery from myopia. In the choroid,
no changes in the expression of BMP-2 were observed during myopia
development or on recovery from myopia.
Ocular growth and refractive error have been
regulated by inducing hyperopic defocus with negative power lenses,
or myopic defocus with positive power lenses, in several animal
models, including primates (24–27).
Studies have reported that eye growth is regulated by retinal
mechanisms, which act locally within the eye, producing a cascade
of changes that ultimately affect the structure of the sclera
(28,29).
BMP-2 is one of the most widely investigated growth
factors in the BMP family and it is essential for the development
and patterning of the retina (30). In addition, BMP signaling is
neuroprotective for retinal ganglion cells following damage
(31) and is involved in glial
proliferation (32). BMP-2 may act
as a negative growth regulator in the retina and the RPE (33). In the development of myopia, a
decrease in the level of BMP-2 was observed in the retina, whereas
its level increased following recovery from myopia. As this
alteration occurred following significant structural change, it is
likely that the retinal level of BMP-2 is associated with eye
growth and myopia development. A previous study reported that the
retinal gene expression of BMP2 was downregulated in form-deprived
myopia in chickens (34). The
potential involvement of BMP-2 in the signaling cascade, which
mediates the development of myopia remains to be elucidated. During
lense-induced myopia development in tree shrews, choroidal gene
expression of BMP2 is upregulated, compared with control animals
(35,36), and this change may have been part
of a common choroidal response. However, in the present study, the
choroidal expression of BMP-2 in the guinea pig myopia model
remained unchanged.
TGF-β is important in myopia. During the development
of myopia in tree shrews, the expression levels of three TGF-β
isoforms (TGF-β1, TGF-β2 and TGF-β3) in the sclera decrease, and
in vitro, TGF-β regulates collagen production in the sclera
(37). However, the levels of
these three isoforms remain within normal ranges in retinal and
choroidal tissues during myopia development (38). bFGF is another important regulator
in the development of myopia. In the development of myopia, the
expression of bFGF in the sclera decreases significantly (39,40),
whereas its content and concentration remain unchanged in the
retina, RPE and choroid (40). The
ocular injection of bFGF inhibits myopia development (41,42),
however, whether bFGF acts on the sclera or upstream of retina, RPE
or choroid remains to be elucidated. A previous study demonstrated
that the intravitreal injection of bFGF acts either parallel to or
downstream of the dopaminergic amacrine cells, rather than through
them, to prevent myopia development (41), and bFGF intraocular injection
suppresses retinal neuronal apoptosis in myopia (42). The peribulbar injection of bFGF
also regulates scleral remodeling to prevent myopia in guinea pigs
(43). Therefore, the present
study hypothesized that, although TGF-β and bFGF were involved in
scleral regulation during myopia development, they did not have a
primary role in the retinal or choroidal signals.
Although a decrease in the retinal expression of
BMP-2 was observed in myopia development in the present study, its
functional correlation remains to be elucidated. As the retinal and
choroidal components of the retinoscleral signal are unlikely to
rely upon BMP-2 to modulate downstream scleral remodeling, the
signaling cascade relies on multiple signaling pathways to control
eye growth in myopia. The change in retinal expression of BMP-2 may
regulate cellular responses in pathways other than the choroid to
sclera pathway, which results in scleral remodeling and facilitates
eye growth (44). In the RPE
layer, the gene expression of BMP2 is upregulated in eyes treated
with minus lenses, and is downregulated in eyes treated with plus
lenses (45). Certain studies have
shown that the levels of the three forms of TGF-β in the
RPE-choroid complex mice with form-deprived myopia are
significantly elevated (46). In
addition, TGF-β2 content and concentration are significantly higher
in myopic eyes, in the retina-RPE-choroid and the sclera, in chicks
(11). The possible explanation
for role of the RPE-choroid complex in myopia development may be
due to RPE cells, possessing the ability to express and secrete
TGF-β (46), and affect choroidal
function or tranverse the choroid to affect the sclera.
The results of the present study demonstrated that
the retinal expression of BMP2 in the eyes of lens-induced myopia
were downregulated, and this expression was upregulated following
recovery of the myopic eyes in guinea pigs. This suggested that
BMP-2 is involved in the development of myopia in mammals. However,
the protein level of BMP-2 in the choroid remained unchanged in the
myopic eyes, which suggested that BMP-2 has different roles in the
retina and sclera during the development of myopia.
Acknowledgments
This study was supported by the Province Science
Foundation of China (grant no. 10251008901000025).
References
|
1
|
Li HH, Huo LJ, Gao ZY, Zhao F and Zeng JW:
Regulation of scleral fibroblast differentiation by bone
morphogenetic protein-2. Int J Ophthalmol. 7:152–156.
2014.PubMed/NCBI
|
|
2
|
Feldkaemper M and Schaeffel F: An updated
view on the role of dopamine in myopia. Exp Eye Res. 114:106–119.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fischer AJ, McGuire JJ, Schaeffel F and
Stell WK: Light- and focus-dependent expression of the
transcription factor ZENK in the chick retina. Nat Neurosci.
2:706–712. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mertz JR and Wallman J: Choroidal retinoic
acid synthesis: A possible mediator between refractive error and
compensatory eye growth. Exp Eye Res. 70:519–527. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McBrien NA, Lawlor P and Gentle A: Scleral
remodeling during the development of and recovery from axial myopia
in the tree shrew. Invest Ophth Vis Sci. 41:3713–3719. 2000.
|
|
6
|
Rymer J and Wildsoet CF: The role of the
retinal pigment epithelium in eye growth regulation and myopia: A
review. Vis Neurosci. 22:251–261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wildsoet C and Wallman J: Choroidal and
scleral mechanisms of compensation for spectacle lenses in chicks.
Vision Res. 35:1175–1194. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McBrien NA: Regulation of scleral
metabolism in myopia and the role of transforming growth
factor-beta. Exp Eye Res. 114:128–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wallman J and Winawer J: Homeostasis of
eye growth and the question of myopia. Neuron. 43:447–468. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rohrer B and Stell WK: Basic fibroblast
growth factor (bFGF) and transforming growth factor beta (TGF-beta)
act as stop and go signals to modulate postnatal ocular growth in
the chick. Exp Eye Res. 58:553–561. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seko Y, Shimokawa H and Tokoro T:
Expression of bFGF and TGF-beta 2 in experimental myopia in chicks.
Invest Ophthalmol Vis Sci. 36:1183–1187. 1995.PubMed/NCBI
|
|
12
|
Wozney JM: The bone morphogenetic protein
family and osteogenesis. Mol Reprod Dev. 32:160–167. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wordinger RJ and Clark AF: Bone
morphogenetic proteins and their receptors in the eye. Exp Biol Med
(Maywood). 232:979–992. 2007. View Article : Google Scholar
|
|
14
|
McGlinn AM, Baldwin DA, Tobias JW, Budak
MT, Khurana TS and Stone RA: Form-deprivation myopia in chick
induces limited changes in retinal gene expression. Invest
Ophthalmol Vis Sci. 48:3430–3436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakuta H, Takahashi H, Shintani T, Etani
K, Aoshima A and Noda M: Role of bone morphogenic protein 2 in
retinal patterning and retinotectal projection. J Neurosci.
26:10868–10878. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Belecky-Adams T and Adler R: Developmental
expression patterns of bone morphogenetic proteins, receptors, and
binding proteins in the chick retina. J Comp Neurol. 430:562–572.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Q, Zhao G, Xing S, Zhang L and Yang
X: Role of bone morphogenetic proteins in form-deprivation myopia
sclera. Mol Vis. 17:647–657. 2011.PubMed/NCBI
|
|
18
|
Wang Q, Zhao G, Xing S, Zhang L and Yang
X: Role of bone morphogenetic proteins in form-deprivation myopia
sclera. Mol Vis. 17:647–657. 2011.PubMed/NCBI
|
|
19
|
Hu J, Cui D, Yang X, Wang S, Hu S, Li C
and Zeng J: Bone morphogenetic protein-2: A potential regulator in
scleral remodeling. Mol Vis. 14:2373–2380. 2008.PubMed/NCBI
|
|
20
|
Yoshikawa M, Yamashiro K, Miyake M, Oishi
M, Akagi-Kurashige Y, Kumagai K, Nakata I, Nakanishi H, Oishi A,
Gotoh N, et al: Comprehensive replication of the relationship
between myopia-related genes and refractive errors in a large
Japanese cohort. Invest Ophthalmol Vis Sci. 55:7343–7354. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Verhoeven VJ, Hysi PG, Wojciechowski R,
Fan Q, Guggenheim JA, Höhn R, MacGregor S, Hewitt AW, Nag A, Cheng
CY, et al: Genome-wide meta-analyses of multiancestry cohorts
identify multiple new susceptibility loci for refractive error and
myopia. Nat Genet. 45:314–318. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen B, Wang C, Chen W and Ma J: Altered
TGF-β2 and bFGF expression in scleral desmocytes from an
experimentally-induced myopia guinea pig model. Graefes Arch Clin
Exp Ophthalmol. 251:1133–1144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu J, Cui D, Yang X, Wang S, Hu S, Li C
and Zeng J: Bone morphogenetic protein-2: A potential regulator in
scleral remodeling. Mol Vis. 14:2373–2380. 2008.PubMed/NCBI
|
|
24
|
Benavente-Perez A, Nour A and Troilo D:
The effect of simultaneous negative and positive defocus on eye
growth and development of refractive state in marmosets. Invest
Ophthalmol Vis Sci. 53:6479–6487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Graham B and Judge SJ: The effects of
spectacle wear in infancy on eye growth and refractive error in the
marmoset (Callithrix jacchus). Vision Res. 39:189–206. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Norton TT and Siegwart JT Jr: Animal
models of emmetropization: Matching axial length to the focal
plane. J Am Optom Assoc. 66:405–414. 1995.PubMed/NCBI
|
|
27
|
Hung LF, Crawford ML and Smith EL:
Spectacle lenses alter eye growth and the refractive status of
young monkeys. Nat Med. 1:761–765. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Benavente-Pérez A, Nour A and Troilo D:
Axial eye growth and refractive error development can be modified
by exposing the peripheral retina to relative myopic or hyperopic
defocus. Invest Ophthalmol Vis Sci. 55:6765–6773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ashby RS, Zeng G, Leotta AJ, Tse DY and
McFadden SA: Egr-1 mRNA expression is a marker for the direction of
mammalian ocular growth. Invest Ophthalmol Vis Sci. 55:5911–5921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sakuta H, Takahashi H, Shintani T, Etani
K, Aoshima A and Noda M: Role of bone morphogenic protein 2 in
retinal patterning and retinotectal projection. J Neurosci.
26:10868–10878. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ueki Y and Reh TA: Activation of
BMP-Smad1/5/8 signaling promotes survival of retinal ganglion cells
after damage in vivo. PLoS One. 7:e386902012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ueki Y and Reh TA: EGF stimulates müller
glial proliferation via a BMP-dependent mechanism. Glia.
61:778–789. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mathura JR Jr, Jafari N, Chang JT, Hackett
SF, Wahlin KJ, Della NG, Okamoto N, Zack DJ and Campochiaro PA:
Bone morphogenetic proteins-2 and -4: Negative growth regulators in
adult retinal pigmented epithelium. Invest Ophthalmol Vis Sci.
41:592–600. 2000.PubMed/NCBI
|
|
34
|
McGlinn AM, Baldwin DA, Tobias JW, Budak
MT, Khurana TS and Stone RA: Form-deprivation myopia in chick
induces limited changes in retinal gene expression. Invest
Ophthalmol Vis Sci. 48:3430–3436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He L, Frost MR, Siegwart JT Jr and Norton
TT: Gene expression signatures in tree shrew choroid during
lens-induced myopia and recovery. Exp Eye Res. 123:56–71. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He L, Frost MR, Siegwart JT Jr and Norton
TT: Gene expression signatures in tree shrew choroid in response to
three myopiagenic conditions. Vision Res. 102:52–63. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jobling AI, Nguyen M, Gentle A and McBrien
NA: Isoform-specific changes in scleral transforming growth
factor-expression and the regulation of collagen synthesis during
myopia progression. J Biol Chem. 279:18121–18126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jobling AI, Wan R, Gentle A, Bui BV and
McBrien NA: Retinal and choroidal TGF-β in the tree shrew model of
myopia: Isoform expression, activation and effects on function. Exp
Eye Res. 88:458–466. 2009. View Article : Google Scholar
|
|
39
|
Chen BY, Wang CY, Chen WY and Ma JX:
Altered TGF-β2 and bFGF expression in scleral desmocytes from an
experimentally-induced myopia guinea pig model. Graefe's Arch Clin
Exp Ophthalmol. 251:1133–1144. 2013. View Article : Google Scholar
|
|
40
|
Seko Y, Shimokawa H and Tokoro T:
Expression of bFGF and TGF-beta 2 in experimental myopia in chicks.
Invest Ophthalmol Vis Sci. 36:1183–1187. 1995.PubMed/NCBI
|
|
41
|
Rohrer B, Iuvone PM and Stell WK:
Stimulation of dopaminergic amacrine cells by stroboscopic
illumination or fibroblast growth factor (bFGF, FGF-2) injections:
Possible roles in prevention of form-deprivation myopia in the
chick. Brain Res. 686:169–181. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mao J, Liu S, Wen D, Tan X and Fu C: Basic
fibroblast growth factor suppresses retinal neuronal apoptosis in
form-deprivation myopia in chicks. Curr Eye Res. 31:983–987. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tian XD, Cheng YX, Liu GB, Guo SF, Fan CL,
Zhan LH and Xu YC: Expressions of type I collagen, α2 integrin and
β1 integrin in sclera of guinea pig with defocus myopia and
inhibitory effects of bFGF on the formation of myopia. Int J
Ophthalmol. 6:54–58. 2013.
|
|
44
|
McBrien NA: Regulation of scleral
metabolism in myopia and the role of transforming growth
factor-beta. Exp Eye Res. 114:128–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Y, Liu Y and Wildsoet CF:
Bidirectional, optical sign-dependent regulation of BMP2 gene
expression in chick retinal pigment epithelium. Invest Ophthalmol
Vis Sci. 53:6072–6080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu PC, Tsai CL, Gordon GM, Jeong S,
Itakura T, Patel N, Shi S and Fini ME: Chondrogenesis in scleral
stem/progenitor cells and its association with form-deprived myopia
in mice. Mol Vis. 21:138–147. 2015.PubMed/NCBI
|