Introduction
Necrosis was initially considered to be a form of
accidental cell death, which only occurred in response to
physicochemical injuries. Due to modern, genetic and molecular
biological techniques, this concept has changed. Necrosis is now
recog-nized to be programmed by multiple signaling pathways, and is
defined as regulated necrosis in order to distinguish this
genetically controlled, necrotic-like cell death process (1–3). A
range of regulated necrosis initiators have been identified in
recent years, including the necrosome, poly (ADP-ribose) polymerase
1 (PARP1) hyperactivation, mitochondrial permeability transition
(MPT), mitochondrial complex I, nicotinamide adenine dinucleotide
phosphate (NADPH)-oxidases amongst others. In addition to the
well-known receptor-interacting protein 1 (RIP1) complex (the
necrosome), cyclophilin D (CypD)-mediated MPT is another important
initiator of regulated necrosis (2). Mammalian target of rapamycin (mTOR)
is an evolutionarily conserved serine (Ser)/threonine (Thr) protein
kinase. The mTOR signaling pathway promotes translation initiation
and elongation, ribosome biogenesis and autophagy, which
subsequently regulate cell survival and cell growth. Aberrant
activation of the mTOR signaling pathway promotes cell growth and
survival, particularly in malignant cells. Furthermore, although
previous evidence indicates that limited activation of the Akt/mTOR
signaling pathway is anti-apoptotic, numerous recent reports
illustrate an emerging role for the Akt/mTOR signaling pathway as a
death kinase and a key regulator of regulated necrosis (4).
HNK is a pharmacologically active small molecule
isolated from the traditional Chinese medicinal herb, houpu. Recent
evidence demonstrates that it may induce diversified types of
regulated cell death, including extrinsic and intrinsic apoptosis,
regulated necrosis and paraptosis, depending on varying cell types
and concentration of therapeutic agents (5). It has been previously reported that
HNK may trigger a CypD-mediated regulated necrosis in numerous cell
lines at certain concentrations (two-fold higher than its half
maximal inhibitory concentration; IC50) (6). The aim of the current study was to
demonstrate whether HNK triggered uniform necrotic cell death in
HEK-293 cells at a reduced concentration (30 µg/ml). In
addition, the function of Akt/mTOR signaling in the process of
HNK-triggered regulated necrosis was investigated.
Materials and methods
Materials and cell culture
HNK powder was purchased from the National Institute
for the Control of Pharmaceutical and Biological Products (Beijing,
China; >99% purity) and was dissolved as 20 mg/ml in dimethyl
sulfoxide (75 mM). RIP1 inhibitor, Nec-1, and pan-caspase
inhibitor, z-VAD-fmk, were obtained from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). CsA, Hoechst 33342, dimethyl sulfoxide
and VP-16 were purchased from Sigma-Aldrich (St. Louis, MO, USA).
the CCK-8 Cell Counting kit, DNA Ladder Detection kit and
MitoTracker probes (MitoRed) were purchased from Nanjing KeyGEN
Biotech Co., Ltd. (Nanjing, China). M-PER Mammalian Protein
Extraction Reagent, Halt Protease and Phosphatase Inhibitor
Cocktails were purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). The Annexin V-fluorescein isothiocyanate (FITC)
Apoptosis Detection kit II was purchased from BD Biosciences
(Franklin Lakes, NJ, USA). Cleaved caspase-3 and -8, B-cell
lymphoma 2 (Bcl-2), RIP1, phosphorylated (p)-Akt (T308), p-Akt
(S473), Akt, p-eIF4E-binding protein 1 (4E-BP1), 4E-BP1, p-S6
kinase (S6K), S6K and GAPDH antibodies were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Millicell EZ Slides
were purchased from Merck Millipore (Darmstadt, Germany).
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum
(FBS) were purchased from Gibco (Thermo Fisher Scientific, Inc.).
Enhanced BCA Protein Assay Kit (P0009) was purchased from Beyotime
Institute of Biotechnology (Haimen, China). Polyvinylidene
difluoride (PVDF) membranes were purchased from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA). The HEK-293 human embryonic
kidney cell line was obtained from the Cancer Institution of
Zhejiang University (Hangzhou, China). The HEK-293 cells were
cultured in DMEM supplemented with 10% FBS. HEK-293 cella were
cultured at 7°C and passaged every 3–4 days.
Cell toxicity assay
HEK-293 cells were seeded in 96-well culture plates
at density of 6,000 cells/per well. Following culture for 24 h,
cells were pretreated with DMEM/FBS, 25 µM CsA (2 h) or 60
µM Nec-1 (1 h). Then cells were incubated with the drug-free
medium, HNK of different concentrations (30 or 40 µg/ml) for
different durations (1, 2 and 4 h). Subsequently, the CCK-8 kit was
added to the culture medium at 10 µl per 100 µl
medium. Following culture with CCK-8 for 1 h, the optical density
at a wavelength of 450 nm (OD450) was tested using a
spectrophotometer (Model 680; Bio-Rad Laboratoeis, Inc.). The
relative cell viability was calculated using the OD450
values.
Flow cytometric analysis for cell
apoptosis and necrosis
HEK-293 cells were cultured in 6-cm culture dishes
at density of 1.2×106 in 3 ml medium for 24 h. Cells
were pretreated with drug-free medium, 25 µM CsA (2 h), 60
µMNec-1 (1 h) or 50 µM z-VAD-fmk (0.5 h). The cells
were treated with drug-free medium or 30 µg/ml HNK (liquid
volume, 3 ml) for varying durations. Following treatment, the cells
were harvested and stained with Annexin V-FITC/propidium iodide
(PI) and assayed by flow cytometry, as previously described
(7,8).
DNA ladder detection
Cells were treated as described above for flow
cytometric analysis. DNA extractions from HEK-293 cells from each
treatment group were performed according to the manufacturer's
instructions of the DNA Ladder Detection Kit. HL-60 cells that had
been treated with 20 µg/ml VP-16 for 6 h served as a
positive control.
Chromatin condensation assessment using
Hoechst 33342 staining
Cells were cultured on Millicell EZ Slides at a
density of 4×104 cells/slide and treated as described
above for flow cytometric analysis. Following treatment, cells were
stained with Hoechst 33342 and MitoRed according to the
manufacturer's instructions. The cells were examined under a
confocal microscope (TCS SP5; Leica Camera, Wetzlar, Germany), as
previously described (8).
Western blot analysis
Cells were treated and harvested as described above
for flow cytometric analysis. Proteins were extracted using
M-PER® Mammalian Protein Extraction Reagent supplemented
with Halt Protease and Phosphatase Inhibitor Cocktail. Protein
concentrations were determined using the BCA method. Samples (20
µg of each) were loaded in 10 or 8% sodium dodecyl sulfate
polyacrylamide gel and electrophoresis was performed using PVDF
membranes (100–150 V; 0.5–2 h), followed by western blotting.
Images were obtained by chemiluminesence using ECL Plus Western
Blotting Substrate (Thermo Fisher Scientific, Inc.). The procedures
were performed as previously described (8).
Statistical analysis
SPSS version 20.0 (IBM SPSS, Amronk, NY, USA) was
used to perform all statistical analyses. All the experiments were
performed in triplicate, and comparable results were obtained from
the three different experimental setups. The data are presented as
means ± standard deviation. Paired student's t-test was used
in single group comparisons and P<0.01 was considered to
indicate a statistically significant difference.
Results
HNK triggers necrotic cell death in
HEK-293 cells
In the current study, 30 µg/ml HNK, a lower
concentration than previously reported (40 µg/ml) (6), was used to demonstrate its effect on
HEK-293 cells. HEK-293 cells were incubated with 30 µg/ml
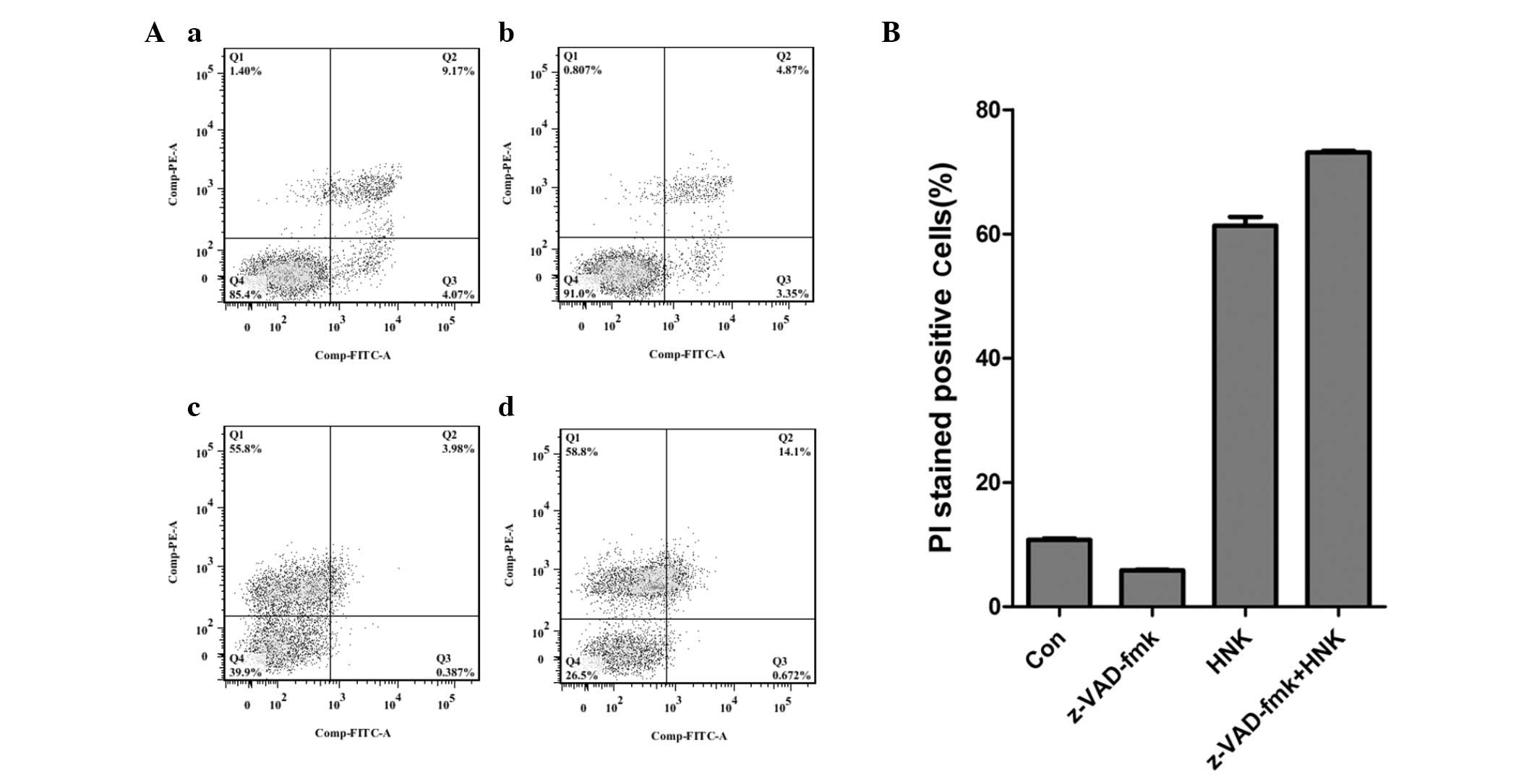
HNK for 4 h, and labeled with Annexin V-FITC and PI (Fig. 1). Cell viability was significantly
reduced following treatment with 30 µg/ml HNK. And the
majority of HNK-triggered cell deaths were represented by positive
staining for PI, which indicated necrotic cell death (Fig. 1Ac and B). Subsequently, whether the
PI-stained cells had undergone necrosis or were composed of
necrotic cells and undergoing advance-stage apoptosis was
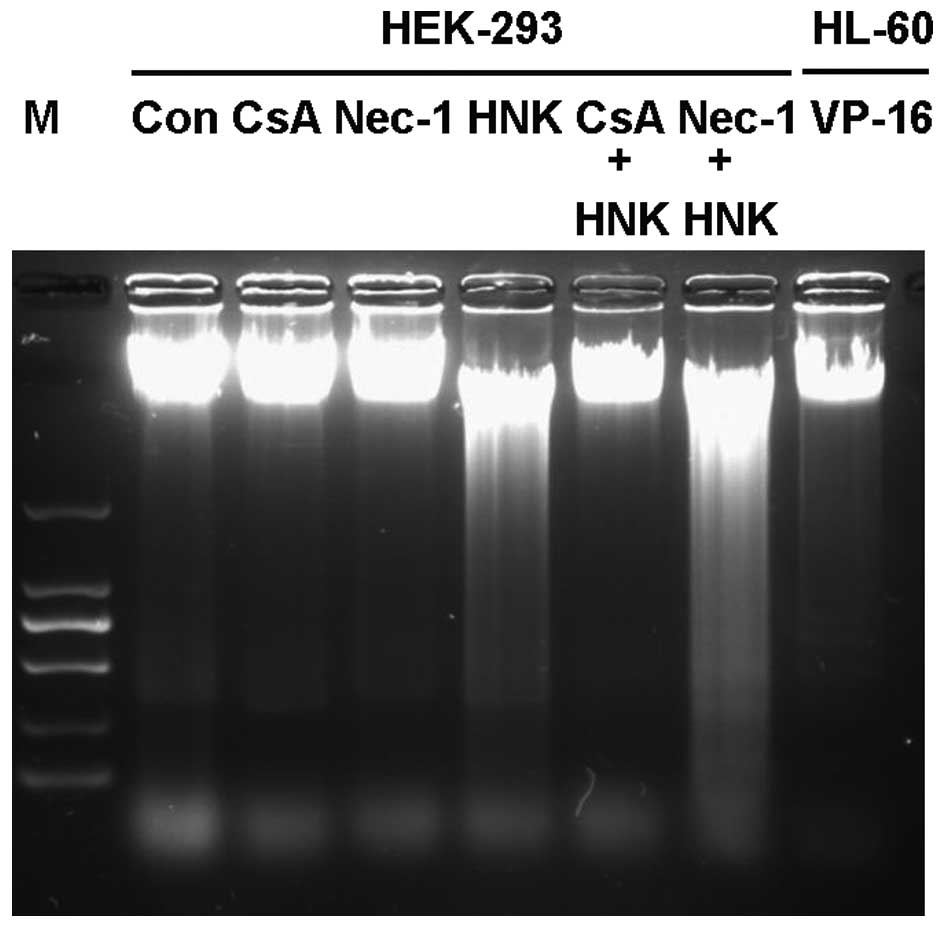
investigated. Internucleosomal DNA fragmentation is a morphological
characteristic of apoptosis at the advanced stage (or degradation
phase), which can be detected as a ladder pattern by
electrophoresis of isolated DNA. Apoptotic bodies are another
morphological characteristic of apoptosis and are detectable using
nuclei staining with Hoechst 33342. DNA laddering and apoptotic
bodies are frequently used to determine the existence of apoptosis.
DNA laddering was performed on DNA extracted from HEK-293 cells
after exposure to 30 µg/ml HNK. No obvious ladder pattern
was observed, however a diffuse fractured pattern was detected
following HNK exposure (Fig. 2).
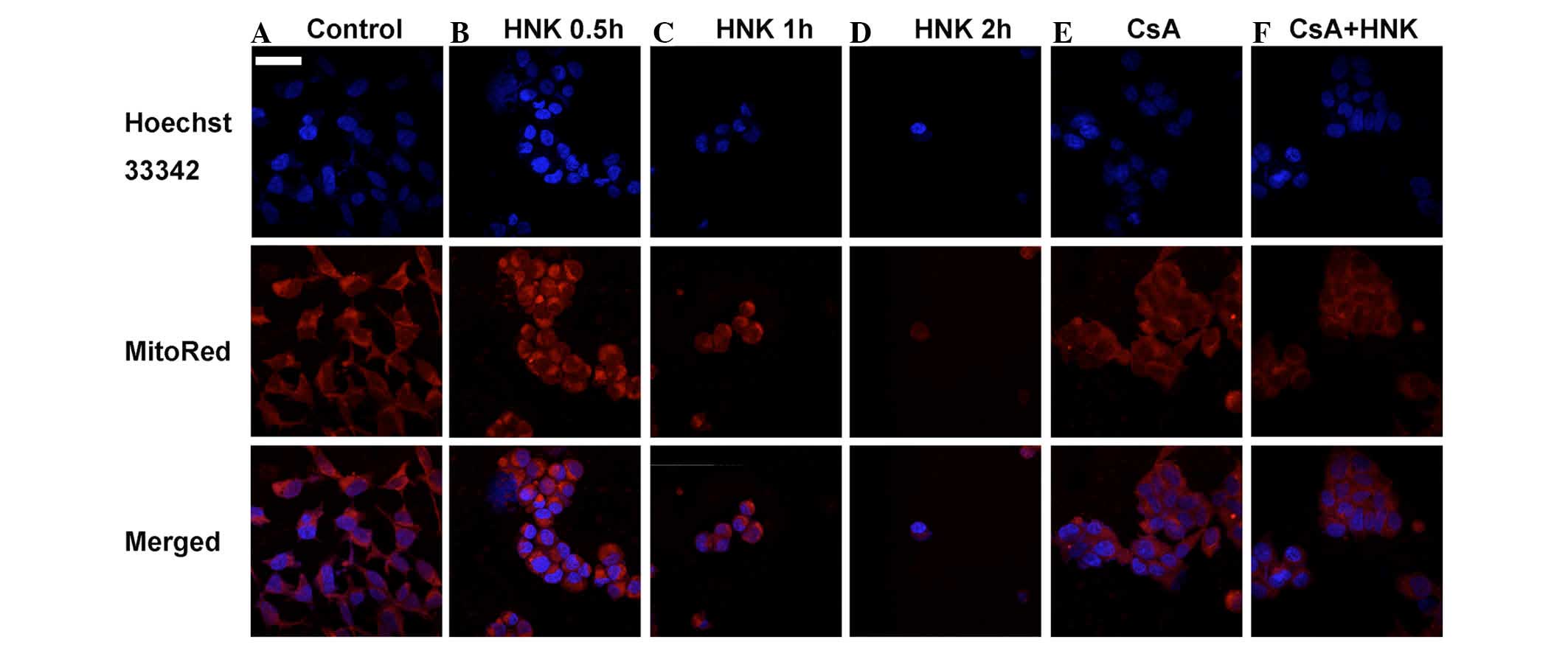
Confocal imaging of Hoechst 33342-stained nuclei was used to
determine apoptotic bodies during the process of HNK-triggered
necrotic cell death. Confocal imaging did not detect any nuclear
chromatin condensation or apoptotic bodies in the HEK-293 cells
after exposure to 30 µg/ml HNK for 0.5, 1 or 2 h (Fig. 3). In addition, DNA laddering and
Hoechst 33342 staining revealed no morphological manifestation of
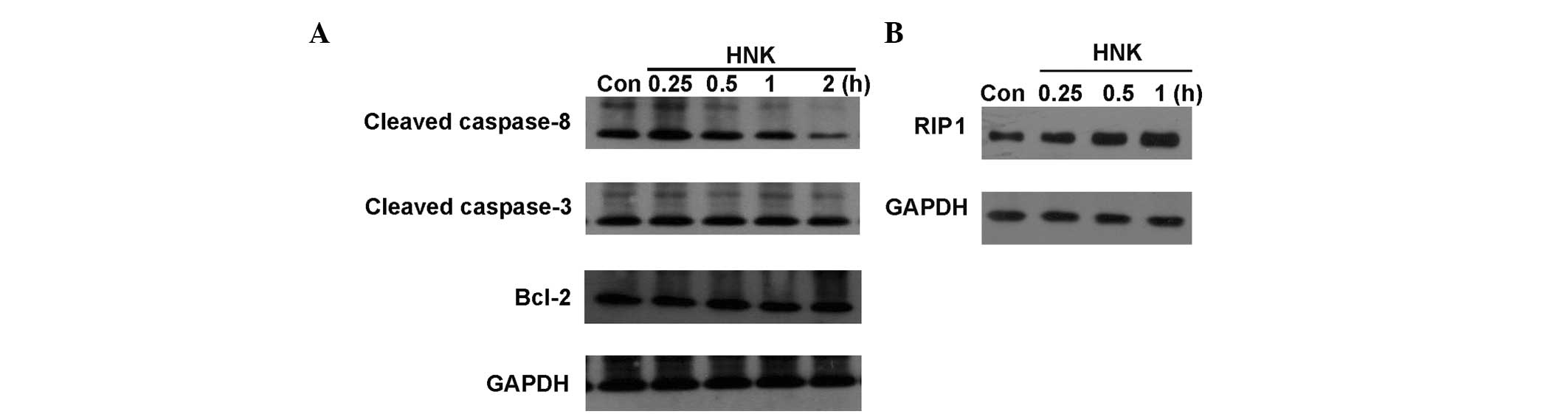
apoptosis in HNK-triggered cell death of HEK-293 cells. Caspase-8
is an important initiator caspase, and it is activated during the
initiation phase of extrinsic apoptosis. Activated caspase-8 leads
to the release of its active fragments, p18 and p10. It cleaves and
activates downstream effector caspases (including caspase-3, -6,
and -7), which in turn execute apoptosis. The present study
demonstrated that in the ultra early stage of HNK-induced necrosis
(0.25 h), the cleaved caspase-8 level was increased. Subsequently,
its expression level decreased in parallel with the increase in
necrosis. Caspase-3 is a critical activator of intrinsic and
extrinsic apoptosis. Activation of caspase-3 requires proteolytic
processing of its inactive zymogen into activated p17 and p12
fragments. And active level of cleaved caspase-3 is frequently used
as a chemical manifestation to determine apoptosis. Western blot
analysis revealed that the protein levels of cleaved caspase-3 had
not elevated though the process of HNK-triggered cell death (0.25,
0.5, 1 and 2 h; Fig. 4). Bcl-2 is
a pro-survival protein of the Bcl-2 family. It exerts a survival
function in response to a wide range of apoptotic stimuli via
inhibition of mitochondrial cytochrome C release. In the process of
HNK-triggered cell death, no obvious changes in expression levels
of Bcl-2 were detected with increasing treatment durations using
western blotting (Fig. 4).
Conversely, pan-caspase inhibitor, z-VAD-fmk did not reverse
HNK-induced cell death (Fig. 1),
which also demonstrated that HNK-induced cell death was
caspase-independent and did not result from apoptosis. Therefore,
the morphological and chemical manifestations reveal that apoptosis
was not induced in HEK-293 cells by HNK at a dose of 30
µg/ml. However, 30 µg/ml HNK did trigger simple
necrotic cell death in HEK-293 cells.
CsA blocks HNK-triggered regulated
necrotic cell death
Regulated necrosis is defined as a genetically
controlled cell death process, which is morphologically
characterized by cytoplasmic granulation, and organelle and/or
cellular swelling (2). Previous
reports demonstrated that morphological manifestations of
HNK-induced regulated necrotic cell death included increased cell
volume (due to swelling of cytoplasmic organelles), dilatation of
mitochondria and the endoplasmic reticulum, vacuoles and nuclear
modifications (6,7). Among the morphological manifestations
(vacuoles presenting as autophagic vacuoles containing a ruptured
plasma membrane, manifesting as membranous whorls), mitochondrial
dilatation was the most characteristic. MitoRed was used to stain
the mitochondria of HEK-293 cells, and confocal imaging was
performed to detect morphological changes of the mitochondria.
Intense staining of the mitochondria was observed in the early
stage of HNK-induced necrosis, and gradually weakened during the
necrotic process (Fig. 3). This
indicated that the mitochondria underwent swelling and subsequently
disintegration during HNK-induced necrosis from the early to late
stages. MPT is an initiator of the schematic steps of regulated
necrosis (2). It was previously
reported that CypD, a vital protein involved in MPT, was a critical
regulator in HNK-induced regulated necrotic cell death (6–8).
CypD levels gradually increased in parallel with HNK-triggered
regulated necrosis. Furthermore, CypD inhibitors (CsA) or CypD
siRNA depletion completely blocked CypD, which significantly
attenuated HNK-induced necrotic cell death (6,8). In
the current study, 25 µM CsA pretreatment prior to HNK
treatment was performed to determine whether CsA pretreatment
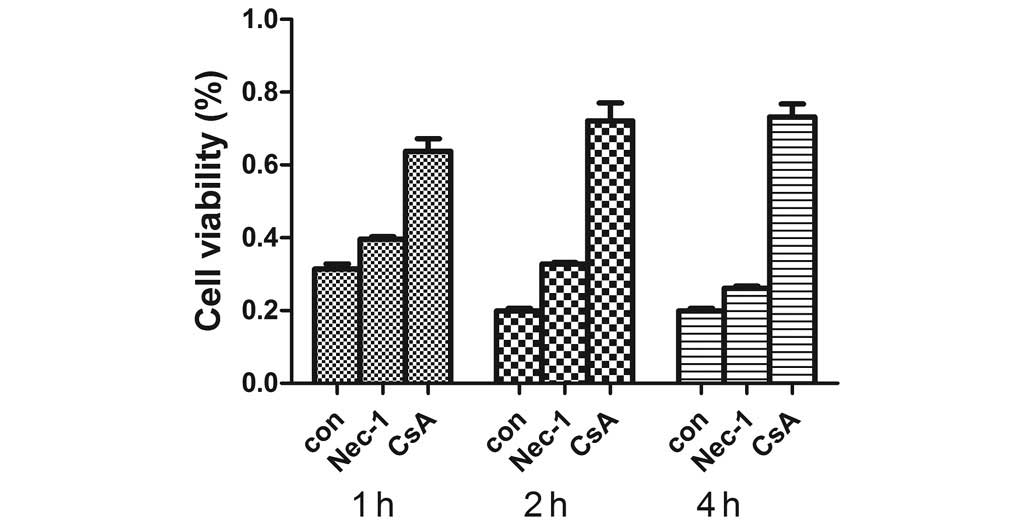
inhibits HNK-induced necrosis. The results indicated that 25
µM CsA pretreatment for 2 h significantly increased cell
viabilities following HNK treatment for 1, 2 and 4 h (Fig. 5). In addition, it completely
blocked HNK-induced necrotic cell death, which returned the levels
of PI-stained cell fractions to the control level. The PI-stained
cell fractions in the control, CsA, HNK and CsA + HNK groups were
10.79±0.19, 4.16±0.07, 61.33±1.42 and 3.33±0.09%, respectively
(P<0.001; Fig. 6). Conversely,
25 µM CsA pretreatment blocked DNA diffuse fracture
(Fig. 2) and mitochondrial
dilatation (Fig. 3). The results
indicated that HNK-triggered regulated necrotic cell death could be
completely blocked by CsA, which indirectly indicates that CypD was
a central initiator during the process of HNK-induced regulated
necrosis in HEK-293 cells.
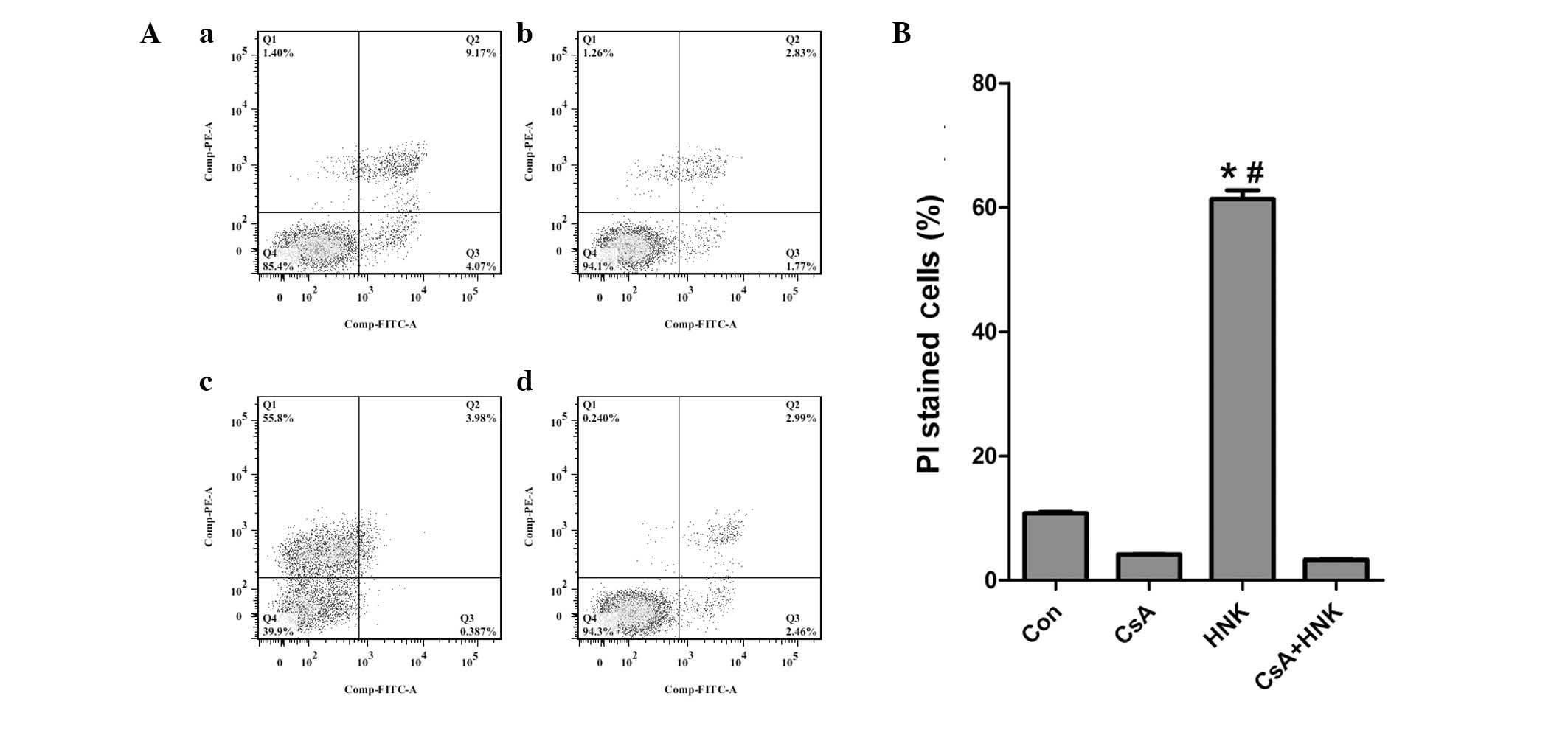 | Figure 6CsA pretreatment markedly inhibited
HNK-triggered regulated necrosis. HEK-293 cells were treated with
drug-free medium or HNK (30 µg/ml) for 4 h in the presence
or absence of CsA pretreatment (25 µM; 2 h). Cells were
labeled with Annexin V-FITC and PI, and flow cytometric analysis
was performed. (A) CsA pretreatment markedly reversed HNK-triggered
necrotic cell death: (a) Con; (b) CsA; (c) HNK; (d) CsA + HNK. (B)
Analysis of ratios of PI-stained cells in the four groups. Results
are representative of three independent experiments. The values are
presented as the mean ± standard deviation.#P<0.001
vs. Con group; *P<0.001 vs. CsA + HNK group. Q1,
AV(−)PI(+); Q2, AV(+)PI(+); Q3, AV(+)PI(−); Q4, AV(-)PI(-); CsA,
cyclosporin A; HNK, honokiol; FITC, fluorescein isothiocyanate; PI,
propidium iodide; Con, control. |
Necrostatin-1 (Nec-1) partly alleviates
HNK-induced necrosis at the early stage
The most characteristic form of regulated necrosis
is termed necroptosis. In human cells, the RIP1-RIP3-mixed lineage
kinase domain-like (MLKL)-PGAM family member 5, Ser/Thr protein
phosphatase, mitochondrial (PGAM5)-dynamin-related protein 1 (Drp1)
axis drives tumor necrosis factor (TNF)-induced necroptosis via
mitochondrial fission (9).
Necroptosis is characterized as RIP1-dependent and can be
specifically inhibited by RIP1 inhibitor, Nec-1. In previous
studies, RIP1 expression levels were demonstrated to be
downregulated in HNK-induced regulated necrosis in the MCF-7 breast
cancer cell line throughout treatment durations of 1–4 h (8). In the current study, RIP1 expression
levels and functions were detected at the ultra early stage in
HNK-induced regulated necrosis in HEK-293 cells. Contrary to our
previous report, RIP1 expression levels were observed to be
upregulated in the ultra early stage (0.25–1 h) of regulated
necrosis (Fig. 4). Subsequent
results revealed that HNK-induced cell death was partly reversed by
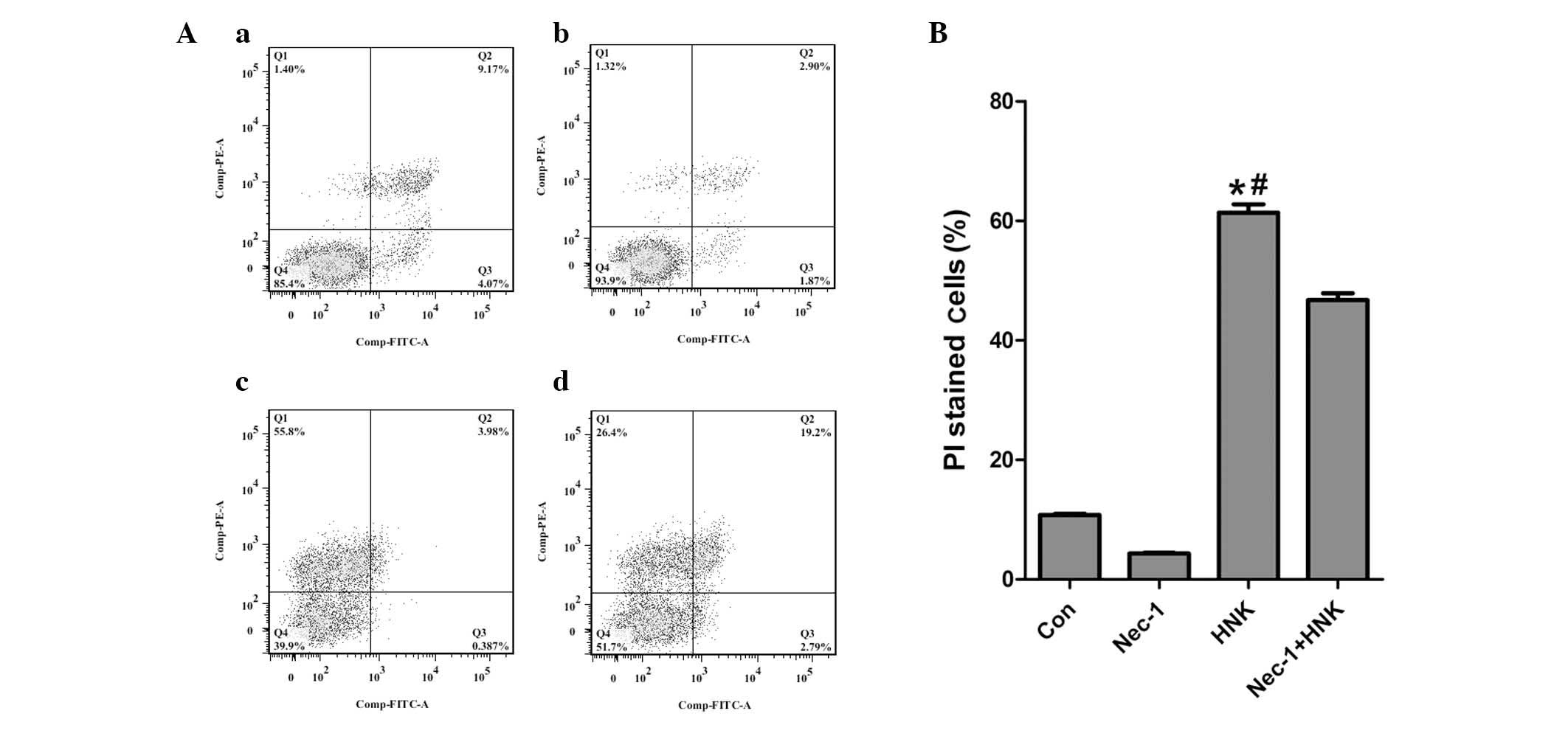
pretreatment with 60 µM Nec-1 for 1 h [PI-stained cell
fractions in the control, Nec-1, HNK and Nec-1 + HNK groups were
10.79±0.19, 4.36±0.12, 61.33±1.42 and 46.73±1.15%, respectively
(P<0.001; Fig. 7)]. In
addition, the inhibition effects of Nec-1 on HNK-induced regulated
necrosis gradually reduced with the elongation of HNK incubating
time (1–4 h; Fig. 5). The results
indicated that RIP1 probably participated in the initiation stage
of HNK-induced regulated necrosis. However, it was not a pivotal
initiator and its effects in the process of HNK-induced regulated
necrosis were limited.
mTOR signaling pathways are inhibited
during HNK-triggered regulated necrosis
mTOR is a highly conserved Ser/Thr kinase, which
integrates diverse signals to control cell growth, proliferation,
survival and metabolism. mTOR assembles into two functionally
different complexes, mTORC1 and mTORC2, with distinct inputs and
downstream effects. mTORC1 regulates cell growth by promoting
translation, ribosome biogenesis and autophagy via phosphorylation
of its substrates, including 4E-BP1 and ribosomal S6K. mTORC2
promotes cell cycle entry, cell survival, actin cytoskeleton
polarization and anabolic output though phosphorylation of its
substrates, the Ser/Thr protein kinases, Akt, protein kinase A
(PKA) and PKC (10,11). Previous findings demonstrate that
aberrant activation of the mTOR signaling pathway is vital in
tumorigenesis, and promotes growth and survival of various types of
cancer cells (12). Due to its
high biological relevance to cancer, different therapeutic
strategies have been developed to target this signaling cascade,
such as mTOR inhibitors Everolimus and Temsirolimus (13). In the current study, the activation
levels of the mTOR signaling pathway during HNK-triggered regulated
necrosis in HEK-293 cells was determined. Western blot analysis was
performed to determine the phosphorylation and expression levels of
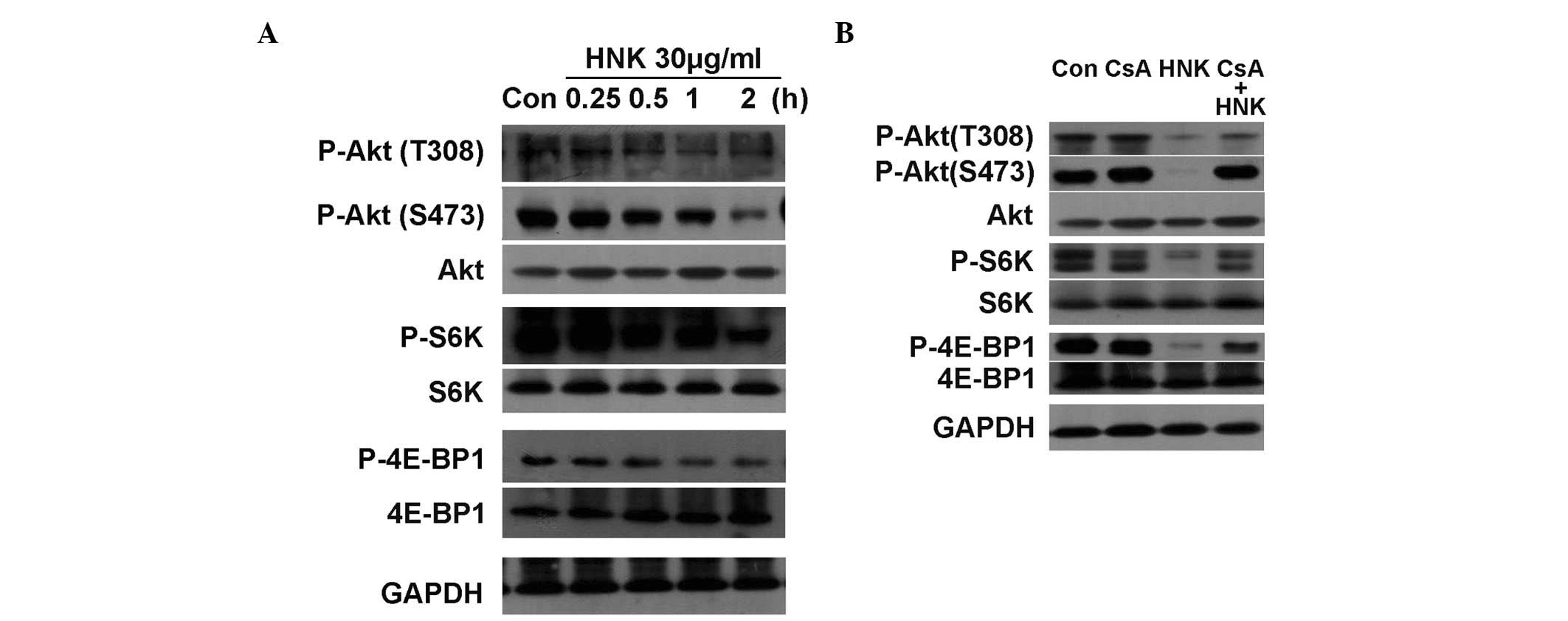
core mTOR substrates, Akt, 4E-BP1 and S6K. The phosphorylation
levels of 4E-BP1 and S6K were observed to gradually decline with
the elongation of incubating duration (0.25, 0.5, 1 and 2 h) during
the process of HNK-triggered regulated necrosis. Phosphorylation of
Akt on Thr308 and Ser473 were also gradually downregulated.
Conversely, the protein expression levels of Akt, 4E-BP1 and S6K
were stable (Fig. 8A). Figs. 5 and 6 demonstrate that CsA markedly inhibited
HNK-induced regulated necrosis. Therefore whether pretreatment with
CsA could reverse dephosphorylation of mTOR substrates was
investigated. Pretreatment with CsA prominently reversed
dephosphorylation of Akt on Ser473, however, only partially
reversed dephosphorylation of 4E-BP1, S6K and Akt on Thr308
(Fig. 8B). Collectively, the above
results reveal that the mTOR signaling pathways were inhibited
during the process of HNK-triggered regulated necrosis in HEK-293
cells. Furthermore, CypD may act downstream of mTOR signaling
pathways, as CsA blocked HNK-triggered regulated necrosis, although
it only partially reversed the dephosphorylation of mTOR
substrates.
Discussion
HNK is a pharmacologically active small molecule,
which is isolated from the traditional Chinese medicinal herb,
houpu. A previous study evidenced its antitumor effects (5). The antitumor function of HNK that has
been investigated most is cell death induction (5). HNK may induce diverse types of
regulated cell death, including extrinsic apoptosis,
caspase-dependent and -independent intrinsic apoptosis, regulated
necrosis and paraptosis depending on the cell type and varying
concentrations of therapeutic agents.
Necrosis was initially considered as a form of
accidental cell death, which only occurred in response to
physicochemical insults (2).
However, recent genetic and molecular biological evidence reveals
that necrosis is regulated by multiple signaling pathways (2). The term regulated necrosis is defined
as a genetically controlled cell death process, which is
morphologically characterized by cytoplasmic granulation, and
organelle and/or cellular swelling (2). A variety of regulated necrosis
initiators have been identified since the introduction of this
concept. Initiators of regulated necrosis include the necro-some,
PARP1 hyperactivation, MPT, mitochondrial complex I, NADPH oxidases
amongst others (2). Furthermore,
regulated necrosis may result from CypD-mediated MPT (14). Previous studies have demonstrated
HNK triggered regulated necrotic cell death in various cell lines
(MCF-7, HL-60 and HEK-293) at certain concentrations (two-fold
higher than its IC50), and this HNK-triggered regulated
necrosis was demonstrated to be CypD-mediated MPT pore dependent
(6). CypD inhibitors (CsA) or CypD
siRNA depletion completely blocked CypD, which significantly
attenuated HNK-induced regulated necrosis. Subsequent
investigations revealed HNK triggered a cell death mode transition
from early stage apoptosis to regulated necrosis in a time- and
dose-dependent manner, and this cell death mode transition was also
highly regulated by CypD (8). In
the current study, 30 µg/ml HNK, a lower concentration than
a previously reported necrotic-induction concentration of 40
µg/ml, was used (6) to
demonstrate whether HNK is able to trigger a similar type of
regulated necrosis in HEK-293 cells. The results of the present
study demonstrated that the majority of HEK-293 cells underwent
cell death, characterized by positive staining for PI, which
suggested these cells lost their membrane integrity and underwent
necrosis (PI-positive) or late-stage apoptosis (Annexin V-positive
and PI-positive). Subsequently, morphological and biochemical
methods were used to distinguish late-stage apoptosis from
HNK-induced cell death. Chromatin condensation and chromosomal DNA
fragmentation are two significant morphological changes that are
observed in late-stage apoptosis. In the present study, DNA
laddering demonstrated a diffuse DNA fractured pattern following
HNK exposure, rather than DNA laddering fragmentation (2). In addition, nuclear chromatin
condensation and apoptotic bodies were not detected in HEK-293
cells following HNK exposure. These preliminary morphological
assessments confirmed that the HNK-induced cell death (indicated by
positive staining for PI) was not due to late-stage apoptosis.
Conversely, initiator caspases (caspase-8) and effector caspases
(caspase-3) were not observed to be activated during this process
of cell death. Pan-caspase inhibitor, z-VAD-fmk also failed to
inhibit HNK-induced cell death. These results indicate that 30
µg/ml HNK-induced cell death was caspase-independent. The
above-mentioned findings indicate that HNK-induced cell death was
not due to late-stage apoptosis, but was solely necrosis.
Subsequently, it was detected that pretreatment with CsA completely
blocked HNK-induced necrosis. DNA diffuse fragmentation and
mitochondrial morphological changes were reversed by CsA. These
results indicated that CypD-mediated MPT was an important initiator
in HNK-triggered regulated necrosis, which was previously reported
(6,8). Comparable to its effects in other
disease models, such as ischemia-reperfusion injury (14–18),
the CypD-mediated MPT-associated signaling pathway was particularly
important in HNK-induced regulated necrosis. In addition to the
CypD-mediated MPT-associated signaling pathway, the
RIPK1-RIPK3-MLKL-PGAM5-Drp1 axis is the most intensively
investigated signaling pathway associated with a classic type of
regulated necrosis, termed necroptosis. Current evidence
demonstrates that necroptosis is initiated by RIP1 phosphorylation,
which activates RIP3 and subsequently phosphorylates the MLKL
protein (1,3,19).
Additionally, necroptosis requires the activity of RIP1, which was
indicated by the protection conveyed by the RIP1 kinase inhibitor,
Nec-1. In the current study, the aim was to demonstrate whether
RIP1 exerted a marked effect in HNK-induced regulated necrosis in
HEK-293 cells. Inconsistent with our previous report (8), RIP1 expression levels were
upregulated in the ultra early stage (0.25–1 h) of regulated
necrosis. Subsequent results revealed that although HNK-induced
cell death was partly reversed by pretreatment with Nec-1, the
inhibition effects of Nec-1 gradually receded with the elongation
of HNK incubating time. Conversely, Nec-1 did not reverse
HNK-induced DNA diffuse fragmentation. These results indicate that
although the expression levels of RIP1 were upregulated in the
ultra early stage of regulated necrosis, HNK-induced regulated
necrosis was RIP1-independent. Collectively, these results strongly
indicate that CypD-mediated MPT, but not the RIP1-associated
complex, was a vital initiator in HNK-triggered regulated necrosis.
However, the downstream signaling pathways of CypD-mediated MPT in
HNK-triggered regulated necrosis remain unknown.
mTOR is an evolutionarily conserved Ser/Thr protein
kinase, which controls cell growth in response to energy,
nutrients, growth factors and other environmental precipitating
factors. The deregulated mTOR signaling pathway presents in
numerous types of human disease with altered metabolism, including
diabetes and cancer (20). mTOR
assembles into two functionally and structurally distinct
complexes, mTORC1 and mTORC2 (10,20).
mTORC1 activity is though phosphorylation and activation of S6K, or
phosphorylation and deactivation 4E-BP1, which promotes translation
initiation and elongation, ribosome biogenesis and autophagy, and
subsequently regulates cell growth (21). mTORC2 promotes cell cycle entry,
cell survival, actin cytoskeleton polarization and anabolic output
though phosphorylation of its substrates, the Ser/Thr protein
kinases, Akt, PKA and PKC 10,11). Aberrant activation of the mTOR
signaling pathway promotes cell growth and survival, particularly
in malignant cells (12). Previous
reports demonstrate that HNK downregulates expression levels and
phosphorylation of Akt, S6K, and 4E-BP1 during the process of
HNK-induced apoptosis (22–25).
Anti-apoptotic activity of the Akt/mTOR signaling pathway via
numerous mechanisms, including inhibition of caspase-9, BCL2
associated agonist of cell death and glycogen synthase kinase 3β
(GSK-3β), and induction of mitochondrial hexokinase and nuclear
factor κ B-dependent anti-apoptotic gene expression, has been well
documented (4). The present study
aimed to investigate whether CypD-mediated MPT exerts its effect by
inhibiting mTOR signaling pathways. Phosphorylation levels of
mTORC1 and mTORC2 substrates (including S6K, 4E-BP1 and Akt) were
observed to be downregulated. Conversely, the protein expression
levels of these substrates were identified to be stable, which
revealed that the transcription and translation processes of these
proteins had not been influenced. These results indicate that the
mTOR signaling pathway was inhibited by dephosphorylation of core
substrates during the process of HNK-triggered regulated necrosis
in HEK-293 cells. Furthermore, these results are consistent with
previous reports that HNK inhibits Akt/mTOR signaling that is
accompanied by cell apoptosis. To the best of our knowledge, the
current study is the first to report that the regulation of
Akt/mTOR signaling is analogous with HNK-triggered regulated
necrosis. There is evidence to indicate that limited activation of
Akt is anti-apoptotic, however, numerous recent reports illustrate
an emerging role for Akt as a death kinase and a key regulator of
programmed necrosis (4). Sustained
Akt activity promotes programmed necrosis via a number of distinct
mechanisms, such as oxygen consumption, enhanced ROS generation
(26) and upregulation of
antioxidant defenses via phosphorylation of forkhead box subclass O
transcription factors (27). Liu
et al (4) recently
demonstrated that Akt and mTOR mediate TNFα/z-VAD-fmk-induced
regulated necrosis in neurons. The study revealed that
RIP1-RIP3-pAkt assembly is a vital node in TNFα/z-VAD-fmk-induced
regulated necrosis. Activation of Akt/mTOR signaling pathways,
including phosphorylation of Akt (T308 and S473), GSK-3β, mTOR and
S6K, participate in the initiation of TNFα/z-VAD-fmk-induced
regulated necrosis in neurons. Furthermore, inhibition of Akt/mTOR
signaling pathways, via a specific inhibitor or siRNA knockdown of
Akt and mTOR, halves TNFα/z-VAD-fmk-induced regulated necrosis. The
study concluded that Akt and mTOR participate in regulation of
TNFα/z-VAD-fmk-induced regulated necrosis in neurons (4). These findings were not consistent
with those of the present study. The discordant effects of Akt and
mTOR may depend on the exact stimulus used to initiate necroptosis
in unique cell types. Specifically, the functions of Akt and mTOR
in CypD-mediated MPT-initiated regulated necrosis and the RIP1-RIP3
complex-initiated regulated necrosis may differ. Furthermore,
pretreatment with CsA was found to prominently inhibit
HNK-triggered regulated necrosis, as well as reverse
dephosphorylation of Akt in Ser473/Thr308, 4E-BP1 and S6K. This
indicates that the mTOR signaling pathway was effective downstream
of the CypD-mediated MPT and prior to the onset of plasma membrane
breakdown in the HNK-induced regulated necrosis process. Although
it is known that the mTOR signaling pathway is downstream of
CypD-mediated MPT, the exact signaling pathway that exists between
them remains unknown. The aim of future studies is to identify the
exact mechanisms between CypD-mediated MPT and inhibition of the
downstream mTOR signaling pathway.
In conclusion, it was found that CypD-mediated MPT,
but not the RIP1-associated complex, is a vital initiator in
HNK-triggered regulated necrosis. The present study demonstrated
for the first time, to the best of our knowledge, that the mTOR
signaling pathway is inhibited and exists downstream of the
CypD-mediated MPT in the HNK-induced regulated necrosis process.
These results provide novel insight into CypD-mediated,
HNK-triggered regulated necrosis.
Acknowledgments
The present study was supported by grants from
Zhejiang Provincial Natural Science Foundation of China (grant nos.
LQ14H160010, LY14H160019 and LY14H160020), the National Natural
Science Foundation of China (grant nos. 81201640) and the
Department of Education of Zhejiang Province of China (grant nos.
Y201225802).
References
|
1
|
Moujalled DM, Cook WD, Murphy JM and Vaux
DL: Necroptosis induced by RIPK3 requires MLKL but not Drp1. Cell
Death Dis. 5:e10862014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vanden Berghe T, Linkermann A,
Jouan-Lanhouet S, Walczak H and Vandenabeele P: Regulated necrosis:
The expanding network of non-apoptotic cell death pathways. Nat Rev
Mol Cell Biol. 15:135–147. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J and Chan FK: Cell biology. RIPK3
takes another deadly turn. Science. 343:1322–1323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Q, Qiu J, Liang M, Golinski J, van
Leyen K, Jung JE, You Z, Lo EH, Degterev A and Whalen MJ: Akt and
mTOR mediate programmed necrosis in neurons. Cell Death Dis.
5:e10842014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tian W, Xu D and Deng YC: Honokiol, a
multifunctional tumor cell death inducer. Pharmazie. 67:811–816.
2012.PubMed/NCBI
|
|
6
|
Li L, Han W, Gu Y, Qiu S, Lu Q, Jin J, Luo
J and Hu X: Honokiol induces a necrotic cell death through the
mitochondrial permeability transition pore. Cancer Res.
67:4894–4903. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian W, Deng Y, Li L, He H, Sun J and Xu
D: Honokiol synergizes chemotherapy drugs in multidrug resistant
breast cancer cells via enhanced apoptosis and additional
programmed necrotic death. Int J Oncol. 42:721–732. 2013.
|
|
8
|
Tian W, Xu D, Han W, He H, Cai H, Chen H,
Zhou M, Chen J and Deng YC: Cyclophilin D modulates cell death
transition from early apoptosis to programmed necrosis induced by
honokiol. Int J Oncol. 42:1654–1663. 2013.PubMed/NCBI
|
|
9
|
Remijsen Q, Goossens V, Grootjans S, Van
den Haute C, Vanlangenakker N, Dondelinger Y, Roelandt R, Bruggeman
I, Goncalves A, Bertrand MJ, et al: Depletion of RIPK3 or MLKL
blocks TNF-driven necroptosis and switches towards a delayed RIPK1
kinase-dependent apoptosis. Cell Death Dis. 5:e10042014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimobayashi M and Hall MN: Making new
contacts: The mTOR network in metabolism and signalling crosstalk.
Nat Rev Mol Cell Biol. 15:155–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang H, Rudge DG, Koos JD, Vaidialingam B,
Yang HJ and Pavletich NP: mTOR kinase structure, mechanism and
regulation. Nature. 497:217–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beauchamp EM and Platanias LC: The
evolution of the TOR pathway and its role in cancer. Oncogene.
32:3923–3932. 2013. View Article : Google Scholar
|
|
13
|
Hortobagyi GN, Chen D, Piccart M, Rugo HS,
Burris HA III, Pritchard KI, Campone M, Noguchi S, Perez AT, Deleu
I, et al: Correlative analysis of genetic alterations and
everolimus benefit in hormone receptor-positive, human epidermal
growth factor receptor 2-negative advanced breast cancer: Results
from BOLERO-2. J Clin Oncol. 26–Oct;2015.Epub ahead of print.
|
|
14
|
Linkermann A, Bräsen JH, Darding M, Jin
MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H,
et al: Two independent pathways of regulated necrosis mediate
ischemia-reperfusion injury. Proc Natl Acad Sci USA.
110:12024–12029. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baines CP, Kaiser RA, Purcell NH, Blair
NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA,
Dorn GW, et al: Loss of cyclophilin D reveals a critical role for
mitochondrial permeability transition in cell death. Nature.
434:658–662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Physiol Rev.
87:99–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakagawa T, Shimizu S, Watanabe T,
Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T and Tsujimoto Y:
Cyclophilin D-dependent mitochondrial permeability transition
regulates some necrotic but not apoptotic cell death. Nature.
434:652–658. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schinzel AC, Takeuchi O, Huang Z, Fisher
JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA and Korsmeyer
SJ: Cyclophilin D is a component of mitochondrial permeability
transition and mediates neuronal cell death after focal cerebral
ischemia. Proc Natl Acad Sci USA. 102:12005–12010. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Sun L, Su L, Rizo J, Liu L, Wang
LF, Wang FS and Wang X: Mixed lineage kinase domain-like protein
MLKL causes necrotic membrane disruption upon phosphorylation by
RIP3. Mol Cell. 54:133–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
From growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View
Article : Google Scholar
|
|
22
|
Park EJ, Min HY, Chung HJ, Hong JY, Kang
YJ, Hung TM, Youn UJ, Kim YS, Bae K, Kang SS and Lee SK:
Down-regulation of c-Src/EGFR-mediated signaling activation is
involved in the honokiol-induced cell cycle arrest and apoptosis in
MDA-MB-231 human breast cancer cells. Cancer Lett. 277:133–140.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagalingam A, Arbiser JL, Bonner MY,
Saxena NK and Sharma D: Honokiol activates AMP-activated protein
kinase in breast cancer cells via an LKB1-dependent pathway and
inhibits breast carcinogenesis. Breast Cancer Res. 14:R352012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Crane C, Panner A, Pieper RO, Arbiser J
and Parsa AT: Honokiol-mediated inhibition of PI3K/mTOR pathway: A
potential strategy to overcome immunoresistance in glioma, breast
and prostate carcinoma without impacting T cell function. J
Immunother. 32:585–592. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaushik G, Ramalingam S, Subramaniam D,
Rangarajan P, Protti P, Rammamoorthy P, Anant S and Mammen JM:
Honokiol induces cytotoxic and cytostatic effects in malignant
melanoma cancer cells. Am J Surg. 204:868–873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nogueira V, Park Y, Chen CC, Xu PZ, Chen
ML, Tonic I, Unterman T and Hay N: Akt determines replicative
senescence and oxidative or oncogenic premature senescence and
sensitizes cells to oxidative apoptosis. Cancer Cell. 14:458–470.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kops GJ, Dansen TB, Polderman PE, Saarloos
I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH and Burgering
BM: Forkhead transcription factor FOXO3a protects quiescent cells
from oxidative stress. Nature. 419:316–321. 2002. View Article : Google Scholar : PubMed/NCBI
|