Introduction
Pain facilitation has been reported to induce
numerous nervous system-mediated sickness responses (1). Inflammation is crucial in the
development and maintenance of persistent pain and inflammatory
mediators are often altered regionally in acute pain (2); however, chronic pain patients have
demonstrated a significant increase in pro-inflammatory cytokines,
including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β,
IL-2, and IL-6 in plasma, which is associated with increased pain
intensity (3,4). Prolonged upregulation of inflammatory
mediators in the injury epicenter, and regions above and below the
lesion may be involved in chronic neuropathic pain in spinal cord
injury (5). The systemic
inflammatory response is also associated with pain and other
symptoms in a cohort of cancer patients (6). Thus, anti-inflammatory intervention
may be a useful therapeutic strategy in the management of
pathological pain.
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a
non-flavonoid polyphenol present in grapes and red wine, which has
been demonstrated to elicit a broad spectrum of biological
responses in vitro and in vivo, including
anticarcinogenic effects and neuroprotective and cardioprotective
activities (7,8). Previous studies have indicated that
resveratrol is a potential therapeutic agent to attenuate bone
cancer pain (9) and diabetic
neuropathic pain (10). Injection
of resveratrol into the cerebral ventricles also exerts evident
central antalgic effects (11).
Resveratrol has been demonstrated to interact with multiple
molecular targets, a number of which are associated with
inflammation and immunity (12).
Thus, the current study hypothesized that resveratrol may also
attenuate systemic inflammation-induced pain. The aim of the
present study was to investigate the effect of resveratrol on
inflammatory pain induced by the bacterial endotoxin,
lipopolysaccharide (LPS), and investigate its underlying molecular
mechanism.
Materials and methods
Reagents
LPS (from Escherichia coli 055:B5),
resveratrol, sodium pentobarbital and EX-257 were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Dimethyl sulfoxide (DMSO),
acetic acid, paraformaldehyde, 0.1 M phosphate buffered saline
(PBS; pH 7.4), paraffin, xylene, ethanol, hematoxylin and
3,3-diaminobenzidine were obtained from Sinopharm Chemical Reagent
Co., Ltd. (Shanghai, China). Bovine serum albumin and 1% Triton
X-100 were obtained from Shanghai Sangon Biological Engineering
Technology & Services Co., Ltd. (Shanghai, China).
Radioimmunoprecipitation lysis buffer, citrate buffer and BeyoECL
Plus kit were purchased from Beyotime Institute of Biotechnology
(Haimen, China). Primary antibodies for glial fibrillary acidic
protein (GFAP; mouse monoclonal; sc-33673), sirtuin 1 (SIRT1;
rabbit monoclonal; sc-15404), and β-actin (mouse monoclonal;
sc-47778) were obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Primary antibodies for ionized calcium binding
adapter molecule 1 (Iba-1; rabbit polyclonal; 019-19741) were
purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Biotin-labeled goat anti-rabbit IgG (A0277) and biotin-labeled goat
anti-mouse IgG (A0286) secondary antibodies used in
immunohistochemistry were obtained from Beyotime Institute of
Biotechnology. Goat anti-rabbit IgG (7074) and horse anti-mouse IgG
(7074) used in western blotting were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The enzyme-linked
immunosorbent assay (ELISA) kits for TNF-α and IL-6 were obtained
from R&D Systems, Inc. (Minneapolis, MN, USA).
Mice
A total of 120 male ICR mice (age, 8–10 weeks;
weight, 22–24 g) were obtained from the Experimental Animal Center
of Zhejiang University (Hangzhou, China). Five mice per cage were
housed in transparent plastic cages in controlled conditions at
20–24°C with 40–60% humidity and a 12 h light/dark cycle. All mice
were allowed ad libitum access to water and food. All
experiments were conducted in accordance with the Guide for the
Care and Use of Laboratory Animals published by National Institutes
of Health (Bethesda, MD, USA) (13). All experimental protocols were
approved by the Ethics Committee on Animal Experimentation of
Zhejiang University.
Mice were divided into the following groups (n=12):
i) Control group, which received the same volume of 0.1% DMSO
vehicle diluted in saline (0.1 ml/10 g body weight); ii) LPS group,
which received an intraperitoneal (i.p.) injection of LPS (0.25,
0.5, or 1 mg/kg) for 5 consecutive days; iii) LPS + resveratrol
group, which received an i.p. injection with resveratrol (5, 10 or
20 mg/kg) 30 min prior to administration of LPS (1 mg/kg); iv)
resveratrol group, which received an i.p. injection of resveratrol
(20 mg/kg) for 5 consecutive days; v) LPS + resveratrol + EX-257
group, which received EX-257 (2 mg/kg) by i.p. injection 30 min
prior to resveratrol (20 mg/kg) administration, which was followed
by injection of LPS; and vi) EX-257 group, which received an i.p.
injection of EX-257 (2 mg/kg). Behavioral assessments, histology
and molecular biology analysis were performed 6 h after the final
LPS injection of the experimental groups, or after the vehicle
injection on the control group at the same time point. Resveratrol
and EX-257 were dissolved in DMSO and diluted in saline. The
concentration of DMSO was <0.1%, which exerted no marked
physiological action or pharmacological anti-inflammatory
effect.
Acetic acid-induced writhing test
The mice received an i.p. injection of 0.7% acetic
acid (0.1 ml/10 g) and were then placed into separate plastic
animal cages. The number of writhes was counted for 20 min after
the i.p. injection according to the method described by Zhao et
al (14). Writhing was defined
as the contraction of abdominal muscles, which were accompanied by
the stretching of the hind limbs.
Tail-flick test
To examine thermal hyperalgesia, the tail-flick test
was employed according to the method described by Sora et al
(15). Briefly, mice were
restrained in conical polypropylene tubes with an opening, and
their tails were immersed ~2 cm into a 50±0.2°C water bath. The
period from the immersion to the removal of the whole tail from the
water was recorded as tail-flick latency.
Measurement of cytokines
Blood samples (1 ml) were collected in test tubes
containing ethylenediaminetetraacetic acid (1 mg/ml) and
centrifuged immediately at 1,000 x g for 10 min at room
temperature. The plasma was stored at −80°C for analysis for
cytokines. The plasma TNF-α and IL-6 levels were measured using
ELISA kits according to manufacturer's protocols.
Immunohistochemistry
Mice were anesthetized with sodium pentobarbital (60
mg/kg, i.p.) and perfused with PBS via the ascending aorta to
remove blood in the tissue prior to the fix-perfusion of 4%
paraformaldehyde in 0.1 M PBS (pH 7.4). The mice were then
sacrificed by exsanguination. Spinal cord segments (lumbar (L)4-L5)
were removed, post-fixed in the same fixative for 72 h, and then
embedded in paraffin. For immunohistochemistry, each specimen was
cut into 5-µm sections. The sections were deparaffinized in
glass centrifuge tubes with two 10 ml changes of xylene each 10
min. Then, sections were rehydrated at room temperature in a
sequence of decreasing concentrations of ethanol by 2 min
incubations in 10 ml of the following: 100% Ethanol 2 times, 90%
ethanol 2 times, 70% ethanol and 50% ethanol. After pretreatment
with citrate buffer to enhance immunoreactivity, the sections were
blocked in 5% bovine serum albumin for 1 h. The sections were
incubated with anti-GFAP antibody (1:500) or anti-Iba-1 (1:500)
overnight at 4°C. After washing with PBS 3 times, the sections were
incubated with biotin-conjugated secondary antibodies (1:200) for 1
h at room temperature. Sections were counterstained with
hematoxylin to label nuclei. The staining was visualized using
3,3-diaminobenzidine, and observed with a BX51 microscope (Olympus
Corporation, Tokyo, Japan). To quantify GFAP-positive and
Iba-1-positive cells, images were captured of the dorsal horn of
the spinal cord (magnification, ×40). Immunoreactive astrocytes and
microglial cells with clearly identifiable nuclei were counted
manually by an investigator who was blinded to the treatment and
experienced in the field.
Western blotting
The spinal cords segments (dorsal part of L4–L5)
were collected and homogenized in radioimmunoprecipitation lysis
buffer containing 1% Triton X-100. Following centrifugation at
10,000 × g at 4°C for 30 min, the supernatant was collected and
frozen at −80°C until use. Lysates from the spinal cord were mixed
with sodium dodecyl sulfate sample buffer, heated at 100°C for 5
min and separated on 10% SDS-PAGE gels at 200 V for 40 min prior to
transfer to a nitrocellulose membrane. Following blocking for 1 h
with 5% skim milk, the membrane was incubated with primary
antibodies (1:1,000) at 4°C overnight, followed by incubation with
HRP-conjugated secondary antibodies (1:2,000) for 45 min at room
temperature. All reactions were detected by the enhanced
chemiluminescence detection method. The enhanced chemiluminescence
signal was captured using a G:BOX Chemi XR5 imaging system
(Syngene, Cambridge, UK). The band density was analyzed with
Quality One software (version 4.6.2; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and normalized to β-actin.
Statistical analysis
Data were expressed as the mean ± standard error of
the mean and analyzed by one-way analysis of variance with a
Bonferroni post hoc test as required using Prism 5.0 (GraphPad
Software, Inc., La Jolla, CA). P<0.05 was considered to indicate
a statistically significant difference.
Results
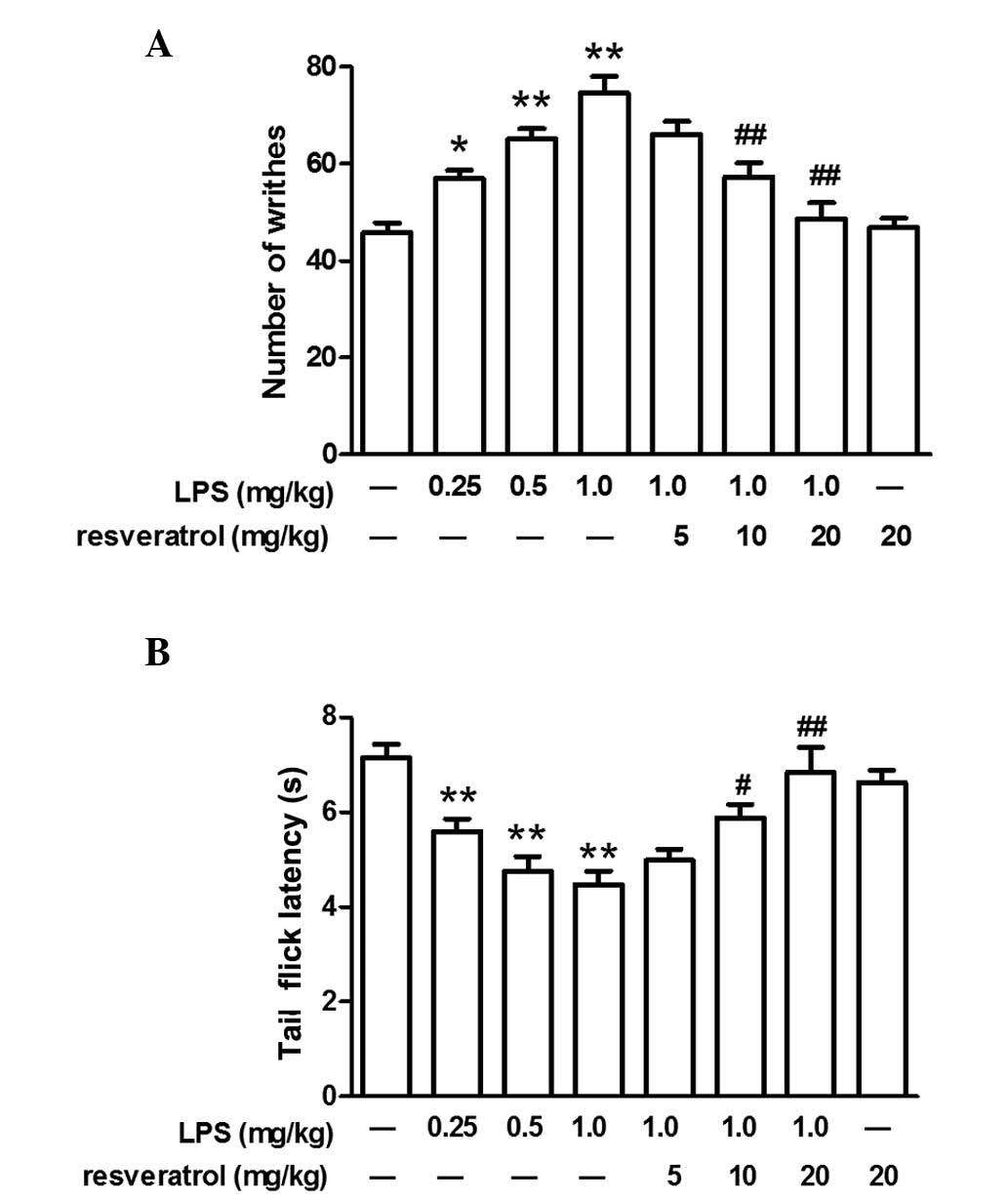
LPS increased the number of writhes and
decreased the latency of tail flicks
LPS-induced hyperalgesia in mice was evaluated by
the acetic acid-induced writhing test and tail-flick test.
Following the administration of LPS (0.25, 0.5, or 1.0 mg/kg, i.p.)
for 5 days, the number of writhes increased and the tail-flick
latency decreased in a dose-dependent manner (P<0.05; Fig. 1). As mice that were exposed to the
highest concentration of LPS (1.0 mg/kg) exhibited marked
hyperalgesia, this concentration of LPS was used for subsequent
experiments. Administration of LPS once per day for 5 days did not
alter body weight and although treatment with LPS induced slight
behavioral alterations, one day after the final injection these
behaviors, including grip tone, motor activity and swimming speed,
were comparable with that of control group (data not shown).
Resveratrol partly inhibited LPS-induced
hyperalgesia at 10 or 20 mg/kg
Compared with the mice injected with LPS (1.0
mg/kg), resveratrol (10 or 20 mg/kg) partly inhibited the
LPS-induced hyperalgesia in the acetic acid-induced writhing test
and tail-flick test, however, a lower dose of resveratrol (5 mg/kg)
did not demonstrate any marked inhibitory effect on LPS-induced
hyperalgesia. Injection of resveratrol alone did not influence the
pain behavioral response in mice (P<0.05; Fig. 1).
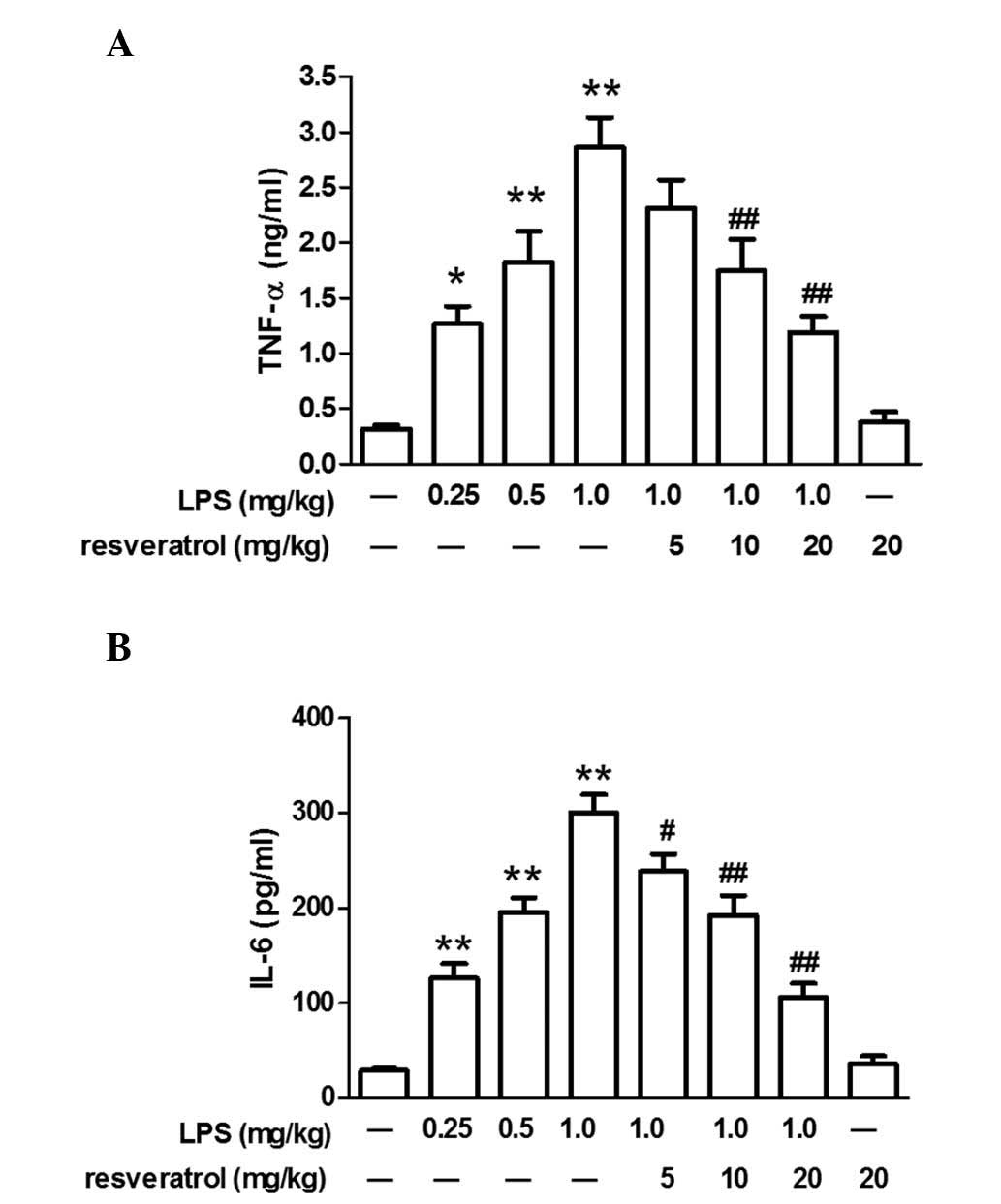
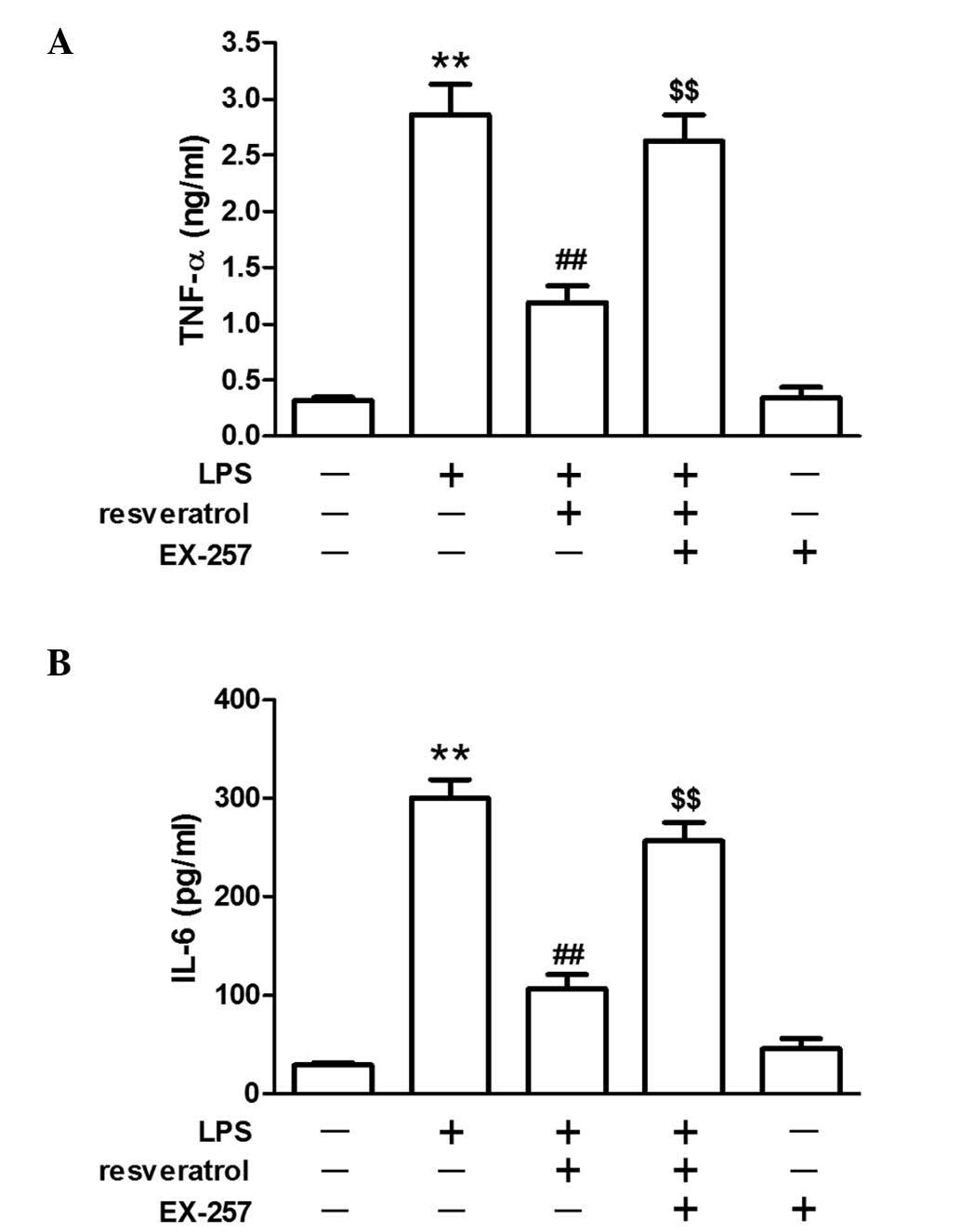
Resveratrol blocked LPS-induced
inflammation in mice
LPS injection significantly increased plasma TNF-α
and IL-6 levels in mice (P<0.05). Resveratrol (10 or 20 mg/kg)
treatment reduced changes in IL-6 or TNF-α expression levels
induced by LPS (P<0.01; Fig.
2).
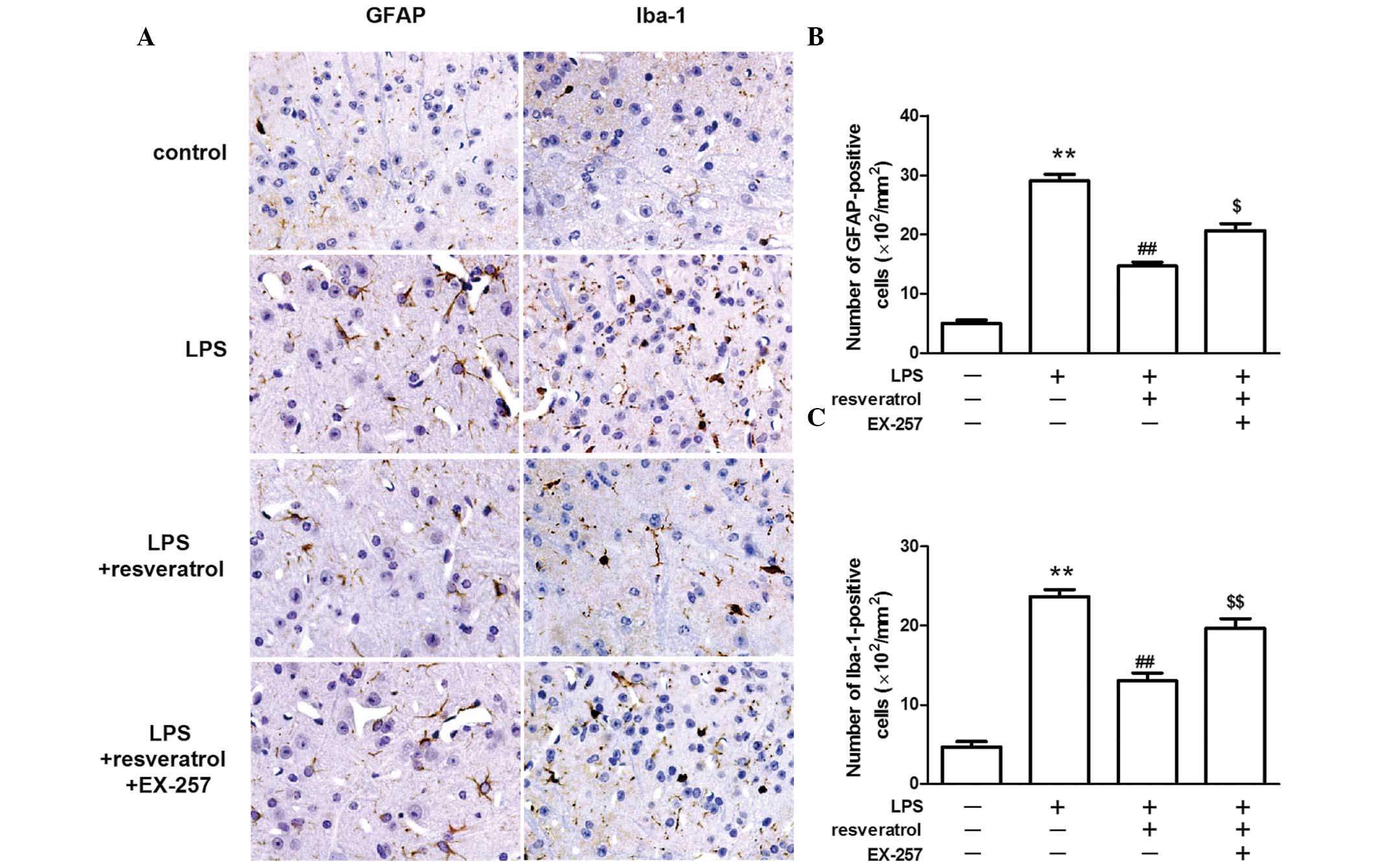
Resveratrol reduced the number of GFAP
and Iba-1 positive cells in LPS treated mice
The astrocyte-specific activation marker, GFAP and
the microglia-specific activation marker, Iba-1 were detected by
immunohistochemical staining. GFAP and Iba-1 staining was weak in
the control group, however, the staining was markedly increased in
the dorsal horns of the spinal cord following the injection of LPS.
The number of GFAP-positive and Iba-1-positive cells in the spinal
cord was markedly lower in the LPS + resveratrol group, when
compared with the LPS treated group (P<0.01; Fig. 3).
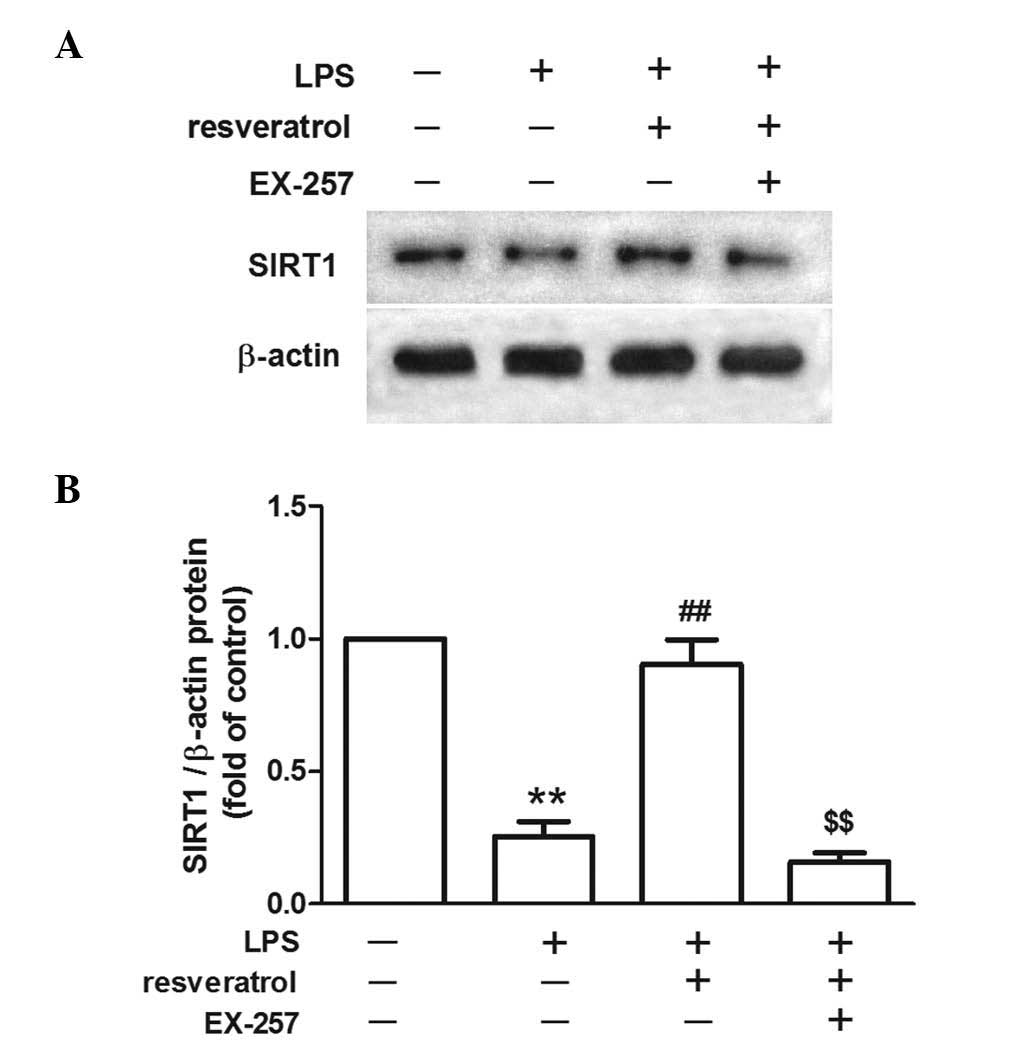
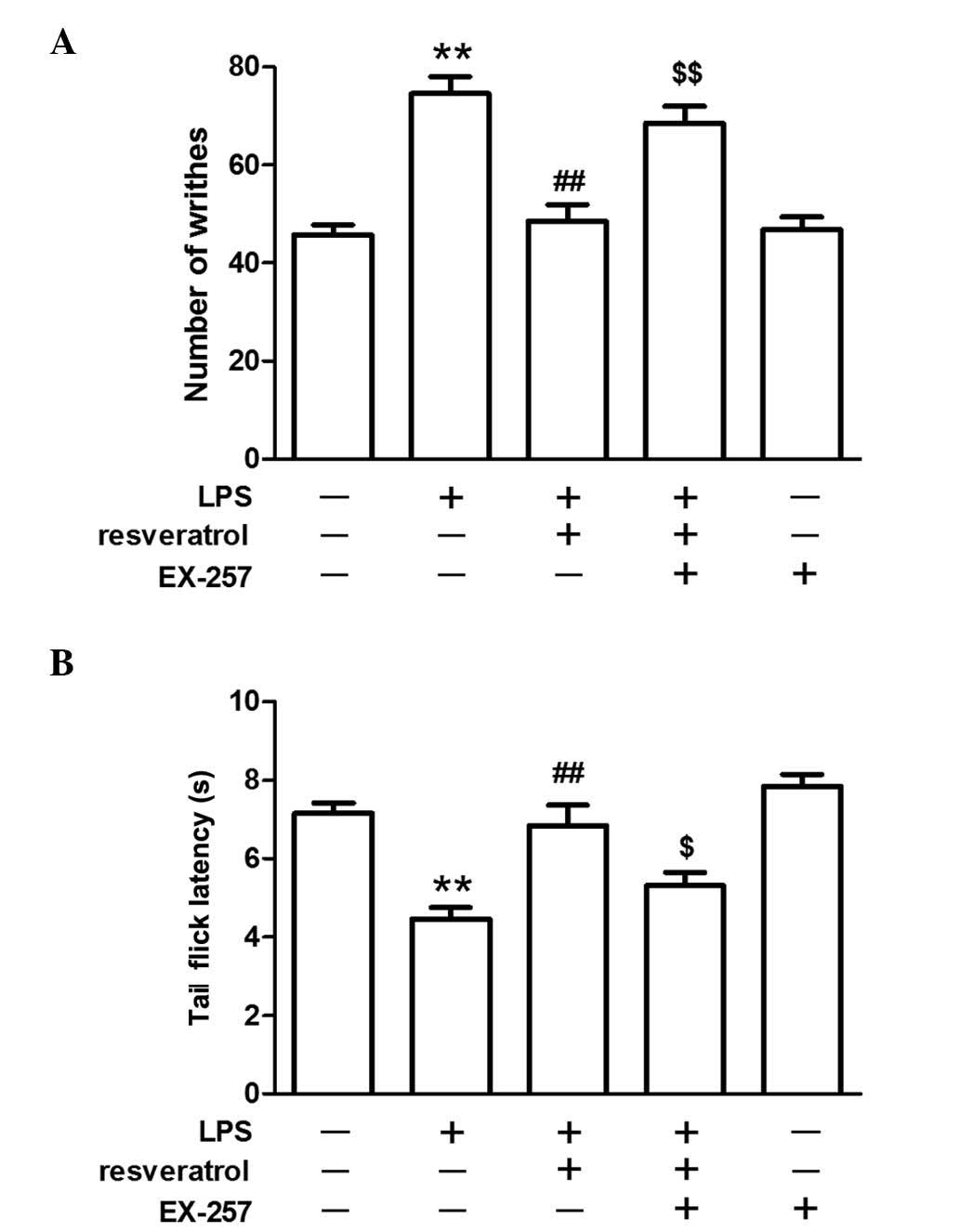
The inhibitory effect of resveratrol on
LPS-induced hyperalgesia was blocked by a SIRT1 inhibitor
LPS injection reduced SIRT1 protein expression in
the spinal cord. Resveratrol increased SIRT1 protein expression
levels in the LPS treated mice (P<0.01; Fig. 4). EX-257, a selective SIRT1
inhibitor, blocked the resveratrol-induced analgesic effect
(P<0.05; Fig. 5), and EX-257
inhibited the anti-inflammatory effect exerted by resveratrol, and
increased astrocyte and microglial activation, when compared with
the LPS + resveratrol group (P<0.05; Figs. 3 and 6).
Discussion
In a number of chronic pain conditions, including
neuropathic pain, cancer and obesity-associated pain, and other
chronic inflammatory conditions, systemic inflammation is important
in the pathophysiology of hyperalgesia, which may be mediated by
the neural response in the spinal cord or brain (5,6,16).
In the present study, subacute i.p. injections of LPS were
administered daily for five consecutive days to assess the
hyperalgesia. Two different hyperalgesic tests were used, the
acetic acid-induced writhing test and the tail flick test. In the
acetic acid-induced abdominal writhing test (a visceral pain
model), acetic acid nociceptive activity may be the result of a
release of cytokines, including TNF-α, IL-1 and IL-8 (17). In the present study, LPS increased
the number of writhing events, which was consistent with the
increased level of TNF-α and IL-6 in the plasma. However, the
tail-flick tests were performed to assess whether resveratrol
possesses central analgesic properties. In the tail-flick test, a
thermal stimulus is focused on the tail skin of the animal to
activate nociceptors in the superficial layers of the skin. The
activation of peripheral nociceptors triggers a complex series of
processes at the spinal level and results in the tail flick
response. The latency of the tail-flick was decreased by LPS
administration.
Glial cells, predominantly astrocytes and microglia,
are important cell populations in the central nervous system
(18). The glial cells are
involved in maintaining homeostasis of the brain via regulation of
pH and ionic balance, neurotransmitter uptake and degradation, and
neuroinflammation modulation in physiological and
pathophysiological conditions (19,20).
Increasing evidence indicates that the glial cells are crucial in
the generation and maintenance of chronic pain (21,22).
Ji et al (23) reported
that spinal astrocytic activation contributes to mechanical
allodynia in a rat chemotherapy-induced neuropathic pain model.
Activation of astrocytes in the anterior cingulate cortex has been
demonstrated to be an affective component of pain in an
inflammatory pain model (24).
Astrocytic activation in the spinal cord also contributes to the
development and maintenance of pain in rat models of chronic
pancreatitis, chronic constriction of the sciatic nerve, and bone
cancer (25–27). Furthermore, activated spinal
microglia are key in neuropathic pain (28–30).
GFAP is a specific astrocytic activation marker and Iba-1 is a
specific microglial activation marker in the central nervous system
(31). Although LPS may induce
satellite glial cell activation in dorsal root ganglia (32), recent evidence suggests the
importance of glial activation in the spinal cord (33,34).
In the present study, GFAP and Iba-1 expression levels were
observed to be increased following administration of LPS for 5
days. The activation of glial cells may a result of the release of
inflammatory cytokines in the rat dorsal horn by LPS (35,36).
Pharmacological inhibition of glial reactions may be a potential
therapeutic strategy to treat inflammatory pain in animal
models.
SIRT1 is a nicotinamide adenine
dinucleotide-dependent histone deacetylase, which is involved in
lifespan extension, age-associated disease delay, metabolism and
apoptosis regulation (37–40). Recently, SIRT1 was demonstrated to
regulate algesthesia. Upregulation of the expression of SIRT1 in
the spinal dorsal horn reversed chronic morphine antinociceptive
tolerance (41). Increased spinal
SIRT1 expression attenuated mechanical allodynia and thermal
hyperalgesia in a chronic constriction injury model (42,43),
and SIRT1 has also been observed to be crucial in inflammatory
diseases, including obesity, type 2 diabetes mellitus, and
atherosclerosis (44,45). The present study showed that SIRT1
was downregulated in mice injected with LPS, suggesting that SIRT1
is important in modulating the development and progression of pain
in subacute systemic inflammation. Thus, upregulation of SIRT1
appears to be a promising therapeutic strategy for systemic
inflammation-induced pain.
SIRT1 has been reported to be one of the key
targeted molecule of resveratrol and involved in its multiple
biological effects (46–48). Resveratrol has potential
anti-oxidative and anti-inflammatory properties and has been
indicated to attenuate bone cancer pain (9). Chronic treatment with resveratrol for
4 weeks decreased the serum concentration of TNF-α and attenuated
diabetic neuropathic pain (10).
Systemic or spinal administration of resveratrol significantly
suppresses morphine-induced microglial activation in the spinal
cord (49). However, whether
resveratrol relieved inflammation-induced pain remains to be
elucidated. In the present study, it was observed that resveratrol
inhibited the LPS-induced increase in serum inflammatory cytokines
levels and attenuated inflammatory hyperalgesia in a dose-dependent
manner. Furthermore, resveratrol inhibited the activation of glial
cells in the dorsal horns of the spinal cord. The biological effect
of resveratrol may be mediated by SIRT1-dependent (50) or SIRT1-independent (51) mechanisms, and phosphatidylinositol
3-kinase is essential for resveratrol-mediated expression of SIRT1
(50). The present study
demonstrated that resveratrol increased the expression levels of
SIRT1, and inhibition of SIRT1 expression by EX-257 blocked the
resveratrol-induced analgesic effect.
A limitation of the present study is that the acute
effect of resveratrol following the five treatments with LPS was
not evaluated. Furthermore, whether the anti-hyperalgesia effect of
resveratrol is more effective by intrathecal injection remains to
be elucidated. However, the results of the present study suggest
that resveratrol attenuates systemic inflammation-induced pain via
upregulation of SIRT1. The protective effect of SIRT1 may occur via
promotion of p65 deacetylation, regulation of mitogen activated
protein kinase pathway, or inhibition of nuclear factor-kB activity
(52,53).
In conclusion, subacute administration of LPS
induced the activation of glial cells and hyperalgesia. Resveratrol
inhibited the activation of glial cells and attenuated inflammatory
hyperalgesia in a SIRT1-dependent manner. The present study
suggests that the beneficial role of resveratrol will help provide
a potential therapeutic strategy in the treatment of pathological
inflammatory pain.
Acknowledgments
The present study was supported by the Science and
Technology Department of Zhejiang Province (grant no. 2014C37027
and 2014C37067) and the National Natural Science Foundation of
China (grant no. 81371953).
References
|
1
|
Watkins LR and Maier SF: Immune regulation
of central nervous system functions: From sickness responses to
pathological pain. J Intern Med. 257:139–155. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schinkel C, Gaertner A, Zaspel J, Zedler
S, Faist E and Schuermann M: Inflammatory mediators are altered in
the acute phase of posttraumatic complex regional pain syndrome.
Clin J Pain. 22:235–239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koch A, Zacharowski K, Boehm O, Stevens M,
Lipfert P, von Giesen HJ, Wolf A and Freynhagen R: Nitric oxide and
pro-inflammatory cytokines correlate with pain intensity in chronic
pain patients. Inflamm Res. 56:32–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang YH, Peng CH, Liu HT and Kuo HC:
Increased pro-inflammatory cytokines, C-reactive protein and nerve
growth factor expressions in serum of patients with interstitial
cystitis/bladder pain syndrome. PLoS One. 8:e767792013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sandhir R, Gregory E, He YY and Berman NE:
Upregulation of inflammatory mediators in a model of chronic pain
after spinal cord injury. Neurochem Res. 36:856–862. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laird BJ, McMillan DC, Fayers P, Fearon K,
Kaasa S, Fallon MT and Klepstad P: The systemic inflammatory
response and its relationship to pain and other symptoms in
advanced cancer. Oncologist. 18:1050–1055. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun AY, Wang Q, Simonyi A and Sun GY:
Resveratrol as a therapeutic agent for neurodegenerative diseases.
Mol Neurobiol. 41:375–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pangeni R, Sahni JK, Ali J, Sharma S and
Baboota S: Resveratrol: Review on therapeutic potential and recent
advances in drug delivery. Expert Opin Drug Deliv. 11:1285–1298.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng W, Zhao Y, Liu H, Fan Q, Lu FF, Li
J, Yin Q and Yan CD: Resveratrol attenuates bone cancer pain
through the inhibition of spinal glial activation and CX3CR1
upregulation. Fundam Clin Pharmacol. 28:661–670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sharma S, Kulkarni SK and Chopra K: Effect
of resveratrol, a polyphenolic phytoalexin, on thermal hyperalgesia
in a mouse model of diabetic neuropathic pain. Fundam Clin
Pharmacol. 21:89–94. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Falchi M, Bertelli A, Galazzo R, Viganò P
and Dib B: Central antalgic activity of resveratrol. Arch Ital
Biol. 148:389–396. 2010.
|
|
12
|
Švajger U and Jeras M: Anti-inflammatory
effects of resveratrol and its potential use in therapy of
immune-mediated diseases. Int Rev Immunol. 31:202–222. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Institute of Laboratory Animal Resources
(US); Committee on Care Use of Laboratory Animals National
Institutes of Health (US); Division of Research Resources: Guide
for the care and use of laboratory animals. 8th edition. National
Academies Press; Washington, DC: 2011
|
|
14
|
Zhao H, Luo F, Li H, Zhang L, Yi Y and Wan
J: Antinociceptive effect of tetrandrine on LPS-induced
hyperalgesia via the inhibition of IKKβ phosphorylation and the
COX-2/PGE2 pathway in mice. PLoS One. 9:e945862014.
View Article : Google Scholar
|
|
15
|
Sora I, Takahashi N, Funada M, Ujike H,
Revay RS, Donovan DM, Miner LL and Uhl GR: Opiate receptor knockout
mice define mu receptor roles in endogenous nociceptive responses
and morphine-induced analgesia. Proc Natl Acad Sci USA.
94:1544–1549. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clark MR: Targeting systemic inflammation
in patients with obesity-related pain: How best to prevent acute
pain from becoming chronic? J Fam Pract. 62(9 Suppl CHPP): S3–S9.
2013.PubMed/NCBI
|
|
17
|
Ribeiro RA, Vale ML, Thomazzi SM,
Paschoalato AB, Poole S, Ferreira SH and Cunha FQ: Involvement of
resident macrophages and mast cells in the writhing nociceptive
response induced by zymosan and acetic acid in mice. Eur J
Pharmacol. 387:111–118. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jha MK, Seo M, Kim JH, Kim BG, Cho JY and
Suk K: The secretome signature of reactive glial cells and its
pathological implications. Biochim Biophys Acta. 1834:2418–2428.
2013. View Article : Google Scholar
|
|
19
|
Gwak YS, Kang J, Unabia GC and Hulsebosch
CE: Spatial and temporal activation of spinal glial cells: Role of
gliopathy in central neuropathic pain following spinal cord injury
in rats. Exp Neurol. 234:362–372. 2012. View Article : Google Scholar :
|
|
20
|
Parpura V, Heneka MT, Montana V, Oliet SH,
Schousboe A, Haydon PG, Stout RF Jr, Spray DC, Reichenbach A,
Pannicke T, et al: Glial cells in (patho)physiology. J Neurochem.
121:4–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Watkins LR and Maier SF: Beyond neurons:
Evidence that immune and glial cells contribute to pathological
pain states. Physiol Rev. 82:981–1011. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barragán-Iglesias P, Pineda-Farias JB,
Cervantes-Durán C, Bravo-Hernández M, Rocha-González HI, Murbartián
J and Granados-Soto V: Role of spinal P2Y6 and P2Y11 receptors in
neuropathic pain in rats: Possible involvement of glial cells. Mol
Pain. 10:292014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ji XT, Qian NS, Zhang T, Li JM, Li XK,
Wang P, Zhao DS, Huang G, Zhang L, Fei Z, et al: Spinal astrocytic
activation contributes to mechanical allodynia in a rat
chemotherapy-induced neuropathic pain model. PLoS One.
8:e607332013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen FL, Dong YL, Zhang ZJ, Cao DL, Xu J,
Hui J, Zhu L and Gao YJ: Activation of astrocytes in the anterior
cingulate cortex contributes to the affective component of pain in
an inflammatory pain model. Brain Res Bull. 87:60–66. 2012.
View Article : Google Scholar
|
|
25
|
Feng QX, Wang W, Feng XY, Mei XP, Zhu C,
Liu ZC, Li YQ, Dou KF and Zhao QC: Astrocytic activation in
thoracic spinal cord contributes to persistent pain in rat model of
chronic pancreatitis. Neuroscience. 167:501–509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cui J, He W, Yi B, Zhao H, Lu K, Ruan H
and Ma D: mTOR pathway is involved in ADP-evoked astrocyte
activation and ATP release in the spinal dorsal horn in a rat
neuropathic pain model. Neuroscience. 275:395–403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen W, Hu XM, Liu YN, Han Y, Chen LP,
Wang CC and Song C: CXCL12 in astrocytes contributes to bone cancer
pain through CXCR4-mediated neuronal sensitization and glial
activation in rat spinal cord. J Neuroinflammation. 11:752014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vega-Avelaira D, Ballesteros JJ and
López-García JA: Inflammation-induced hyperalgesia and spinal
microglia reactivity in neonatal rats. Eur J Pain. 17:1180–1188.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu ZZ, Berta T and Ji RR: Resolvin E1
inhibits neuropathic pain and spinal cord microglial activation
following peripheral nerve injury. J Neuroimmune Pharmacol.
8:37–41. 2013. View Article : Google Scholar :
|
|
30
|
Moss A, Beggs S, Vega-Avelaira D, Costigan
M, Hathway GJ, Salter MW and Fitzgerald M: Spinal microglia and
neuropathic pain in young rats. Pain. 128:215–224. 2007. View Article : Google Scholar
|
|
31
|
Guo J, Jia D, Jin B, Xu F, Yuan X and Shen
H: Effects of glial cell line-derived neurotrophic factor
intrathecal injection on spinal dorsal horn glial fibrillary acidic
protein expression in a rat model of neuropathic pain. Int J
Neurosci. 122:388–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Blum E, Procacci P, Conte V and Hanani M:
Systemic inflammation alters satellite glial cell function and
structure. A possible contribution to pain. Neuroscience.
274:209–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao FL, Xu M, Wang Y, Gong KR and Zhang
JT: Tanshinone IIA attenuates neuropathic pain via inhibiting glial
activation and immune response. Pharmacol Biochem Behav. 128:1–7.
2015. View Article : Google Scholar
|
|
34
|
Silva GD, Lopes PS, Fonoff ET and Pagano
RL: The spinal anti-inflammatory mechanism of motor cortex
stimulation: Cause of success and refractoriness in neuropathic
pain? J Neuroinflammation. 12:102015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu MD, Zhao LX, Wang XT, Gao YJ and Zhang
ZJ: Ligustilide inhibits microglia-mediated proinflammatory
cytokines production and inflammatory pain. Brain Res Bull.
109:54–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Clark AK, Staniland AA, Marchand F, Kaan
TK, McMahon SB and Malcangio M: P2X7-dependent release of
interleukin-1beta and nociception in the spinal cord following
lipopolysaccharide. J Neurosci. 30:573–582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luo XY, Qu SL, Tang ZH, Zhang Y, Liu MH,
Peng J, Tang H, Yu KL, Zhang C, Ren Z and Zhang ZS: SIRT1 in
cardiovascular aging. Clin Chim Acta. 437:106–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Herskovits AZ and Guarente L: SIRT1 in
neurodevelopment and brain senescence. Neuron. 81:471–483. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Leibiger IB and Berggren PO: Sirt1: A
metabolic master switch that modulates lifespan. Nat Med. 12:34–36.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kalle AM, Mallika A, Badiger J, Alinakhi,
Talukdar P and Sachchidanand: Inhibition of SIRT1 by a small
molecule induces apoptosis in breast cancer cells. Biochem Biophys
Res Commun. 401:13–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He X, Ou P, Wu K, Huang C, Wang Y, Yu Z
and Guo Q: Resveratrol attenuates morphine antinociceptive
tolerance via SIRT1 regulation in the rat spinal cord. Neurosci
Lett. 566:55–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yin Q, Lu FF, Zhao Y, Cheng MY, Fan Q, Cui
J, Liu L, Cheng W and Yan CD: Resveratrol facilitates pain
attenuation in a rat model of neuropathic pain through the
activation of spinal Sirt1. Reg Anesth Pain Med. 38:93–99. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shao H, Xue Q, Zhang F, Luo Y, Zhu H,
Zhang X, Zhang H, Ding W and Yu B: Spinal SIRT1 activation
attenuates neuropathic pain in mice. PLoS One. 9:e1009382014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Winnik S, Stein S and Matter CM: SIRT1-an
anti-inflammatory pathway at the crossroads between metabolic
disease and atherosclerosis. Curr Vasc Pharmacol. 10:693–696. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xie J, Zhang X and Zhang L: Negative
regulation of inflammation by SIRT1. Pharmacol Res. 67:60–67. 2013.
View Article : Google Scholar
|
|
46
|
Joe IS, Jeong SG and Cho GW:
Resveratrol-induced SIRT1 activation promotes neuronal
differentiation of human bone marrow mesenchymal stem cells.
Neurosci Lett. 584:97–102. 2015. View Article : Google Scholar
|
|
47
|
Li L, Sun Q, Li Y, Yang Y, Yang Y, Chang
T, Man M and Zheng L: Overexpression of SIRT1 Induced by
resveratrol and inhibitor of miR-204 suppresses activation and
proliferation of microglia. J Mol Neurosci. 56:858–867. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sin TK, Tam BT, Yung BY, Yip SP, Chan LW,
Wong CS, Ying M, Rudd JA and Siu PM: Resveratrol protects against
doxorubicin-induced cardiotoxicity in aged hearts through the
SIRT1-USP7 axis. J Physiol. 593:1887–1899. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Han Y, Jiang C, Tang J, Wang C, Wu P,
Zhang G, Liu W, Jamangulova N, Wu X and Song X: Resveratrol reduces
morphine tolerance by inhibiting microglial activation via AMPK
signalling. Eur J Pain. 18:1458–1470. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zong Y, Sun L, Liu B, Deng YS, Zhan D,
Chen YL, He Y, Liu J, Zhang ZJ, Sun J and Lu D: Resveratrol
inhibits LPS-induced MAPKs activation via activation of the
phosphatidylinositol 3-kinase pathway in murine RAW 264.7
macrophage cells. PLoS One. 7:e441072012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Centeno-Baez C, Dallaire P and Marette A:
Resveratrol inhibition of inducible nitric oxide synthase in
skeletal muscle involves AMPK but not SIRT1. Am J Physiol
Endocrinol Metab. 301:E922–E930. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang H, Zhang W, Pan H, Feldser HG, Lainez
E, Miller C, Leung S, Zhong Z, Zhao H, Sweitzer S, et al: SIRT1
activators suppress inflammatory responses through promotion of p65
deacetylation and inhibition of NF-kappaB activity. PLoS One.
7:e463642012. View Article : Google Scholar
|
|
53
|
Park GJ, Kim YS, Kang KL, Bae SJ, Baek HS,
Auh QS, Chun YH, Park BH and Kim EC: Effects of sirtuin 1
activation on nicotine and lipopolysaccharide-induced cytotoxicity
and inflammatory cytokine production in human gingival fibroblasts.
J Periodontal Res. 48:483–492. 2013. View Article : Google Scholar
|