Introduction
Gap junctions (GJs) are plasma membrane channels,
which directly connect the cytoplasms of neighboring cells. GJs are
composed of two hemichannals, each of which contains six connexin
(Cx) proteins for docking to its counterpart in the coupled cell
membrane and form a GJ channel (1). GJs provide the direct cell-cell
transfer of ions, metabolites and other small molecules, thereby
mediating intimate intercellular molecular signaling (2). GJ intercellular communication (GJIC)
is essential in diverse processes, including cell growth,
differentiation and the maintenance of homeostasis (3,4).
Several studies have shown important roles of GJIC in cancer
biology (5–7).
It has been reported that the toxicities of
cisplatin and oxaliplatin are increased by the presence of GJIC
between the target cells (8,9).
This enhanced toxicity may be due to the transmission of 'death
signals' among adjacent cells via GJs. This effect has been
observed in ionizing radiation, in which cells that are not
irradiated, but adjacent to irradiated cells, also become damaged
or die (10,11). In addition, the
enhancement/maintenance of GJIC may enhance the efficacy of cancer
treatment, whereas the inhibition of GJIC is likely to decrease the
toxicity of chemotherapeutic agents.
Propofol (2,6-diisopropylphenyl) is the most widely
used intravenous general anesthetic agent for the induction and
maintenance of anesthesia (12),
and it is often used during chemotherapy. It has been reported that
propofol mediates protective effects against cisplatin-induced
injury, including the upregulation of endothelial adhesion
molecules in human umbilical vein endothelial cells (13) and the attenuation of toxic
oxidative stress (14). In
addition, propofol has been shown to suppress GJIC composed of Cx32
in various cell lines (15,16).
Our previous studies demonstrated that tramadol and flurbiprofen,
two commonly used analgesics, depressed the cytotoxicity of
cisplatin via inhibiting GJIC (17). In addition, propofol has been
observed to depress the toxicity of X-ray irradiation through
inhibition of GJs in Cx32-transfected HeLa cells (18), which suggested that the inhibition
of GJIC is one of the possible mechanisms underlying the effects of
anesthetic agents against toxic effects during chemotherapy and
radiotherapy. However, there remains a lack of evidence of the
effects of propofol on the regulation of GJs composed of Cx43 or
Cx26, and its chemotherapeutic efficiency.
In the present study, U87 glioma and
Cx26-transfected HeLa cells were selected to investigate whether
the effects of propofol on the cytotoxicity of cisplatin are
mediated by alterations in GJ function. The results of the present
study may help elucidate a novel mechanism underlying the effects
of analgesics in counteracting chemotherapeutic efficiency.
Materials and methods
Materials
Propofol and intralipid (10% soybean oil, 2.25%
glycerol and 1.2% purified egg phosphatide) were purchased from Sun
Yat-Sen Memorial Hospital (Guangzhou, China). G-418, hygromycin and
doxycycline were from Calbiochem (San Diego, CA, USA).
Calcein-acetoxymethyl ester (calcein-AM) and cell culture reagents
were from Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Cisplatin and primary and secondary antibodies for use in
western blotting were purchased from Sigma-Aldrich (St. Louis, MO,
USA). All other reagents were purchased from Sigma-Aldrich, unless
stated otherwise.
Cell lines and cell culture
The human U87 glioma cell line was obtained from
American Tissue Culture Collection (Manassas, VA, USA), and the
cells (3×104 cells/well) were cultured at 37°C for 48 h
to 70-100% confluence in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum. The HeLa cell line
expressing the Cx26 gene under the control of a bidirectional
tetracycline-inducible promoter, was provided by Dr Andrew L.
Harris (Department of Pharmacology and Physiology, New Jersey
Medical School, University of Medicine and Dentistry of New Jersey,
NJ, USA) and has been described previously (19). The Cx26-expressing HeLa cells were
grown at 37°C in DMEM supplemented with 10% fetal bovine serum, 100
μg/ml G418 sulfate and 200 μg/ml hygromycin B. The
Cx26 coding sequence was followed by an influenza hemagglutinin
(HA) epitope tag at the C-terminus, which was incorporated using a
Tet-On inducible expression system and one-step anti-haemagglutinin
immunoaffinity purification, as previously described (19). Expression of Cx26 was induced by
exposure to 1 μg/ml doxycycline for 48 h at 37°C.
Sulforhodamine B (SRB) assay
Cell viability was assessed using an SRB assay
(20). The cells were seeded in
96-well plates (~3×104) and exposed to various
concentrations of propofol (1, 5, 30 and 100 μM) for 48 h at
37°C. The medium was then removed, and the cells were fixed with
10% (wt/vol) cold trichloroacetic acid for 1 h at 4°C, following
which the excess dye was removed by washing repeatedly with 1%
(vol/vol) acetic acid. The protein-bound dye was dissolved in 10 mM
Tris base solution for determination of the optical density (OD) at
564 nm using an Epoch™ microplate reader (BioTek Instruments, Inc.,
Winooski, VT, USA).
Standard colony-forming assay
The toxicity of cisplatin was evaluated using a
standard colony-forming assay, as described previously (9). The cells were seeded at a high
density of 30,000 cells/cm2 and grown to 90% confluence,
followed by drug treatment. Propofol was added to the cells at 15
μM 4 h prior to cisplatin treatment. Following exposure to
20 μM cisplatin for 1 h at 37°C, the cells were washed with
phosphate-buffered saline (PBS), trypsinized, counted and reseeded
into six-well dishes at a density of 500 cells/well. A low density
group was also included, in which the cells were seeded at 500
cells/cm2 in six-well dishes and treated with cisplatin.
In the two culture groups, the cells were incubated for another 5-8
days at 37°C, and were then fixed and stained with 4% crystal
violet in ethanol. Cells were counted using an Olympus CKX41
inverted microscope (Olympus Corporation, Tokyo, Japan), and
colonies containing ≥50 cells were counted. Colony formation was
normalized to the number of colonies formed by vehicle-treated (10
μg/ml lipid emulsion) cells. The surviving fraction was
calculated as follows: Surviving fraction (%) = colonies in
cisplatin-treated group/colonies in non-treated group × 100%.
῾Parachute᾽ dye-coupling assay
A dye-coupling assay was used to examine GJ
function, and was performed as described previously (19,21).
Cells were grown to confluence, and the donor cells were labeled
with 5 μM calcein-AM, which is converted into calcein in the
intracellular plasma to permeate through GJs to the adjacent cells,
for 30 min at 37°C. Donor cells were then trypsinized and seeded
onto the receiver cells at a 1:500 donor:receiver ratio. These
cells were allowed to attach to the monolayer of the receiver cells
to form GJs for 4 h at 37°C, and were then monitored under a
fluorescence microscope (Olympus IX71; Olympus Corporation). The
average number of receiver cells containing calcein per donor cell
was determined and normalized to that of the vehicle cultures, and
thus considered to be a measurement of the degree of GJ
function.
Western blot analysis
The cells were washed with cold PBS three times and
then harvested using lysis buffer (22). The cell lysate was sonicated and
then centrifuged at 14,167 × g for 30 min at 4°C. Proteins were
quantified using a DC protein assay kit (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Subsequently, 25 μg proteins from
each sample were separated by SDS-PAGE and then transferred onto a
nitrocellulose membrane. Membranes were blocked with 5% milk for 1
h at room temperature and incubated with the mouse monoclonal
primary antibodies against Cx43 (C8093; diluted 1:3,000 in 5%
milk), HA IgG [H9658; diluted 1:1,000 in Tris-buffered saline with
Tween 20 (TBST)] and β-actin (A1978; diluted 1:10,000 in 5% milk)
at 4°C overnight. Membranes were subsequently washed three times
with TBST for 10 min and incubated with mouse anti-goat secondary
antibody (88704) against Cx43 (diluted 1:6,000 in 5% milk), HA lgG
(diluted 1:2,000 in TBST), and β-actin (diluted 1:10,000 in 5%
milk) for 1 h at room temperature. The membranes subsequently were
washed three times with TBST for 10 min. Immunopositive bands were
visualized using the Amersham ECL™ Plus Western Blotting Detection
kit (GE Healthcare Life Sciences) and protein expression levels
were quantified using a GeneGenius Bio Imaging system (version 1.2;
Syngene, Frederick, MD, USA).
Statistical analysis
Differences between groups were statistically
analyzed using an unpaired Student's t-test and the results
are presented as the mean ± standard error of the mean using Sigma
Plot 10.0 software (Jandel Scientific, San Rafael, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of propofol on cisplatin
cytotoxicity
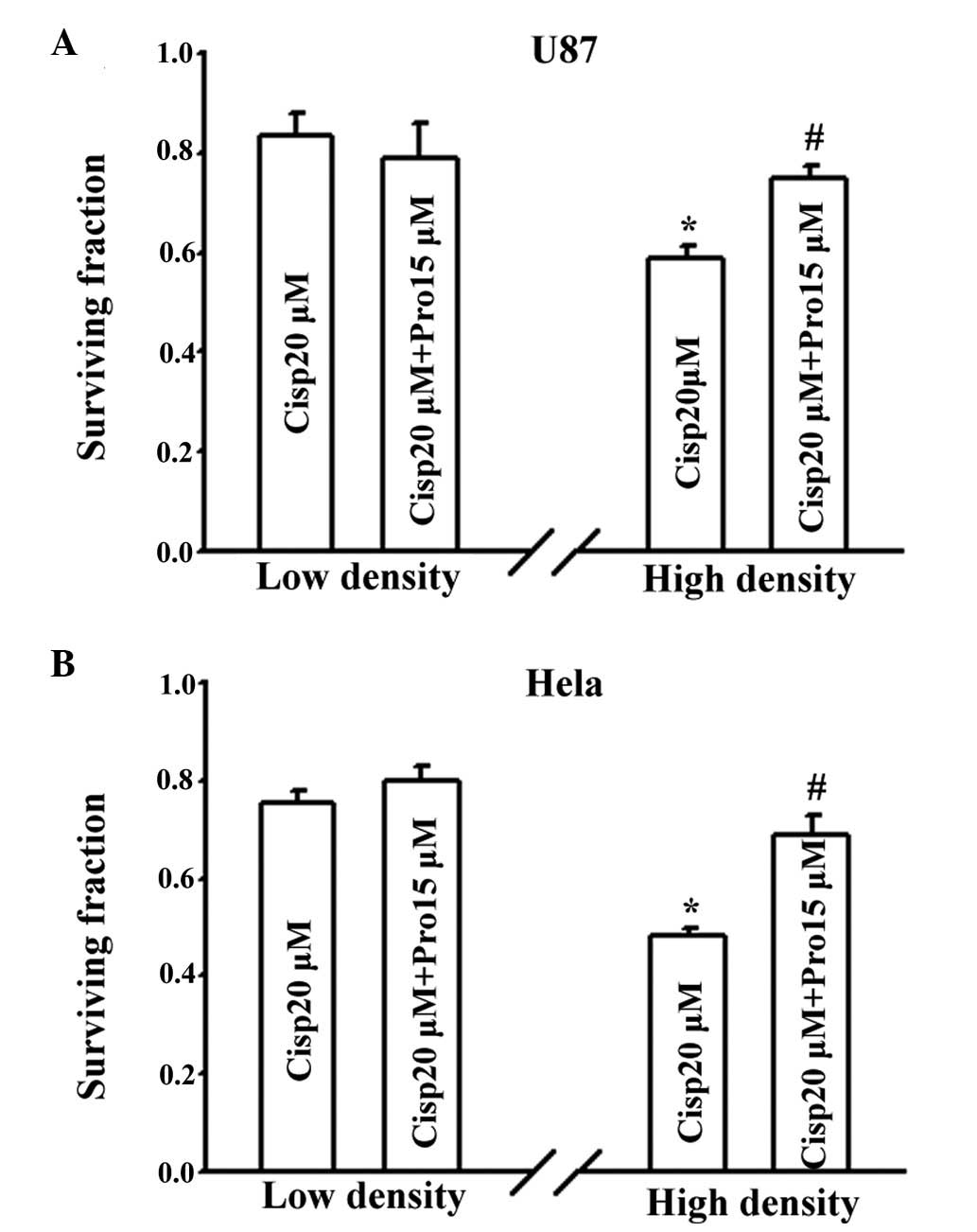
As shown in Fig. 1,
the effects of propofol on cisplatin toxicity were determined in
the U87 and Cx26-transfected HeLa cells. Propofol was used at a
concentration of 15 μM, which is the 50% effective
concentration (EC50) in humans (23), for 4 h prior to treatment with 20
μM cisplatin for 1 h. A clinical concentration (10
μg/ml) of lipid emulsion was selected as a solvent control
to exclude the possible effect of lipids on the effects of
propofol. Cisplatin significantly reduced the surviving fraction of
cells at low (without GJs) and high density (with GJs) cultures in
the two cells, and the survival rate was higher in the low-density
cultures, compared with the high-density cultures. At a low
density, pretreatment with propofol had no effect on cell viability
in either cell type; however, propofol markedly increased the
clonogenic survival of the cisplatin-treated cells in the
high-density cultures. In the U87 cell, 4 h treatment with 15
μM propofol increased the surviving fraction between
0.59±0.02 and 0.75±0.03 (P<0.05), and in the HeLa cells, the
viability of the cells increased between 0.48±0.02 and 0.69±0.04
(P<0.05). These results indicated that propofol decreased
cisplatin toxicity only when GJs were formed.
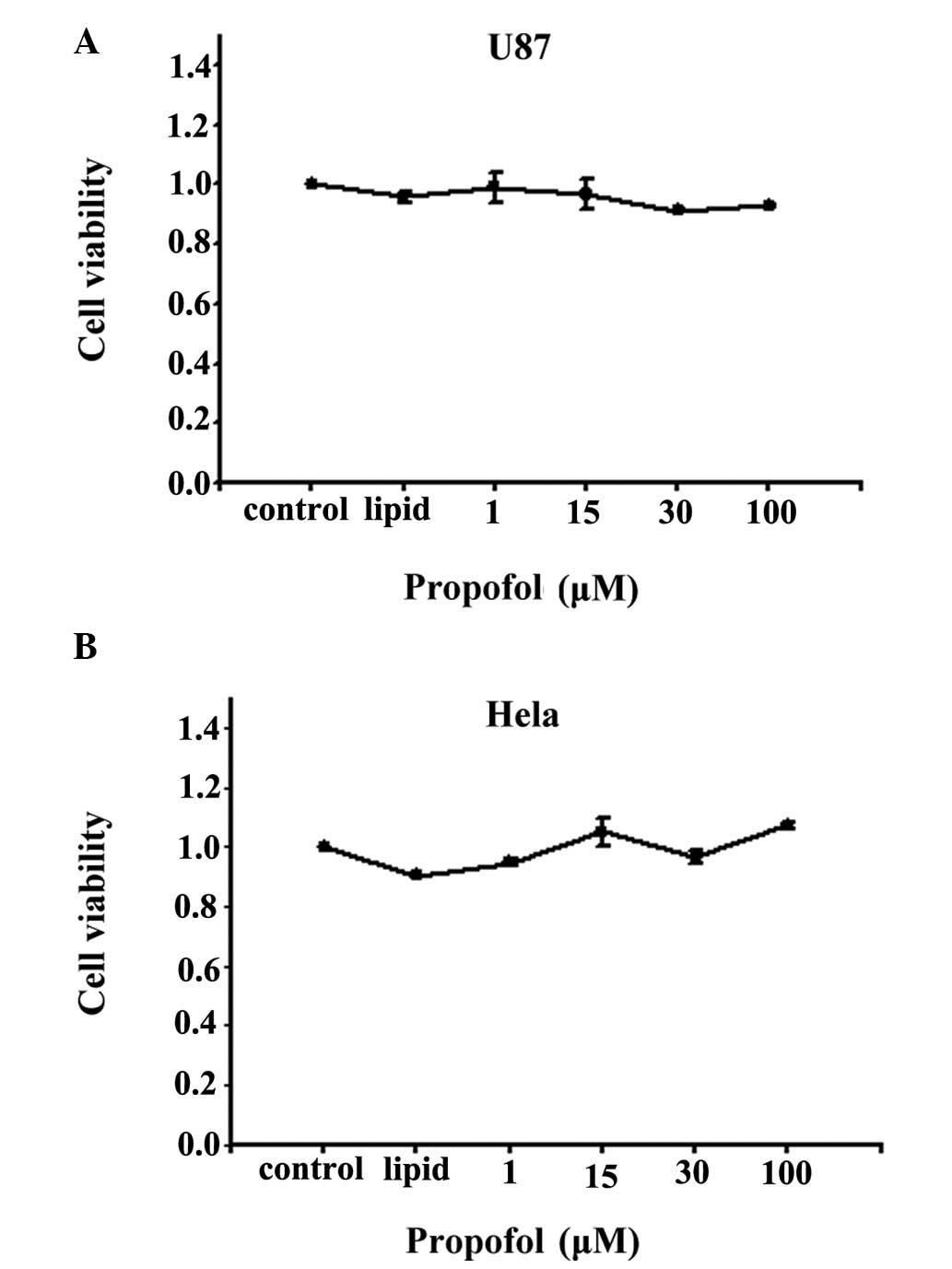
Cell viability measurement
The observation that propofol decreased the toxicity
of cisplatin only in high density conditions, in which GJs were
formed, suggested that the effect of propofol on cisplatin was
mediated by GJs. In order to exclude the effects of cell
viability on cisplatin toxicity and on GJ function, the present
study first examined the effects of propofol on cell viability
using an SRB assay. As shown in Fig.
2, propofol had no significant effect on cell viability, even
at 100 μM for 48 h, in either the U87 cells or the
Cx26-expressing HeLa cells. The viability of the cells under all
experimental conditions was >85%, and no alterations in either
cell morphology or adhesion were apparent (data not shown). As a
result, the concentration of propofol used in the following
experiments was between 1 and 100 μM.
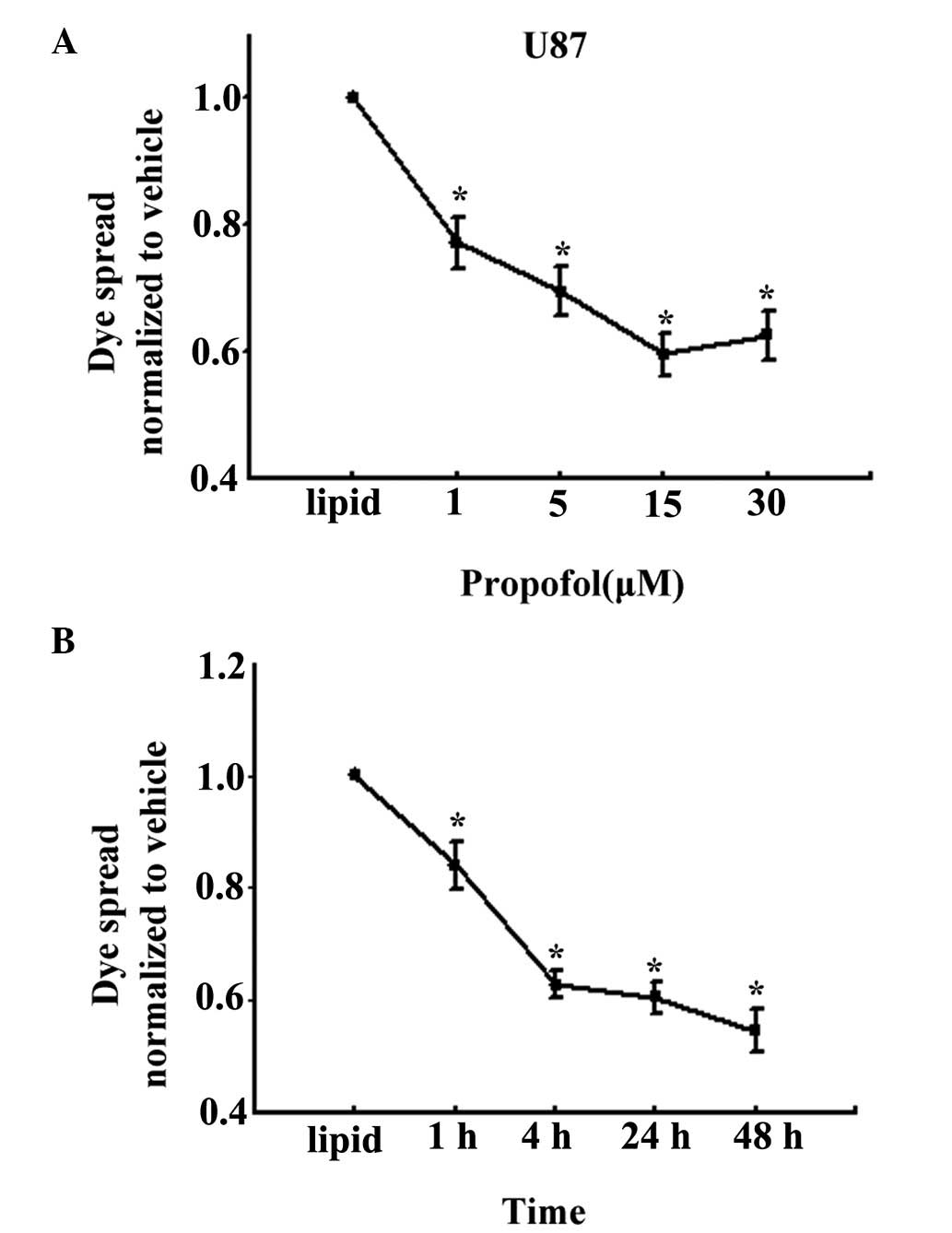
Effect of propofol on GJ function
To test the hypothesis that propofol depresses the
toxicity of cisplatin due to the presence of GJs, the effects of
propofol on dye coupling between confluent U87 or Cx26-transfected
HeLa cells were examined. GJ function was assessed using the
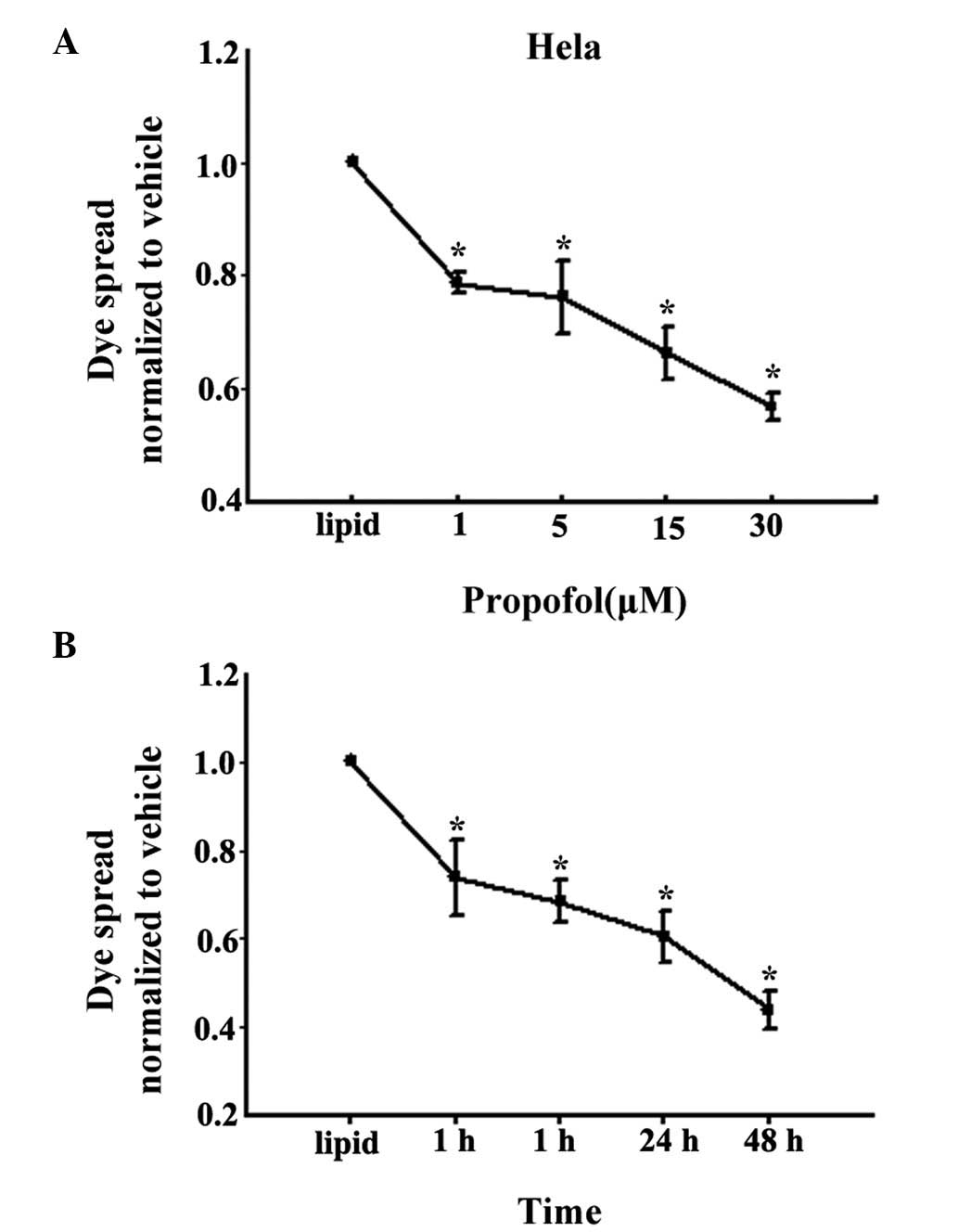
parachute dye-coupling assay. As shown in Fig. 3A, treatment with 1, 5, 15 and 30
μM propofol for 4 h led to marked inhibition of the spread
of dye between the donor cells and receiver cells, which occurred
in a concentration-dependent manner, in the U87 cells. Treatment
with 15 μM propofol inhibited GJ function by ~40%, compared
with the lipid-treated cells. Furthermore, as shown in Fig. 3B, 15 μM propofol inhibited
GJ function following treatment for 1, 4, 24 and 48 h, and the
inhibition rate was the highest at 48 h at almost 55%.
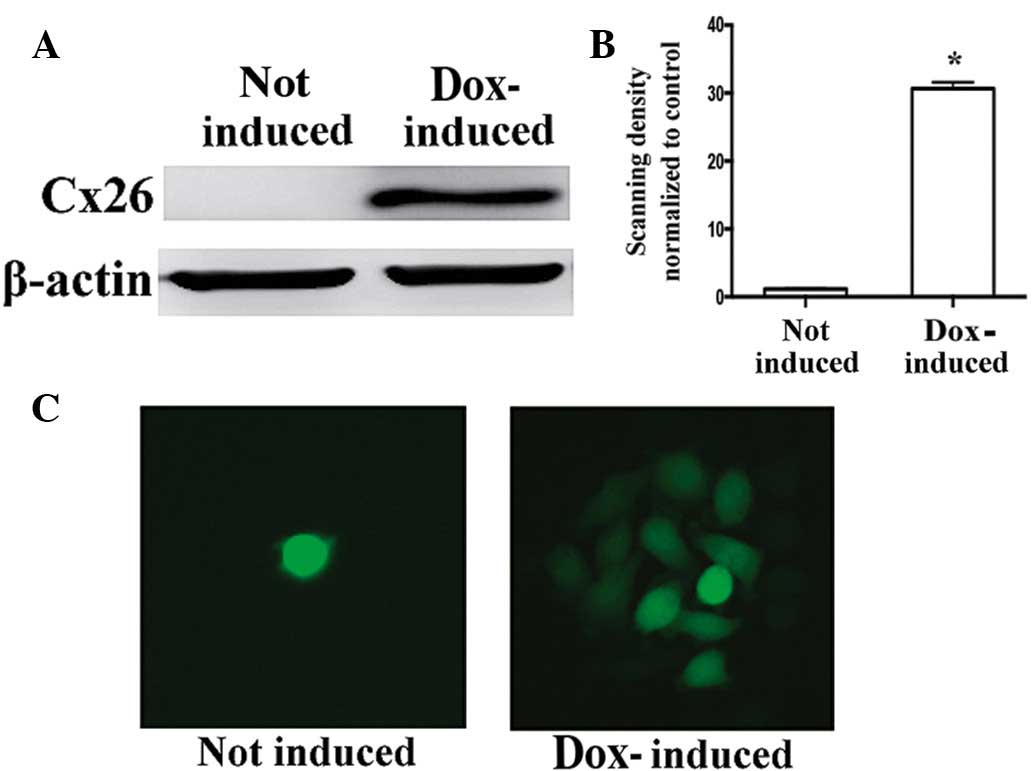
In the Cx26-transfected HeLa cells, the induction of
Cx26 expression with doxycycline was examined by western blot
analysis (Fig. 4A and B), and the
emergence of GJIC was examined using the parachute dye coupling
assay (Fig. 4C). Treatment with 1,
5, 15 and 30 μM propofol for 4 h significantly inhibited the
function of the GJs composed of Cx26, which occurred in a
concentration-dependent manner (Fig.
5A). Treatment with 15 μM propofol inhibited GJ function
by ~35% at 4 h and 60% at 48 h, compared with the lipid-treated
cells (Fig. 5B). The above results
indicated that propofol inhibited the function of GJs composed of
Cx43 or Cx26 in a concentration- and time-dependent manner.
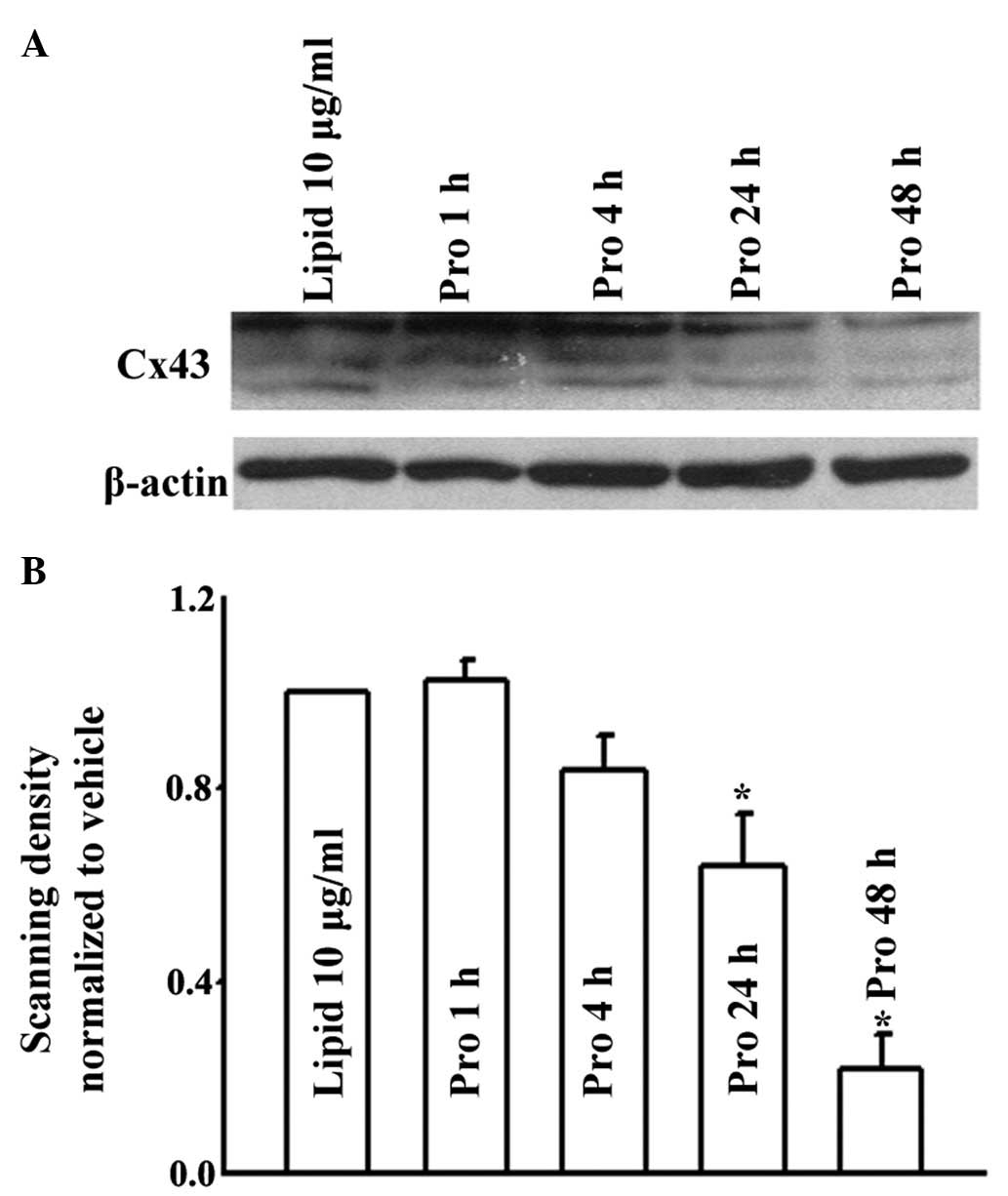
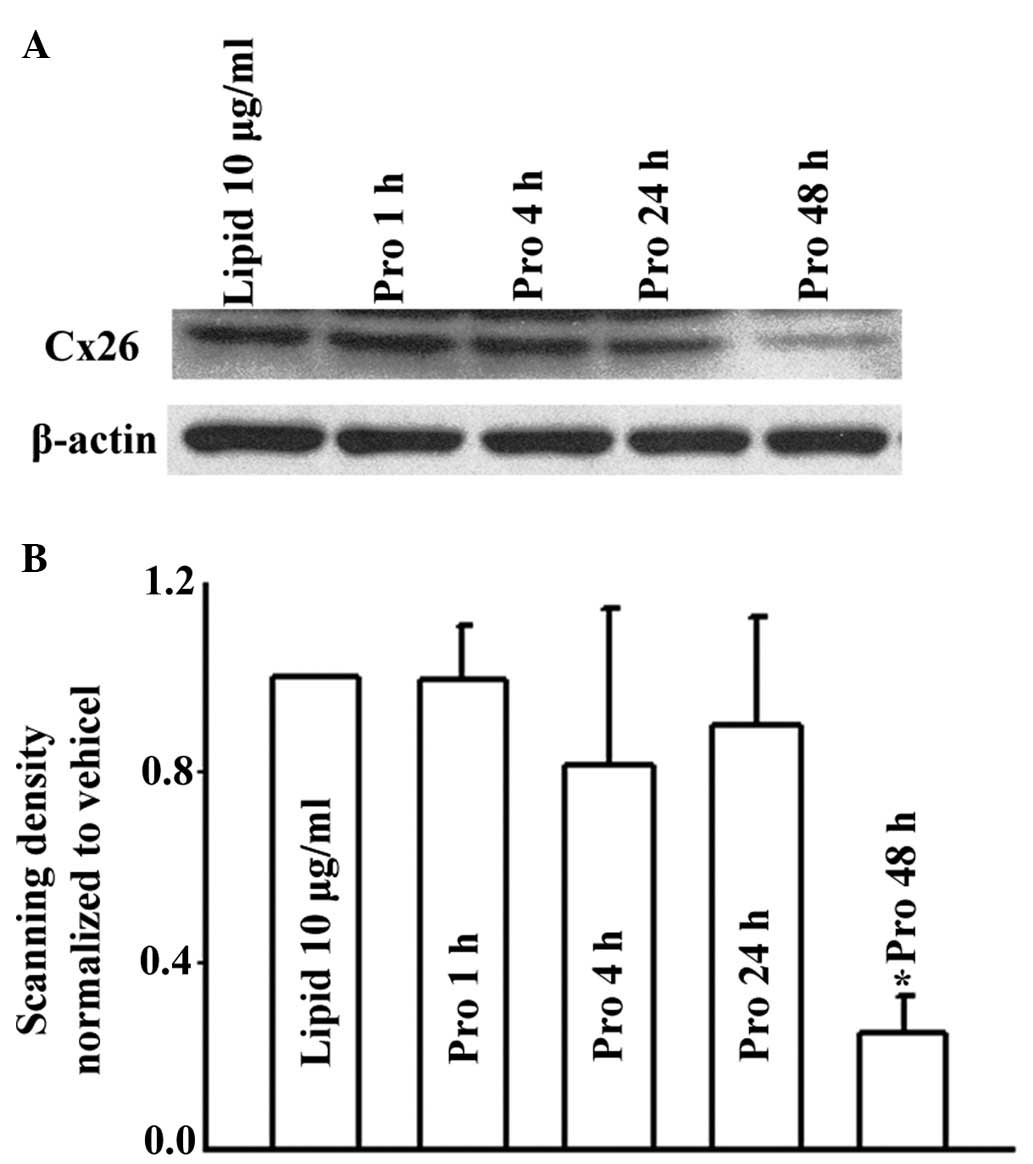
Effects of propofol on expression levels
of Cx43 and Cx26
Changes in the number of GJs affected by the
expression of Cx is one of the mechanisms by which propofol has
been suggested to alter GJ function. In the present study, the
expression levels of Cx43 and Cx26 were determined using western
blot analysis. As shown in Fig. 6,
treatment of the U87 cells with 15 μM propofol for 1 and 4 h
did not alter the expression levels of Cx43. However, by prolonging
the treatment duration to 24 and 48 h, propofol treatment led to
decreases in the levels of Cx43, compared with the lipid emulsion
group. Similarly, in the HeLa cells, 15 μM propofol
decreased the expression of Cx26 only when the cells were treated
for 48 h (Fig. 7). Therefore,
propofol reduced the function of GJs composed of Cx43 or Cx26 by
decreasing the expression levels of Cx in long treatment durations.
Consequently, these results confirmed the hypothesis that propofol
depresses cisplatin toxicity by the inhibition of GJ function via
altering the expression of Cxs.
Discussion
The present study demonstrated that propofol, at
clinically relevant concentrations, significantly inhibited the
function of the GJs formed by Cx43 or Cx26, and reduced the
cytotoxicity of cisplatin by the inhibition of GJIC. The results
showed that propofol inhibited the function of GJ channels, and
decreased the expression levels of Cx43 or Cx26 in long-term
treatment. This revealed a novel mechanism underlying the effects
of analgesics in counteracting chemotherapeutic efficiency.
Cisplatin cytotoxicity was decreased when GJIC was
inhibited and enhanced when GJIC was upregulated, as previously
demonstrated (8,24). In the present study, the cells were
cultured in two conditions: Low density, in which GJs were not
formed, and high density, which allowed the cells to contact each
other to form GJs. As shown in Fig.
1, cisplatin toxicity was increased in the high-density culture
with GJs, compared with the low-density culture without GJs, which
was consistent with previous reports (8,17,18,22).
In addition, pretreatment with propofol at its EC50 significantly
decreased cispaltin toxicity in the high-density culture in the U87
cells and Cx26-transfected HeLa cells, indicating that propofol
depressed cisplatin toxicity only in the presence of GJs.
Substantial evidence has suggested that GJIC is
reduced or absent in numerous types of carcinoma (7,25–27),
however, GJIC remains preserved in certain types of cancer
(28,29), and during the invasion and
metastatic stages, an upregulation of GJIC has been observed in
certain cancer cells with nominally defective GJs (30–32).
In these GJs, which are derived from Cx26, Cx32 or Cx43, the effect
of propofol on GJIC and how it is likely to impact the therapeutic
efficacy of cisplatin requires consideration.
The present study demonstrated that treatment of the
U87 or Cx26-transfected HeLa cells with propofol (15 μM) for
1 h had no effect on the expression levels of Cx43 or Cx26.
However, in contrast to our previous studies on Cx32 (17,18),
the expression levels of Cx43 or Cx26 altered when the duration of
propofol exposure was extended to 24 or 48 h. These results
indicated that long-term propofol exposure decreased GJIC,
predominantly via downregulation of the protein levels of Cx43 or
Cx26. However, the reduction of GJIC following short-term (1 and 4
h) treatment with propofol may occur via a different manner. It is
possible that short-term treatment with propofol caused aberrant
localization of the Cx proteins without reducing their expression
levels, as reported previously (27,33),
which requires further investigation.
In conclusion, the present study demonstrated that
propofol affects the function of GJs formed of various types of
Cxs, and demonstrated that propofol depressed the cytotoxicity of
cisplatin in U87 glioma cells and Cx26-transfected HeLa cells
through the inhibition of GJ activity. The results further
indicated that long-term treatment with propofol decreased GJIC,
predominantly via reductions in the expression levels of Cx43 and
Cx26. Although the effects of propofol on chemotherapy and
radiotherapy, and the underlying molecular mechanisms require
further investigation, the results of the present study suggest
that GJIC is one of the possible mechanisms.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81373439 and
81473234), the Joint Fund of the National Nature Science Foundation
of China (grant no. U1303221), the Grant for the Construction of
Technique Plate for Evaluation of the Pharmacodynamics of New Drugs
in Xinjiang from the Department of Science and Technology of
Xinjiang province (grant no. 201233150), the Department of Science
and Technology of Guangzhou, China (grant no. 201300000158) and the
Grant for Development of Science and Technology from Department of
Science and Technology of Guangzhou, China (grant no.
201300000158).
References
|
1
|
Sosinsky GE and Nicholson BJ: Structural
organization of gap junction channels. Biochim Biophys Acta.
1711:99–125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harris AL: Connexin channel permeability
to cytoplasmic molecules. Prog Biophys Mol Biol. 94:120–143. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maeda S and Tsukihara T: Structure of the
gap junction channel and its implications for its biological
functions. Cell Mol Life Sci. 68:1115–1129. 2011. View Article : Google Scholar
|
|
4
|
Vinken M, Vanhaecke T, Papeleu P, Snykers
S, Henkens T and Rogiers V: Connexins and their channels in cell
growth and cell death. Cell Signal. 18:592–600. 2006. View Article : Google Scholar
|
|
5
|
Cronier L, Crespin S, Strale PO, Defamie N
and Mesnil M: Gap junctions and cancer: New functions for an old
story. Antioxid Redox Signal. 11:323–338. 2009. View Article : Google Scholar
|
|
6
|
Kandouz M and Batist G: Gap junctions and
connexins as therapeutic targets in cancer. Expert Opin Ther
Targets. 14:681–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naus CC and Laird DW: Implications and
challenges of connexin connections to cancer. Nat Rev Cancer.
10:435–441. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Q, You T, Yuan D, Han X, Hong X, He
B, Wang L, Tong X, Tao L and Harris AL: Cisplatin and oxaliplatin
inhibit gap junctional communication by direct action and by
reduction of connexin expression, thereby counteracting cytotoxic
efficacy. J Pharmacol Exp Ther. 333:903–911. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jensen R and Glazer PM:
Cell-interdependent cisplatin killing by Ku/DNA-dependent protein
kinase signaling transduced through gap junctions. Proc Natl Acad
Sci USA. 101:6134–6139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Azzam EI, de Toledo SM and Little JB:
Direct evidence for the participation of gap junction-mediated
intercellular communication in the transmission of damage signals
from alpha-particle irradiated to nonirradiated cells. Proc Natl
Acad Sci USA. 98:473–478. 2001.
|
|
11
|
Mesnil M, Piccoli C, Tiraby G, Willecke K
and Yamasaki H: Bystander killing of cancer cells by herpes simplex
virus thymidine kinase gene is mediated by connexins. Proc Natl
Acad Sci USA. 93:1831–1835. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weiser TG, Regenbogen SE, Thompson KD,
Haynes AB, Lipsitz SR, Berry WR and Gawande AA: An estimation of
the global volume of surgery: A modelling strategy based on
available data. Lancet. 372:139–144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu M, Chen J, Yin H, Jiang H, Wen M and
Miao C: Propofol protects human umbilical vein endothelial cells
from cisplatin-induced injury. Vascul Pharmacol. 61:72–79. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taheri Moghadam G, Hosseini-Zijoud SM,
Heidary Shayesteh T, Ghasemi H and Ranjbar A: Attenuation of
cisplathin-induced toxic oxidative stress by propofol. Anesth Pain
Med. 4:e142212014. View Article : Google Scholar
|
|
15
|
Wentlandt K, Carlen PL, Kushnir M, Naus CC
and El-Beheiry H: General anesthetics attenuate gap junction
coupling in P19 cell line. J Neurosci Res. 81:746–752. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang F, Li S, Gan X, Wang R and Chen Z:
Propofol inhibits gap junctions by attenuating sevoflurane-induced
cytotoxicity against rat liver cells in vitro. Eur J Anaesthesiol.
31:219–224. 2014. View Article : Google Scholar
|
|
17
|
He B, Tong X, Wang L, Wang Q, Ye H, Liu B,
Hong X, Tao L and Harris AL: Tramadol and flurbiprofen depress the
cytotoxicity of cisplatin via their effects on gap junctions. Clin
Cancer Res. 15:5803–5810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Y, Liu B, Wang Q, Yuan D, Yang Y,
Hong X, Wang X and Tao L: Propofol depresses the cytotoxicity of
X-ray irradiation through inhibition of gap junctions. Anesth
Analg. 112:1088–1095. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koreen IV, Elsayed WA, Liu YJ and Harris
AL: Tetracycline-regulated expression enables purification and
functional analysis of recombinant connexin channels from mammalian
cells. Biochem J. 383:111–119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Papazisis KT, Geromichalos GD, Dimitriadis
KA and Kortsaris AH: Optimization of the sulforhodamine B
colori-metric assay. J Immunol Methods. 208:151–158. 1997.
View Article : Google Scholar
|
|
21
|
Goldberg GS, Bechberger JF and Naus CC: A
pre-loading method of evaluating gap junctional communication by
fluorescent dye transfer. Biotechniques. 18:490–497.
1995.PubMed/NCBI
|
|
22
|
Hong X, Wang Q, Yang Y, Zheng S, Tong X,
Zhang S, Tao L and Harris AL: Gap junctions propagate opposite
effects in normal and tumor testicular cells in response to
cisplatin. Cancer Lett. 317:165–171. 2012. View Article : Google Scholar
|
|
23
|
White M and Kenny GN: Intravenous propofol
anaesthesia using a computerised infusion system. Anaesthesia.
45:204–209. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Wang Q, Zhang S, Zhang Y and Tao
L: Baicalein increases the cytotoxicity of cisplatin by enhancing
gap junction intercellular communication. Mol Med Rep. 10:515–521.
2014.PubMed/NCBI
|
|
25
|
Mesnil M, Crespin S, Avanzo JL and
Zaidan-Dagli ML: Defective gap junctional intercellular
communication in the carcinogenic process. Biochim Biophys Acta.
1719:125–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loewenstein WR and Kanno Y: Intercellular
communication and the control of tissue growth: Lack of
communication between cancer cells. Nature. 209:1248–1249. 1966.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leithe E, Sirnes S, Omori Y and Rivedal E:
Downregulation of gap junctions in cancer cells. Crit Rev Oncog.
12:225–256. 2006. View Article : Google Scholar
|
|
28
|
Plante I, Stewart MK, Barr K, Allan AL and
Laird DW: Cx43 suppresses mammary tumor metastasis to the lung in a
Cx43 mutant mouse model of human disease. Oncogene. 30:1681–1692.
2011. View Article : Google Scholar
|
|
29
|
Krutovskikh VA, Piccoli C and Yamasaki H:
Gap junction intercellular communication propagates cell death in
cancerous cells. Oncogene. 21:1989–1999. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Czyz J: The stage-specific function of gap
junctions during tumourigenesis. Cell Mol Biol Lett. 13:92–102.
2008. View Article : Google Scholar
|
|
31
|
Kanczuga-Koda L, Sulkowska M, Koda M,
Rutkowski R and Sulkowski S: Increased expression of gap junction
protein-connexin 32 in lymph node metastases of human ductal breast
cancer. Folia Histochem Cytobiol. 45(Suppl 1): S175–S180. 2007.
|
|
32
|
Saito-Katsuragi M, Asada H, Niizeki H,
Katoh F, Masuzawa M, Tsutsumi M, Kuniyasu H, Ito A, Nojima H and
Miyagawa S: Role for connexin 26 in metastasis of human malignant
melanoma: Communication between melanoma and endothelial cells via
connexin 26. Cancer. 110:1162–1172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang N, Wang Q, Wu DP, Zhang SZ, Zhang Y
and Tao L: Differential effects of paclitaxel and docetaxel on gap
junctions affects their cytotoxicities in transfected HeLa cells.
Mol Med Rep. 8:638–644. 2013.PubMed/NCBI
|