Introduction
Bone is a living organ, which undergoes remodeling
throughout life (1). This
remodeling involves the removal of mineralized bone by osteoclasts,
followed by formation of the bone matrix through osteoblasts, which
subsequently becomes mineralized (2). The major regulators of bone
remodeling include growth hormone (GH), parathyroid hormone,
insulin-like growth factor-1, transforming growth factor (TGF)-β
and bone morphogenetic proteins (BMPs) (1,3).
BMP-2, a member of the TGF-β superfamily, was originally identified
as an inducer of ectopic bone formation (4). The binding of BMP-2 to
serine/threonine kinase receptors induces small mothers against
decapentaplegic (Smad) phosphorylation, and the phosphorylated
Smads then translocate from the cytoplasm to the nucleus to
regulate gene transcription (5).
The osteogenic differentiation of mesenchymal stem
cells (MSCs) is characterized by the expression of genes, including
Runt-related transcription factor 2 (Runx2) and alkaline
phosphatase (ALP), followed by extracellular matrix synthesis and
mineralization (6). Runx2 is
essential in osteoblast differentiation during bone development and
remodeling, binding a conserved promoter sequence (R/TACCRCA) and
transactivating genes encoding osteogenic proteins, including
collagen α1, osteopontin (OPN), bone sialoprotein (BSP) and
osteocalcin (OCN) (7,8).
Multiple isotypes of BMP, including BMP-2 and BMP-7,
induce MSCs to undergo osteogenic differentiation (9–12).
BMP-7 and BMP-2 are currently used clinically to induce new bone
formation in spine fusion and long bone non-union fractures
(13).
Methylsulfonylmethane (MSM) is a simple, volatile
organic sulfur, which is non-toxic to humans and contains a
compound also known as dimethyl sulfone. Intake of MSM is possible
through the consumption of fruits, vegetables, grains and animals
(14–17). This compound affects the
differentiation of MSCs into osteoblasts by affecting the Janus
kinase 2/signal transducer and activator of transcription (STAT)5b
signaling pathway (18,19). However, although MSM and BMP-2
affect the bone formation pathway, the effects of the combination
of the two on osteoblastic differentiation have received little
investigation.
In the present study, the roles of MSM in BMP
signaling and the differentiation of MSCs were analyzed. It was
hypothesized that MSM enhances BMP-2 signaling and the process of
osteoblast differentiation, with Smad1/5/8 and Runx2 as major
targets. MSM has potential to be used as a therapeutic agent to
treat bone-depleting conditions, such as osteoporosis, where the
mineralization of bone is impaired due to decreased osteoblast
differentiation.
Materials and methods
MSC isolation and cell culture
MSCs were isolated from the long bone (femur and
tibia) of 12 six-week-old male balb/c mice (Orient Bio, Inc.,
Seongnam, Korea) following euthanization in a CO2
chamber. The bone marrow was flushed out using needles and
α-minimal essential medium (α-MEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) without fetal bovine serum (FBS), and
unless otherwise stated, the cells were maintained in α-MEM
containing 10% FBS medium at 37°C with 5% CO2 for 7
days. The medium was replaced at 4 day intervals.
Antibodies and reagents
Modified α-MEM containing FBS was purchased from
Gibco; Thermo Fisher Scientific, Inc. Smad1/5/8 (cat. no. sc-6031),
phosphorylated (p)-Smad1/5/8 (cat. no. 9511S) and β-actin (cat. no.
sc-47778) antibodies, and the horseradish peroxidase
(HRP)-conjugated rabbit anti-rabbit immunoglobulin G secondary
antibodies (cat. no. sc-2004) were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA) and Cell Signaling
Technology, Inc. (Beverly, MA, USA). The anti-actin antibody, MSM,
Alizarin red S, ascorbic acid phosphate, β-glycerophosphate
disodium salt hydrate, L-glutamine and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide were
obtained from Sigma-Aldrich (St. Louis, MO, USA). Tri-RNA reagent
was purchased from Favogen Biotech Corp. (Kaohsiung, Taiwan). The
kit used for reverse transcription-polymerase chain reaction
(RT-PCR) analysis was obtained from Bioline, Ltd. (London, UK). The
ALP, BSP, OCN, Runx2 and 18S primers for use in RT-PCR were
purchased from Bioneer (Daejeon, Korea), and enhanced
chemiluminescence (ECL) and detection kits were purchased from GE
Healthcare (Piscataway, NJ, USA). The Coomassie Protein Assay kit
and Restore Western Blot Stripping Buffer were purchased from
Pierce Biotechnology, Inc. (Rockford, IL, USA).
Western blot analysis
The MSCs were cultured in the osteogenic medium with
MSM (20 mM) and BMP-2 (100 ng/ml) during the initiation of
osteoblast differentiation. The cells were lysed in whole lysis
buffer, containing 50 mM Tris-HCl (pH 7.5), 5 mM EDTA, 150 mM NaCl,
1% Triton X-100, and protease and phosphatase inhibitors. Lysates
were incubated on ice for 40 min and then centrifuged at 15,000 × g
for 10 min at 4℃. The supernatant was collected and the protein
concentrations were determined using a Coomassie protein assay. An
equivalent quantity (20 µg) of protein extract from each
sample was electrophoresed on 10% sodium dodecyl sulfate
polyacrylamide gels and transferred onto nitrocellulose membranes.
The membranes were blocked for 1 h with 5% bovine serum albumin
(Merck Millipore, Darmstadt, Germany) in 20 mM Tris-HCl (pH 7.6)
137 mM NaCl and 0.16 Tween 20 (T-TBS), and incubated overnight at
4°C with primary antibodies (Smad1/5/8, p-Smad1/5/8 or β-actin;
dilution, 1:1,000). The membranes were then washed three times in
T-TBS and incubated with the corresponding anti-rabbit or
anti-mouse immunoglobulin G HRP-conjugated secondary antibodies
(1:2,000) in T-TBS with 0.5% bovine serum albumin for 1 h under
agitation at room temperature. Following incubation, the membranes
were washed three times in T-TBS, following which the membranes
were developed using the ECL PLUS kit and analyzed using an LAS
4000 machine (Fujifilm, Tokyo, Japan).
RT-PCR
The bone marrow MSCs were cultured in osteogenic
medium, and the mRNA expression levels were measured following
treatment with MSM (20 mM) and BMP-2 (100 ng/ml) at day 5 for ALP,
day 14 for BSP and day 21 for OCN. Total RNA was prepared using
Tri-RNA reagent, and cDNA was synthesized using the SensiFAST™ cDNA
Synthesis kit (Bioline, Ltd.), according to the manufacturer's
protocol. The PCR analysis was performed on aliquots of cDNA to
detect Runx2 (301 bp), ALP (454 bp), BSP (81 bp) and OCN (113 bp).
The PCR primer sequences were as follows: Runx2, sense
5′-TCACTACCAGCCACCGAGAC-3′ and antisense 5′-ACGCCATAGTCCCTCCTTT-3′;
ALP, sense 5′-TGGAGCTTCAGAAGCTCAACACCA-3′ and antisense
5′-ATCTCGTTGTCTGAGTACCAGTCC-3′; BSP, sense
5′-CAGAGGAGGCAAGAGTCACT-3′ and antisense
5′-CTGTCTGGGTGCCAACACTG-3′; OCN, sense 5′-AAGCAGGAGGGCAATAAGGT-3′
and antisense 5′-CAAGCAGGGTTAAGCTCACA-3′. For control purposes, the
levels of 18S mRNA were measured using the following primer: Sense
5′-CGGCTACCACATCCAAGGAA-3′ and antisense 5′-CCGGCGTCCCCTCTTAATC-3′.
The PCR reaction was performed as follows: 25 cycles at 94°C for 30
sec, 56°C for 30 sec and 72°C for 45 sec. Following amplification,
the PCR products were analyzed on a 1.5% agarose gel stained with
ethidium bromide. mRNA levels were semi-quantified using Multigauge
software (version 3.1; Fujifilm Corporation, Tokyo, Japan) with 18S
as the reference gene.
Mineralization assay
Alizarin Red staining and von Kossa staining of the
MSC cultures were performed 21 days following the MSM (20 mM),
BMP-2 (100 ng/ml) and combination treatments to evaluate the
effects of MSM and BMP-2 on the matrix mineralization of MSCs. The
cells were washed with phosphate buffered saline (PBS), fixed with
4% paraformaldehyde in PBS for 15 min, and rinsed with distilled
water three times. For Alizarin Red S staining, 40 mM Alizarin Red
S (pH 4.2) stain was added to the plates and incubated for 10 min
at room temperature; the plates were then rinsed three times with
distilled water and washed with PBS to reduce non-specific
staining. For the von Kossa staining, the fixed cells were stained
with freshly prepared 5% silver nitrate under ultraviolet light for
1 h to detect phosphate deposits. Background color was removed
using 5% sodium thiosulfate, and the cells were rinsed three times
with distilled water. The cells were observed using phase contrast
microscopy (Olympus DP71; Olympus Corporation, Tokyo, Japan), and
images were captured using DPC controller software and a Nikon
digital camera (Nikon, Tokyo, Japan).
Immunofluorescence microscopy
Immunofluorescence microscopy of the MSC cultures
was performed 5 days following the MSM (20 mM), BMP-2 (100 ng/ml),
and combination treatments to evaluate the effects of MSM and BMP-2
on Smad1/5/8. The cells were permeabilized with Triton X-100 (0.1%)
for 15 min on ice, and blocked with 1% horse serum (Thermo Fisher
Scientific, Inc.) in PBS for 1 h, followed by incubation in a
closed humid chamber with the p-Smad1/5/8 antibody in wash buffer
(1% horse serum and 0.1% triton X-100 in PBS), and subsequent
incubation with the Alexa Fluor 594 (rabbit) secondary antibody,
(Invitrogen; Thermo Fisher Scientific, Inc.) in wash buffer. For
nuclear staining, the cells were incubated with TO-PRO3 (Molecular
Probes; Thermo Fisher Scientific, Inc.) in wash buffer for 5 min
and rinsed with wash buffer. The slides were then observed under a
fluorescence microscope (LSM710; Carl Zeiss, Inc., Oberkochen,
Germany).
Statistical analysis
The results of the experiments are expressed as the
mean ± standard error of the mean. Statistical analysis was
performed using one-way analysis of variance on the SAS program
(version 9.3; SAS Institute, Inc., Cary, NC, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
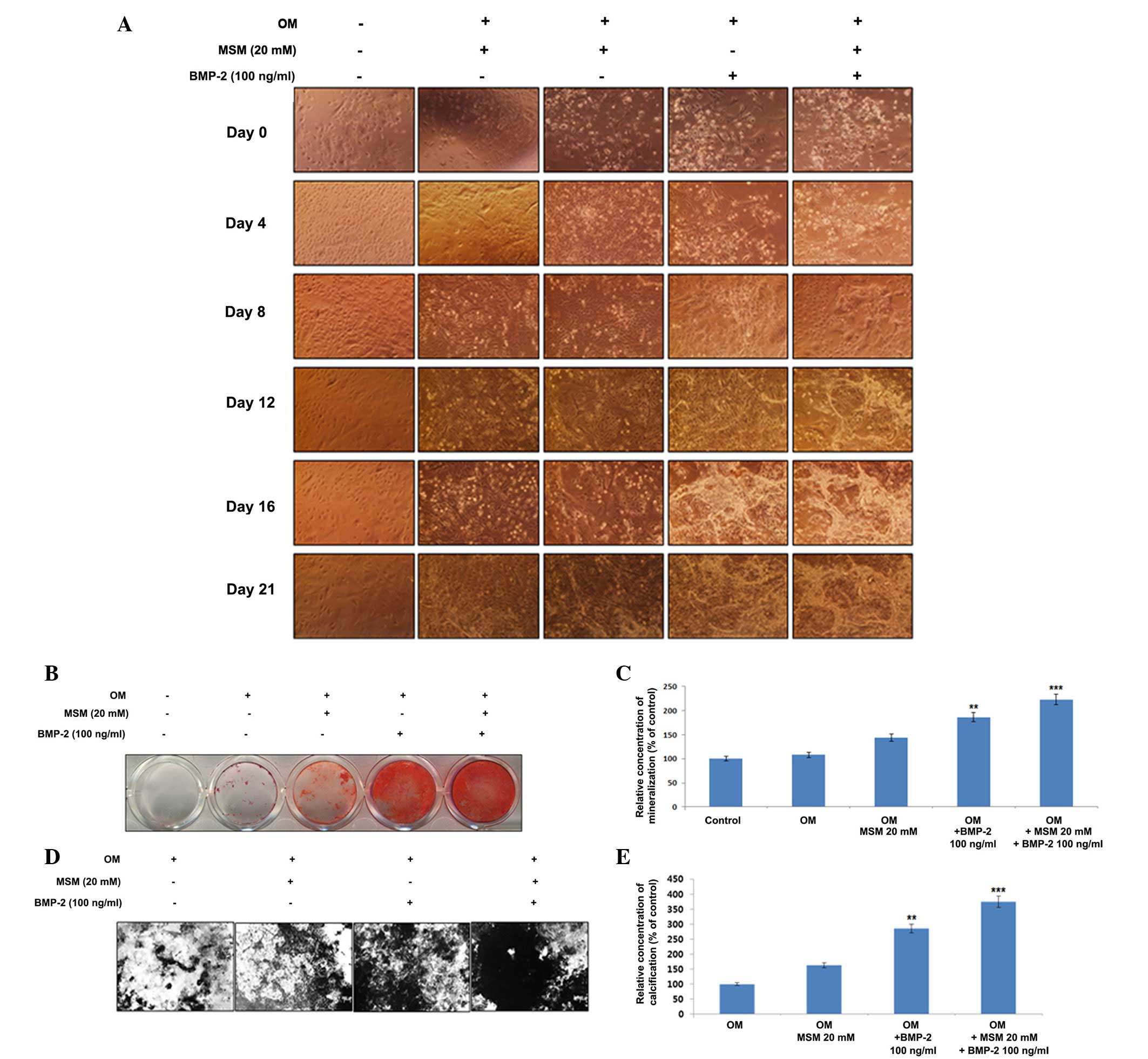
Osteogenic differentiation and
mineralization are increased in MSCs treated with a combination of
MSM and BMP-2
The present study examined the combination effects
of MSM and BMP-2 on the differentiation of primary bone marrow
MSCs. The cells were cultured with the combination of MSM and BMP-2
for 21 days, following which alterations in morphology were
observed (Fig. 1A). Alizarin Red
staining was used to visualize the precipitated calcium
incorporated into the matrix; it stains intracellular calcium,
calcium-binding proteins and proteoglycans, and is thus useful for
evaluating the early phases of differentiation. Extracellular
calcium deposition by mature osteoblasts was confirmed using von
Kossa staining, which detects the phosphate of the calcium
phosphate from the secreted matrix. The cells undergoing osteoblast
differentiation and mineralization stained positive, enabling
comparison in the third week of differentiation. As shown in
Fig. 1B, compared with the cells
treated with BMP-2 alone, the combination of MSM and BMP-2
increased the area of mineralization, visualized by the Alizarin
Red staining for calcium. The relative concentration of
mineralization with respect to the control is shown in Fig. 1C. Similar results were verified by
the von Kossa staining (Fig. 1D).
The percentage of calcification relative to the control is shown in
Fig. 1E.
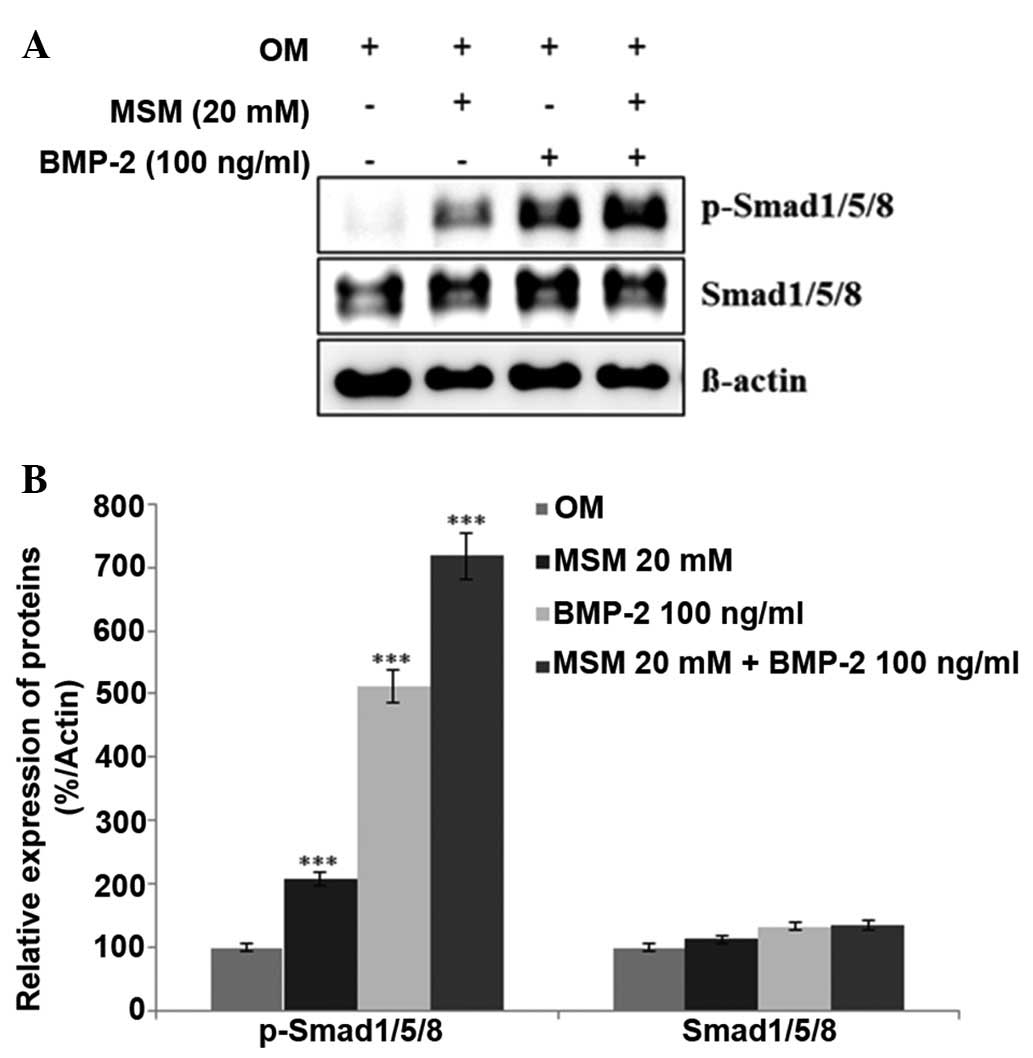
Expression levels of BMP
signaling-associated proteins are increased in MSCs treated with a
combination of MSM and BMP-2
The expression levels of various proteins involved
in BMP signaling were assessed using western blot analysis. It was
hypothesized that treatment with a combination of MSM and BMP-2
leads to a more marked increase in the expression levels of
Smad1/5/8 in MSCs, compared with treatment with BMP-2 alone. As
shown in Fig. 2, the combination
treatment increased the expression of p-Smad1/5/8 more markedly,
compared with the MSCs treated with either BMP-2 or MSM alone. This
result confirmed that the combination of MSM and BMP-2 involved the
BMP signaling pathways in the MSCs.
mRNA expression levels of Runx2 and
osteogenic markers are promoted in MSCs treated with a combination
of MSM and BMP-2
The present study analyzed the mRNA expression
levels of Runx2, ALP, BSP and OCN in the different treatment
groups. Runx2 is a key transcription factor associated with
osteoblast differentiation. During the differentiation process, the
expression of the early-stage osteogenic differentiation marker,
ALP, middle-stage markers, BSP and OPN, and late-stage marker, OCN,
increased. However, RT-PCR analysis of the expression of OPN showed
no change, compared with the control. The mRNA levels of Runx2 and
osteogenic-specific markers in the MSCs were increased following
combined treatment with MSM and BMP-2, compared with the levels
following the individual treatments (Fig. 3).
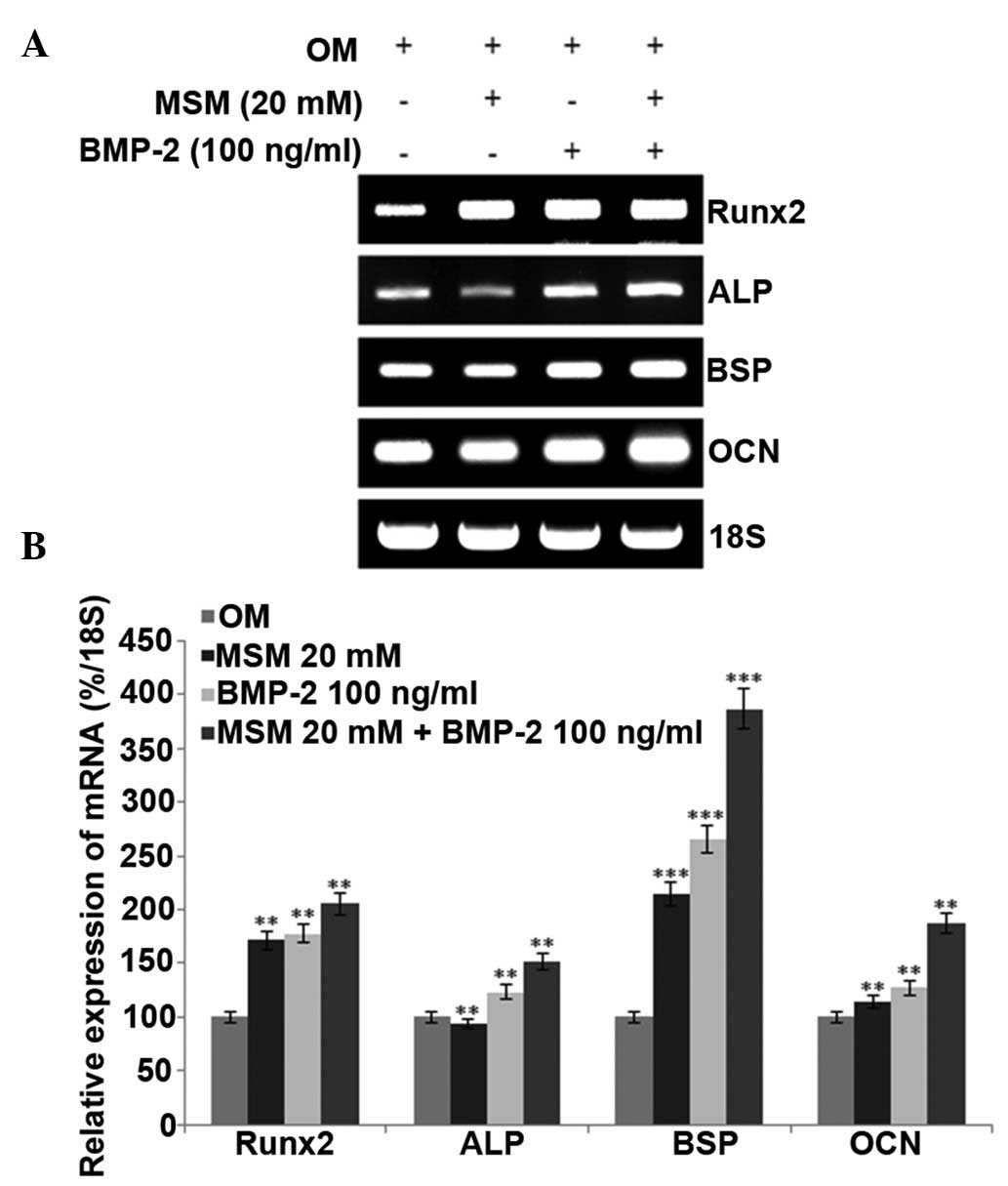 | Figure 3Combined treatment with MSM and BMP-2
enhances the expression of osteoblast differentiation markers in
MSCs. (A) Bone marrow MSCs were cultured in OM, and the mRNA
expression levels of ALP and Runx2 were detected at day 5, BSP was
detected at day 14, and OCN was detected at day 21 following
treatment with MSM and BMP-2. Reverse transcription-polymerase
chain reaction analysis was performed using primers for ALP, BSP,
OCN, Runx2 and 18S. Total RNA was isolated from the MSCs using an
RNeasy kit. 18S was used as a control. (B) mRNA levels of ALP, BSP,
OCN and Runx2 were determined using densitometric analysis and
normalized to the level of 18S. Data shown are representative of
three independent experiments and expressed as the mean ± standard
error of the mean. One-way analysis of variance was used to detect
significant differences (**P<0.01 and
***P<0.001, vs. OM). MSCs, mesenchymal stem cells;
OM, osteogenic medium; MSM, methylsulfonylmethane; BMP-2; bone
morphogenetic protein-2; Runx2, Runt-related transcription factor
2; ALP, alkaline phosphatase; BSP, bone sialoprotein; OCN,
osteocalcin. |
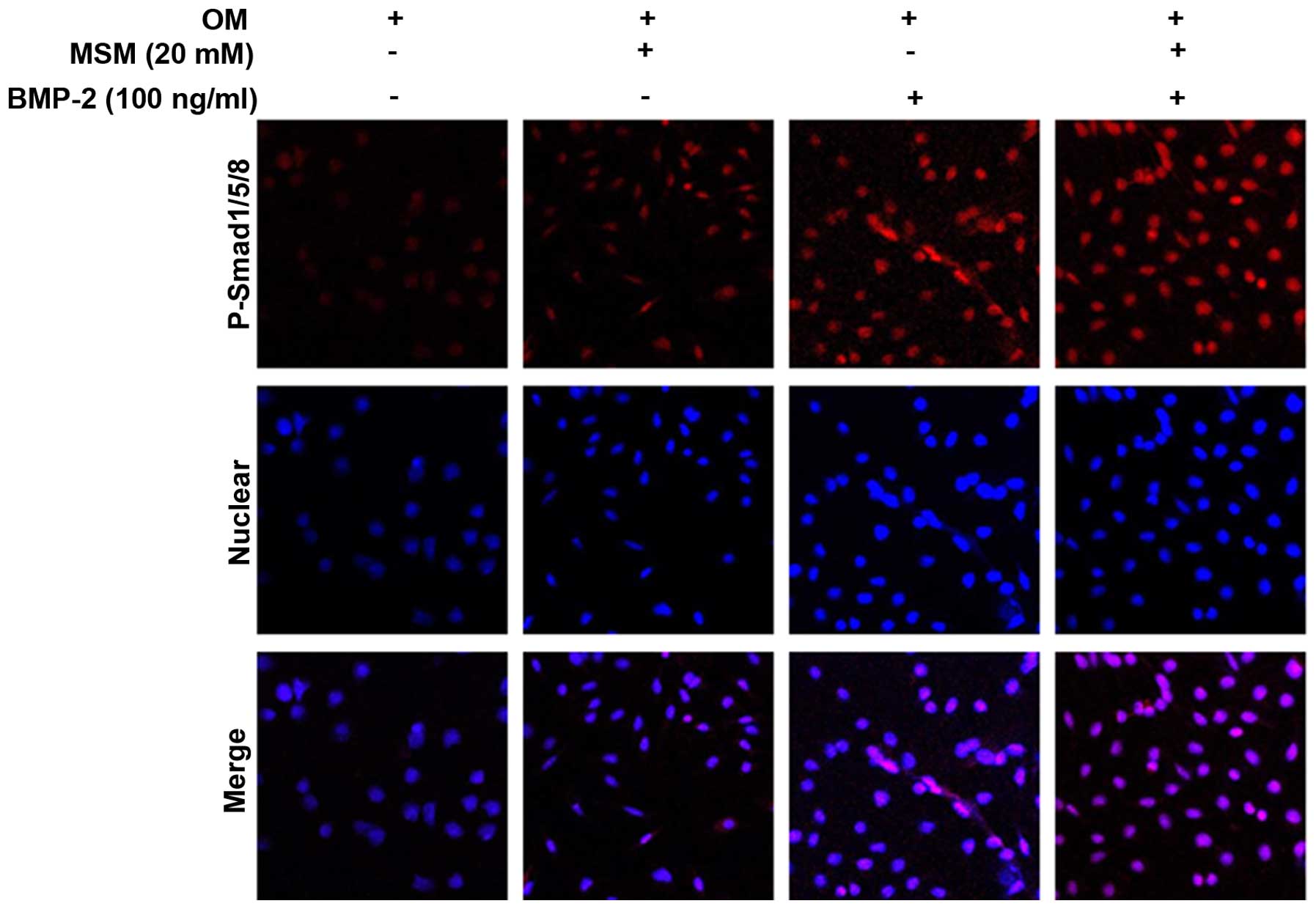
Enhanced complex translocation to the
nucleus and changes in the expression of target genes are
associated with combined treatment with MSM and BMP-2
Phosphorylation of the Smad1/5/8 protein enhances
the expression of osteoblastic marker genes. The present study
performed p-Smad1/5/8 localization analysis using
immunofluorescence microscopy in the MSCs. The cells were cultured
with a combination of MSM and BMP-2 for 5 days, as shown in
Fig. 4, and this combination
resulted in the upregulation of p-Smad1/5/8 localization from the
cytosol to the nucleus.
Discussion
MSM is a natural organic sulfur from pine tree
extract, which is consumed as a 'functional food' with no reported
side-effects (15). In a previous
study, it was reported that STAT5b is involved in the MSM-induced
osteoblastic differentiation of MSCs and is essential in the
MSM-induced GH signaling of C3H10T1/2 cells (18). In addition, it has been found that
Hwanggeumchal sorghum extract induces the expression of BMP-7 in
osteoblastic cells (3). BMP-2 is
important in bone formation, bone regeneration, fracture healing
and osteophyte formation (20–23);
thus, the present study hypothesized that the combination of MSM
and BMP-2 affects the gene expression of osteoblast markers and
osteoblastic differentiation.
In the present study, the effect of MSM on BMP-2
activity in the differentiation of MSCs into osteoblasts was
investigated. Among BMPs, BMP-2 is involved in bone mineralization,
an essential step in the late stage of osteoblast differentiation
(24). BMP-2 signals by binding
with BMP receptor type II and phosphorylates BMP receptor type I.
This phosphorylation activates Smad1/5/8, an important protein for
the activation of downstream proteins responsible for
differentiation.
In the present study, an increase in the
phosphorylation of Smad1/5/8 was observed when MSM and BMP-2 were
used as a combination treatment. This combination increased the
expression of p-Smad1/5/8 in MSCs more markedly, compared with
either BMP-2 or MSM alone, indicting that the ability of MSM to
enhance the effects of BMP-2 in osteoblast differentiation.
Interleukin-6R can trigger the phosphorylation of
Smad4, which leads to formation of a heterocomplex with Smad1/5/8
and translocation to the nucleus. In the nucleus, this Smad complex
binds with DNA and other transcription factors, including Dlx5 and
Runx2, promoting their activity (25,26).
This binding activates the transcription and translation of
multiple proteins involved in the differentiation of MSCs into
osteoblasts. Runx2 regulates its target genes, including OPN, BSP
and OCN, by binding and transactivating the promoter region
(27). The role of Runx2 in
regulating target genes is not always positive, and negative
regulation by Runx2 has also been reported (27).
To confirm the ability of MSM to enhance
BMP-2-mediated osteoblast differentiation, the present study
analyzed multiple molecular markers. The combination treatment
resulted in transcriptional upregulation of the receptor molecules
involved in BMP-2 signaling. Additionally, ALP, BSP and OCN were
markedly increased following the combination treatment, compared
with the effects of BMP-2 or MSM alone. These elevated expression
levels of the early-stage, middle-stage and late-stage marker
genes, ALP, BSP and OCN, respectively, suggested that MSM enhanced
the effects of BMP-2 in MSCs.
Osteoblast differentiation is estimated by the rate
of calcium mineralization in MSCs. The role of BMP-2 in bone
mineralization has been previously reported, and the present study
confirmed the ability of MSM to enhance BMP-2 activity. This
ability was further confirmed in the present study using
calcification assays. Bone mineralization/calcification is the
process mediated by osteoblastic cells, and the level of calcium
deposition can be used to detect the level of osteoblastic cells
differentiation from MSCs (28).
In the present study, the rates of mineralization of the MSCs
following treatment with BMP-2, MSM, and the two in combination
were examined using the Alizarin Red and von Kossa staining assays.
The results showed a high rate of mineralization in the
combination-treated group, compared with either BMP-2 or MSM alone.
Morphological analysis of the MSCs at 21 days also showed calcium
deposition, which indicated differentiation. The calcium
mineralization was suggested the ability of MSM to synergize with
BMP-2 to induce osteoblast differentiation in the MSCs. The
analysis of p-Smad1/5/8 using confocal microscopy confirmed the
osteoblast differentiation.
In conclusion, the present study demonstrated that
the combination of MSM and BMP-2 can be used to stimulate BMP-2
signaling, thereby promoting osteoblast differentiation. MSM
positively regulated BMP-2-induced osteoblastic differentiation via
Smad1/5/8, suggesting that MSM has a significant role in bone
formation. Thus, the results of the current study that MSM may
potentially be useful as a candidate therapeutic agent for
bone-depleting conditions, such as osteoporosis, were osteoblast
differentiation and bone mineralization are impaired. Further
investigations are required to elucidate the implications of
MSM-induced BMP-2 signaling using in vivo models of bone
resorption mechanisms.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Science, ICT & Future Planning (grant
no. 2013R1A1A3013276) and was partially supported by the Next
Generation BioGreen 21 Program RDA, Republic of Korea (grant no.
PJ001104401).
Abbreviations:
|
MSM
|
methylsulfonylmethane
|
|
MSC
|
mesenchymal stem cell
|
|
BMP-2
|
bone morphogenetic protein 2
|
|
Runx2
|
Runt-related transcription factor
2
|
References
|
1
|
Hadjidakis DJ and Androulakis II: Bone
remodeling. Ann NY Acad Sci. 1092:385–396. 2006. View Article : Google Scholar
|
|
2
|
Zuo C, Huang Y, Bajis R, Sahih M, Li YP,
Dai K and Zhang X: Osteoblastogenesis regulation signals in bone
remodeling. Osteoporos Int. 23:1653–1663. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Joung YH, Lim EJ, Darvin P, Jang JW, Park
KD, Lee HK, Kim HS, Cho BW, Park T, Chung S, et al: Hwanggeumchal
sorghum extract enhances BMP7 and GH signaling through the
activation of Jak2/STAT5B in MC3T3-E1 osteoblastic cells. Mol Med
Rep. 8:891–896. 2013.PubMed/NCBI
|
|
4
|
Wozney JM: The potential role of bone
morphogenetic proteins in periodontal reconstruction. J
Periodontol. 66:506–510. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen D, Zhao M and Mundy GR: Bone
morphogenetic proteins. Growth Factors. 22:233–241. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dieudonne FX, Sévère N, Biosse-Duplan M,
Weng JJ, Su Y and Marie PJ: Promotion of osteoblast differentiation
in mesenchymal cells through Cbl-mediated control of STAT5
activity. Stem Cells. 31:1340–1349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schroeder TM, Jensen ED and Westendorf JJ:
Runx2: A master organizer of gene transcription in developing and
maturing osteoblasts. Birth Defects Res C Embryo Today. 75:213–225.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Long F: Building strong bones: Molecular
regulation of the osteoblast lineage. Nat Rev Mol Cell Biol.
13:27–38. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katagiri T, Yamaguchi A, Ikeda T, Yoshiki
S, Wozney JM, Rosen V, Wang EA, Tanaka H, Omura S and Suda T: The
non-osteogenic mouse pluripotent cell line, C3H10T1/2, is induced
to differentiate into osteoblastic cells by recombinant human bone
morphogenetic protein-2. Biochem Biophys Res Commun. 172:295–299.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang EA, Israel DI, Kelly S and Luxenberg
DP: Bone morpho-genetic protein-2 causes commitment and
differentiation in C3H10T1/2 and 3T3 cells. Growth Factors.
9:57–71. 1993. View Article : Google Scholar
|
|
11
|
Puleo DA: Dependence of mesenchymal cell
responses on duration of exposure to bone morphogenetic protein-2
in vitro. J Cell Physiol. 173:93–101. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ducy P, Zhang R, Geoffroy V, Ridall AL and
Karsenty G: Osf2/Cbfa1: A transcriptional activator of osteoblast
differentiation. Cell. 89:747–754. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gautschi OP, Frey SP and Zellweger R: Bone
morphogenetic proteins in clinical applications. ANZ J Surg.
77:626–631. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morton JI and Siegel BV: Effects of oral
dimethyl sulfoxide and dimethyl sulfone on murine autoimmune
lymphoproliferative disease. Proc Soc Exp Biol Med. 183:227–230.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Horváth K, Noker PE, Somfai-Relle S,
Glávits R, Financsek I and Schauss AG: Toxicity of
methylsulfonylmethane in rats. Food Chem Toxicol. 40:1459–1462.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim YH, Kim DH, Lim H, Baek DY, Shin HK
and Kim JK: The anti-inflammatory effects of methylsulfonylmethane
on lipopolysaccharide-induced inflammatory responses in murine
macrophages. Biol Pharm Bull. 32:651–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caron JM, Bannon M, Rosshirt L, Luis J,
Monteagudo L, Caron JM and Sternstein GM: Methyl sulfone induces
loss of metastatic properties and reemergence of normal phenotypes
in a metastatic cloudman S-91 (M3) murine melanoma cell line. PLoS
One. 5:e117882010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Joung YH, Lim EJ, Darvin P, Chung SC, Jang
JW, Do Park K, Lee HK, Kim HS, Park T and Yang YM: MSM enhances GH
signaling via the Jak2/STAT5b pathway in osteoblast-like cells and
osteoblast differentiation through the activation of STAT5b in
MSCs. PLoS One. 7:e474772012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Darvin P, Joung YH and Yang YM:
JAK2-STAT5B pathway and osteoblast differentiation. JAKSTAT.
2:e249312013.
|
|
20
|
Takaoka K, Nakahara H, Yoshikawa H,
Masuhara K, Tsuda T and Ono K: Ectopic bone induction on and in
porous hydroxyapatite combined with collagen and bone morphogenetic
protein. Clin Orthop Relat Res. 250–254. 1988.PubMed/NCBI
|
|
21
|
Nakase T, Nomura S, Yoshikawa H, Hashimoto
J, Hirota S, Kitamura Y, Oikawa S, Ono K and Takaoka K: Transient
and localized expression of bone morphogenetic protein 4 messenger
RNA during fracture healing. J Bone Miner Res. 9:651–659. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakase T, Miyaji T, Tomita T, Kaneko M,
Kuriyama K, Myoui A, Sugamoto K, Ochi T and Yoshikawa H:
Localization of bone morphogenetic protein-2 in human
osteoarthritic cartilage and osteophyte. Osteoarthritis Cartilage.
11:278–284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaneko K, Higuchi C, Kunugiza Y, Yoshida
K, Sakai T, Yoshikawa H and Nakata K: Hyaluronan inhibits
BMP-induced osteoblast differentiation. FEBS Lett. 589:447–454.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prall WC, Haasters F, Heggebö J, Polzer H,
Schwarz C, Gassner C, Grote S, Anz D, Jäger M, Mutschler W and
Schieker M: Mesenchymal stem cells from osteoporotic patients
feature impaired signal transduction but sustained osteoinduction
in response to BMP-2 stimulation. Biochem Biophys Res Commun.
440:617–622. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shea CM, Edgar CM, Einhorn TA and
Gerstenfeld LC: BMP treatment of C3H10T1/2 mesenchymal stem cells
induces both chondrogenesis and osteogenesis. J Cell Biochem.
90:1112–1127. 2013. View Article : Google Scholar
|
|
26
|
Kim HJ, Park JW, Lee KH, Yoon H, Shin DH,
Ju UI, Seok SH, Lim SH, Lee ZH, Kim HH and Chun YS: Plant
homeodomain finger protein 2 promotes bone formation by
demethylating and activating Runx2 for osteoblast differentiation.
Cell Res. 24:1231–1249. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Westendorf JJ, Zaidi SK, Cascino JE,
Kahler R, van Wijnen AJ, Lian JB, Yoshida M, Stein GS and Li X:
Runx2 (Cbfa1, AML-3) interacts with histone deacetylase 6 and
represses the p21 (CIP1/WAF1) promoter. Mol Cell Biol.
22:7982–7992. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mbalaviele G, Sheikh S, Stains JP, Salazar
VS, Cheng SL, Chen D and Civitelli R: Beta-catenin and BMP-2
synergize to promote osteoblast differentiation and new bone
formation. J Cell Biochem. 94:403–418. 2005. View Article : Google Scholar
|