Introduction
Congenital heart disease (CHD) is a common, complex
illness with high rates of morbidity and mortality, the genetic
etiology of which emains to be fully elucidated (1,2).
Worldwide, the incidence of moderate and severe CHD is ~6/1,000
live births, however, if small muscular ventricular septal defects
(VSDs) and other minor lesions are included, the incidence is
~75/1,000 live births (3).
Appropriately, 1% of patients with CHD require intervention
(4), and ~13% of patients with CHD
show recognizable chromosomal variants (5,6). The
majority of adult patients with show a variety of cardiac
complications, including coronary heart disease, arrhythmias and
heart failure (7). Although
extensive genetic investigations and high resolution technologies
have revealed subtle defects in familiar and sporadic cases of CHD
(2,8), the subtle genetic causes and
molecular mechanisms of CHD remain to be fully elucidated.
In embryonic development, the heart is the first
formed organ, which is strictly controlled by gene regulatory
networks, including transcription factors, signaling pathways,
epigenetic factors and microRNAs (2,9,10).
During the last few decades, several cardiac enriched transcription
factors have been identified, and a variety of CHD-causing
mutations have been identified in those factor genes, which provide
molecular markers and models for the analysis of heart development
and the molecular mechanisms underlying CHD (6,11).
For example, NKX2-5 is a member of the NK-2 class homeodo main
proteins, and is among the earliest markers of cardiac
specification. Drosophila embryos fail to form a heart in
the presence of a mutant NKX gene (12) and, if the functions of the NKX
family members are inhibited, vertebrate embryos also fail to form
a heart (13). The zinc finger
binding proteins, GATA binding protein (GATA)-4, -5 and -6, are
also members of cardiac-enriched transcription factors, and are
expressed early in heart development, being involved in the initial
steps of cardiomyocyte differentiation (14). Mutations in GATA-4 have been
identified to be associated with CHD-(15), and a variety of other CHD causing
gene mutations have been identified, includingCITED2 (16), CFC1 (17) and TBX1 (18). These gene are critical in cardiac
development; mutations in these genes lead to cardiovascular
malformations and contribute to CHD (19).
In non-precardiac mesoderm, the expression of the
heart-specific transcription factors, NKX2-5 and GATA-4, can be
induced by BMP-2 and -4, and inhibition of BMP-2 and -4 signaling
inhibits the late expression of NKX2-5 and affects the cardiac
differentiation (20). In
zebrafish, the BMPs also have a direct role in development of heart
tube looping (21), and in
Xenopus, BMP-2 and -4 are broadly expressed in the anterior
endoderm and cardiac mesoderm, prior to cardiac differentiation in
heart development (22–24). The inhibition of BMP signaling in
mice lacking Sma6, leads to the development of hyperplasia in heart
valves and defects in the aorticopulmo nary septum (25).
BMP-2 and -4 have important functions at multiple
stages of cardiogenesis, particularly in the initial induction of
heart development. However, the exact nature of these roles, and
the association between BMP-2 and -4, with CHD remains to be
elucidated. To determine whether there is an association between
BMP-2 and -4, and CHD, the gene sequences of BMP-2 and -4 were
compared between 230 Chinese Han patients with CHD and 160 control
individuals, focusing on the rs1049007, rs235768 and rs17563 SNP
genetic variations. The results revealed no correlation between
these candidate SNPs and the risk of CHD, and the BMP-2 rs1049007,
rs235768 and BMP-4 rs17563 genetic variations may not be risk
factors for CHD.
Materials and methods
Study population
A total of 230 patients with CHD and 160 control
subjects with no reported cardiac phenotypes were recruited for the
present study from the Second Affiliated Hospital of Harbin Medical
University (Harbin, China), as shown in Table I. Written informed consent was
obtained from each participant and the study was reviewed by the
Ethics Committee of Harbin Medical University, consistent with the
1975 Declaration of Helsinki (26,27).
The Ethics Committee approved the study. Detailed records on the
medical history, physical examination and chest X-ray examination,
electrocardiogram and ultrasonic echocardio gram were obtained.
 | Table IClinical characteristics of the study
populations. |
Table I
Clinical characteristics of the study
populations.
| Parameter | CHD | Control |
|---|
| Sample (n) | 230 | 160 |
| Male/Female
(n) | 142/88 | 105/55 |
| Age (years) | 16.18±10.22 | 7.88±11.96 |
BMP-2 rs1049007 and rs235768, and BMP-4
rs17563 SNP genotyping analysis and statistical methods
Whole genomic DNA was extracted from peripheral
blood leukocytes using a QIAamp DNA Blood Mini Kit (cat. no. 51104;
Qiagen, Hilden, Germany) (28).
The genotypes for the rs1049007, rs235768 and rs17563 SNPs
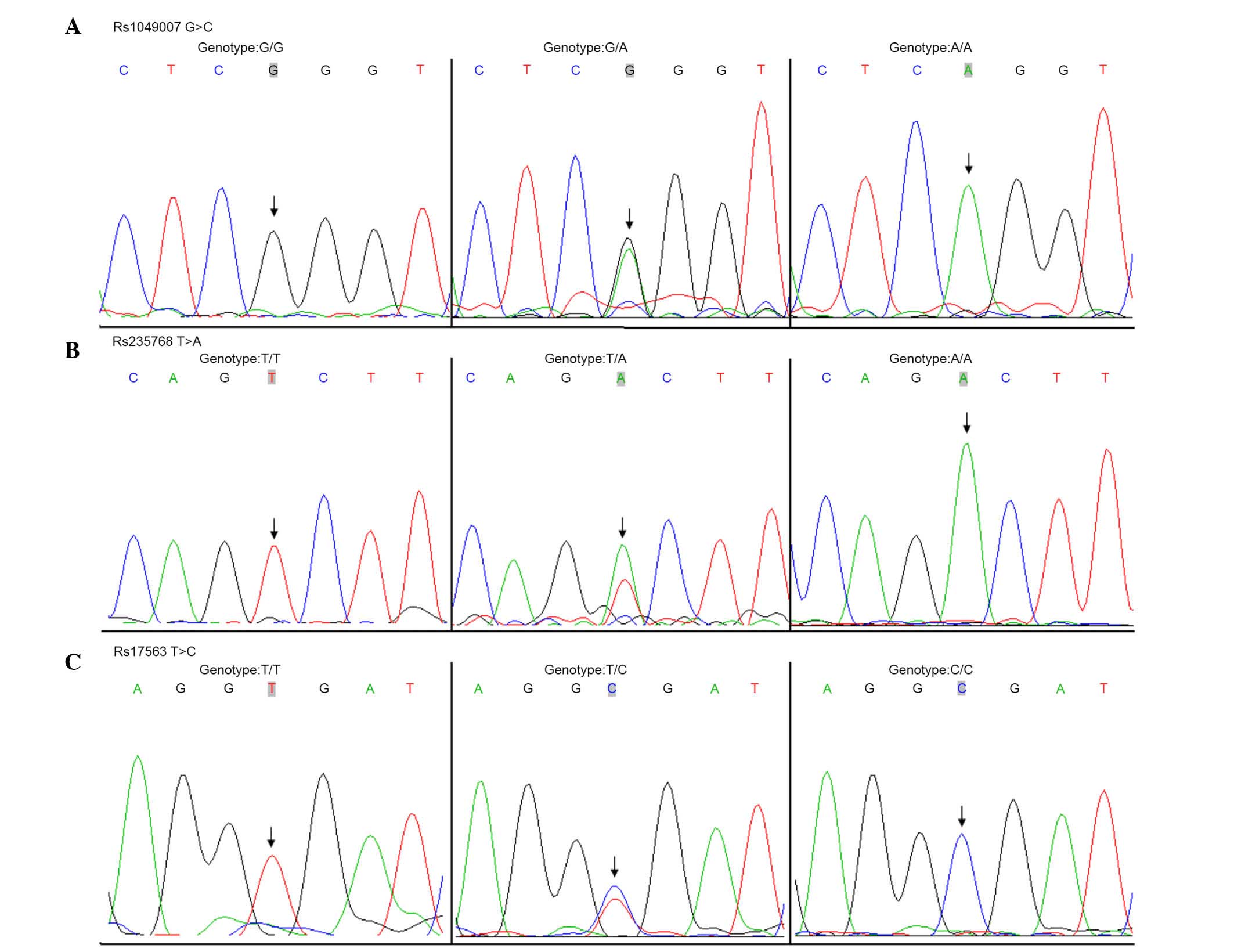
associated with the BMP-2 and -4 genes, respectively (Fig. 1), were determined using a two stage
method. First, rs1049007, rs235768 and rs17563 (Table II) were amplified using standard
procedures (2,6,29),
following which the PCR products were sequenced (Genewiz, Inc.,
South Plainfield, NJ, USA) to determine the genotype (Fig. 2). The statistical analyses were
performed using χ2 tests (descriptive statistic
crosstalk) to calculate the odds ratios and P values, implemented
using SPSS software (version 13.0; SPSS, Inc., Chicago, IL, USA).
In addition, Online Encyclopedia for Genetic Epidemiology studies
(OEGE; http://www.oege.org/software) online
software was used to perform the Hardy Weinberg equilibrium test
for the CHD and control populations.
 | Table IIPolymerase chain reaction primers
used for BMP genotyping sequence analysis. |
Table II
Polymerase chain reaction primers
used for BMP genotyping sequence analysis.
| Gene | Single nucleotide
polymorphism | Primer | Size (bp) | Temperature
(°C) |
|---|
| BMP2 | rs1049007 | Forward
CGGGACCCGCTGTCTTCT | 455 | 60.5 |
| | Reverse
TGGAAACGTCCGCTGGTG | | |
| rs235768 | Forward
CCCACGGAGGAGTTTATC | 275 | 52.5 |
| | Reverse
GCCACTTCCACCACGAAT | | |
| BMP4 | rs17563 | Forward
CCCCACTTATCTGCTCCT | 500 | 52.8 |
| | Reverse
AGTTTGGCTGCTTCTCCC | | |
Multiple sequence alignment
From the National Center for Biotechnology
Information website (http://www.ncbi.nlm.nih.gov/), the BMP-2 and -4
protein sequences of various species were obtained. Using Vector
NTI software (Suite 9; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), multiple-sequence alignments of these proteins were
performed.
Results
Patients
Clinical diagnosis of the recruited patients was
confirmed at the Second Affiliated Hospital of Harbin Medical
University. There were no histories of other systemic abnormalities
in the patients with CHD, and their mothers had no history of
taking medicines or contracting infections during pregnancy
(30).
BMP genotyping and statistical
analysis
To confirm the hypothesis that there are possible
associations between BMPs, which inhibit the late expression of
NKX2-5 and affects cardiac differentiation (20), and CHD, the present study performed
SNP analyses. No significant differences were found between the
patients with CHD and the 160 CHD free control individuals in
relation to the risk of CHD (Tables
III and IV). The present
study also performed Hardy-Weinberg equilibrium tests for the
patients with CHD and control individuals, and found that the
χ2 value was 3.06 for the patients with CHD and 2.86 for
the CHD free control individuals. The results were concordant with
the Hardy-Weinberg equilibrium.
 | Table IIIGenotype and allele frequencies of
the rs1049007, rs235768 and rs17563 SNPs in 230 Chinese Han
patients with CHD and 160 non CHD control individuals. |
Table III
Genotype and allele frequencies of
the rs1049007, rs235768 and rs17563 SNPs in 230 Chinese Han
patients with CHD and 160 non CHD control individuals.
| SNP | Group | n | Genotype frequency,
n (%) | Allele frequency, n
(%) |
|---|
| rs1049007 | | | G/G | A/G | A/A | G | A |
| CHD | 230 | 155 (67.4) | 68 (29.6) | 7 (3.0) | 378 (82.2) | 82 (17.8) |
| Control | 160 | 103 (64.4) | 54 (33.8) | 3 (1.9) | 260 (81.3) | 60 (18.8) |
| rs235768 | | | T/T | T/A | A/A | T | A |
| CHD | 230 | 142 (61.7) | 80 (34.8) | 8 (3.5) | 364 (79.1) | 96 (20.9) |
| Control | 160 | 97 (60.6) | 57 (35.6) | 6 (3.8) | 251 (78.4) | 69 (21.6) |
| rs17563 | | | T/T | T/C | C/C | T | C |
| CHD | 230 | 114 (49.6) | 96 (41.7) | 20 (8.7) | 324 (70.4) | 136 (29.6) |
| Control | 160 | 85 (53.1) | 62 (38.8) | 13 (8.1) | 232 (72.5) | 88 (27.5) |
 | Table IVrs1049007, rs235768 and rs17563 SNPs
within BMP 2 and 4 are not associated with risk of congenital heart
disease. |
Table IV
rs1049007, rs235768 and rs17563 SNPs
within BMP 2 and 4 are not associated with risk of congenital heart
disease.
| Genotyped SNP | Gene |
Genotype/allele | Pearson's
χ2
| Pearson's R
|
|---|
| χ2 | Min counta | df | Asymp. P-value
(2-sided) | Pearson's
R-value | Asymp. SEb | Approx. T
valuec | Approx. P
valued |
|---|
| rs1049007 | BMP2 | Genotype | 1.160 | 4.10 | 2 | 0.560 | 0.017 | 0.050 | 0.337 | 0.737d |
| | Allele | 0.108 | 58.26 | 1 | 0.742 | 0.012 | 0.036 | 0.329 | 0.743d |
| rs235768 | BMP2 | Genotype | 0.568 | 5.74 | 2 | 0.972 | 0.012 | 0.051 | 0.239 | 0.811d |
| | Allele | 0.054 | 67.69 | 1 | 0.816 | 0.008 | 0.036 | 0.233 | 0.816d |
| rs17563 | BMP4 | Genotype | 0.479 | 13.54 | 2 | 0.787 | 0.032 | 0.051 | 0.623 | 0.534d |
| | Allele | 0.393 | 91.90 | 1 | 0.531 | 0.022 | 0.036 | 0.626 | 0.531d |
Conservation of proteins in
evolution
The comparison of the BMP-2 and -4 protein sequences
between different species included birds, fishes, rodents and
mammals, including Homo sapiens, Pan troglodytes and
Macaca mulatta. The results of the multiple sequence alignment
analysis showed that the conservation of the rs235768 and rs17563
variations were high; the rs1049007 variation did not alter the
protein sequence. The conservation of the 190Ser and 152Val
residues in BMP-2 and -4, respectively, were high and were located
in highly conserved regions of the proteins (Fig. 3).
Discussion
BMP4, a member of the transforming growth factor
(TGF) β family, is capable of causing human embryonic stem cells
(hESCs) to differentiate, and this differentiation can occur
without extensive generation of mesoderm and endoderm (31). When inhibiting the fibroblast
growth factor (FGF)2 pathway and maximizing BMP4 signaling, BMP4
can direct the hESCs to differentiate towards syncytiotrophoblasts
(32,33). The differentiation program induced
by BMP4 involves rapid induction, and occurs prior to the
expression of caudal type homeobox 2 and several other mesoderm
marker genes (32,34). BMP2 is a homodimeric
disulfide-bonded protein and is also a member of the TGF-β family
(35–37). Despite substantial differences in
amino acid sequences with cystine knot growth factors, it has a
similar monomer structure with the factor (38), and has a similar dimer structure
with the TGF-β family (39,40).
Several previous studies have shown that BMP-2 and
-4 are implicated in the formation of the heart from the overlying
mesoderm (41). The expression of
the early cardiac markers, NKX2-5 and GATA-4, can be induced by
BMP-2 within anterior mesodermal cells, which are located close to
the heart forming region (20,42).
Following pretreatment with FGFs, BMP-4 can increase the activity
of the posterior mesoderm cells (20,43).
If the expression of BMP-2 and -4 are inhibited, cardiac differ
entiation can be inhibited (20,44).
Therefore, BMP-2 and -4 are essential in cardiogenesis, and the
inductive functions of BMP-2 and -4 may result from the
interactions between BMP and other extracellular signaling
molecules (45). BMP signaling is
important in the formation of different early cell types from
hESCs, including the mesoderm (46), endoderm (18) and trophoblasts (47). However, BMP signaling is not
required for the expression of early markers of cardiac
specification, including NKX2-5, and GATA-4, -5 and -6 (41,48).
Therefore, the present study focussed on the rs1049007, rs235768
and rs17563 SNP genetic variations, the aim of which was to analyze
the association between BMP-2 and -4, and CHD.
Previously, Qian et al found that the
rs762642 polymorphism in BMP4 may increase susceptibility to
sporadic CHD, and that the polymorphism contributes to the
susceptibilities to atrial septal defects and VSDs (49). In addition, it was found that the
rs17563 SNP in BMP4 was not associated with the risk of CHD or
types of CHD (49). These previous
results do not conflict with the results of the present study. The
rs762642 SNP is located within the intron between the first and
second exon of BMP4, which may affect the function of the promoter
and enhancer regions of the gene. Although the rs17563 SNP within
the fourth exon resulted in an amino acid change within the CDS
region of the gene, the SNP may not be as important as the rs762642
SNP for the function of BMP4.
In conclusion, the present study compared the gene
sequences of BMP-2 and -4 between 230 Chinese Han patients with CHD
and 160 control individuals, focusing on the rs1049007, rs235768
and rs17563 SNP genetic variations. The meta analysis assisted in
clarifying the associations between these SNPs with CHD, the
results of which revealed that there were no correlations between
these candidate SNPs and the risk of CHD in the Chinese population.
The risk of developing CHD in individuals with these variants of
the BMP-2 and -4 genes may be low; the results of the present study
demonstrated that these variations in the BMP-2 and -4 genes were
not associated with CHD in the Chinese Han population.
Acknowledgments
This study was supported by a grant from the
Heilongjiang Innovation Research Foundation for Graduate Studies
(grant no. YJSCX2014-10HYD) to Dr Fei-Feng Li and grants from the
National Natural Science Foundation of China (grant nos.
NSFC81271786, 81110378, 30970119 and 81030029) to Dr Shu Lin
Liu.
References
|
1
|
Zomer AC, Uiterwaal CS, van der Velde ET,
Tijssen JG, Mariman EC, Verheugt CL, Vaartjes I, Pieper PG,
Meijboom FJ, Grobbee DE and Mulder BJ: Mortality in adult
congenital heart disease: Are national registries reliable for
cause of death? Int J Cardiol. 152:212–217. 2011. View Article : Google Scholar
|
|
2
|
Li FF, Zhou J, Zhao DD, Yan P, Li X, Han
Y, Li XS, Wang GY, Yu KJ and Liu SL: Characterization of SMAD3 gene
variants for possible roles in ventricular septal defects and other
congenital heart diseases. PLoS One. 10:e01315422015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoffman JI and Kaplan S: The incidence of
congenital heart disease. J Am Coll Cardiol. 39:1890–1900. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoffman JI, Kaplan S and Liberthson RR:
Prevalence of congenital heart disease. Am Heart J. 147:425–439.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pierpont ME, Basson CT, Benson DW Jr, Gelb
BD, Giglia TM, Goldmuntz E, McGee G, Sable CA, Srivastava D and
Webb CL; American Heart Association Congenital Cardiac Defects
Committee; Council on Cardiovascular Disease in the Young: Genetic
basis for congenital heart defects: Current knowledge: A scientific
statement from the American heart association congenital cardiac
defects committee, council on cardiovascular disease in the young:
Endorsed by the American academy of pediatrics. Circulation.
115:3015–3038. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng X, Zhou J, Li FF, Yan P, Zhao EY, Hao
L, Yu KJ and Liu SL: Characterization of nodal/TGF lefty signaling
pathway gene variants for possible roles in congenital heart
diseases. PLoS One. 9:e1045352014. View Article : Google Scholar
|
|
7
|
van der Bom T, Zomer AC, Zwinderman AH,
Meijboom FJ, Bouma BJ and Mulder BJ: The changing epidemiology of
congenital heart disease. Nat Rev Cardiol. 8:50–60. 2011.
View Article : Google Scholar
|
|
8
|
Richards AA and Garg V: Genetics of
congenital heart disease. Curr Cardiol Rev. 6:91–97. 2010.
View Article : Google Scholar :
|
|
9
|
Buckingham M, Meilhac S and Zaffran S:
Building the mammalian heart from two sources of myocardial cells.
Nat Rev Genet. 6:826–835. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Weerd JH, Koshiba Takeuchi K, Kwon C
and Takeuchi JK: Epigenetic factors and cardiac development.
Cardiovasc Res. 91:203–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fishman MC and Olson EN: Parsing the
heart: Genetic modules for organ assembly. Cell. 91:153–156. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Azpiazu N and Frasch M: Tinman and
bagpipe: Two homeo box genes that determine cell fates in the
dorsal mesoderm of Drosophila. Genes Dev. 7:1325–1340. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grow MW and Krieg PA: Tinman function is
essential for vertebrate heart development: Elimination of cardiac
differen tiation by dominant inhibitory mutants of the tinman
related genes, XNkx2 3 and XNkx2 5. Dev Biol. 204:187–196. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Charron F and Nemer M: GATA transcription
factors and cardiac development. Semin Cell Dev Biol. 10:85–91.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Butler TL, Esposito G, Blue GM, Cole AD,
Costa MW, Waddell LB, Walizada G, Sholler GF, Kirk EP, Feneley M,
et al: GATA4 mutations in 357 unrelated patients with congenital
heart malformation. Genet Test Mol Biomarkers. 14:797–802. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sperling S, Grimm CH, Dunkel I, Mebus S,
Sperling HP, Ebner A, Galli R, Lehrach H, Fusch C, Berger F and
Hammer S: Identification and functional analysis of CITED2
mutations in patients with congenital heart defects. Hum Mutat.
26:575–582. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang B, Wang J, Liu S, Han X, Xie X, Tao
Y, Yan J and Ma X: CFC1 mutations in Chinese children with
congenital heart disease. Int J Cardiol. 146:86–88. 2011.
View Article : Google Scholar
|
|
18
|
Teo AK, Ali Y, Wong KY, Chipperfield H,
Sadasivam A, Poobalan Y, Tan EK, Wang ST, Abraham S, Tsuneyoshi N,
et al: Activin and BMP4 synergistically promote formation of
definitive endoderm in human embryonic stem cells. Stem Cells.
30:631–642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong W, Gottlieb S, Collins J, Blescia A,
Dietz H, Goldmuntz E, McDonald McGinn DM, Zackai EH, Emanuel BS,
Driscoll DA and Budarf ML: Mutation analysis of TBX1 in non deleted
patients with features of DGS/VCFS or isolated cardiovascular
defects. J Med Genet. 38:E452001. View Article : Google Scholar
|
|
20
|
Ladd AN, Yatskievych TA and Antin PB:
Regulation of avian cardiac myogenesis by activin/TGFbeta and bone
morpho genetic proteins. Dev Biol. 204:407–419. 1998. View Article : Google Scholar
|
|
21
|
Chen JN: Left right pattern of cardiac
BMP4 may drive asymmetry of the heart in zebrafish. Development.
124:4373–4382. 1997.PubMed/NCBI
|
|
22
|
Wang S, Krinks M, Kleinwaks L and Moos M
Jr: A novel Xenopus homologue of bone morphogenetic protein 7 (BMP
7). Genes Funct. 1:259–271. 1997. View Article : Google Scholar
|
|
23
|
Hawley SH, Wunnenberg Stapleton K,
Hashimoto C, Laurent MN, Watabe T, Blumberg BW and Cho KW:
Disruption of BMP signals in embryonic Xenopus ectoderm leads to
direct neural induction. Genes Dev. 9:2923–2935. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hemmati Brivanlou A and Thomsen GH:
Ventral mesodermal patterning in Xenopus embryos: Expression
patterns and activities of BMP 2 and BMP 4. Dev Genet. 17:78–89.
1995. View Article : Google Scholar
|
|
25
|
Galvin KM, Donovan MJ, Lynch CA, Meyer RI,
Paul RJ, Lorenz JN, Fairchild Huntress V, Dixon KL, Dunmore JH,
Gimbrone MA Jr, et al: A role for smad6 in development and
homeostasis of the cardiovascular system. Nat Genet. 24:171–174.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tyebkhan G: Declaration of Helsinki: The
ethical cornerstone of human clinical research. Indian J Dermatol
Venereol Leprol. 69:245–247. 2003.
|
|
27
|
Palacios R: Post trial access and the new
version of the Declaration of Helsinki. Colomb Med (Cali).
44:206–207. 2013.
|
|
28
|
Gebril OH and Meguid NA: HFE gene
polymorphisms and the risk for autism in Egyptian children and
impact on the effect of oxidative stress. Dis Markers. 31:289–294.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abanmi A, Al Harthi F, Zouman A, Kudwah A,
Jamal MA, Arfin M and Tariq M: Association of Interleukin 10 gene
promoter polymorphisms in Saudi patients with vitiligo. Dis
Markers. 24:51–57. 2008. View Article : Google Scholar
|
|
30
|
Fung A, Manlhiot C, Naik S, Rosenberg H,
Smythe J, Lougheed J, Mondal T, Chitayat D, McCrindle BW and Mital
S: Impact of prenatal risk factors on congenital heart disease in
the current era. J Am Heart Assoc. 2:e0000642013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Amita M, Adachi K, Alexenko AP, Sinha S,
Schust DJ, Schulz LC, Roberts RM and Ezashi T: Complete and
unidirec tional conversion of human embryonic stem cells to
trophoblast by BMP4. Proc Natl Acad Sci USA. 110:E1212–E1221. 2013.
View Article : Google Scholar
|
|
32
|
Sudheer S, Bhushan R, Fauler B, Lehrach H
and Adjaye J: FGF inhibition directs BMP4 mediated differentiation
of human embryonic stem cells to syncytiotrophoblast. Stem Cells
Dev. 21:2987–3000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kraushaar DC, Rai S, Condac E, Nairn A,
Zhang S, Yamaguchi Y, Moremen K, Dalton S and Wang L: Heparan
sulfate facilitates FGF and BMP signaling to drive mesoderm
differentiation of mouse embryonic stem cells. J Biol Chem.
287:22691–22700. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Greber B: When BMP meets FGF. Cell Stem
Cell. 9:91–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Isaacs NW: Cystine knots. Curr Opin Struct
Biol. 5:391–395. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Griffith DL, Keck PC, Sampath TK, Rueger
DC and Carlson WD: Three dimensional structure of recombinant human
osteogenic protein 1: Structural paradigm for the transforming
growth factor beta superfamily. Proc Natl Acad Sci USA. 93:878–883.
1996. View Article : Google Scholar
|
|
37
|
Vallejo LF and Rinas U: Folding and
dimerization kinetics of bone morphogenetic protein 2, a member of
the transforming growth factor β family. FEBS J. 280:83–92. 2013.
View Article : Google Scholar
|
|
38
|
McDonald NQ and Hendrickson WA: A
structural superfamily of growth factors containing a cystine knot
motif. Cell. 73:421–424. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Scheufler C, Sebald W and Hülsmeyer M:
Crystal structure of human bone morphogenetic protein 2 at 2.7 A
resolution. J Mol Biol. 287:103–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shibata Y, Suzuki D, Wurihan, Yamada A,
Maruyama N, Fujisawa N, Kamijo R and Miyazaki T: Lysyl oxidase like
2 reinforces unsatisfactory ossification induced by bone morpho
genetic protein 2: Relating nanomechanical properties and molecular
changes. Nanomedicine. 9:1036–1047. 2013.PubMed/NCBI
|
|
41
|
Walters MJ, Wayman GA and Christian JL:
Bone morphogenetic protein function is required for terminal
differentiation of the heart but not for early expression of
cardiac marker genes. Mech Dev. 100:263–273. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Waldo KL, Kumiski DH, Wallis KT, Stadt HA,
Hutson MR, Platt DH and Kirby ML: Conotruncal myocardium arises
from a secondary heart field. Development. 128:3179–3188.
2001.PubMed/NCBI
|
|
43
|
Lough J, Barron M, Brogley M, Sugi Y,
Bolender DL and Zhu X: Combined BMP 2 and FGF 4, but neither factor
alone, induces cardiogenesis in non precardiac embryonic mesoderm.
Dev Biol. 178:198–202. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Borges L, Iacovino M, Mayerhofer T, Koyano
Nakagawa N, Baik J, Garry DJ, Kyba M, Letarte M and Perlingeiro RC:
A critical role for endoglin in the emergence of blood during
embryonic development. Blood. 119:5417–5428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Leung AW, Kent Morest D and Li JY:
Differential BMP signaling controls formation and differentiation
of multipotent preplacodal ectoderm progenitors from human
embryonic stem cells. Dev Biol. 379:208–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang P, Li J, Tan Z, Wang C, Liu T, Chen
L, Yong J, Jiang W, Sun X, Du L, et al: Short term BMP 4 treatment
initiates mesoderm induction in human embryonic stem cells. Blood.
111:1933–1941. 2008. View Article : Google Scholar
|
|
47
|
Yu P, Pan G, Yu J and Thomson JA: FGF2
sustains NANOG and switches the outcome of BMP4 induced human
embryonic stem cell differentiation. Cell Stem Cell. 8:326–334.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shi Y, Katsev S, Cai C and Evans S: BMP
signaling is required for heart formation in vertebrates. Dev Biol.
224:226–237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Qian B, Mo R, Da M, Peng W, Hu Y and Mo X:
Common variations in BMP4 confer genetic susceptibility to sporadic
congenital heart disease in a han Chinese population. Pediatr
Cardiol. 35:1442–1447. 2014. View Article : Google Scholar : PubMed/NCBI
|