Introduction
MicroRNA (miRNA) is a small non-coding
single-stranded RNA molecule with a length of approximately 22
nucleotides. miRNAs assume several key regulatory functions, such
as cell growth, tissue differentiation, cell proliferation,
embryonic development and cell apoptosis (1). The involvement of miRNAs in various
tumors and cardiovascular diseases has been reported (1,2). An
abnormal miRNA level has been reported in many diseases including
myocardial infarction (2). Results
from those studies demonstrated that the abnormal expression was
found uniquely in the case of specific miRNAs and their
overexpression was used as genotype markers (3).
Early stage myocardial infarction is a serious
health condition and timely diagnosis and treatment have an
important impact on the quality of life of patients. Functional
genomic research revealed that microRNA-208a (miR-208a) was solely
expressed in the cardiac muscle cells, and released into the
bloodstream after the occurrence of myocardial damage (4). However, to the best of our knowledge,
no study reported the expression levels of miR-208a in myocardial
infarction.
In this study, we examined the expression level of
miR-208a in rat myocardial infarction tissue and the level of
miR-208a in the serum. We also studied the possible effects of the
miR-208a level on the cAMP-PKA pathway in myocardial injuries.
Materials and methods
Establishment of the early stage
myocardial infarction rat model
Male Sprague-Dawley rats weighing 200 to 220 g, were
obtained from the Experimental Animal Center of Fengxian Hospital
(Shanghai, China), were divided into two groups. Rats in the sham
operation group were given thoracotomy without ligation of coronary
artery, while rats in the myocardial infarction group were fed for
another 8 weeks after ligation of anterior descending coronary
artery. After administration of chloral hydrate (10%, 350 mg/kg) to
rats in the myocardial infarction group under anesthesia, the neck
skin was incised longitudinally, while limbs and head were fixed in
the supine position, exposing the trachea after blunt separation.
The trachea was cut between the second and third cricoid cartilage
and the animal ventilator was inserted into the trachea after
intubation for artificial respiration. Skin was incised in the 3rd
to 4th intercostal along the rib gap on the left side of the
sternum. Subcutaneous tissue and muscle were then separated layer
by layer. The chest was entered in the 3rd to 4th intercostal to
expose the heart. Initial portion of the left anterior descending
coronary artery was located between the left atrial appendage
margin and the pulmonary cone. The left anterior descending
coronary artery was ligated with a wisp of myocardia. After
ligation, the left ventricular wall turned pale, and ventricular
wall motion decreased.
The rat model
The ST segment of lead II in ECG monitor was
significantly higher. Chest was monitored for 10 min and then
closed layer by layer after thorough hemostasis. Spontaneous
breathing was recovered and the trachea as well as the neck
incisions were sutured. Rats in the sham operation group were only
treated with open thoracic puncture needle without ligation of the
coronary artery. After the experiment, rats in the sham operation
group were in good condition with smooth hair and were active and
agile. The nutritional status of rats in the myocardial infarction
group was poor. The hair was messy and sparse, and the movement of
the rats was slow with less activity.
Ethics approval for the animal experiments was
received from the Medical Ethics Committee of Fengxian
Hospital.
Experimental method
Total RNA extraction, serum collecting
and reverse transcription reactions
Serum extraction
Peripheral venous blood was collected from the two
groups and EDTA anticoagulant was added to the samples. Samples
were centrifuged at 4,000 × g for 5 min at 4°C. After
centrifugation, the serum was kept at −80°C. TRIzol (1 ml; Gibco,
Carlsbad, CA, USA) was added to approximately 100 mg of tissue or
100 µl of serum. Samples were homogenized and total RNA was
extracted. Extracted RNA was dissolved in 30 µl DEPC water
and reverse transcription kit (Thermo Fisher Scientific, Waltham,
MA, USA) was used for reverse transcription.
PCR reaction conditions
PCR was set for 40 cycles (denaturation at 95°C for
20 sec, renaturation at 60°C for 20 sec and extension at 70°C for 1
sec). Primers used were: miR-208a forward,
5′-GTCATCTAGAAAGCTTGATGCAGGAAA GAGCTTTGG-3′ and reverse,
5′-TGACAGATCTCAGCTGA CATCCTCTAGGCTGGGGTT-3′ (5); U6 control forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse 5′-AACGCT
TCACGAATTTGCGT-3′.
PCR was conducted with ABI PCR, model 7900 (Applied
Biosystems, Foster City, CA, USA). The relative quantitative
analysis was performed using the 2−ΔΔCt method (6).
Cell transfection
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA)
was used for myocardial cell line transfection using miR-208a
stimulant (mimic; Guangzhou Ruibo Biotechnology Co., Ltd.,
Guangzhou, China). The interference efficiency was evaluated after
48 h.
Evaluation of protein expression by western
blotting
HCM cells were collected and treated with 1X SDS
cell lysis buffer (Beyotime Inc., Shanghai, China). After SDS-PAGE,
the proteins were transferred to the PVDF membrane (Millipore,
Shanghai, China) at 110 V for 90 min. The membranes were blocked at
37°C for 100 min and then incubated with polyclonal goat anti-human
PKA (R&D Systems, Inc., Minneapolis, MN, USA cat no. AF4177,
1:500 dilution) and monoclonal mouse anti-human cAMP antibody
(R&D Systems, Inc., Minneapolis, MN, USA, cat no. MAB2146,
1:500 dilution) at 4°C overnight. Subsequently, the membranes were
rinsed and incubated with anti-goat secondary (PKA, cat. no.
ab339770) and HRP-mouse secondary (cAMP, cat. no. ab157532)
antibodies (1:2,000; Abcam, Cambridge, MA, USA) at 37°C for 30 min.
The reference protein used in this study was rabbit polyclonal to
human β-actin (ab8227, 1:500) (Sigma, St. Louis, MO, USA).
Statistical analysis
Data were analyzed using SPSS 19.0 software (SPSS
software Inc., Chicago, IL, USA). The differences between the two
groups were compared using the Student's t-test. P<0.05 was
considered statistically significant.
Results
Expression levels of miR-208a in the rat
myocardial tissues
The expression levels of miR-208a in myocardial
tissue in rats with early myocardial infarction were significantly
higher than those in control group (P<0.05) (Table I).
 | Table IExpression levels of miR-208a in rat
myocardial tissues. |
Table I
Expression levels of miR-208a in rat
myocardial tissues.
| Group (n) | miR-208a miRNA | t/P-value |
|---|
| Control (12) | 1.21±0.31 | |
| Study (12) | 4.21±1.41 | 7.20/0.000a |
miR-208a levels in the serum
For rats with subacute myo cardial infarction we
detected higher levels of miR-208a in the serum. The difference
observed between the myocardial infarction and the control group
was statistically significant (P<0.05) (Table II).
 | Table IImiR-208a levels in the serum. |
Table II
miR-208a levels in the serum.
| Group (n) | miR-208a miRNA | t/P-value |
|---|
| Control (12) | 1.29±0.41 | |
| Study (12) | 3.24±1.39a | 4.66/0.000 |
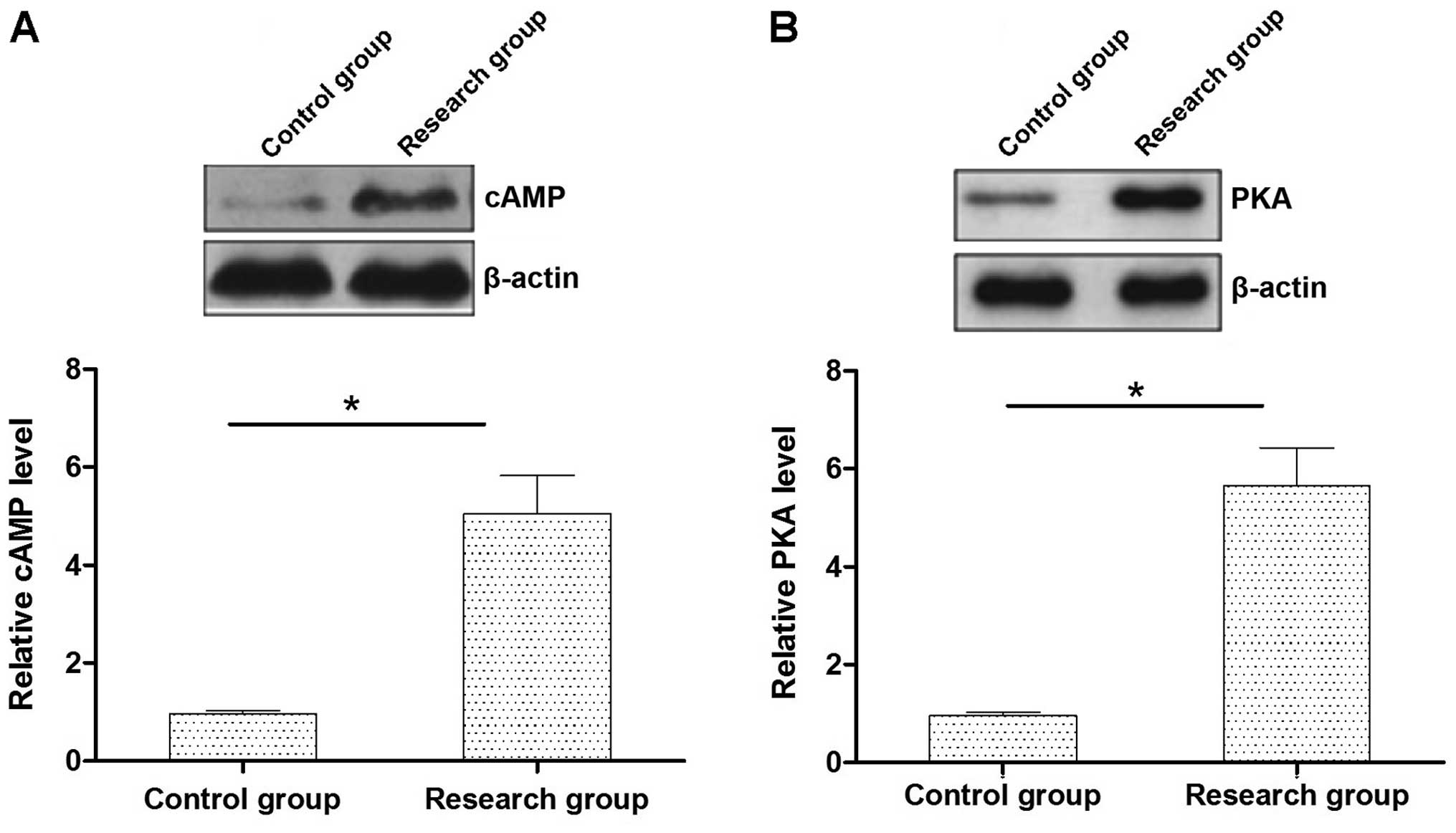
cAMP-PKA expression levels in the rat
myocardial tissues
The expression levels of cAMP-PKA protein in the
myocardial tissue in rats with early myocardial infarction were
significantly higher than those in the control group (P<0.05)
(Fig. 1).
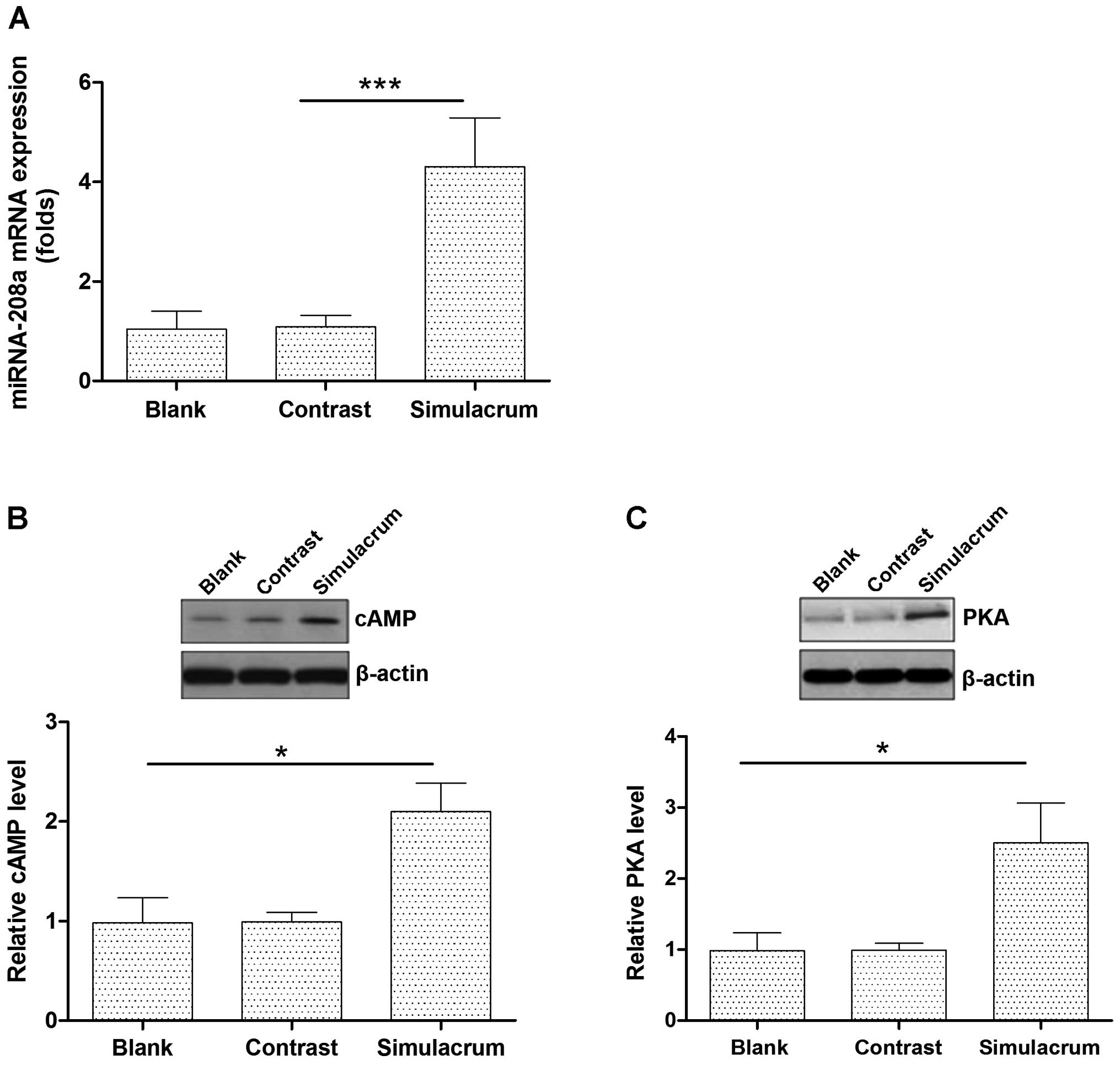
The effects of miR-208a overexpression on
the cAMP-PKA expression levels in cardiac muscle cells
Transfecting cells with the miR-208a stimulation
increased the expression levels of the miR-208a (Fig. 2A). High levels of miR-208a
expression significantly upregulated the cAMP-PKA protein
expression (Fig. 2B and C).
The differences observed between the experimental
and control groups were statistically significant.
Discussion
Acute myocardial infarction is a multi-factor
related disease on the basis of atherosclerosis. Its clinical
manifestation is intense and persistent chest pain. Resting or
taking nitrates usually cannot induce complete remission. Acute
myocardial infarction is often accompanied by an increase in serum
myocardial enzyme and troponin activity. We also observed severe
and dangerous electrocardiogram changes. Acute myocardial
infarction can cause severe damage to patients and has a high
disability rate as well as high mortality rate (7). Effective and timely medical
intervention is an important element to reduce the devastating
impact of myocardial infarction and to improve the survival rates
of the patients suffering from this condition. At present,
laboratory diagnosis of acute myocardial infarction is obtained by
measuring serum myocardial damage markers (myocardial enzymes and
troponin) (2). However, there are
some shortcomings, such as poor specificity and short duration.
Therefore, it is imperative to identify a more specific marker for
the treatment and prognosis of acute myocardial infarction.
miRNA is a class of single-stranded short chain
non-encoding small RNA fragments with regulatory effects on human
genes. miRNAs is important in the process of cell differentiation
and apoptosis (8–12). In recent years, the importance of
miRNA as an endogenous factor in myocardial ischemia has been
demonstrated. Prior studies showed that miRNA was involved in the
pathological process of myocardial infarction and fibrosis process
after myocardial infarction. For example, it was found that
miR-199a levels in a rat model of acute myocardial infarction
tissue decreased significantly and were involved in the regulation
of myocardial hypoxia by regulating the hypoxia-inducible factor
(HIF-1α) expression level (13,14).
Rane et al found that miR-21, miR-214 and miR-223 in the
myocardial infarction margin in patients with myocardial infarction
increased significantly, while miR-29b and miR-149 significantly
decreased (14). Previous genomic
studies demonstrated that miR-208a exists in the human body, and
its expression in patient peripheral blood following myocardial
infarction was increased (4). To
the best of our knowledge, there are only few studies that focus on
the level of miR-208a in animal models for acute myocardial
infarction and the mechanism of myocardial injury.
In the present study, the expression levels of
miR-208a in the serum as well as the tissue were detected in an
acute myocardial infarction rat model. The aim was to examine the
function and mechanism of miR-208a involvement in the pathogenesis
of acute myocardial infarction. The levels of miR-208a in the
myocardial tissue and the serum were significantly higher in rats
with acute myocardial infarction suggesting that miR-208a may be
involved in the occurrence and development of acute myocardial
infarction. We hypothesized that miR-208a is released into the
blood after myocardial infarction. This is consistent with a
previous study conducted on patients with myocardial infarction
(4).
The cAMP-PKA pathway is a part of the receptor G
protein and cAMP-PKA signaling pathway, which plays a key role in
mediating cell responses to various stimuli (15). The cAMP-PKA pathway can mediate a
variety of physio logical and pathological functions, especially in
cardiovascular disease, such as the expansion of coronary artery
and enhanced myocardial contractility (16). The results of this study showed
that cAMP-PKA protein levels in myocardial tissue were
significantly higher in rats with subacute myocardial infarction
compared to those in sham-operated rats. This findings showed that
myocardial injury was involved in the occurrence of myocarditis by
promoting the cAMP-PKA protein signaling pathway. Furthermore, the
effect of miR-208a on the cAMP-PKA protein signaling pathway in
myocardial cells was studied by overexpressing the miR-208a in
human myocardial cells. It was found that the overexpression of
miR-208a, significantly upregulated the cAMP-PKA expression. This
result suggests that miR-208a is probably involved in the
inflammatory process of acute myocardial infarction by affecting
the cAMP-PKA signaling pathway.
In conclusion, we have demonstrated that the
expression of miR-208a increased during the acute myocardial
infarction and may be involved in the development of myocardial
infarction by regulating the cAMP-PKA signaling pathway. We also
suggest that our study showed that the detection of miR-208a in the
serum of patients with subacute myocardial infarction can be used
as a potential diagnostic indicator/marker for myocardial
injuries.
References
|
1
|
Li Z, Ying X, Chen H, Ye P, Shen Y, Pan W
and Zhang L: MicroRNA-194 inhibits the epithelial-mesenchymal
transition in gastric cancer cells by targeting FoxM1. Dig Dis Sci.
59:2145–2152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu
Q, Deitch EA, Huo Y, Delphin ES and Zhang C: MicroRNA-145, a novel
smooth muscle cell phenotypic marker and modulator, controls
vascular neointimal lesion formation. Circ Res. 105:158–166. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suárez Y, Fernández-Hernando C, Pober JS
and Sessa WC: Dicer dependent microRNAs regulate gene expression
and functions in human endothelial cells. Circ Res. 100:1164–1173.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Callis TE, Pandya K, Seok HY, Tang RH,
Tatsuguchi M, Huang ZP, Chen JF, Deng Z, Gunn B, Shumate J, et al:
MicroRNA-208a is a regulator of cardiac hypertrophy and conduction
in mice. J Clin Invest. 119:2772–2786. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
7
|
Newby AC: Metalloproteinases promote
plaque rupture and myocardial infarction: A persuasive concept
waiting for clinical translation. Matrix Biol. 44–46:157–66. 2015.
View Article : Google Scholar
|
|
8
|
Zhang Y, Zhou ZG, Wang L, Zhang P, Wang
MJ, Cui CF, Guan JT, Chen KL and Zhan L: Clinicopathological
significance of microRNA-21 and miR-125 expression in colorectal
cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 12:623–626. 2009.In
Chinese. PubMed/NCBI
|
|
9
|
Du J, Yang S, An D, Hu F, Yuan W, Zhai C
and Zhu T: BMP-6 inhibits microRNA-21 expression in breast cancer
through repressing deltaEF1 and AP-1. Cell Res. 19:487–496. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K
and Yang GH: MicroRNA-21 (miR-21) represses tumor suppressor PTEN
and promotes growth and invasion in non-small cell lung cancer
(NSCLC). Clin Chim Acta. 411:846–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bae HJ, Noh JH, Kim JK, Eun JW, Jung KH,
Kim MG, Chang YG, Shen Q, Kim SJ, Park WS, et al: MicroRNA-29c
functions as a tumor suppressor by direct targeting oncogenic SIRT1
in hepatocellular carcinoma. Oncogene. 33:2557–2567. 2014.
View Article : Google Scholar
|
|
12
|
Fan DN, Tsang FH, Tam AH, Au SL, Wong CC,
Wei L, Lee JM, He X, Ng IO and Wong CM: Histone lysine
methyltransferase, suppressor of variegation 3–9 homolog 1,
promotes hepatocellular carcinoma progression and is negatively
regulated by microRNA-125b. Hepatology. 57:637–647. 2013.
View Article : Google Scholar
|
|
13
|
Zhu H and Fan GC: Role of microRNAs in the
reperfused myocardium towards post-infarct remodelling. Cardiovasc
Res. 94:284–292. 2012. View Article : Google Scholar :
|
|
14
|
Rane S, He M, Sayed D, Vashistha H,
Malhotra A, Sadoshima J, Vatner DE, Vatner SF and Abdellatif M:
Downregulation of miR-199a derepresses hypoxia-inducible
factor-1alpha and Sirtuin 1 and recapitulates hypoxia
preconditioning in cardiac myocytes. Circ Res. 104:879–886. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raghunandan R and Ingram VM:
Hyperphosphorylation of the cytoskeletal protein Tau by the
MAP-kinase PK40erk2: regulation by prior phosphorylation with
cAMP-dependent protein kinase A. Biochem Biophys Res Commun.
215:1056–1066. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fantidis P: The role of intracellular
3′5′-cyclic adenosine mono-phosphate (cAMP) in atherosclerosis.
Curr Vasc Pharmacol. 8:464–472. 2010. View Article : Google Scholar
|