Introduction
Telomerase is essential for maintaining telomeres,
and consists of an RNA template and a catalytic protein subunit,
telomerase reverse transcriptase (TERT), which adds a six-base DNA
repeat sequence (TTAGGG) to the telomere (1). With decreasing telomerase activity,
the ends of chromosomes become shortened as somatic cells age or
mature (2,3). Extensive efforts have been made to
clarify the molecular mechanisms underlying telomerase activation.
Human (h) TERT is a critical determinant of telomerase activation
within the cell (4,5). Although a number of transcription
factors have been identified as regulators of the hTERT promoter
(6), the specific factors
responsible for determining telomerase activity in cells remain to
be fully elucidated.
Previously, the nucleolar protein, GLTSCR2, was
shown to be a putative tumor suppressor gene, as it is capable of
inducing phosphatase and tensin homolog-dependent apoptotic cell
death and inhibiting tumor growth (7–9).
However, the biological function and molecular mechanisms of
GLTSCR2 remain to be fully elucidated. In our previous study, it
was reported that GLTSCR2 functions as a DNA damage response
protein in the ataxia telangiectasia mutated (ATM) -Chk2 and ataxia
telangiectasia and Rad3-related protein (ATR) -Chk1 pathways
(10). Notably, increasing
evidence indicates that several DNA damage response proteins are
involved in telomere maintenance. Mutations in proteins involved in
the response to DNA damage result in telomere dysfunction and
subsequent chromosomal instability, suggesting extensive functional
interactions between telomere maintenance and DNA damage response
mechanisms (11–14). In the present study, the effect of
GLTSCR2 on telomerase activity in various cell types was examined.
Subsequent to this, the effect of the knockdown of GLTSCR2 on
cellular morphology and growth rate was investigated. The present
study revealed a novel biological function of GLTSCR2 in
maintaining chromosomal stability via telomerase regulation.
Materials and methods
Cell culture and treatment
The SK-Hep-1 cells, T98G cells and human umbilical
vein endothelial cells (HUVECs) were obtained from the American
Type Culture Collection (Rockville, MD, USA). The cells were
cultured in a humidified 5% CO2 incubator maintained at
37°C, either in Dulbecco's modified Eagle's medium (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA; for SK-Hep-1 and
T98G cells) or in endothelial growth medium-2 (HUVECs; Thermo
Fisher Scientific, Inc) supplemented with 10% fetal bovine serum
(Thermo Fisher Scientific, Inc).
Antibodies and reagents
The anti-GLTSCR2 polyclonal antibody was produced by
the present group by immunizing a rabbit with keyhole limpet
hemocyanin-conjugated amino-acid residues 78–193 (CTRAKPGPQDTVERPF)
of human GLTSCR2. The antibody was purified from the immune serum
by affinity chromatography (8).
The rabbit polyclonal anti-human TERT (1:1,000; SC-7212) and the
rabbit polyclonal anti-tubulin antibodies (1:1,000; SC-9104) were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). All other reagents were obtained from Sigma-Aldrich (St.
Louis, MO, USA), unless otherwise specified.
Construction of the Tet-Off
adenoviral-mediated system
A GFP-GLTSCR2 construct consisting of full-length
GLTSCR2 cDNA fused to a C-terminal GFP tag was first cloned into
pTRE-Shuttle2 vector (Clontech Laboratories, Inc., Mountain View,
CA, USA), which contains the tetracyclineresponsive element (TRE)
upstream of the CMV minimal promoter. The resulting TRE-GFP-GLTSCR2
expression unit was excised from the pTRE-Shuttle2 vector using
I-Ceu I and PI-Sce I resection enzymes, and then ligated to Swa
I-digested Adeno-X System 1 Viral DNA (Clontech). The resulting
recombinant Adeno-XGFP-GLTSCR2 vector (Ad-GFP/GLT) was packaged
into infectious adenoviral particles by transfection of HEK293
cells, and recombinant adenovirus was harvested by lysing
transfected cells. In the Tet-off system, the tet-responsive
transcriptional activator is expressed and binds to the TRE in the
absence of doxycycline (Clontech; stored in aliquots and kept at
−20°C) to activate transcription of GLTSCR2. As doxycycline is
added to the culture medium at 37°C, transcription from the TRE is
turned off in a dose dependent manner. To transiently express
GLTSCR2, the cells were plated in a 6-well plate at 70% confluence
(5×105 cells) and coinfected with a recombinant
adenovirus, [Ad-green fluorescent protein (GFP)/GLT], and a
regulation virus (Adeno-X Tet-Off virus) with a multiplicity of
infection (MOI) of 100 in serum-free media for 12 h. Fresh complete
medium was subsequently added.
Generation of stable GLTSCR2-knockdown
cell lines
For knockdown of the expression of GLTSCR2, feline
leukemia virus-based lentiviral GLTSCR2 shRNA vectors were
purchased from GeneCopoeia, Inc. (Rockville, MD, USA). The
GLTSCR2-target sequence was 5′-GAGACCGGTTCAAGAGCTT-3′, and the
scrambled (Scr) sequence was 5′-CGATACTGAACGAATC-3′. Cells were
seeded at 5×105 cells in 30 mm dishes and lentiviral
stocks of the GLTSCR2 shRNA or the control shRNA vector were then
incubated with separate sets of cells with an MOI of 10. After 48
h, clones of GLTSCR2-knockdown cells were selected for using
puromycin (1 µg/ml; Sigma-Aldrich) treatment. Protein
expression levels were analyzed using western blot and
immunocytochemical analyses.
Telomere repeat amplification protocol
(TRAP)
A TRAP assay was used to detect telomerase activity,
in which a TRAPEZE® XL telomerase detection kit (EMD
Millipore, Billerica, MA, USA) was used, according to the
manufacturer's protocol. Briefly, cell pellets were resuspended in
200 µl of 3- [cholamidoprophyl) -dimethylammonio]
-1-propane-sulfonate lysis buffer and incubated for 30 min on ice.
After centrifugation at 13,000 g for 20 min at 4°C, aliquots of the
supernatant were rapidly frozen and store −80°C (15). The telomeric DNA from the cell
extracts was amplified by 36 cycles of polymerase chain reaction
(PCR) with a denaturation at 94°C for 30 sec, annealing at 59°C for
30 sec, and elongation at 72°C for 1 min. 20 µl of the PCR
product were subjected to electrophoresis on a non-denaturing 10%
polyacrylamide gel (Sigma-Aldrich) The experiment was repeated four
times.
Reverse transcription semi-quantitative
PCR (RT-PCR) analysis
Total RNA (1 µg) was extracted using Trizol
reagent (Invitrogen) from the cultured cells and converted into
cDNA using a cDNA synthesis kit (Takara Bio, Inc., Otsu, Japan),
according to the manufacturer's protocol. hTERT and tubulin mRNA
amplification were performed with the following primers: hTERT
forward, 5′-AGAGTGTCTGGAGCAAGTTGC-3′, hTERT reverse,
5′-CGTAGTCCATGTTCACAATCG-3′; tubulin forward,
5′-CATGTATCTTCCATACCCTG-3′, tubulin reverse,
5′-CTGAAGGTATTCATGATGCG-3′. The cycling conditions were as follows:
Denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec,
elongation at 72°C for 30 sec. The resulting PCR products were
separated on 2% agarose gels (Sigma-Aldrich) and visualized using
ethidium bromide staining (0.5 µg/ml; Sigma-Aldrich). The
quantification of the transcriptional gene expression was performed
using the Gel Doc EZ system Image Lab™ software (Bio-Rad
Laboratories, Inc., Richmond, CA, USA) and normalized to the
expression of tubulin as an endogeneous control. The method used
for quantification was semi-quantitative evaluation using
densitometric analysis (16)
Luciferase assay
The pGL2 luciferase plasmid (Promega Corporation,
Madison, WI, USA) was used. The hTERT promoter sequence was
amplified by PCR to generate a 1580 bp fragment from human genomic
DNA using upstream primers (5′-AGCGATACCTATTGAATGCC-3′) containing
a KpnI site, together with a single downstream primer
(5′-TCTCCTCGCGGCGCGAGTTT-3′) containing a HindIII site.
Luciferase activity was measured in samples containing equivalent
quantities of protein using a luminometer and luciferase assay
reagents (Promega Corporation). The protein content of cell lysates
was determined using BCA protein assay reagent (Pierce
Biotechnology, Inc., Rockford, IL, USA) in clear 96-well plates
(Nunc, Wiesbaden, Germany), and colorimetric assay was performed
using an VersaMax ELISA Microplate reader (Molecular Devices, LLC,
Sunnyvale, CA, USA). The equivalent quantities of protein were used
in the subsequent luciferase activity assay.
Giemsa staining and slide
preparation
Fixed slides were immersed in 5% Giemsa
(Sigma-Aldrich) for 20 min. The slides were then rinsed briefly in
dH2O and air-dried. For long-term storage, the slides
were mounted with a drop of Permount (Thermo Fisher Scientific,
Inc.) and coverslips.
Senescence-associated β-galactosidase
staining
The cells were fixed with 2% formaldehyde/0.2%
glutaraldehyde solution (both Cell Signaling Technology, Inc.,
Danvers, MA, USA) and stained overnight at 37°C with 1 mg/ml X-gal
(Cell Signaling Technology, Inc.), 40 mM citric
acid/Na2HPO4 (pH 6.0; Sigma-Aldrich), 5 mM
potassium ferrocyanide/ferricyanide (Cell Signaling Technology,
Inc.), 150 mM NaCl and 2 mM MgCl2, both
Sigma-Aldrich.
Statistical analysis
In all cases, the results are presented as the mean
± standard deviation. Statistical analysis was performed using SPSS
software, version 12.0 (SPSS, Inc., Chicago, IL, USA). A
two-tailed, unpaired Student's t-test was used for data
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
To determine the effect of GLTSCR2 on telomerase
activity, the present study first measured the level of telomerase
activity in the cultured SK-Hep-1 cells. Initially, the SK-Hep-1
cells were infected with a doxycycline-inducible (Tet-Off system)
adenovirus, expressing either GFP-tagged GLTSCR2 (Ad-GLT/GFP) or
GFP (Ad-GFP) as a control, and placed in media containing different
concentrations of doxycycline (0, 10, 20 and 50 ng/ml) for 48 h.
Telomerase activity was measured using a TRAP assay, as described
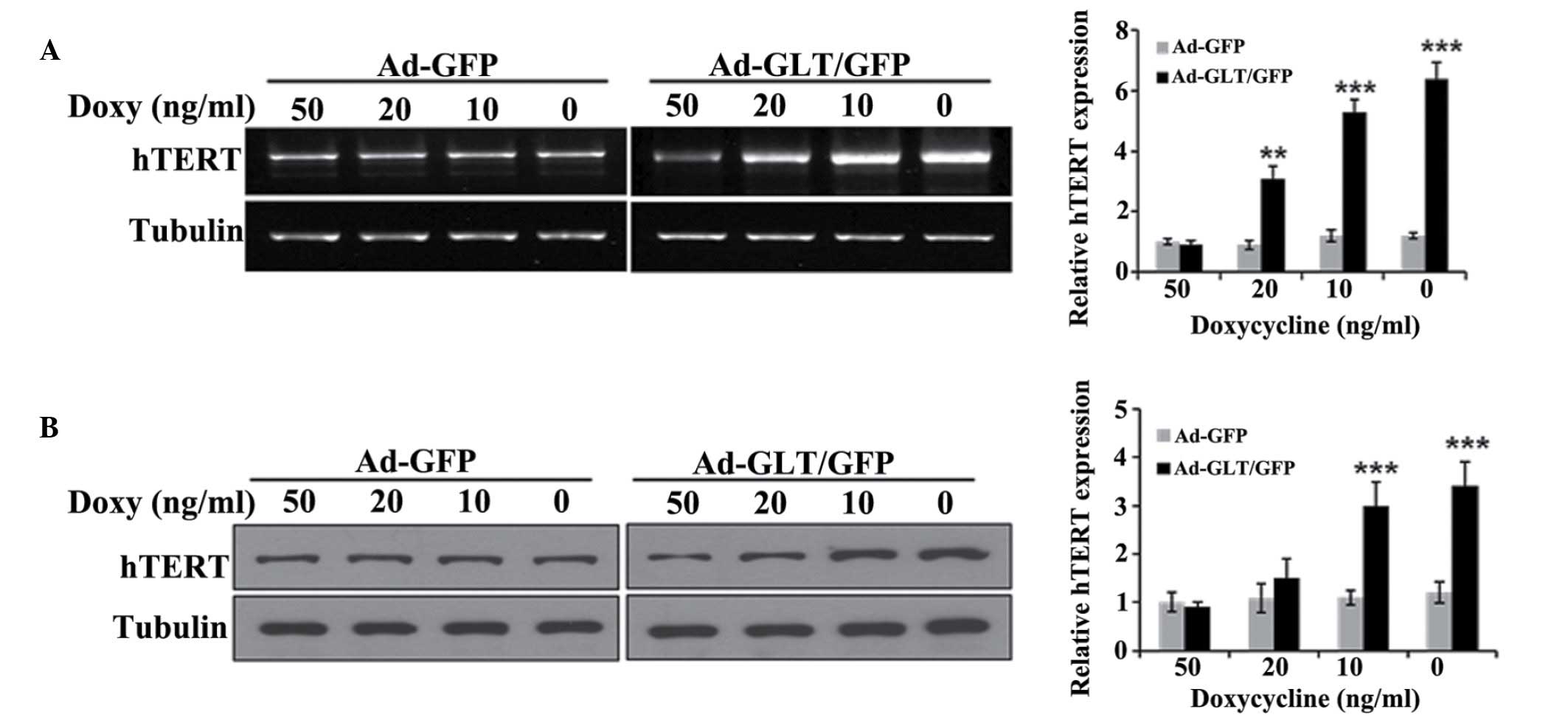
above. As shown in Fig. 1A, the
Ad-GLT/GFP cells showed significantly increased telomerase activity
in proportion to the expression levels of GLTSCR2, compared with
the Ad-GFP cells. The effects of GLTSCR2 in the T98G glioblastoma
cells were similar to those observed in the SK-Hep-1 cells
(Fig. 1B).
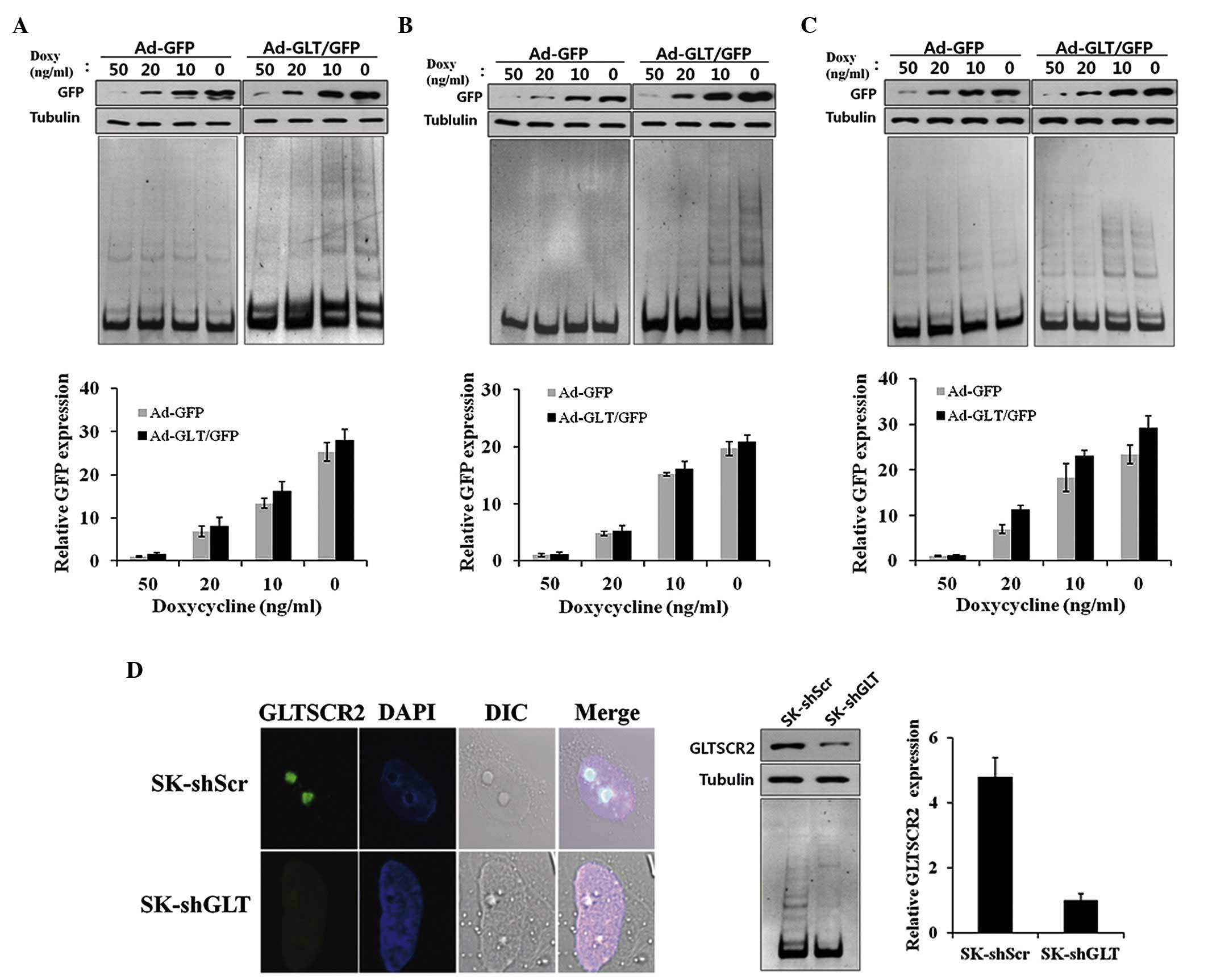 | Figure 1GLTSCR2 increases telomerase activity.
(A) SK-Hep-1 cells, (B) T98 G cells and (C) human umbilical vein
endothelial cells were infected with Ad-GFP or Ad-GLT/GFP, and
incubated with decreasing concentrations of doxycycline (50, 20, 10
and 0 ng/ml). (D) SK-Hep-1 cells were stably infected with
lentivirus carrying either GLTSCR2-targeting SK-shGLT or SK-shScr,
immunostained with anti-GLTSCR2 and DAPI, and viewed under an
DIC-equipped confocal microscope (Left, magnification ×630). Cells
were harvested, and lysates were examined using western blotting
with the indicated antibodies. Tubulin was used as a loading
control. TRAP assays were performed using subconfluent
proliferating cultures of cells. GLT, glioma tumor-supressor;
GLTSCR2, glioma tumor-suppressor candidate region gene 2; GFP,
green fluorescent protein; sh, short hairpin RNA; Scr, scambled;
Doxy, doxycycline. |
The majority of cancer cells express high levels of
telomerase activity. However, human primary normal cells usually
exhibit a low level of telomerase activity (17,18).
If GLTSCR2 is an inducer of telomerase activity, its expression may
induce telomerase activity in primary normal cells. Thus, the
present study examined the effect of GLTSCR on telomerase activity
in HUVECs. The HUVECs were infected with Ad-GLT/GFP or Ad-GFP for
48 h, and the TRAP assay was performed. Telomerase activity was low
and almost undetectable in the Ad-GFP HUVEC cells. However,
telomerase activity in the Ad-GLT/GFP HUVEC cells was significantly
increased and correlated with the expression level of GLTSCR2,
which was similar to the results observed in the SK-Hep-1 and T98G
cells (Fig. 1C).
To further demonstrate the effect of GLTSCR2 on
telomerase activity, the SK-Hep-1 cells were stably infected with a
lentivirus carrying either GLTSCR2-targeting shGLT or shScr.
Subsequent immunofluoresence and immunoblotting confirmed that the
expression of GLTSCR2 was significantly reduced, by >80%, in the
cells stably infected with shGLT (Fig.
1D). In contrast to the GLTSCR2-overexpressing SK-Hep-1 cells,
the SK-shGLT cells showed lower telomerase activity, compared with
the control (SK-shScr) cells (Fig.
1D). These results suggested that GLTSCR2 exerted a positive
effect on telomerase activity.
To investigate the mechanism by which GLTSCR2
increases telomerase activity, the present study examined whether
GLTSCR2 affected the expression of hTERT, a catalytic subunit of
telomerase and a critical determinant of telomerase activity. The
SK-Hep-1 cells were infected with Ad-GLT/GFP or Ad-GFP for 48 h.
The mRNA and protein expression levels of hTERT were then evaluated
using RT-PCR analysis and western blotting, respectively. Compared
with the Ad-GFP control cells, the Ad-GLT/GFP cells exhibited
increased mRNA and protein levels of hTERT (Fig. 2A and B).
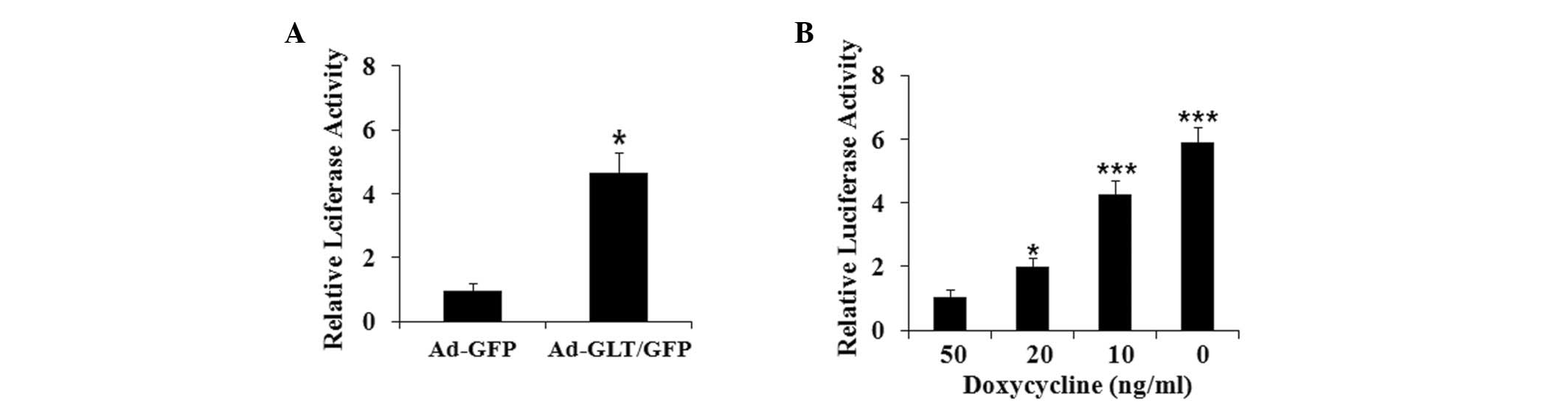
To investigate the mechanism underlying the
GLTSCR2-induced expression of hTERT, the present study examined the
effect of GLTSCR2 on the activity of the hTERT gene promoter by
using the hTERT promoter-luciferase reporter plasmid, pGL2-hTERT,
in the SK-Hep-1 cells. The SK-Hep-1 cells were transiently
transfected for 24 h with pGL2-hTERT, and were then infected with
either Ad-GLT/GFP or Ad-GFP for 24 h to allow for the expression of
GLTSCR2. As shown in Fig. 3A,
Ad-GLT/GFP markedly induced hTERT promoter activity, whereas Ad-GFP
had minimal or no effect on hTERT promoter activity. The induction
of pGL2-hTERT activity by GLTSCR2 was dose-dependent (Fig. 3B). Together, these data
demonstrated consistent positive regulation of the hTERT gene by
GLTSCR2 in the SK-Hep-1 cells.
Telomeres are essential for maintaining chromosome
stability, and extensive telomere shortening can lead to abnormal
nuclear morphologies, including micronuclei, nucleoplasmic bridges
or nuclear buds as a marker of chromosome instability (19). Subsequently, the effect of the
knockdown of GLTSCR2 on abnormal nuclear morphology was examined.
The presence of micronuclei, nucleoplasmic bridges and nuclear buds
increased in the T98G-shGLT cells (Fig. 4A).
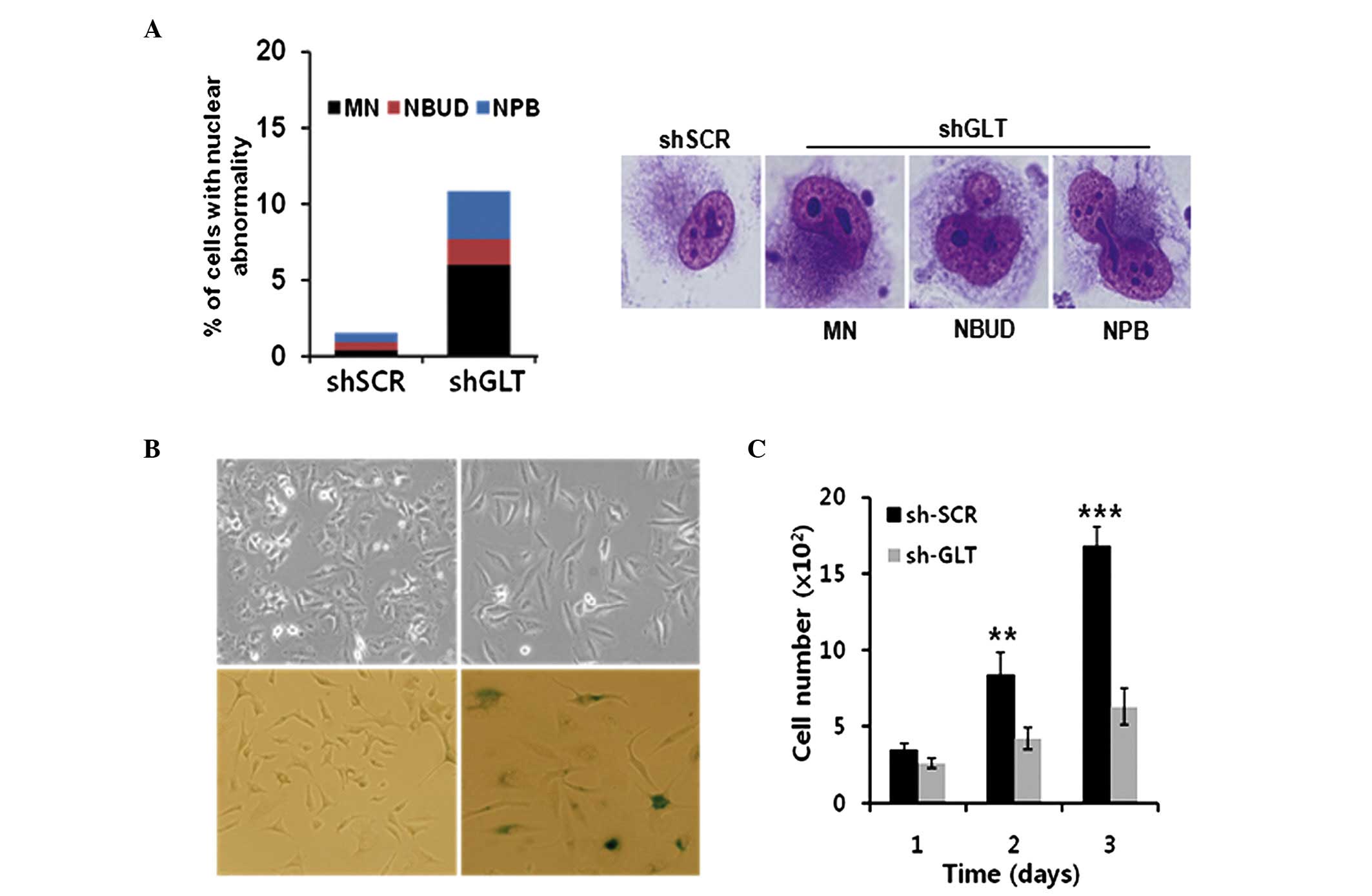 | Figure 4Knockdown of GLTSCR2 induces abnormal
nuclear morphology and cellular senescence. The T98G cells were
stably infected with a lentivirus carrying either GLTSCR2-targeting
shGLT or scrambled shRNA. (A) T98G-shGLT cells showed nuclear
abnormalities, visualized by staining with Giemsa, including MN,
NBUD and NPB, magnification ×630. A minimum of 300 cells were
counted and the count was performed three times. (B) Top and bottom
panels show morphological changes and cellular senescence by
β-galatosidase staining, in the T98G-shScr and T98G-shGLT groups.
Magnification, ×100. (C) Growth rates of the cells were determined
by total cell count at the time points indicated. Data are
presented as the mean ± standard deviation of three independent
experiment. sh, short hairpin RNA; GLT, glioma tumor-supressor;
GLTSCR2, glioma tumor-suppressor candidate region gene 2; Scr,
scambled; MN, micronucleus; NBUD, nuclear buds; NPB. nucleoplasmic
bridges. **P<0.01, ***P<0.001 vs. shSCR
1 day. |
The present study also examined the effects of the
knockdown of GLTSCR2 on cellular morphology and growth rate. The
T98G-shGLT cells presented with significant morphological changes,
characterized by an elongated and enlarged flat shape, resembling
the morphology of senescent cells, compared with parental T98 G
cells (Fig. 4B). The growth rate
was reduced in the T98G-shGLT cells (Fig. 4C). Additionally, the T98G-shGLT
cells entered cellular senescence, as determined by
senescence-associated β-galactosidase staining (Fig. 4B). Taken together, these results
suggested that GLTSCR2 is crucially involved in the positive
regulation of telomerase and chromosome stability.
Discussion
In the present study, to investigate the effect of
GLTSCR2 on telomerase activity, human SK-Hep-1 and T98G cancer
cells, and normal HUVECs were infected with Ad-GLT/GFP in a
doxycycline-inducible (Tet-Off system) manner. Subsequently,
changes in telomerase activity and the expression of hTERT were
determined. GLTSCR2 significantly increased telomerase activity in
the cancer and normal cells. This increase was consistent with an
increase in the expression pf hTERT, as determined by
semi-quantitative RT-PCR analysis, western blot analysis and
promoter assays for hTERT. Although the molecular mechanism
underlying the regulation of telomerase activity by GLTSCR2 remains
to be fully elucidated and currently under investigation, these
results suggested that GLTSCR2 is crucially involved in the
positive regulation of telomerase maintenance.
Several other studies have been performed to
identify the transcriptional factors critical for hTERT regulation
(20–22), however, no specific factors have
been identified, which are responsible for determining telomerase
activity in cells. It is now widely accepted that a number of
nuclear factors are involved in the regulation of hTERT
transcription(23,24). Furthermore, hTERT undergoes a
regulated subnuclear translocation between the nucleoplasm and the
nucleolus (25). hTERT nucleolar
transportation may be an essential step for the biogenesis of
telomerase in human cells (26,27).
The telomere-capping and stabilizing proteins, telomeric repeat
binding factor (TRF)1 and TRF2, also accumulate in the nucleolus,
and they are modulated by nucleolar proteins (28). Within these complex regulatory
mechanisms, GLTSCR2 appears to be involved. The present study
suggested that the GLTSCR2 nucleolar protein is a crucial
determinant of telomerase activity in normal and cancer cells.
Human primary cells usually exhibit low levels of
telomerase activity. However, the majority of cancer cell lines
express high levels of telomerase activity, which may support
continued cell proliferation (17,18).
In GLTSCR2-overexpressing SK-Hep-1 cells and control cells, no
significant differences in cell proliferation were observed (data
not shown), although telomerase activity was significantly
increased in the former. By contrast, in the GLTSCR2-knockdown
cells, cell proliferation was significantly lower, compared with
that in the control cells. These results indicated that it is
unlikely that cell proliferation contributed to the increased
telomerase activity observed in the GLTSCR2-overexpressing cell
lines. However, in the GLTSCR2-knockdown cells, decreased
telomerase activity may have impeded proliferation. Thus, GLTSCR2,
but not the rate of cell proliferation, appears to be important in
telomerase activity.
Reports have indicated that the cellular DNA damage
response (DDR) ensures genomic stability and protects against
genotoxic stresses. By contrast, defects in the DDR contribute to
genomic instability (10). For
example, defects in ATM, a key DNA damage signaling molecule, are
associated with telomere loss, telomeric fusions and
extrachromosomal telomeric DNA appearance in cells from patients
with ataxia telangiectasia and ATM-deficient mice (12). In addition, the TRF1 and TRF2
proteins, which stabilize telomeres, are known to be important in
the DDR. In particular, the inhibition of the DDR by dominant
negative alleles or by the genetic deletion of TRF2 in mouse cells
gives rise to a potent checkpoint response and frequent end-to-end
fusions (29). Furthermore, TRF2
is rapidly phosphorylated in response to DNA damage, likely via an
ATM-kinase-mediated pathway, which is critical for telomere
maintenance (12,30). Upon phosphorylation, TRF2 rapidly
localizes to sites of DNA damage, specifically double-strand
breaks, where it acts as an early component of the DNA repair
response system (14). GLTSCR2
mediates the activation of ATM-Chk2 and ATR-Chk1 in response to DNA
damage (10). In the present
study, it was shown that the siRNA-based knockdown of GLTSCR2 led
to abnormal nuclear morphology, including the presence of
micronuclei, nucleoplasmic bridges and nuclear buds as a indicator
of chromosome instability, which indicated an impaired response of
the cells to DNA damage.
In conclusion, the results of the present study
suggested that GLTSCR2 is a crucially involved in the positive
regulation of telomerase and chromosome stability. The precise role
of GLTSCR2 in the regulation of telomerase remains to be
elucidated. However, GLTSCR2-induced telomerase regulation may
provide important clues regarding the role of GLTSCR2 in chromosome
stability. Continued investigations into other GLTSCR2-associated
proteins involved in the DDR are critical to determine the extent
of overlap between telomere maintenance and DNA repair response
signaling pathways.
Acknowledgments
The present study was supported by grants from the
National Research Foundation of Korea funded by the Korean
government (Ministry of Science, ICT and Future Planning; grant
nos. 2011-0030072 and NRF-2013R1A2A2A01009006).
References
|
1
|
Oh BK, Yoon SM, Lee CH and Park YN: Rat
homolog of PinX1 is a nucleolar protein involved in the regulation
of telomere length. Gene. 400:35–34. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bekaert S, Derradji H and Baatout S:
Telomere biology in mammalian germ cells and during development.
Dev Biol. 274:15–30. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wills LP and Schnellmann RG: Telomeres and
telomerase in renal health. J Am Soc Nephrol. 22:39–41. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakamura TM, Morin GB, Chapman KB,
Weinrich SL, Andrews WH, Linger J, Harley CB and Cech TR:
Telomerase catalytic subunit homologs from fission yeast and human.
Science. 277:955–959. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meyerson M, Counter CM, Eaton EN, Ellisen
LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ,
Liu Q, et al: hEST2, the putative human telomerase catalytic
subunit gene, is up-regulated in tumor cells and during
immortalization. Cell. 90:785–797. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hashimoto M, Kyo S, Hua X, Tahara H,
Nakajima M, Takakura M, Sakaguchi J, Maida Y, Nakamura M, Ikoma T,
et al: Role of menin in the regulation of telomerase activity in
normal and cancer cells. Int J Oncol. 33:330–340. 2008.
|
|
7
|
Okahara F, Itoh K, Nakagawara A, Murakami
M, Kanaho Y and Maehama T: Critical role of PICT-1, a tumor
suppressor candidate, in phosphatidylinositol 3,4,5-trisphosphate
signals and tumorigenic transformation. Mol Biol Cell.
17:4888–4895. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yim JH, Kim YJ, Ko JH, Cho YE, Kim SM, Kim
JY, Lee S and Park JH: The putative tumor suppressor gene GLTSCR2
induces PTEN-modulated cell death. Cell Death Differ. 14:1872–1879.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okahara F, Ikawa H, Kanaho Y and Maehama
T: Regulation of PTEN phosphorylation and stability by a tumor
suppressor candidate protein. J Biol Chem. 279:45300–45303. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JY, Seok KO, Kim YJ, Bae WK, Lee S and
Park JH: Involvement of GLTSCR2 in the DNA damage response. Am J
Pathol. 179:1257–1264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Metcalfe JA, Parkhill J, Campbell L,
Stacey M, Biggs P, Byrd PJ and Taylor AM: Accelerated telomere
shortening in ataxia telangiectasia. Nat Genet. 13:350–353. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hande MP, Balajee AS, Tchirkov A,
Wynshaw-Boris A and Lansdorp PM: Extra-chromosomal telomeric DNA in
cells from Atm(−/−) mice and patients with ataxia-telangiectasia.
Hum Mol Genet. 10:519–528. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kishi S, Zho XZ, Ziv Y, Khoo C, Hill DE,
Shiloh Y and Lu LP: Telomeric protein Pin2/TRF1 as an important ATM
target in response to double strand DNA breaks. J Biol Chem.
276:29282–29291. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bradshaw PS, Stavropoulos DJ and Meyn MS:
Human telomeric protein TRF2 associates with genomic double-strand
breaks as an early response to DNA damage. Nat Genet. 37:193–197.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Banerjee PP and Jagadeesh S:
Non-radioactive assay methods for the assessment of telomerase
activity and telomere length. Methods Mol Biol. 1288:305–316. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marone M, Mozzetti S, De Ritis D, Pierelli
L and Scambia G: Semiquentitative RT-PCR analysis to assess the
expression levels of multiple transcripts from the same sample.
Biol Proced Online. 16:19–25. 2001. View
Article : Google Scholar
|
|
17
|
Hahn WC, Stewart SA, Brooks MW, York SG,
Eaton E, Kurachi A, Beijersbergen RL, Kmoll JH, Meyerson M and
Weinberg RA: Inhibition of telomerase limits the growth of human
cancer cells. Nat Med. 5:1164–1170. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim NW, Piatyszek MA, Prowse KR, Harley
CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL and Shay
JW: Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gisselsson D, Björk J, Höglund M, Mertens
F, Dal Cin P, Akerman M and Mandahl N: Abnormal nuclear shape in
solid tumors reflects mitotic instability. Am J Pathol.
158:199–206. 2011. View Article : Google Scholar
|
|
20
|
Horikawa I and Barrett JC: Transcriptional
regulation of the telomerase hTERT gene as a target for cellular
and viral oncogenic mechanisms. Carcinogenesis. 24:1167–1176. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Y, Cheng D, Wang S and Zhu J: Dual
roles of c-Myc in the regulation of hTERT gene. Nucleic Acids Res.
42:10385–10398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Daniel M, Peek GW and Tollefsbol TO:
Regulation of the human catalytic subunit of telomerase (hTERT).
Gene. 498:135–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chebel A, Rouault JP, Urbanowicz I,
Baseggio L, Chien WW, Salles G and Ffrench M: Transcriptional
activation of hTERT, the human telomerase reverse transcriptase, by
nuclear factor of activated T cells. J Biol Chem. 284:35725–35734.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gladych M, Wojtyla A and Rubis B: Human
telomerase expression regulation. Biochem Cell Biol. 89:359–376.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wong JM, Kusdra L and Collins K:
Subnuclear shuttling of human telomerase induced by transformation
and DNA damage. Nat Cell Biol. 4:731–736. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Y, Chen Y, Zhang C, Huang H and
Weissman SM: Nucleolar localization of hTERT protein is associated
with telomerase function. Exp Cell Res. 277:201–209. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mitchell JR, Cheng K and Collins K: A box
H/ACA small nucleolar RNA-lie domain at the human telomerase RNA
3′end. Mol Cell Biol. 19:567–576. 1999. View Article : Google Scholar
|
|
28
|
Lin J, Jin R, Zhang B, Yang PX, Chen H,
Bai YX, Xie Y, Huang C and Huang J: Characterization of a nobel
effect of hPinX1 on hTERT nucleolar localization. Biochem Biophys
Res Commum. 353:946–952. 2007. View Article : Google Scholar
|
|
29
|
Denchi EL: Give me a break: How telomeres
suppress the DNA damage response. DNA Repair (Amst). 8:1118–1126.
2009. View Article : Google Scholar
|
|
30
|
Tanaka H, Mendonca MS, Bradshaw PS, Hoelz
DJ, Malkas LH, Meyn MS and Gilley D: DNA damage-induced
phosphorylation of the human telomerase-associated protein TRF2.
Proc Natl Acad Sci USA. 102:15539–15544. 2005. View Article : Google Scholar
|