Introduction
A period of ischemia is required for a number of
surgical procedures on the liver, including when dealing with
hepatic trauma, resecting large intrahepatic lesions and
transplanting. Ischemia causes tissue damage, however the liver is
subjected to a further insult when restoring the blood supply,
which is termed ischemia-reperfusion (I-R) injury (1). In the hepatic I-R period, several
cellular function changes, including generation of reactive oxygen
species (ROS), inflammatory cytokines and chemokines, may result in
cell injury, triggering the apoptotic pathway and leading to organ
damage or dysfunction (2). As
numerous studies have reported (3–5) that
generation of ROS and oxidant stress are the most vital mechanisms
in I-R injury, the ROS scavengers should be considered as
therapeutic agents for hepatic I-R. Under normal conditions,
endogenous antioxidants, including vitamins C and E, and the
antioxidant enzymes superoxide dismutase (SOD), catalase and
glutathione peroxidase (GPX) have the capacity to scavenge ROS
products. However, in ischemic conditions, these defense mechanism
fail to protect tissues from oxidative damage because of
overproduction of oxygen radicals, inactivation of antioxidant
enzymes and consumption of antioxidants in the ischemic tissue
(6). SOD (7), allopurinol (8), and N-acetylcysteine (9) have all been previously demonstrated
to attenuate hepatic I-R injury.
GPX is a mammalian selenium-containing antioxidant
enzyme, which scavenges peroxides and H2O2
using glutathione (GSH), and is associated with various
ROS-mediated diseases (10). Due
to its poor stability, limited availability and difficulty in
preparation, various artificial GPX mimics have been made as free
radical scavengers, including ebselen (11). Based on the structure of the active
site in bovine GPX (12), the
present study constructed a novel selenocysteine-containing 7-mer
peptide (H-Arg-Sec-Gly-Arg-Asn-Ala-Gln-OH), with GPX activity that
reaches 13 U/µmol, which is 13 times that of ebselen. The
7-mer peptide is advantageous due to low molecular weight, improved
cell membrane permeability, good water-solubility and stability
compared with other previous GPX mimics. Encouraged by these
advantages, the current study evaluated the effects of the 7-mer
peptide on the liver damage induced by hepatic I-R injury using a
rat model.
Materials and methods
Animals
All the animal experiments were performed using a
protocol approved by the Animal Care and Research Committee of
Heilongjiang University of Chinese Medicine (Harbin, China). Clean,
healthy adult male Wistar rats, weighing 200–250 g, were obtained
from the Laboratory Animal Center of Heilongjiang University of
Chinese Medicine, provided with standard pellet food and tap water
in individual cages with 12-h light/dark cycle at 22+2°C.
Animal administration and induction of
liver I-R model
Rats (n=48) were equally and randomly assigned into
four experimental groups as follows: Sham operation group (control
group, n=12), I-R group (physiological saline-treatment for 5 min
prior to I-R, n=12), the 7-mer + I-R group (15 µmol/kg 7-mer
peptide treatment for 5 min prior to I-R, n=12), the 7-mer peptide
control group (15 µmol/kg 7-mer peptide treatment without
I-R, n=12).
The model of partial hepatic I-R was performed using
a published protocol (13). All
rats were fasted for 12 h preceding the operation but provided with
access to drinking water. The rats were anesthetized with an
intraperitoneal injection of 40 mg/kg pentobarbital sodium (8%;
Sigma-Aldrich, St. Louis, MO, USA) and placed supinely on a heating
pad (36–37°C). At 5 min prior to occlusion, the 7-mer peptide (15
µmol/kg body weight) or physiological saline alone was
intravenously injected into the tongue. A midline incision was made
on the abdominal wall of the rats. The left portal vein and hepatic
artery were occluded with a micro-clip for 60 min, then the clip
was removed to initiate hepatic reperfusion. The sham control group
underwent the same protocol without vascular occlusion. Occlusion
was verified visually by the change in liver color to a paler shade
and upon reperfusion to a blush. Rats were sacrificed by cervical
dislocation under anesthesia (8% pentobarbital sodium) 2 h after
reperfusion, blood samples and liver tissue (from the ischemic
lobe) were taken for analysis.
Alanine aminotransferase (ALT; cat. no. C009-1),
aspartate aminotransferase (ASTcat. no. C010-1), lactate
dehydrogenase (LDH; cat. no. A020-1), myeloperoxidase (MPO; cat.
no. A044), malondialdehyde (MDA cat. no. A003-1) and nitric oxide
(NO; cat. no. A012) detection kits were purchased from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). Rabbit
anti-rat B-cell CLL/lymphoma-2 (Bcl-2; cat. no. ZA-0536) and Bcl-2
associated X protein (Bax; cat. no. ZA-0611) monoclonal antibodies
were purchased from Zhongshan Jinqiao Biotechnology Co., Ltd.
(Beijing, China). All other chemicals used were of analytical or
reagent grade.
GPX-like activity assay of the 7-mer
peptide
The 7-mer peptide (H-Arg-Sec-Gly-Arg-Asn-Ala-Gln-OH)
was synthesized by GL Biochem, Ltd. (Shanghai, China). The
catalytic activity was determined by the method of Wilson (14). The reaction was carried out at 37°C
in 700 µl reaction solution containing 50 mM pH 7.0 sodium
phosphate buffer, 1 mM EDTA, 1 mM sodium azide, 1 mM GSH, 0.25 mM
nicotinamide adenine dinucleotide phosphate (NADPH), 1 U of GSH
reductase, 10–50 µM of the mimic. The reaction was initiated
by addition of 0.5 mM H2O2 or CuOOH. The
activity was determined by the decrease of NADPH absorption at 340
nm using a Lambda 750 UV/Visable/Near Infrared spectrophotometer
(PerkinElmer, Inc., Waltham, MA, USA). Background absorption was
run without mimic and was subtracted. The activity unit was defined
as the amount of the mimic that utilized 1 µmol NADPH per
min. The activity was expressed in U/µmol of the GPX
mimic.
Blood chemistry assay
Following the reperfusion period, 5 ml blood was
collected from the abdominal aorta. The blood sample was
centrifuged at 1200 × g for 10 min at room temperature to separate
serum for analysis. Liver injury was assessed by measuring the
levels of ALT (a specific marker of hepatic parenchymal injury),
AST (a nonspecific marker of hepatic injury) and LDH (a marker of
nonspecific cellular injury) in the serum. ALT, AST and LDH were
assayed using assay kits (Nanjing Jiancheng Bioengineering
Institute) according to the manufacturer's instructions with a DG 8
standard biochemistry automatic analyzer (Nanjing Huadong
Electronics Group Medical Equipment Co., Ltd., Nanjing, China).
MPO, MDA, NO assay
Liver tissue (~1 cm3) was obtained 2 h
after reperfusion. The samples were homogenized in 10 ml ice-cold
physiological saline and centrifuged at 12,000 × g for 20 min at
4°C to separate the supernatant, then liver MPO activity, MDA
content, protein content and serum NO content were measured using
assay kits (Nanjing Jiancheng Bioengineering Institute) according
to the manufacturer's instructions.
GPX activity assay
GPX activity was determined using the method of
Wilson (14) using the supernatant
of tissue luminal content. The enzymatic reaction contained the
following components: NADPH, reduced GSH and GSH reductase, and was
initiated by addition of H2O2. The change in
absorbance at 340 nm was monitored by a Lambda 750
UV-Visible-Near-Infrared spectrophotometer (PerkinElmer, Inc.).
Activity was recorded as U/g protein in liver tissue.
Immunohistochemical assay
For immunohistochemical analysis, tissue samples
were obtained 2 h after reperfusion, fixed in 4% paraformaldehyde
for 30 min at room temperature and embedded in paraffin.
Paraffin-embedded tissue sections (5 µm) were cut from each
specimen and then immersed in xylene followed by rehydration in
graded alcohol. The sections were washed with the
phosphate-buffered saline (PBS) for 5 min, fixed in cold acetone
for 10 min, washed with PBS 3×3 min and then incubated with 3%
H2O2 in distilled water for 5 min to quench
any endogenous peroxidase activity and subsequently immersed in PBS
for 5 min. The tissue sections were then blocked with normal goat
serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA,
USA) for 10 min and incubated at 37°C for 2 h with monoclonal
rabbit anti-rat Bcl-2 and Bax antibodies (diluted 1:200) at a
concentration of 5 µg/ml, then washed with PBS 3 times for 3
min each time The expression of Bcl-2 and Bax proteins were
detected by the labeled streptavidin biotin (LSAB) method using an
LSAB kit (Abcam, Cambridge, UK) consisting of a blocking reagent,
biotinylated link antibody and peroxidase-labeled streptavidin
reagents. The peroxidase binding sites were detected using 3′,
3′-diaminobenzidine and images were acquired using an IX-81
microscope (Olympus Corporation, Tokyo, Japan).
Reverse transcription-polymerase chain
reaction (RT-PCR) assay
The tissue samples taken from the rats were
immediately frozen in liquid nitrogen and then stored at −80°C
until use. Total RNA was extracted from liver tissue by the acid
guanidinium thiocyanatephenol-chloroform method and concentration
determined by UV spectrophotometer. RT was performed on RNA (1
µg) and cDNA amplified using One-Step RT-PCR kit (Takara
Biotechnology Co., Ltd., Dalian, China) with the following
conditions: 45°C for 30 min; 94°C for 5 min; 40 cycles at 94°C for
30 sec, 55°C for 30 sec, 72°C for 2 min; and final step of 72°C for
10 min. Primers used in the PCR reactions were as follows: Bcl-2,
sense 5′-CCCCTTCATCCAAGAATGC-3′, antisense
5′-TTCCACAAAGGCATCCCAG-3′, producing a 623 bp product; Bax, sense
5′-CCACCAGCTCTGAACAGTTCA-3′, anti-sense 5′-TGAGGACTCCAGCCACAAAG-3′,
producing a 506 bp product. The PCR reaction products were
separated by electrophoresis on 0.8% agarose gel and stained with
ethidium bromide. Digital images were assessed with image analysis
software and mRNA expressions were evaluated by the band intensity
ratios of Bcl-2 and Bax and presented as percentage of β-actin
levels.
Histological analysis
Liver tissue samples were obtained following
reperfusion and immediately fixed in 4% paraformaldehyde for 4 h.
The samples were subsequently transferred into 70% ethanol then
embedded in paraffin, sectioned (4 µm), and stained with
hematoxylin (for 5 min) and eosin (for 3 min) for examination. The
samples were imaged using an IX-81 microscope.
Electron microscopy analysis
Samples of small bowel tissue were obtained
following reperfusion and immediately fixed in 2.5% glutaraldehyde
at 4°C until they were subsequently fixed in 1% osmic acid. The
fixed tissue was dehydrated in graded alcohol, embedded in Epon 812
epoxy resin, and cut into 4 µm-thin sections using the LKB
super-thin sectioning machine. The sections were stained with
acetate double oxygenic uranium (30 min) and citrate lead (8 min)
for examination under a JEM-1200 EX transmissive electron
microscope.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed using one-way
analysis of variance and post-hoc Student-Newman-Keuls test for
multi-group comparisons with SPSS software (version 19.0; IBM SPSS,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Characterization of the designed 7-mer
peptide
The key for GPX imitation is to generate high
affinity for GSH. Based on the main amino residues in active site
of the bovine GPX, the sequence of the 7-mer peptide was designed
as follows: H-Arg-Sec-Gly-Arg-Asn-OH; this sequence increases Gly
flexibility, Sec is the catalytic site of the 7-mer peptide, Arg
and Asn form salt bridges and a hydrogen bond with the GSH
molecule. Glutamine (Glu) is the most abundant amino acid in the
plasma and is important for modulating inflammatory responses,
oxidative stress and apoptosis (15). It has previously been reported that
Glu protected the gut, heart and skeletal muscle against I-R injury
by preserving the GSH content in the tissues (16). However, the limited solubility and
poor stability hampered the application of Glu as a therapeutic for
I-R injury. Aiming to solve this problem, alanine (Ala) was
employed to enhance the stability and solubility of Glu in the form
of alanyl-Glu dipeptide, which had previously been demonstrated to
protect rats from hepatic I-R injury (17). Thus, in the current study, Ala-Gln
was added to the C-terminal of the peptide in order to improve the
activity and stability of the 7-mer peptide.
GPX-like activity of the 7-mer
peptide
The activity of the 7-mer peptide-catalyzed
reduction of H2O2 by GSH is presented in
Table I. The GPX activity of the
7-mer peptide was 13 U/µmol, which is ~13 times that of
ebselen (0.99 U/µmol), a well-known GPX mimic.
 | Table IGPX activities of the 7-mer peptide
and other GPX mimics. |
Table I
GPX activities of the 7-mer peptide
and other GPX mimics.
| Species | Substrate | Activity
(U/µmol) |
|---|
| 7-mer peptide |
H2O2 | 13.0 |
| Ebselen |
H2O2 | 0.99 |
|
Seleno-cyclodextrin |
H2O2 | 7.4 |
| Native GPX |
H2O2 | 5780 |
Effect of the 7-mer peptide on liver
injury
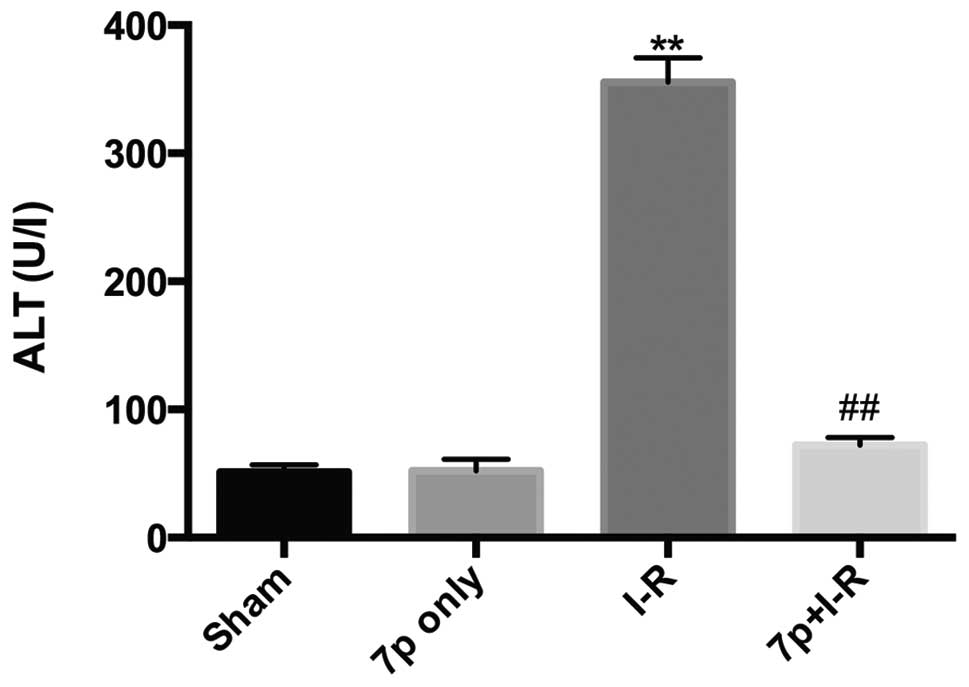
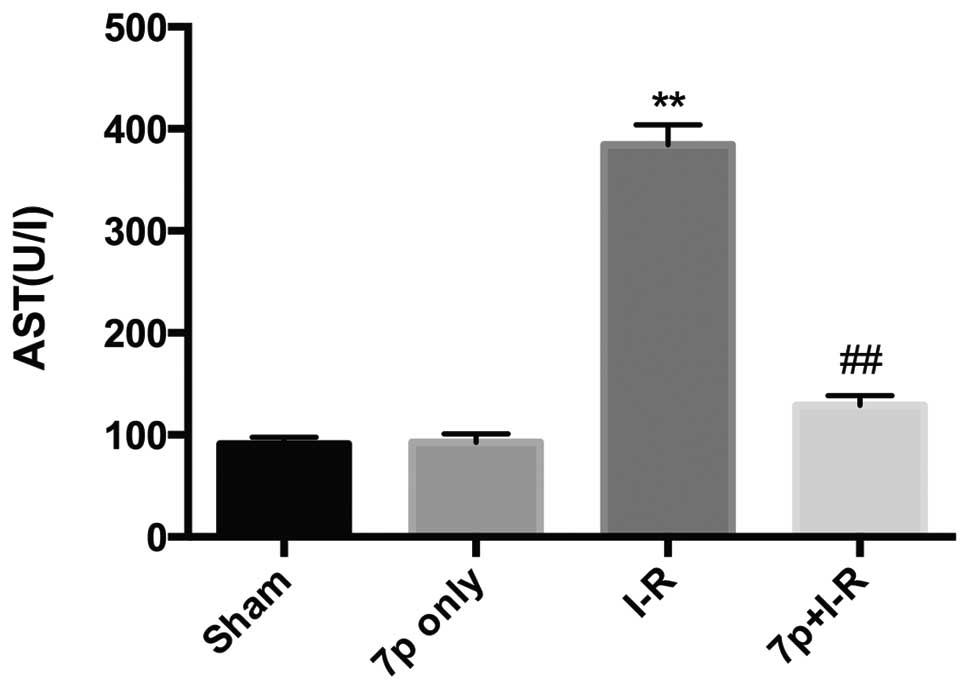
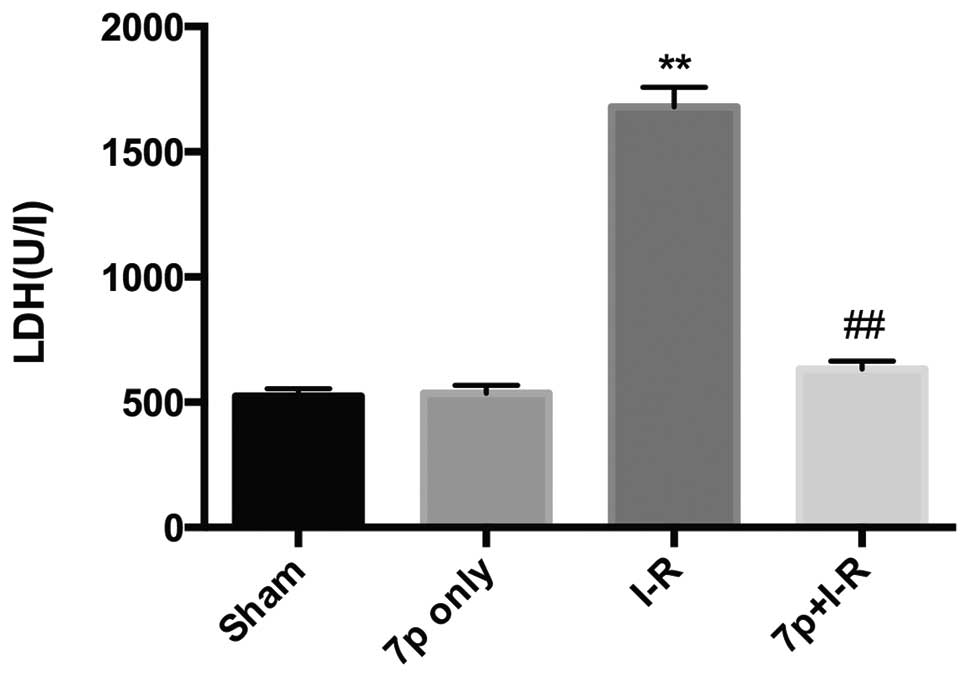
Liver injury was assessed by measuring serum levels
of ALT, AST and LDH. Compared with sham-operated rats, the ALT
(Fig. 1; P<0.01), AST (Fig. 2; P<0.01), LDH (Fig. 3; P<0.01) levels in serum were
significantly increased in I-R liver, which demonstrated the
development of hepatocellular injury. Whereas, for the I-R rats
pretreated with the 7-mer peptide, no significant increase in ALT,
AST and LDH levels were detected. In the 7-mer peptide only group,
no significant effect on ALT, AST and LDH serum levels was
detected, indicating that the 7-mer peptide exerts no I-R injury to
the rats.
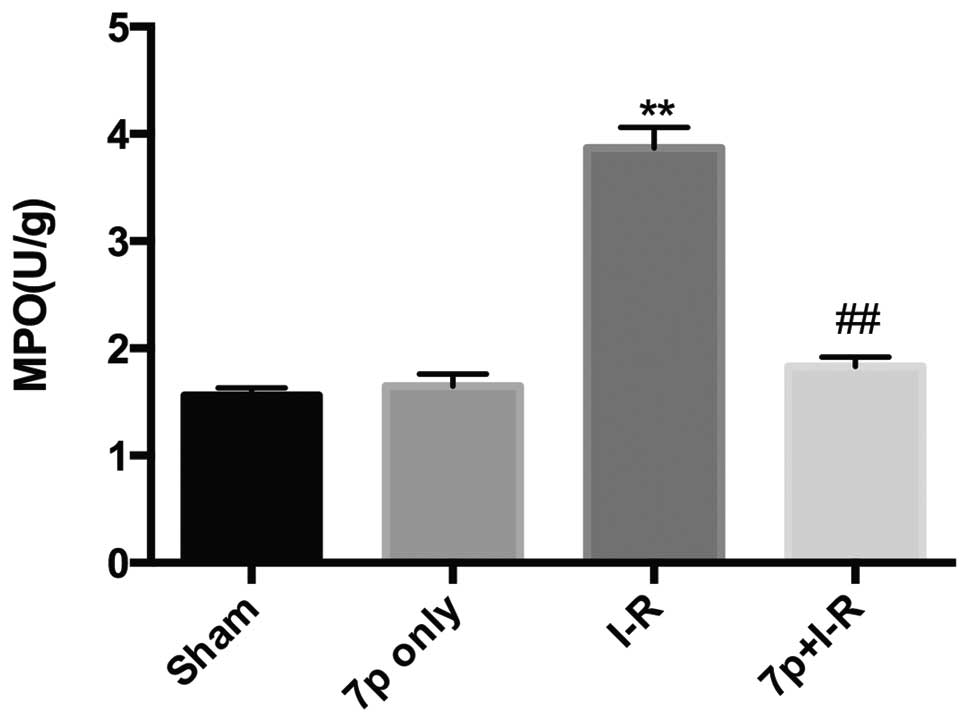
Effect of the 7-mer peptide on hepatic
neutrophil recruitment
MPO activities in liver tissues were analyzed as the
index of hepatic neutrophil recruitment. A unit of MPO activity is
defined as that which degrades 1 µmol
H2O2/min at 25°C. In the I-R group, I-R
caused a significantly increased the MPO activity in the liver
compared with sham control (P<0.01). This increase of MPO
activity following reperfusion in the I-R group was significantly
inhibited by pretreatment of the liver tissues with the 7-mer
peptide (P<0.01; Fig. 4).
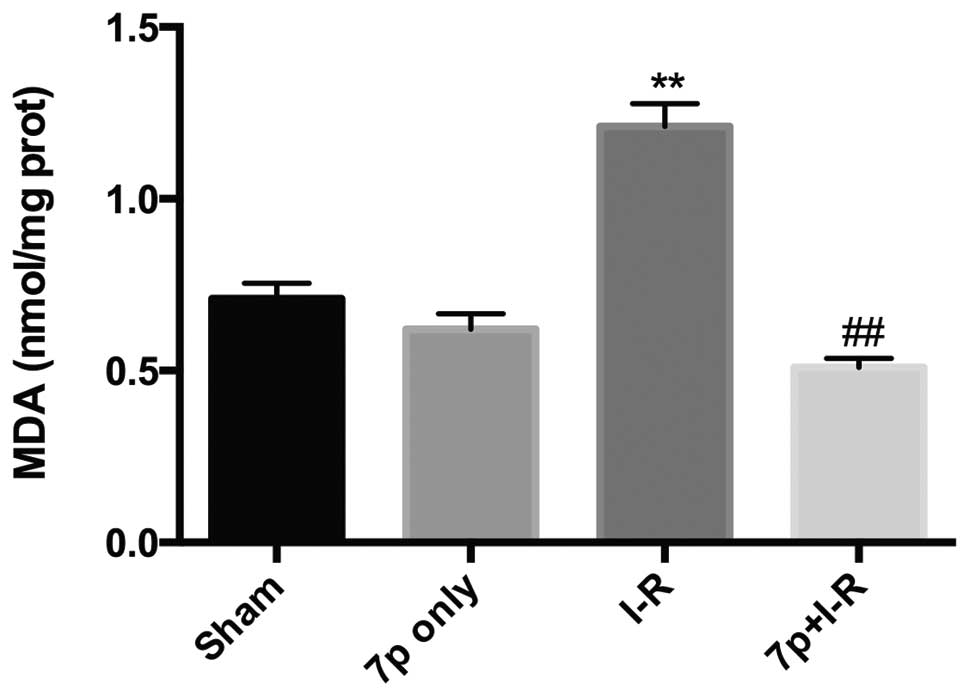
Inhibition of lipid peroxidation by the
7-mer peptide
MDA is the final product of lipid peroxidation,
liver tissues were assayed for MDA content as a marker of hepatic
oxidative stress. Hepatic I-R injury significantly increased the
MDA content in the liver compared with the sham control group
(P<0.01). The I-R-induced increase in MDA level was
significantly prevented by the 7-mer peptide treatment (P<0.01;
Fig. 5).
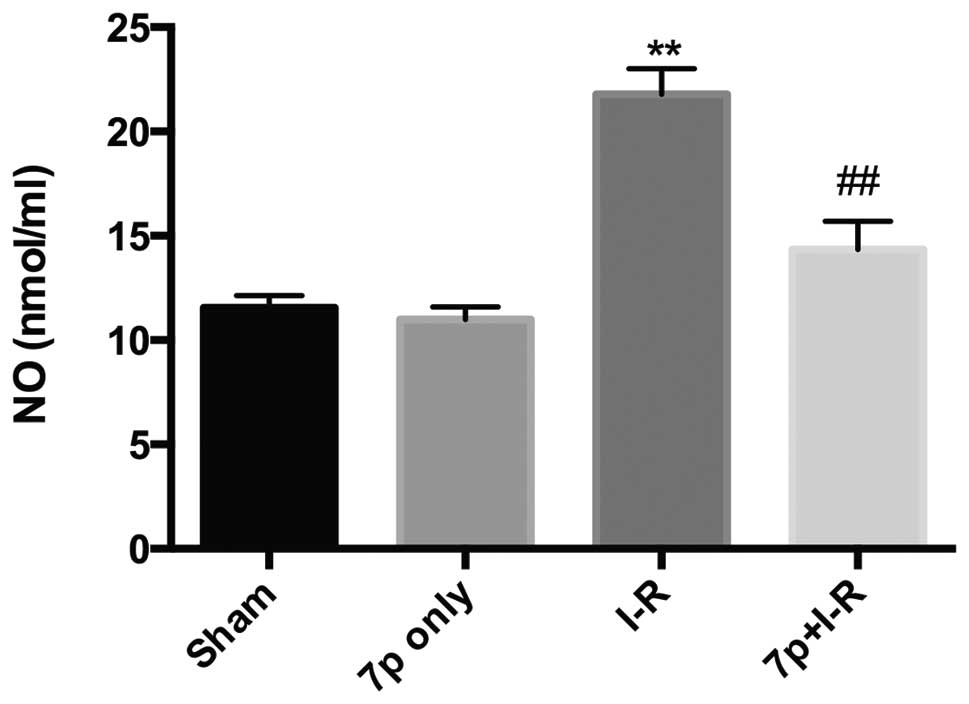
Reduction of NO content by the 7-mer
peptide
NO is a potent vasodilator that reacts with
superoxide to form the oxidant peroxynitrite, which is considered
to be a strong cytotoxic agent. In the current study, NO content
was significantly increased in the hepatic I-R injury group
compared with the sham control group (P<0.01), and the 7-mer
peptide treatment inhibited the increase in NO level caused by I-R
(P<0.01; Fig. 6).
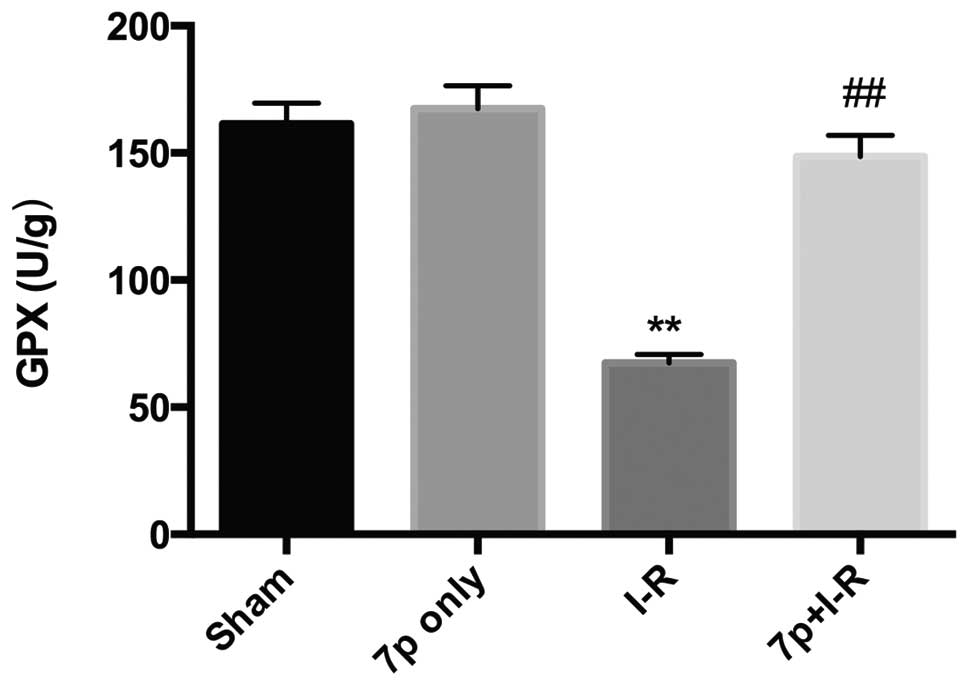
Effect of the 7-mer peptide on liver GPX
activity
GPX, an endogenous antioxidant enzyme, usually
limits damage caused by oxygen derived free radicals, however its
level falls in response to increased free radicals. Following the
2-h reperfusion period in the present study a significant decrease
in GPX activity was observed in the I-R group compared with the
sham control group (P<0.01), whereas the decrease of GPX
activity in the I-R group was inhibited by the 7-mer peptide
treatment (P<0.01; Fig. 7).
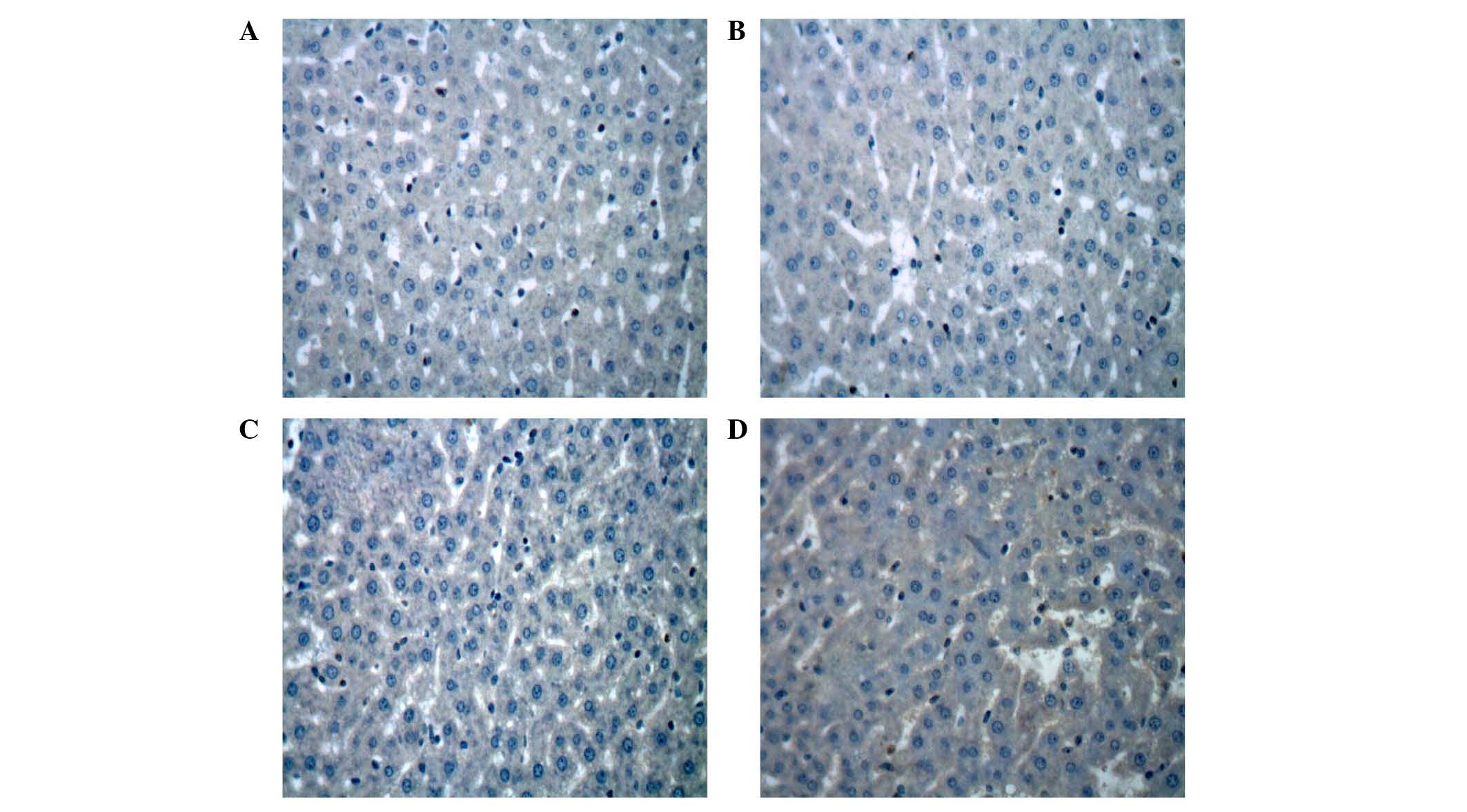
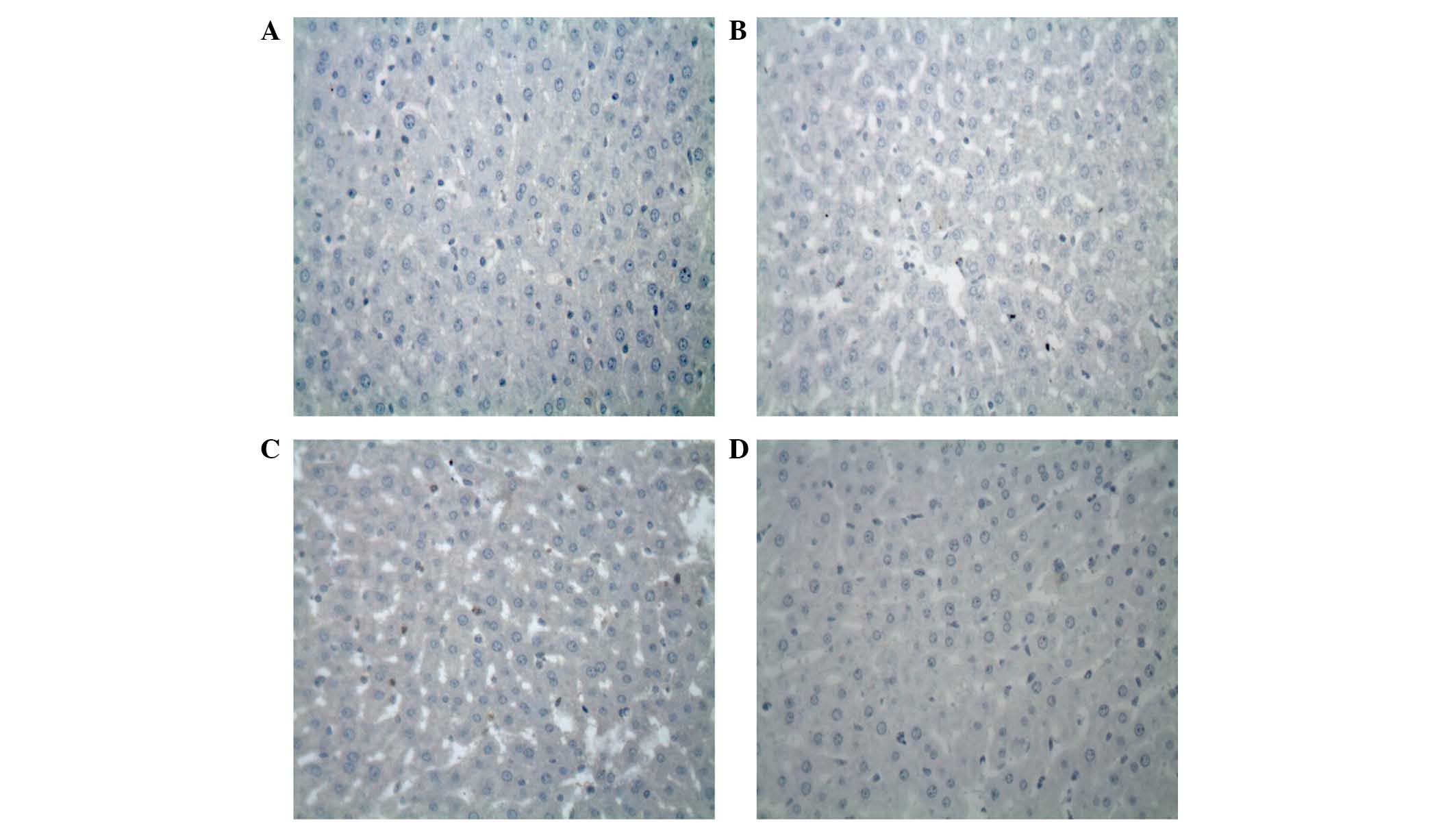
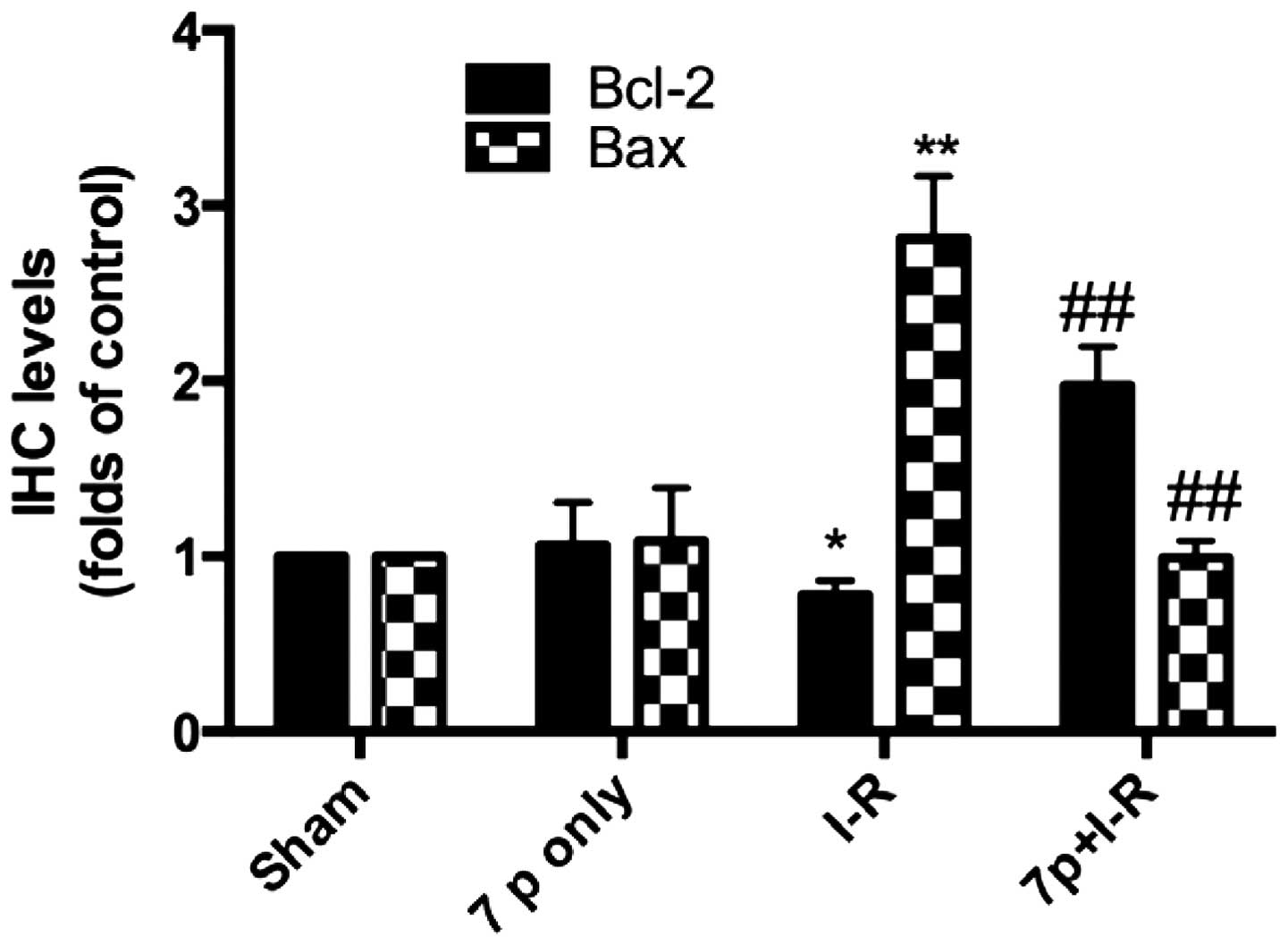
Inhibition of the expression levels of
Bcl-2 and Bax by the 7-mer peptide
The protein expression levels of Bcl-2 and Bax
protein in the liver following I-R were evaluated by
immunohistochemical analysis. In the I-R group, there was weak
expression of Bcl-2 protein (Fig.
8). By contrast, strong immunoreactivity for Bax expression was
observed in the I-R group 2 h after reperfusion compared with the
sham control group (Fig. 9). In
the 7-mer peptide + I-R group, the expression of Bcl-2 was
significantly upregulated compared with the I-R group (P<0.01),
and Bax overexpression was significantly suppressed (P<0.01;
Fig. 10).
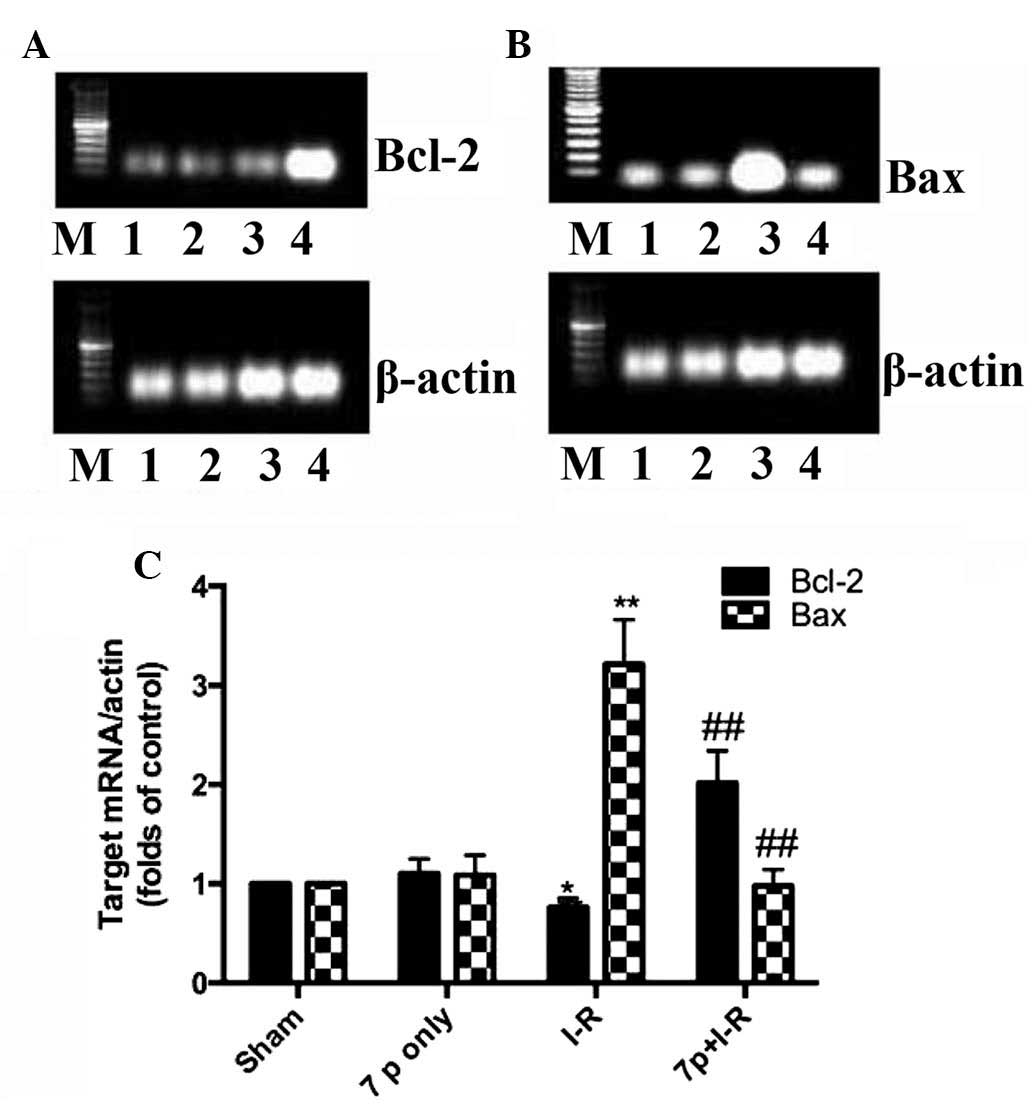
Inhibition of hepatic mRNA expression
levels of Bcl-2 and Bax by the 7-mer peptide
To determine the mRNA expression levels of the
apoptosis regulatory genes, mRNA transcript levels for Bcl-2 and
Bax were assessed by RT-PCR. The band intensity ratios of Bcl-2 and
Bax normalized to β-actin were compared among the sham, I-R and the
7-mer peptide-treated groups. As demonstrated in Fig. 11, hepatic I-R significantly
increased the mRNA expression of Bax compared with sham control
(P<0.01), with the expression of Bcl-2 decreased (P<0.05).
Administration of the 7-mer peptide significantly enhanced the
Bcl-2 mRNA expression compared with the I-R group (P<0.01) and
suppressed hepatic I-R-induced mRNA overexpression of Bax
(P<0.01; Fig. 11).
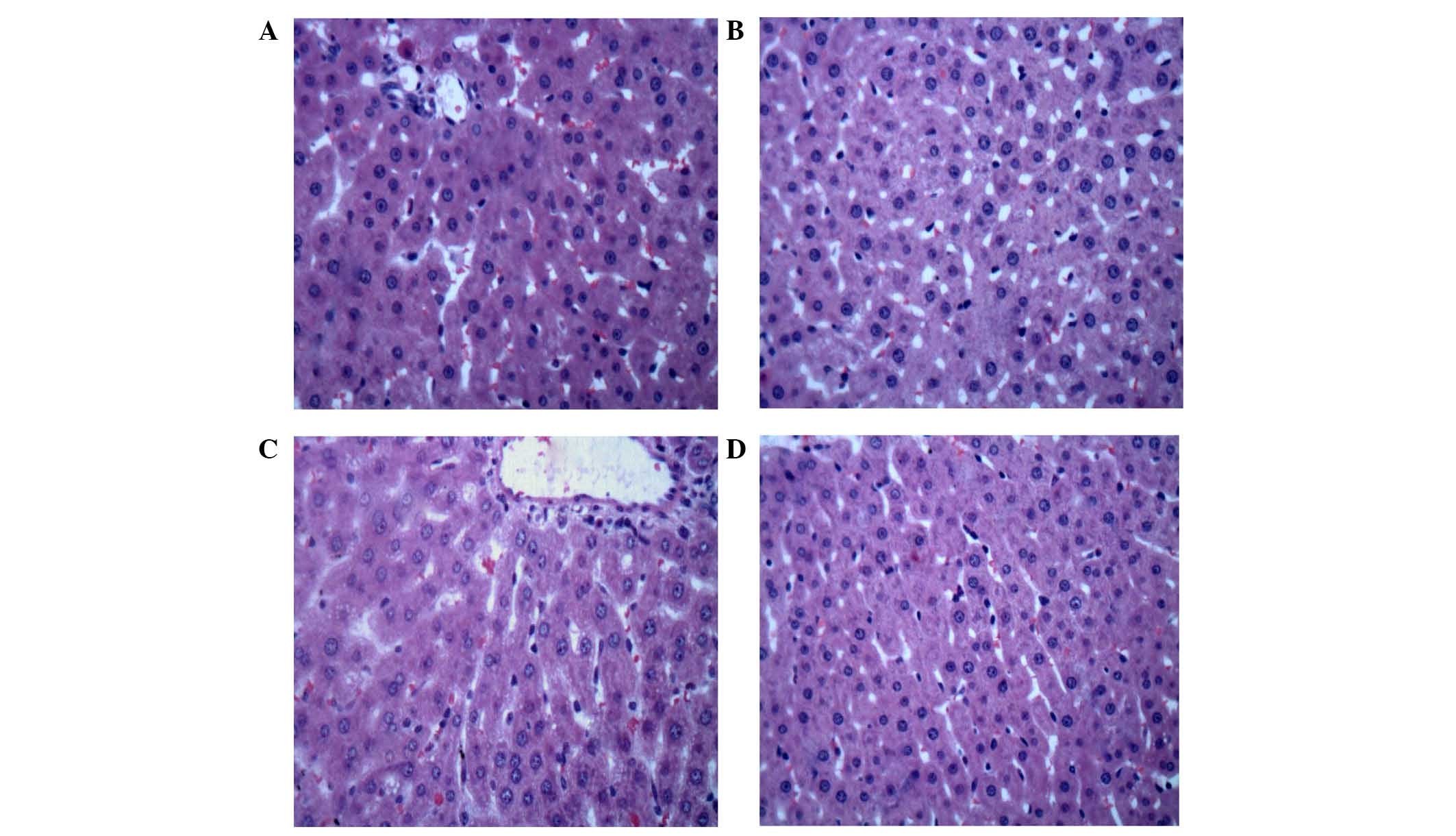
Histological changes
Following 2-h reperfusion, severe swelling induced
by hepatic I-R injury was observed along with abundant fatty and
vacuolation degeneration in hepatocytes compared with the sham
control group. The hepatic sinus compartment became narrow or
disappeared. Hemorrhage and even derangement of cell constitution
were also observed. In the 7-mer peptide-treated I-R group, these
changes were reduced and the hepatic cellular structure remained
clear (Fig. 12).
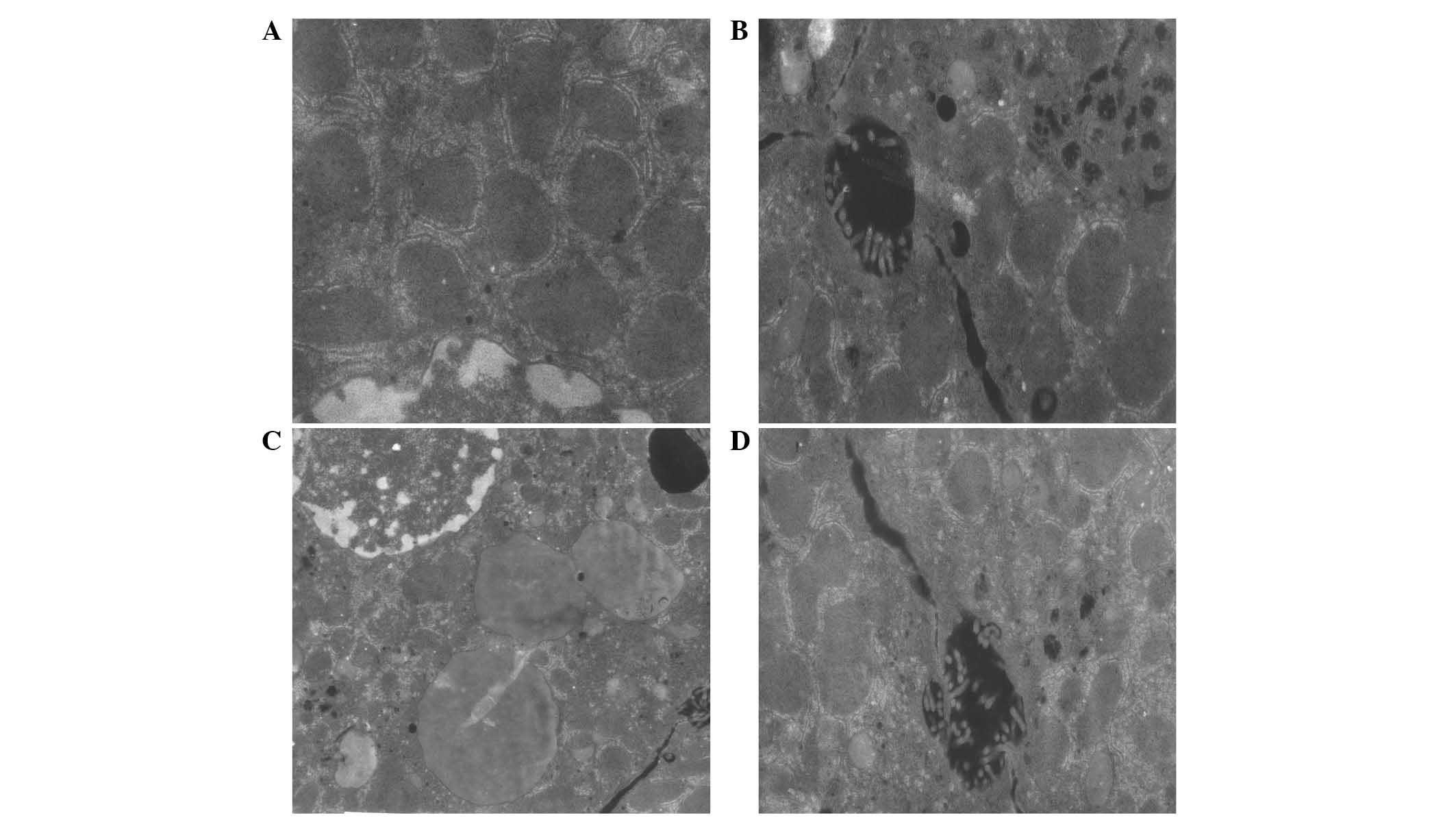
Ultrastructural changes of organelles in
cytoplasm
The ultrastructural changes to the organelles in the
cytoplasm were observed by electron microscopy among the
experimental groups. As demonstrated in Fig. 13, in the I-R injury group, the
rough endoplasmic reticulum was dilated in the majority of cells.
Additionally, the clearance of dying cells was increased and the
mitochondria in the hepatic cytoplasm were severely swollen. The
nuclei were irregular in the I-R injury group, the nucleoli were
visible and agglomeration of heterochromatin was also observed. The
structure of organelles was not clear at 2 h after reperfusion
compared with the sham and 7-mer peptide only groups. In the 7-mer
peptide-treated I-R group, all of the described changes were
observably reduced (Fig. 13).
Discussion
Liver ischemia during arterial occlusion, shock or
organ transplantation is a common cause of hepatocyte death and
liver failure. The ischemic cells manifest distinct biochemical,
structural and functional alterations that finally lead to injury
(18). The are numerous mediators
involved in the pathogenesis of hepatic I-R injury. Among them, ROS
and cell apoptosis are central to this process (19,20).
Previous evidence has supported that ROS are
produced at the moment of reperfusion initiation following hepatic
ischemia, and free radicals are involved in the mechanism of
hepatic graft failure during reperfusion in rats (21). Additionally, oxidative stress is
also demonstrated to be sustained for a long time following
clinical liver transplantation (22). Thus, reduction of hepatic oxidative
stress, including via pretreatment with ROS-scavengers, may
effectively protect liver against I-R injury and transplant
failure.
A novel selenocysteine-containing 7-mer peptide was
constructed in the present study to imitate GPX, an important
antioxidant enzyme, which is an oxygen radical scavenger and has
the capacity to protect cells against oxidative damage. This study
aimed to investigate whether the 7-mer peptide may exert a
protective effect and by consequence be a potential pharmacological
agent for hepatic I-R injury, which has previously been
characterized as a complex process encompassing a number of
mechanisms, including oxidant stress and apoptosis. The results of
the present study indicated that the administration of the 7-mer
peptide attenuated rat liver injury induced by I-R, demonstrated by
the reduction of serum ALT and AST, hepatic MPO activity, MDA
content, NO content, attenuation of histopathological alterations,
inhibition of GPX activity and decreased cell apoptosis. The
results demonstrated that the 7-mer peptide exhibits various
I-R-protective effects via antioxidation and antiapoptosis.
MPO is an enzyme expressed in leukocytes. Tissue MPO
levels may indicate leukocyte infiltration into liver tissue
following I-R (23). According to
the findings of the present study, the 7-mer peptide inhibited the
elevation of MPO activity following reperfusion and consequently
protected against hepatic injury (Fig.
4). Additionally, lipid peroxidation was monitored by measuring
MDA level, which represent from free radical damage to membrane
components of the cells (24).
Treatment with the 7-mer peptide significantly attenuated the
increase of MDA concentration in the tissue (Fig. 5), the effect may be due to its
capacity to eliminate ROS. The increase of superoxide and NO
contents has been previously demonstrated lead to
peroxynitrite-induced injury, and the increased NO production that
occurs during reperfusion by an increase in inducible NO synthase
activity promotes lipid peroxidation and cell damage (25). The current study indicated the
marked elevation in NO level in the hepatic I-R injury was
significantly attenuated by the 7-mer peptide (Fig. 6). This effect on NO generation in
the liver tissue of I-R group supported that generation of NO may
be caused by free radicals under oxidative stress. The 7-mer
peptide, as a novel GPX mimic, protected the liver from the
depletion of GPX activity (Fig.
7).
Oxygen-radical-induced apoptosis has also been
previously reported to be involved in I-R injury and regarded as a
central mechanism of injury during hepatic I-R. A number of genes
regulate the apoptotic process. The family of Bcl-2-associated
proteins are involved in the regulation of apoptosis. Bcl-2, an
anti-apoptotic protein, is known to prevent increased mitochondrial
permeability, release of cytochrome c and various caspases.
As a member of the Bcl-2-associated protein family, Bcl-2 promotes
cell survival through interactions with other Bcl-2 protein family
members (26). Previous studies
have indicated that overexpression of Bcl-2 protein reduced
hepatocellular apoptosis following reperfusion and protected
against hepatic I-R injury (27,28).
Bax, another member of the Bcl-2-associated protein family, forms
homodimers to accelerate cell death or heterodimers with Bcl-2 to
inhibit cell death. Changes in the ratio of Bcl-2 and Bax
expression determine cell survival or death following apoptotic
stimuli (29–31).
The present study examined cell apoptosis via RT-PCR
and immunohistochemistry. According to the results, Bcl-2 was not
observed to be overexpressed in the I-R group compared with the
control, however the Bcl-2 mRNA and protein expression levels were
increased in the 7-mer peptide-treated I-R group. By contrast,
overexpression of Bax in rat liver was observed 2 h after
reperfusion in the I-R group, and the 7-mer peptide pretreatment
returned Bax to normal levels, indicating that the 7-mer peptide
protected against hepatic I-R injury by upregulation of Bcl-2 and
downregulation of Bax to inhibit I-R-induced apoptosis.
In summary, the present study constructed a novel
GPX mimic and demonstrated the protective effect of the 7-mer
peptide on hepatic I-R injury. Its protective mechanisms may be
attributed to its free radical scavenging capacity and
antioxidative activity by reducing the production of ROS and
increasing the activity of GPX. The 7-mer peptide also exhibited
antiapoptotic activity by regulating the expression of apoptotic
genes associated with hepatic I-R. These results suggest that the
7-mer peptide could potentially be used for protecting the liver
against hepatic I-R injury. The 7-mer peptide may be a potent
antioxidant for use in pharmacological investigation.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81573539), the Natural
Science Foundation of Heilongjiang Province (grant no. H2015042),
the Excellent Creative Talents Support Project of Heilongjiang
University of Chinese Medicine (grant no. 2012RCQ12) and the
Research Foundation of Heilongjiang University of Chinese Medicine
(grant no. 201004).
References
|
1
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klune JR and Tsung A: Molecular biology of
liver ischemia/reperfusion injury: Established mechanisms and
recent advancements. Surg Clin North Am. 90:665–677. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taki-Eldin A, Zhou L, Xie HY, Chen KJ, Yu
D, He Y and Zheng SS: Triiodothyronine attenuates hepatic
ischemia/reperfusion injury in a partial hepatectomy model through
inhibition of proinflammatory cytokines, transcription factors, and
adhesion molecules. J Surg Res. 178:646–656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katsumi H, Nishikawa M, Yamashita F and
Hashida M: Prevention of hepatic ischemia/reperfusion injury by
prolonged delivery of nitric oxide to the circulating blood in
mice. Transplantation. 85:264–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katsumi H, Nishikawa M, Yasui H, Yamashita
F and Hashida M: Prevention of ischemia/reperfusion injury by
hepatic targeting of nitric oxide in mice. J Control Release.
140:12–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan PH: Role of oxidants in ischemic
brain damage. Stroke. 27:1124–1129. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mizoe A, Kondo S, Azuma T, Fujioka H,
Tanaka K, Hashida M and Kanematsu T: Preventive effects of
superoxide dismutase derivatives modified with monosaccharides on
reperfusion injury in rat liver transplantation. J Surg Res.
73:160–165. 1997. View Article : Google Scholar
|
|
8
|
Kusumoto K, Morimoto T, Minor T, Uchino J
and Isselhard W: Allopurinol effects in rat liver transplantation
on recovery of energy metabolism and free radical-induced damage.
Eur Surg Res. 27:285–291. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koeppel TA, Lehmann TG, Thies JC, Gehrcke
R, Gebhard MM, Herfarth C, Otto G and Post S: Impact of
N-acetylcysteine on the hepatic microcirculation after orthotopic
liver transplantation. Transplantation. 61:1397–1402. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lubos E, Loscalzo J and Handy DE:
Glutathione peroxidase-1 in health and disease: From molecular
mechanisms to therapeutic opportunities. Antioxid Redox Signal.
15:1957–1997. 2011. View Article : Google Scholar :
|
|
11
|
Sies H: Ebselen, a selenoorganic compound
as glutathione peroxidase mimic. Free Radic Bio Med. 14:313–323.
1993. View Article : Google Scholar
|
|
12
|
Epp O, Landensteine R and Wendel A: The
refined structure of the selenoenzyme glutathione peroxidase at
0.2-nm resolution. Eur J Biochem. 133:51–69. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kohmoto J, Nakao A, Stolz DB, Kaizu T,
Tsung A, Ikeda A, Shimizu H, Takahashi T, Tomiyama K, Sugimoto R,
et al: Carbon monoxide protects rat lung transplants from
ischemia-reperfusion injury via a mechanism involving p38 MAPK
pathway. Am J Transplant. 7:2279–2290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gavish M, Zakut R, Wilckek M and Givol D:
Preparation of a semisynthetic antibody. Biochemistry.
17:1345–1351. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Santos AA, Braga-Neto MB, Oliveira MR,
Freire RS, Barros EB, Santiago TM, Rebelo LM, Mermelstein C, Warren
CA, Guerrant RL and Brito GA: Glutamine and alanyl-glutamine
increase RhoA expression and reduce Clostridium difficile
toxin-a-induced intestinal epithelial cell damage. Biomed Res Int.
2013:1520522013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jia CJ, Dai CL, Zhang X, Cui K, Xu F and
Xu YQ: Alanyl-glutamine dipeptide inhibits hepatic
ischemia-reperfusion injury in rats. World J Gastroenterol.
12:1373–1378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Braga-Neto MB, Oliveira BM, Rodrigues RS,
Noronha FJ, Leitao RF, Brito GA, Lima AA, Guerrant RL and Warren
CA: Protective effects of alanyl-glutamine supplementation against
nelfinavir-induced epithelial impairment in IEC-6 cells and in
mouse intestinal mucosa. Cancer Biol Ther. 13:1482–1490. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dobashi K, Ghosh B, Orak JK, Singh I and
Singh AK: Kidney ischemia-reperfusion: Modulation of antioxidant
defenses. Mol Cell Biochem. 205:1–11. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HJ, Joe Y, Kong JS, Jeong SO, Cho GJ,
Ryter SW and Chung HT: Carbon monoxide protects against hepatic
ischemia/reperfusion injury via ROS-dependent Akt signaling and
inhibition of glycogen synthase kinase 3ß. Oxid Med Cell Longev.
2013:3064212013. View Article : Google Scholar
|
|
20
|
Pan S, Liu L, Pan H, Ma Y, Wang D, Kang K,
Wang J, Sun B, Sun X and Jiang H: Protective effects of
hydroxytyrosol on liver ischemia/reperfusion injury in mice. Mol
Nutr Food Res. 57:1218–1227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Connor HD, Gao W, Nukina S, Lemasters JJ,
Mason RP and Thurman RG: Evidence that free radicals are involved
in graft failure following orthotopic liver transplantation in the
rat-an electron paramagnetic resonance spin trapping study.
Transplantation. 54:199–204. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burke A, FitzGerald GA and Lucey MR: A
prospective analysis of oxidative stress and liver transplantation.
Transplantation. 74:217–221. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang XL, Liu HR, Tao L, Liang F, Yan L,
Zhao RR, Lopez BL, Christopher TA and Ma XL: Role of iNOS-derived
reactive nitrogen species and resultant nitrative stress in
leukocytes-induced cardiomyocyte apoptosis after myocardial
ischemia/reperfusion. Apoptosis. 12:1209–1217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Vries DK, Kortekaas KA, Tsikas D,
Wijermars LG, van Noorden CJ, Suchy MT, Cobbaert CM, Klautz RJ,
Schaapherder AF and Lindeman JH: Oxidative damage in clinical
ischemia/reperfusion injury: A reappraisal. Antioxid Redox Signal.
19:535–545. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Simonsen U, Rodriguez-Rodriguez R,
Dalsgaard T, Buus NH and Stankevicius E: Novel approaches to
improving endothelium-dependent nitric oxide-mediated
vasodilatation. Pharmacol Rep. 61:105–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sheng M, Zhou Y, Yu W, Weng Y, Xu R and Du
H: Protective effect of Berberine pretreatment in hepatic
ischemia/reperfusion injury of rat. Transplant Proc. 47:275–282.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang C, Chen K, Xia Y, Dai W, Wang F, Shen
M, Cheng P, Wang J, Lu J, Zhang Y, et al: N-acetylcysteine
attenuates ischemia-reperfusion-induced apoptosis and autophagy in
mouse liver via regulation of the ROS/JNK/Bcl-2 pathway. PLoS One.
9:e1088552014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao J, Ming Y, Wan Q, Ye S, Xie S, Zhu Y,
Wang Y, Zhong Z, Li L and Ye Q: Gypenoside attenuates hepatic
ischemia/reperfusion injury in mice via anti-oxidative and
anti-apoptotic bioactivities. Exp Ther Med. 7:1388–1392.
2014.PubMed/NCBI
|
|
29
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bartling B, Holtz J and Darmer D:
Contribution of myocyte apoptosis to myocardial infarction? Basic
Res Cardiol. 93:71–84. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin S and Dai CL: Attenuation of
reperfusion-induced hepatocyte apoptosis in associated with
reversed bcl-2/bax ratio in hemi-hepatic artery-preserved portal
occlusion. J Surg Res. 174:298–304. 2012. View Article : Google Scholar
|