Introduction
Tumor growth depends on the formation and
maintenance of a vascular network in neoplastic tissues to ensure
delivery of oxygen and nutrients to malignant cells. The growth of
new blood vessels from existing ones, termed angiogenesis, is a
multifactorial process required for the growth and metastasis of
tumors. Growing tumors have to stimulate new blood vessel formation
in order to obtain sufficient oxygen and nutrients and to discard
of waste products. Inhibition angiogenesis is a promising strategy
in cancer therapy (1,2).
Targeting angiogenesis may add to the therapeutic
effect conventional cancer therapeutic strategies, including
chemotherapeutic agents and radiation therapy, as they are not yet
entirely effective against cancer. Angiogenesis is stringently
controlled via the balance of pro-angiogenic and anti-angiogenic
factors, which are diffusible chemical signal molecules secreted
from tumor cells. Angiogenesis may be initiated by altering the net
balance between positive and negative regulators via increased
production of any one of the positive regulators of angiogenesis,
including vascular endothelial growth factor (VEGF), fibroblast
growth factor-2, interleukin-8, placental growth factor,
trans-forming growth factor (TGF) β, platelet derived growth
factor, or angiopoietins, or downregulation of endogenous
inhibitors of angiogenesis, including endostatin (ES), angiostatin
and thrombospondin (3). Thus, it
is possible to suppress angiogenesis adequately by enabling the
predominance of anti-angiogenic factors surrounding the tumor.
Anti-angiogenic therapy would also allow administration of
anti-cancer therapeutic agents at a lower dose for shorter periods
compared with conventional therapies (4).
ES was first identified in and purified from
conditioned medium of cultured murine hemangioendothelioma cells in
1997 as a 20-kDa non-collagenous, proteolytically cleaved
carboxyl-terminal fragment of collagen XVIII, a basement membrane
and vessel wall protein. Generation of ES from collagen XVIII
involves numerous proteases, including cathepsin L, elastase and
matrilysin (5,6). ES exerts its anti-angiogenic activity
either by inhibiting endothelial cell adhesion, migration, and
proliferation or by inducing apoptosis. ES has been demonstrated to
inhibit endothelial cell proliferation, migration, and survival
partially by blocking VEGF receptor 2 signaling, repressing Wnt
signaling, activating caspase-9 and inhibiting B-cell lymphoma 2,
B-cell lymphoma-extra large by destabilizing the structure of
β-catenin, and by altering the β-catenin/vascular endothelial
cadherin interactions in inter-endothelial cell junctions (5,7–10).
Furthermore, ES has been shown to regulate a range of genes that
suppress angiogenesis, and to inactivate metalloproteinases
(11,12). Matrix metalloproteinases (MMPs) are
proteolytic zinc-dependent endopeptidases involved in cancer
progression. They are important in cancer cell growth, migration,
invasion and metastasis. They degrade basement membranes and
facilitate the invasion of cancer cells. Notably, they may also
generate novel peptides and/or proteins that have different
functions than their precursors (13).
Cell surface neutral endopeptidases, also termed
EC3.4.24.11, enkephalinase, cluster of differentiation (CD)10 or
neprilysin (NEP) is important in the degradation of amyloid β
peptides (Aβ), a characteristic feature of Alzheimer's disease (AD)
(14). NEP is also important in
pulmonary development, inflammation and injury (15). Furthermore, NEP has been
demonstrated to contribute to tumor progression in human lung,
prostate and breast cancer (15–19).
A disintegrin and metalloproteinases (ADAMs) have
been described as membrane-anchored cell surface proteins
consisting of >40 identified family members in the mammalian
genome (20,21). Among all ADAM proteins, only 12
ADAM genes encode for proteinase activities, these are ADAM8, 9,
10, 12, 15, 17, 19, 20, 21, 28, 30 and 33. ADAM10 and 17 are the
most extensively examined family members (21). ADAM17 is known to release soluble
tumor necrosis factor-α (TNF-α) from its membrane precursor, thus
called tumor necrosis factor-α convertase (TACE). It is reported
that ADAM9, ADAM10 and ADAM17 cleave amyloid precursor protein at
the α-secretase processing site by their α-secretase activity
(22).
ADAM10 and NEP enzymes hydrolyze substance P (SP) at
identical sites (23). SP is a
member of the tachykinin family, encoded by the preprotachykinin Al
gene (24) and has been indicated
to be involved in the generation or progression of various
physiological and pathophysiological conditions, including pain and
depression, in addition to a variety of neurodegenerative
disorders, such as AD, Parkinson's disease, Huntington's disease,
and schizophrenia (25–28). In additions to the function of SP
as a neurotransmitter and neuromodulator (29), SP also stimulates cell
proliferation of various normal and neoplastic cell types in
vitro (30). Although SP is
considered to be associated with carcinogenesis, the role of SP
appears to be bidirectional on inflammation, tumor growth and
carcinogenesis as the intact peptide is tumorigenic and induces
inflammation, whereas the hydrolysis fragments produced by
peptidases are antitumorigenic and anti-angiogenic (31).
Previously, we reported that ES inhibits the in
vitro growth of breast cancer cells and potentiates the
anti-tumor effects of radiotherapy (RT) at appropriate doses via
alteration of the quantity of substance P (32). The aim of the present study is to
elucidate whether ES, either alone or in combination with RT, is
able to alter the activity and/or expression level of ADAM-10 and
NEP, which are termed SP degrading proteases.
Materials and methods
Recombinant murine ES
Recombinant murine ES, expressed in Pichia
pastoris, in citrate phosphate buffer (17 mM citric acid, 66 mM
sodium phosphate dibasic, 59 mM sodium chloride, at pH 6.2) was
purchased from Sigma-Aldrich (St. Louis, MO, USA). The ES was
thawed, gently mixed and aliquoted into standard micro Eppendorf
tubes in quantities of 10 μl for daily assays. These
aliquots were stored at −70°C until needed.
Cell lines and in vitro culture
conditions
The 4T1 breast cancer cells and 4THMpc (4T1 heart
metastases post-capsaicin) cell line derived from cardiac
metastases of 4T1 cells were used in the present study. The two
cell lines were provided by Dr Nuray Erin at Akdeniz University,
Medicine Faculty (Antalya, Turkey). The cells were grown in
Dulbecco's modified Eagle's medium/F12 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 5% fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.), 2 mM
L-glutamine, 1 mM sodium pyruvate, and 0.02 mM non-essential amino
acids. The cell lines were maintained at 37°C in a humidified
atmosphere of 5% CO2. Cells were passaged at 80–90%
confluency using a 2 mM EDTA solution in Ca2+
Mg2+ free Dulbecco's phosphate-buffered saline (PBS).
All cell lines used in the current study were tested and
demonstrated to be free of mycoplasma contamination (32).
Radiotherapy
Each cell plate (2-cm thick) was irradiated in the
Co-60 teletherapy unit at a distance of 100 cm. To achieve a
homogeneous dose (+2.5%) at the cell plate, the plate was embedded
in water equivalent bolus material and a 0.5-cm thick bolus
material was placed on the cover of the plate. The optimal dose of
irradiation was found to be 45 Gy at 1.5 cm (in the center of the
plate) and the dose rate at RT was ~145 cGy/min (32).
Determination of the cytotoxic dose
The cytotoxic effect of ES alone or in combination
with RT on 4T1 and 4THMpc mouse breast cancer cell lines was
determined in our previous study using the MTT colorimetric assay
(Promega Corporation, Madison, WI, USA), the trypan blue dye
exclusion method (Sigma-Aldrich) and the LIVE/DEAD® Cell
Viability assay (Invitrogen; Thermo Fisher Scientific, Inc.)
following 24, 48 and 72 h of incubation. An EnzCheck®
Caspase-3 Enzyme Activity assay (Thermo Fisher Scientific, Inc.)
was also performed to determine whether ES and RT, alone or in
combination result in apoptosis (32).
Assay of α-secretase activity
α-Secretase activity was measured using a
fluorimetric SensoLyte™ 520 TACE (α-Secretase) Activity assay kit
(AnaSpec Inc., Fremont, CA, USA) according to the manufacturer's
protocols. Briefly, cells were seeded into sixteen different petri
dishes (100×20 mm) at a density of 3×105 cell/ml. After
24 h, 4 μg/ml ES was added to only eight of sixteen petri
dishes. After 4 h, the control, RT, and ES + RT petri dishes were
irradiated with 45 Gy 60Co. Following incubation for 24
h, cells were homogenized in lysis buffer (containing 50 mM
Tris-HCl pH 7.6, 150 mM NaCl, 5 mM CaCl2, 0.05% Brij-35,
0.02% NaN3, and 1% Triton X-100), and centrifuged at
12,000 × g for 10 min at 4°C. The supernatants were collected and
the protein contents of samples were determined by Bio-Rad Protein
assay kit (Bio-Rad Laboratories, Inc., Hercules, CA) based on the
Bradford method. Equal quantities of total protein (100 μg)
was mixed with assay buffer and TACE substrate to a final volume of
100 μl. The change in fluorescence was continuously
monitored with a luminescence spectrometer (Model LS55;
PerkinElmer, Inc., Beaconsfield, UK) at excitation wavelength of
490 nm and emission wavelength of 520 nm.
Assay of NEP activity
ES and/or RT were administered in the same manner as
described above. At the end of incubation period, mediums were
removed and cells were mechanically harvested using 500 μl
sterile PBS and a cell scraper (BD Biosciences, Franklin Lakes, NJ,
USA). Cells were homogenized in 250 μl of 50 mM Tris-HCl
buffer (pH 7.4). The homogenate was then centrifuged at 1,000 × g
for 10 min at 4°C to remove crude debris and to collect
supernatants. The protein concentration of the samples was
determined with a Bio-Rad Protein assay kit and measured against
bovine serum albumin (Invitrogen; Thermo Fisher Scientific, Inc.)
standards. The activity of NEP was determined as described
previously with minor modifications (33). Briefly, substrate solutions
consisting of 1 mM DAGPNG and 10 mM enalaprile in 50 mM Tris-HCl
with and without the addition of 10 mM phosphoramidon, a NEP
inhibitor (19), were prepared.
The substrate solutions were pre-incubated at 37°C for 20 min. The
samples (50 μg) were then incubated at 37°C for 20 min with
100 ml of each substrate solution. The reaction was stopped by
boiling for 10 min at 90°C. The samples were then diluted 1:10 with
50 mM Tris-HCl and spun for 5 min in a microfuge at 13,190 × g at
4°C. The change in fluorescence of the supernatants was monitored
with a luminescence spectrometer (Model LS55) at an emission
wavelength of 562 nm and an excitation wavelength of 342 nm.
Western blotting
To investigate whether changes in NEP and ADAM10
activities of 4T1 and 4THMpc cells were due to changes in protein
content, cell homogenates were assayed by western blotting as
described previously (32).
Briefly, 25 μg of homogenate protein were separated on a 10%
acrylamide gel by SDS-PAGE and then transferred to polyvinylidene
difluoride membranes (Hybond-P; GE Healthcare Life Sciences, Little
Chalfont, UK) with a semi-dry transfer apparatus. The membranes
were blocked with 5% milk in Tris-buffered saline and then probed
with rabbit polyclonal anti-NEP (1:2,000; EMD Millipore, Billerica,
MA, USA; cat. no. AB5458) or polyclonal anti-ADAM10 (1:1,000; EMD
Millipore; cat. no. AB19026) at room temperature for 1 h. The
membranes were washed four times with Tris-buffered saline and
Tween 20 solution. The primary antibody was detected with
horseradish peroxidase-conjugated goat anti-mouse secondary
antibody (1:10,000 for ADAM10, 1:20,000 for NEP; Santa Cruz
Biotechnology, Santa Cruz, CA; cat. no. sc-2005) and the blots were
visualized with a chemiluminescent substrate (ECL Plus kit; GE
Healthcare Life Sciences) and exposure to film (Sigma-Aldrich).
Kaleidoscope™ protein standards (Bio-Rad Laboratories, Inc.) were
used to determine the molecular weights of the visualized
bands.
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Data analysis was performed using a Instat 3.1
professional statistics software program (Graph Pad Software, Inc.,
La Jolla, CA, USA). Analysis of variance with Dunnett's multiple
comparisons post-test and t-tests (for comparisons between two
groups) were used for intergroup comparisons. The graphs were drawn
using Sigma Plot version 10.0 (Systat Software, Inc., Chicago, IL,
USA) and CorelDRAW version X5 (Corel Corporation, Ontario, ON,
Canada). P<0.05 was considered to indicate a statistically
significant difference.
Results
Determining the cytotoxic effects of ES
alone or in combination with RT
In our initial study, different concentrations of ES
(0.5, 1, 2, 4 and 8 μg/ml) and different fractions of
irradiation (5–45 Gy 60Co), either alone or in
combination, were tested to determine the optimum cytotoxic doses
for 4T1 and 4THMpc breast cancer cells. According to four different
cytotoxicity test results, 4 μg/ml ES and 45 Gy
60Co irradiation exhibited the most notable cytotoxic
effect on the 4T1 and 4THMpc cell lines (32). These optimum doses were used to
determine the possible effects of ES and/or RT on ADAM and NEP
activity in 4T1 and 4THMpc cells in the present study.
Activity of ADAM10 and NEP enzymes
Activity of ADAM10 and NEP were examined at the end
of 24 h following ES and/or RT administration to investigate the
association between the quantity of SP and the activity and/or
quantity of these enzymes. To characterize the activity of ADAM10
responsible for SP cleavage, α-secretase activity in the 4T1 and
4THMpc cell lines was investigated by enzyme-linked immunosorbent
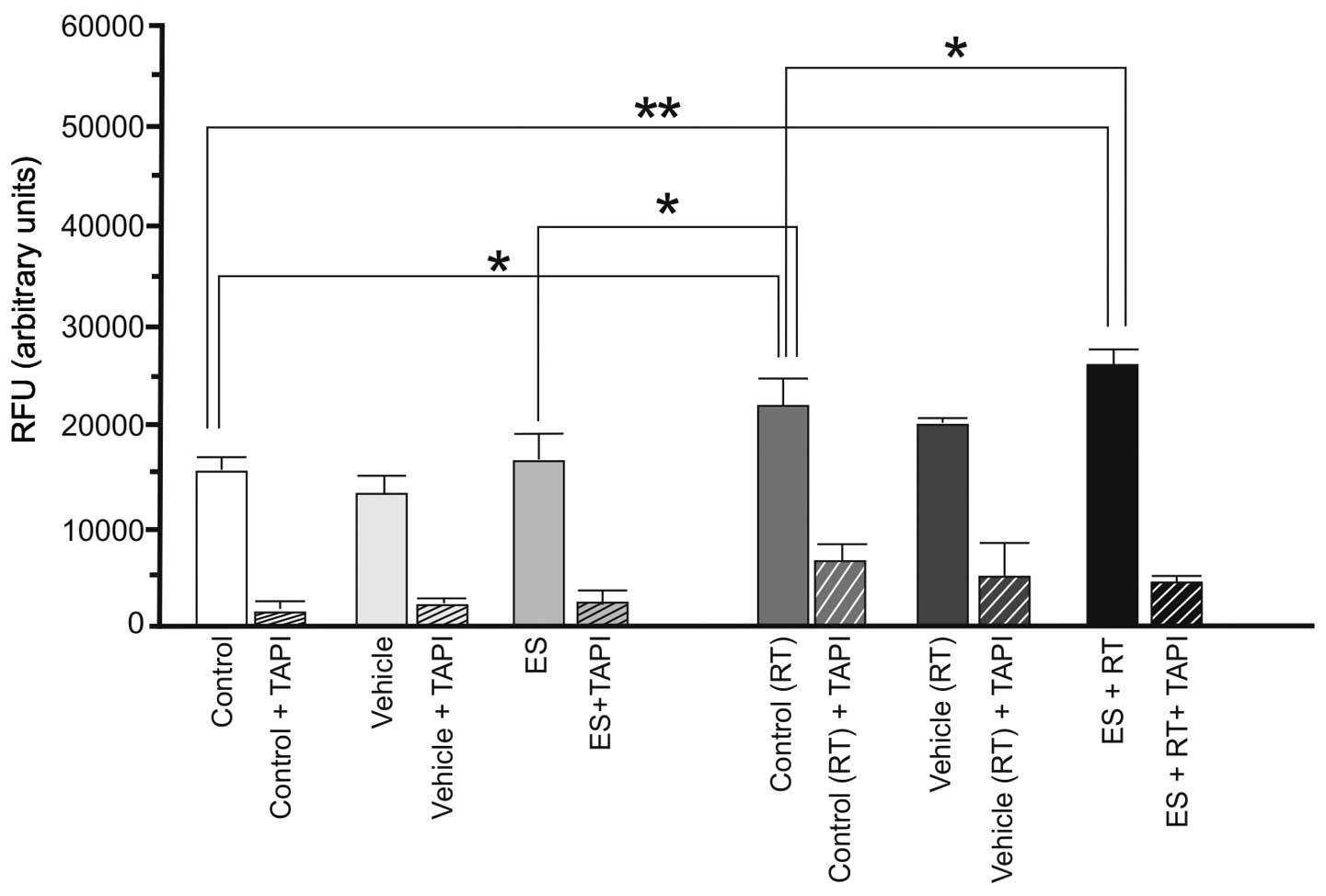
assay using a TACE inhibitor (TAPI). According to the results, ES
alone did not alter basal α-secretase activity of 4T1 cells
(P>0.05). RT alone resulted in a marked increase in α-secretase
activity (P<0.05). However, the increase in enzyme activity was
greater (P<0.01) with administration of ES and RT together
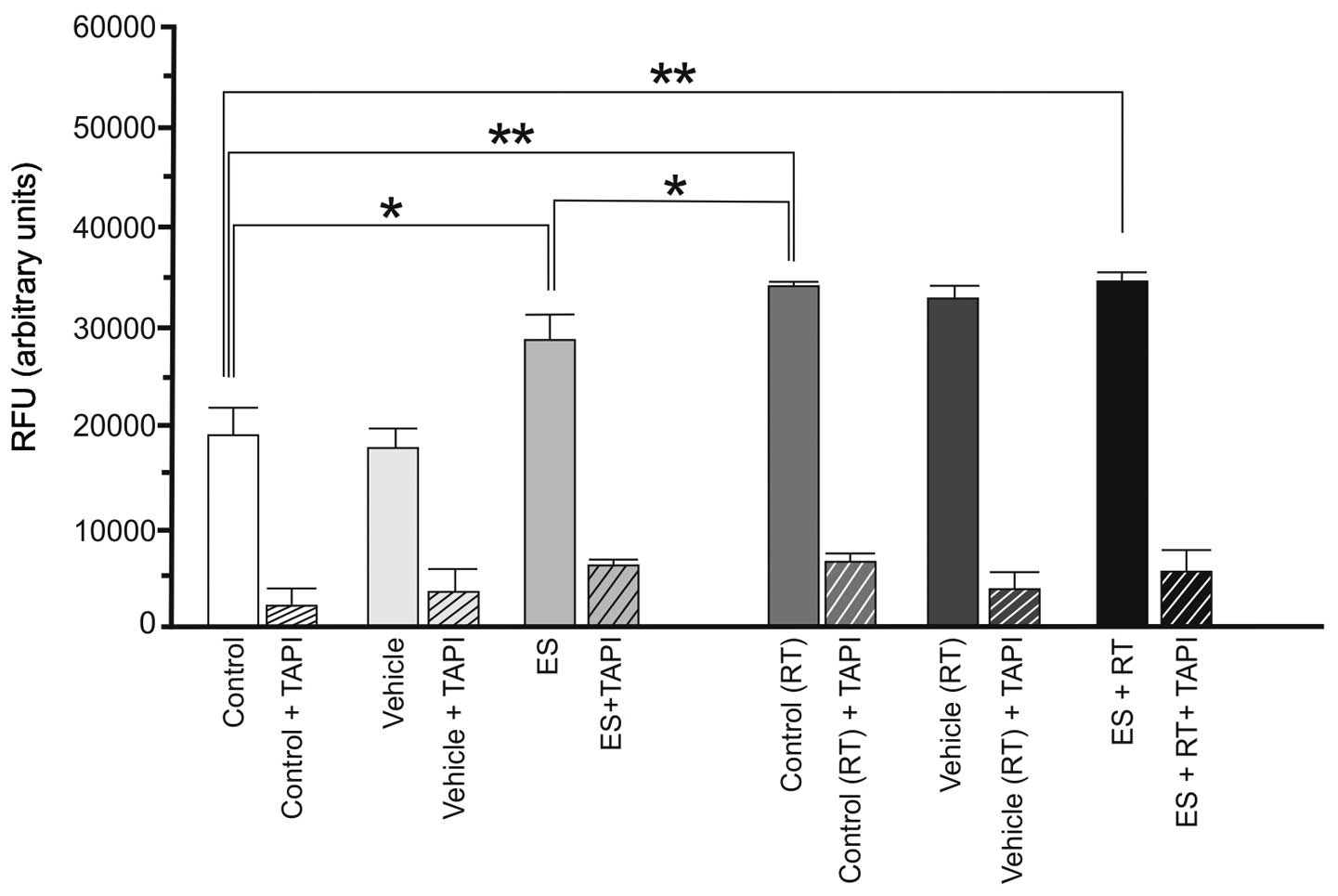
(Fig. 1). The 4THMpc cells treated
with ES, RT or ES + RT were demonstrated to already have a high
basal level of α-secretase compared with the 4T1 cells. By contrast
to the 4T1 cells, ES (P<0.05), RT (P<0.01) and ES + RT
(P<0.01) increased the activity of α-secretase in 4THMpc cells
(Fig. 2).
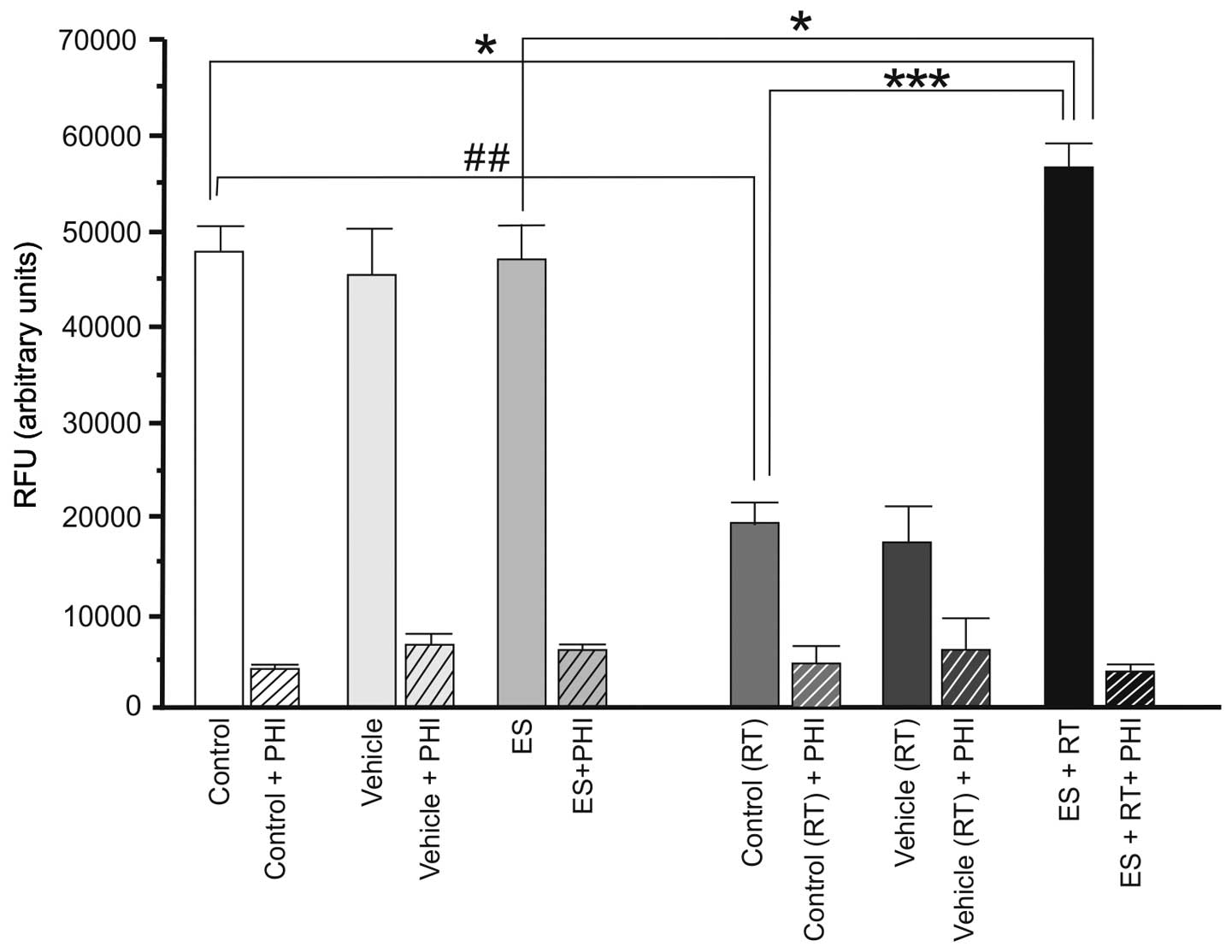
The basal level of NEP enzyme activity was higher in
4T1 cells compared with 4THMpc cells. As presented in Fig. 3, in 4T1 cells ES alone did not
alter NEP activity (P>0.05). However, RT alone resulted in a
significant decrease in NEP activity of 4T1 cells (P<0.01). RT +
ES resulted in a significant increase in NEP activity (P<0.05).
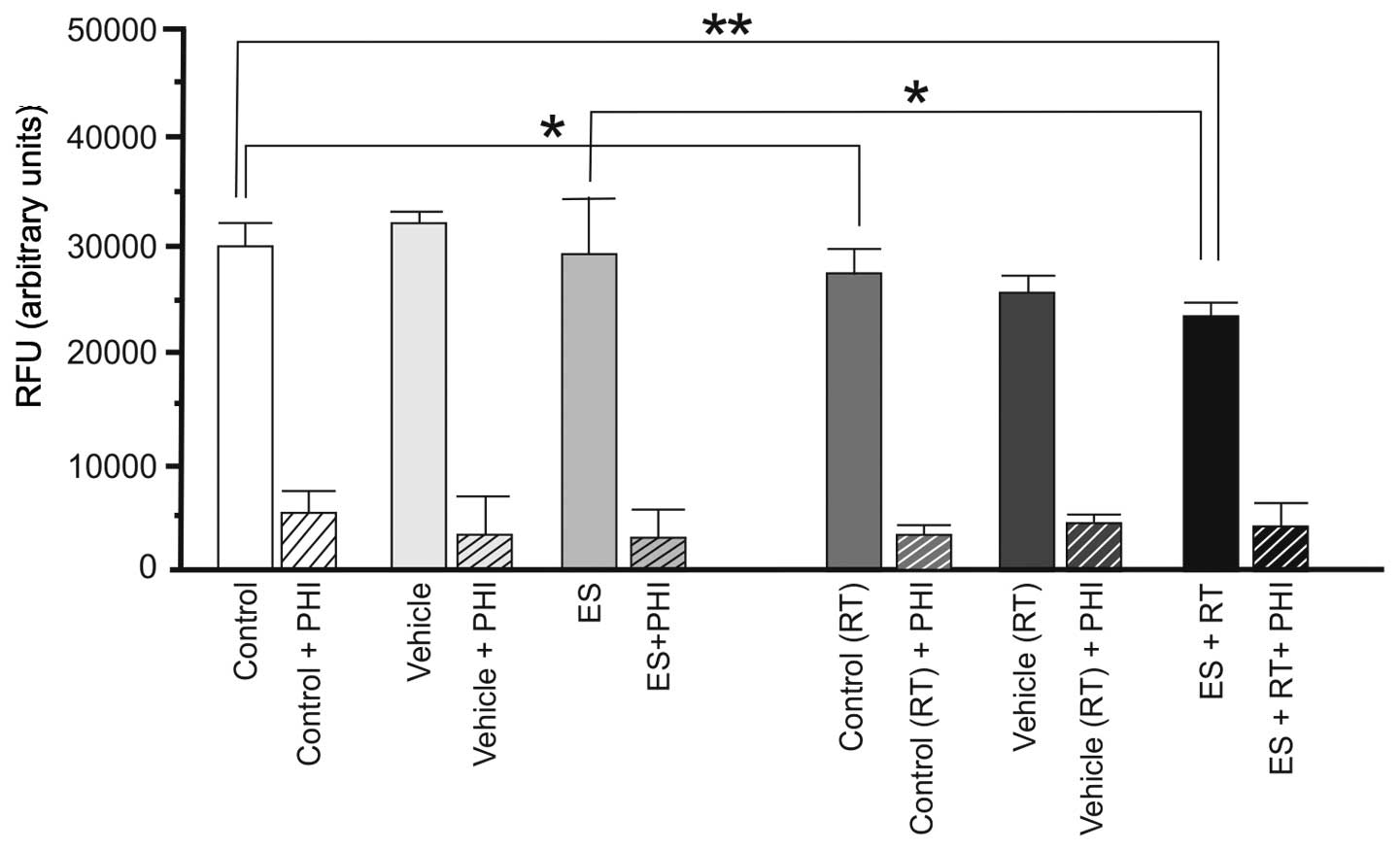
In 4THMpc cells, ES alone did not alter NEP activity (P>0.05),
however RT alone or in combination produced a decrease in NEP
activity (P<0.05; Fig. 4).
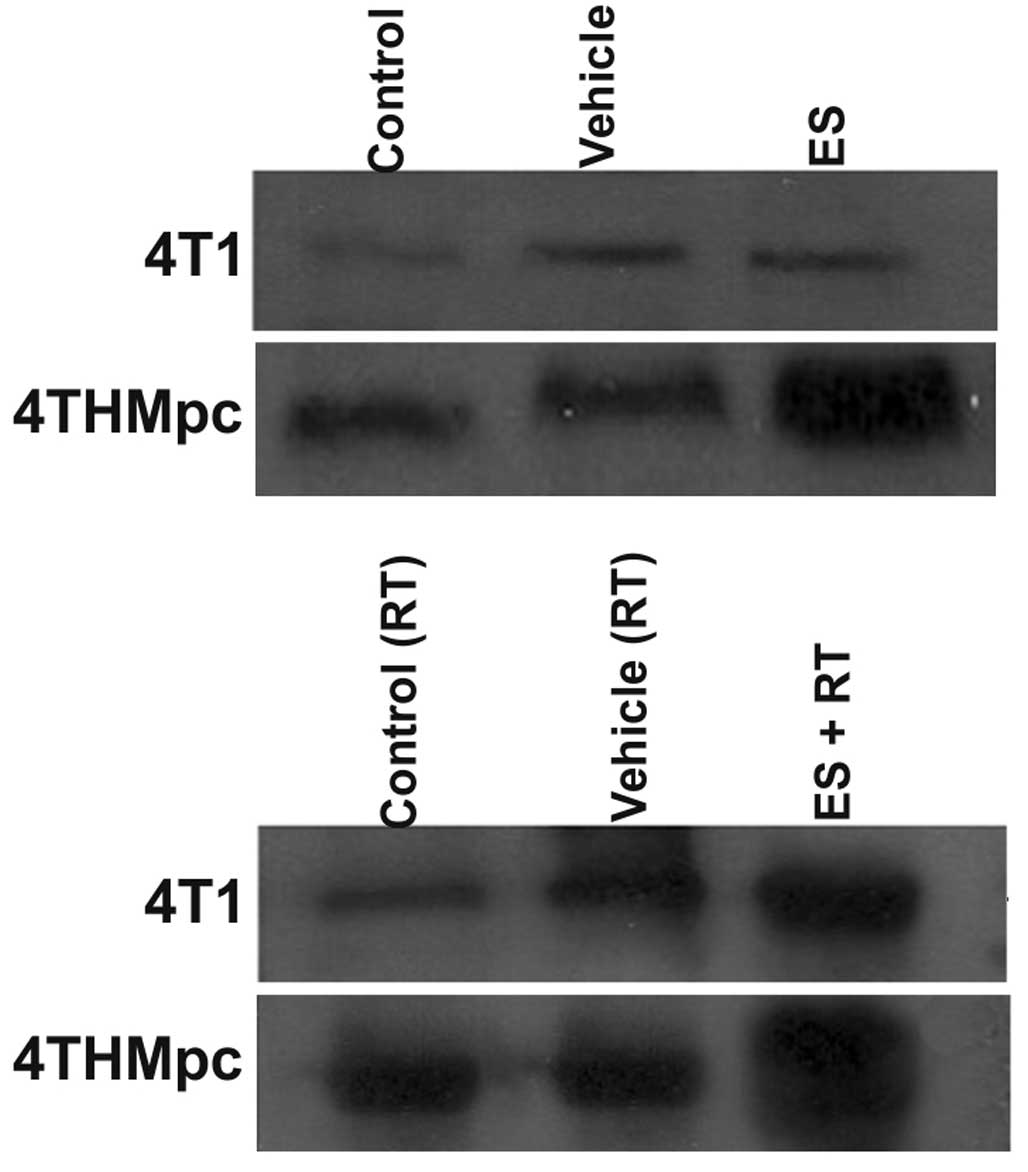
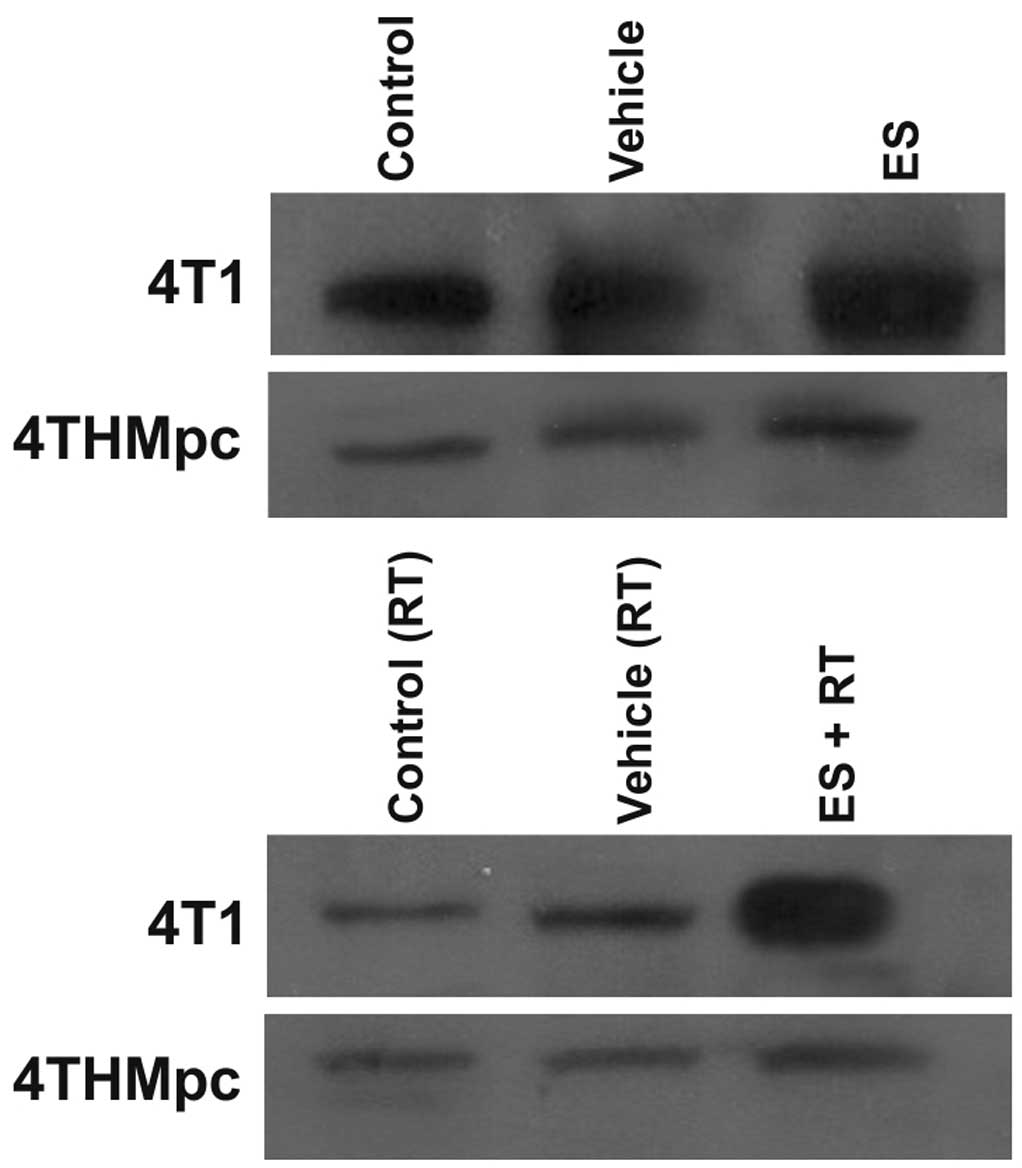
Western blotting
To determine whether changes in NEP and ADAM10
activity in 4T1 and 4THMpc cells were due to changes in NEP and
ADAM10 protein expression levels, cell homogenates were assayed by
western blotting. As presented in Figs. 5 and 6, the density of the bands depend on the
quantity of ADAM10 or NEP, thus, correlating with the activity of
the enzymes. ES alone did not change the amount of ADAM10 in 4T1
cells but RT alone resulted in an increased amount of the enzyme.
However, the increase in the amount of ADAM10 was greater following
combinaiton with ES and RT, compared with either alone. Treatment
of the 4THMpc cells with ES led to an increase in ADAM10 but this
increase was greater in cells treated with the combination of ES
and RT.
In 4T1 cells, ES alone did not alter the amount of
NEP while RT alone resulted in a decrease, the combination of RT
and ES resulted in a marked increase in the amount of NEP. In
4THMpc cells, RT alone or in combination with ES resulted in a
decrease in the amount of NEP; however, ES treatment alone did not
yield any change in NEP.
Discussion
The internal fragment of collagen XVIII, ES, has
been demonstrated to inhibit in vitro endothelial
proliferation, but not tumor cell proliferation, suggesting that ES
has endothelial cell specific activity (34,35).
Previous in vivo studies have indicated that ES inhibits
growth of primary tumors by inhibiting tumor angiogenesis without
directly affecting the growth of tumor cells (5,6).
These in vitro and in vivo studies suggest that ES
does not have a direct cytotoxic effect on tumor cells. However,
Dkhissi et al (36) have
demonstrated that ES has direct anti-proliferative and apoptotic
effects on HT29 and C51 colon cancer cell lines in addition to its
anti-angiogenic effect. Similarly, Hanai et al (37) reported that ES inhibited the
Wnt-dependent signaling pathway by stimulating the degradation of
β-catenin in endothelial cells and in DLD-1 colon cancer cells.
Furthermore, Hajitou et al (38) have demonstrated that ES inhibits
in vitro EF43.Fgf-4 mouse breast cancer cell proliferation
via the inhibition of VEGF expression (38). Due to these different activities,
ES exhibits a multifaceted anti-angiogenic effect. In our initial
study, it was reported that ES at appropriate doses and incubation
periods exerted cytotoxic and apoptotic effects against 4T1 and
4THMpc cells. These results regarding the cytotoxic effects of ES
to tumor cells appeared to be contrary to the general
considerations, however, the results were consistent with findings
that demonstrate direct cytotoxic effects of ES on tumor cells. It
was previously indicated that that the cytotoxic effect of ES on
tumor cells depends on a minimum of four factors, as follows: i)
The concentration of ES, ii) the type of targeted cell, iii) the
time period of incubation and iv) the number of tested cells
(32).
Combinations of different therapies may repress the
multifactor stimulated biological processes of tumors, and result
in cytoxicity. At present, anti-cancer research is investigating
the success of radiotherapy and angiogenesis inhibitors together
(39,40). Different scientists have
demonstrated that combination of radiotherapy and angiogenesis
inhibitors may result in either additive or synergistic effects.
Previous studies suggest that the addition of anti-angiogenic
agents to conventional therapeutic strategies may increase clinical
efficacy (39,41,42).
Previous studies suggest that the addition of anti-angiogenic
agents to conventional therapeutic strategies may increase clinical
efficacy (39,43,44).
As radiation therapy is a conventional cancer treatment strategy,
tumor resistance to ionizing radiation has been previously
investigated. The interactions between exposure to ionizing
radiation and anti-angiogenic treatment may result in an improved
understanding of effective cancer therapy with these treatments
(43).
In the present study, the possible effects of 4
μg/ml ES, either alone or in combination with 45 Gy
60Co irradiation, on the expression levels and activity
of NEP and ADAM-10 were investigated. NEP and ADAM10 degrade SP at
the same point. SP, a member of the tachykinin family is suggested
to be predominantly localized in sensory nerves and around blood
vessels and smooth muscle (20).
The role of SP in cancer development and progression is likely
bidirectional, with intact peptide and hydrolysis products exerting
different effects (20,23,45).
Bidirectional effects of SP on carcinogenesis may be the result of
the counter-balancing effects of SP fragments compared with the
intact peptide, as the intact peptide is tumorigenic and induces
inflammation, whereas fragments produced by peptidases (including,
neprilysin and ADAM10) exert opposing effects (23,46,47).
A disintegrin and metalloproteases (ADAMs) are a
family of metalloproteases (48),
with important functions in a variety of different biological
processes, including the interaction of sperm and egg, cell
migration, wound healing, heart development, immunity, cell
proliferation and angiogenesis (49). ADAM-10 and TACE (ADAM17) have been
proposed as an α-secretase (50).
ADAM10 sheds a number of cell surface proteins, including TNF-α,
epidermal growth factor (EGF), TGF-α, and heparin-binding EGF-like
growth factor. Furthermore, ADAM10 mediates regulated intramembrane
proteolysis of CD44, cadherins and Notch, which produce an
intracellular fragment that can influence gene transcription
following translocation to the nucleus (51). Overexpression of ADAM family
members (51), including ADAM10
(52), has been reported in
different malignancies.
In 4T1 cells, ES alone did not alter the activity or
the protein expression level of ADAM10. RT (45 Gy 60Co)
alone induced a 55% increase in ADAM10 enzyme activity and this
activation increased to 74.5% when combined therapy was used. By
contrast, in the 4THMpc cell line, ES alone induced a 43.3%
increase in ADAM10 enzyme activity, while RT alone and with ES
resulted in an increase of 70.9 and 72.5% in ADAM10 activity,
respectively.
NEP is a zinc-dependent type II integral
membrane-bound metallopeptidase with ubiquitous distribution that
is demonstrated to cleave a variety of peptides, including
natriuretic. peptides, angiotensin-I and -II, endothelin-1, kinins,
adrenomedullin, opioid peptides, bradykinin, bombesin,
calsistonine, neurotensin, substance P, Aβ, enkephalin, and gastrin
(14,52–54).
This enzyme is also observed in various tissues, however, it has
highest abundance in the kidney (53). Recently, it has been suggested that
NEP activity and/or expression levels are decreased in different
carcinomas, including prostate (19), lung (55), renal (56) and stomach and colon adenocarcinomas
(17,53,57).
By contrast, high levels of NEP were also indicated in a malignant
hepatocellular carcinomas (17,58,59).
Furthermore, Burns et al (19) suggested that NEP modulates
bombesin-mediated proliferation in breast cancer cells in
vitro.
In the present study, in 4T1 cells, ES alone
slightly increased NEP activity by 7.54%, however, this is not a
statistically significant result. While RT alone resulted in 60%
decrease, ES + RT resulted in a 16.6% increase in NEP activity. In
the more aggressive 4THMpc cells, administration of ES alone led to
a 14.2% increase in NEP activity. RT treatment and ES + RT in
combination resulted in a 6.6 and 8.3% decrease in enzyme activity,
respectively.
Considering our previous study (32), the present study hypothesized that
ES interfered with angiogenesis indirectly by altering the quantity
of SP in breast cancer cells by modulating the activity of ADAM10
and NEP. To investigate whether cell death was a result of
differences in SP levels due to changes in ADAM10 and NEP activity,
4T1 and 4THMpc cells were treated with 4 μg/ml exogenous SP,
either with ES, RT or alone (32).
The growth inhibition of these two cancer cell lines was also
partially reversed by administration of exogenous SP concomitantly
with ES and RT, whereas exogenous SP alone enhances cell viability
rather than cell death. Further experiments determined that
exogenous SP alone did not result in cell death or changes to the
activity of ADAM10 and NEP. This result indicates that the action
of the ES on 4T1 and 4THMpc cells is associated with the activation
of SP-degrading enzymes, ADAM10 and NEP. The results of the present
study are in agreement with the findings of previous studies, in
which the use of SP antagonists inhibited in vitro growth of
small cell lung cancer and the U373 MG glioma cell line (60–65).
Gross et al (66)
demonstrated that SP reduces caspase-3 activity and TNF-α-induced
apoptosis. Caspase-3 activity was markedly increased following
treatment with ES and RT. If SP had remained unchanged in the 4T1
and 4THMpc cells or the media, it would not have been possible to
record the increase in caspase-3 activity (32). The results of our previous study
and the current study suggest that the cytotoxic activity of ES on
4T1 and 4THMpc cells is a specific, dose-dependent action, which
alters the activity of ADAM10 and NEP, and SP levels. The level of
SP is an important determinants for tumor cells to increase mitotic
signals. Different changes in SP levels following ES, RT and the
combination treatment were recorded (32), suggesting the cytotoxic activity of
ES occurs due to the changes in ADAM10 and NEP activity. ES and RT
used in combination affects the activation of ADAM10 and NEP and
once these peptidases are activated, they may degrade SP at the
same point producing short fragments. Finally, these fragments may
trigger the activation of caspase-3 and result in apoptosis of the
4T1 and 4THMpc cells.
In conclusion, the present study demonstrates for
the first time that ES potentiates the activity of ADAM10 and NEP.
The current study hypothesizes that the recorded cytotoxic activity
of ES on 4T1 and 4THMpc breast cancer cells is likely associated
with altering the activity of these peptidases. Further in
vitro and in vivo studies are required to elucidate the
suggested underlying mechanism and to increase understanding of
whether ES exert cytotoxic effect via the fragmented SP, which
results from activating these peptidases. Furthermore, in addition
to its cytotoxic and anti-angiogenic effects, the ability of ES to
increase the quantity and activity of ADAM10 may be valuable in the
treatment of AD.
Acknowledgments
The present study was supported by The Scientific
and Technological Research Council of Turkey (grant no. 107 T 204).
The authors would like to thank Dr Nina Tuncel and all the
technicians of the Department of Radiation Oncology for their
technical assistance; all the employees of Akdeniz University
Research Unit under the leadership of Professor Olcay Yeğin for
their support throughout the present study; Professor B. Uğur
Yavuzer for her thoughtful comments and suggestions on the western
blotting results; and Ms. Duygu Sahintürk Ünal for her excellent
technical assistance.
References
|
1
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pieraccini S, Saladino G, Sironi M,
Francescato P, Cattaneo MG, Vicentini LM, Speranza G and Manitto P:
A molecular dynamics study of an endostatin-derived peptide with
antiangiogenic activity and of its mutants. Chem Phys Lett.
455:311–315. 2008. View Article : Google Scholar
|
|
3
|
Wang Z, Dabrosin C, Yin X, Fuster MM,
Arreola A, Rathmell WK, Generali D, Nagaraju GP, El-Rayes B,
Ribatti D, et al: Broad targeting of angiogenesis for cancer
prevention and therapy. Semi Cancer Biol. 35(Suppl): S224–S243.
2015. View Article : Google Scholar
|
|
4
|
Shimizu K and Oku N: Cancer
anti-angiogenic therapy. Biol Pharm Bull. 27:599–605. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Reilly MS, Boehm T, Shing Y, Fukai N,
Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR and Folkman J:
Endostatin: An endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zatterstrom UK, Felbor U, Fukai N and
Olsen BR: Collagen XVIII/endostatin structure and functional role
in angiogenesis. Cell Struct Funct. 25:97–101. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dhanabal M, Ramchandran R, Waterman MJ, Lu
H, Knebelmann B, Segal M and Sukhatme VP: Endostatin induces
endothelial cell apoptosis. J Biol Chem. 274:11721–11726. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rehn M, Veikkkola T, Kukk-Valdre E,
Nakamura H, Ilmonen M, Lombardo C, Pihlajaniemi T, Alitalo K and
Vuori K: Interaction of endostatin with integrins implicated in
angiogenesis. Proc Natl Acad Sci USA. 98:1024–1029. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim YM, Hwang S, Kim YM, Pyunn BJ, Kim TY,
Lee ST, Gho YS and Kwon YG: Endostatin blocks vascular endthelial
growth factor-mediated signaling via direct interaction with
KDR/Flk-1. J Biol Chem. 277:27872–27879. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanai J, Dhanabal M, Karumanchi SA,
Albanese C, Waterman M, Chan B, Ramchandran R, Pestell R and
Sukhatme VP: Endostatin causes G1 arrest of endothelial cells
through inhibition of cyclin D1. J Biol Chem. 277:16464–16469.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim YM, Jang JW, Lee OH, Yeon J, Choi EY,
Kim KW, Lee ST and Kwon YG: Endostatin inhibits endothelial and
tumor cellular invasion by blocking the activation and catalytic
activity of matrix metalloproteinase. Cancer Res. 60:5410–5413.
2000.PubMed/NCBI
|
|
12
|
Lee SJ, Jang JW, Kim YM, Lee HI, Jeon JY,
Kwon YG and Lee ST: Endostatin binds to the catalytic domain of
matrix metalloproteinase-2. FEBS Lett. 519:147–152. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Foda HD and Zucker S: Matrix
metalloproteinases in cancer invasion, metastasis and angiogenesis.
Drug Discov Today. 6:478–482. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hellström-Lindahl E, Ravid R and Nordberg
A: Age-dependent decline of neprilysin in Alzheimer's disease and
normal brain: Inverse correlation with A beta levels. Neurobiol
Aging. 29:210–221. 2008. View Article : Google Scholar
|
|
15
|
Bunn PA Jr, Helfrich BA, Brenner DG, Chan
DC, Dykes DJ, Cohen AJ and Miller YE: Effects of recombinant
neutral endopeptidase (EC 3.4.24.11.) on the growth of lung cancer
cell lines in vitro and in vivo. Clin Cancer Res. 4:2849–2858.
1998.PubMed/NCBI
|
|
16
|
Usmani BA, Harden B, Maitland NJ and
Turner AJ: Differential expression of neutral endopeptidase-24.11
(neprilysin) and endothelin-converting enzyme in human prostate
cancer cell lines. Clin Sci (Lond). 103(Suppl 48): 314S–317S. 2002.
View Article : Google Scholar
|
|
17
|
Renneberg H, Albrecht M, Kurek R, Krause
E, Lottspeich F, Aumüller G and Wilhelm B: Identification and
characterization of neutral endopeptidase (EC 3.4.24.11) from human
prostasomes-localization in prostatic tissue and cell lines.
Prostate. 46:173–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Albrecht M, Doroszewicz J, Gillen S, Gomes
I, Wilhelm B, Stief T and Aumüller G: Proliferation of prostate
cancer cells and activity of neutral endopeptidase is regulated by
bombesin and IL-Ibeta with IL-1beta acting as a modulator of
cellular differentiation. Prostate. 58:82–94. 2004. View Article : Google Scholar
|
|
19
|
Burns DM, Walker B, Gray J and Nelson J:
Breast cancer cell associated endopeptidase EC 24.11 modulates
proliferative response to bombesin. Br J Cancer. 79:214–220. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seals DF and Courtneidge SA: The ADAMs
family of metalloproteinases: Multidomain proteins with multiple
functions. Genes Dev. 17:7–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Van der Vorst EP, Keijbeck AA, de Winther
MP and Donners MM: A disintegrin and metalloproteases: Molecular
scissors in angiogenesis, inflammation and atherosclerosis.
Atherosclerosis. 224:302–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mochizuki S and Okada Y: ADAMs in cancer
cell proliferation and progression. Cancer Sci. 98:621–628. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Erin N, Zhao W, Bylander J, Chase G and
Clawson G: Capsaicin induced inactivation of sensory neurons
promotes a more aggressive gene expression phenotype in breast
cancer cells. Breast Cancer Res Treat. 99:351–364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sumner SC, Gallagher KS, Davis DG, Covell
DG, Jernigan RL and Ferretti JA: Conformational analysis of the
tachykinins in solution: Substance P and physalaemin. J Biomol
Struct Dyn. 8:687–707. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kurtz MM, Wang R, Clements MK, Cascieria
MA, Austin CP, Cunningham BR, Chicchia GG and Liu Q:
Identification, localization and receptor characterization of novel
mammalian substance P-like peptides. Gene. 296:205–212. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koon HW and Pothoulakis C:
Immunomodulatory properties of substance P: The gastrointestinal
system as a model. Ann NY Acad Sci. 1088:23–40. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schäffer M, Beiter T, Becker HD and Hunt
TK: Neuropeptides: Mediators of inflammation and tissue repair?
Arch Surg. 133:1107–1116. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Muangman P, Tamura RN, Muffley LA, Isik
FF, Scott JR, Xie C, Kegel G, Sullivan SR, Liang Z and Gibran NS:
Substance P enhances wound closure in nitric oxide synthase
knockout mice. J Surg Res. 153:201–209. 2009. View Article : Google Scholar :
|
|
29
|
Chappa AK, Audus KL and Lunte SM:
Characteristics of substance P transport across the blood-brain
barrier. Pharm Res. 23:1201–1208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Esteban F, Gonzalez-Moles MA, Castro D,
Martin-Jaen Mdel M, Redondo M, Ruiz-Avila I and Muñoz M: Expression
of substance P and neurokinin-1-receptor in laryngeal cancer:
Linking chronic inflammation to cancer promotion and progression.
Histopathology. 54:258–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Erin N and Ulusoy O: Differentiation of
neuronal from non-neuronal substance P. Regul Pept. 152:108–113.
2009. View Article : Google Scholar
|
|
32
|
Arslan Aydemir E, Simsek Oz E, Fidan
Korcum A and Fiskin K: Endostatin enhances radioresponse in breast
cancer cells via alteration of substance P levels. Oncol Lett.
2:879–886. 2011.
|
|
33
|
Carpenter TC and Stenmark KR: Hypoxia
decreases lung neprilysin express and increases pulmonary vascular
leak. Am J Physiol Lung Cell Mol Physiol. 281:L941–L948.
2001.PubMed/NCBI
|
|
34
|
Shichiri M and Hirata Y: Antiangiogenesis
signals by endostatin. FASEB J. 15:1044–1053. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kranenburga O, Kroon-Batenburgb LM,
Reijerkerka A, Wuc YP, Voesta EE and Gebbinka MF: Recombinant
endostatin forms amyloid fibrils that bind and are cytotoxic to
murine neuroblastoma cells in vitro. FEBS Lett. 539:149–155. 2003.
View Article : Google Scholar
|
|
36
|
Dkhissi F, Lu H, Sorıa C, Opolon P,
Griscelli F, Liu H, Khattar P, Mishal Z, Perricaudet M and Li H:
Endostatin exhibits a direct antitumor effect in addition to its
antiangiogenic activity in colon cancer cells. Hum Gene Ther.
14:997–1008. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hanai J, Gloy J, Karumanchi SA, Kale S,
Tang J, Hu G, Chan B, Ramchandran R, Jha V, Sukhatme VP and Sokol
S: Endostatin is a potential inhibitor of Wnt signaling. J Cell
Biol. 158:529–539. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hajitou A, Grignet C, Devy L, Berndt S,
Blacher S, Deroanne CF, Bajou K, Fong T, Chiang Y, Foidart JM and
Noël A: The antitumoral effect of endostatin and angiostatin is
associated with a down-regulation of vascular endothelial growth
factor expression in tumor cells. FASEB J. 16:1802–1804.
2002.PubMed/NCBI
|
|
39
|
Verhoef C, de Wilt JH and Verheul HM:
Angiogenesis inhibitors: Perspectives for medical, surgical and
radiation oncology. Curr Pharma Design. 12:2623–2630. 2006.
View Article : Google Scholar
|
|
40
|
Higgins GS, O'Cathail SM, Muschel RJ and
McKenna WG: Drug radiotherapy combinations: Review of previous
failures and reasons for future optimism. Cancer Treat Rev.
41:105–113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Teicher BA: A systems approach to cancer
therapy. (Antioncogenics + standard cytotoxics→mechanism(s) of
interaction). Cancer Metastasis Rev. 15:247–272. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zips D, Krause M, Hessel F, Westphal J,
Brüchner K, Eicheler W, Dörfler A, Grenman R, Petersen C, Haberey M
and Baumann M: Experimental study on different combination
schedules of VEGF-receptor inhibitor PTK787/ZK222584 and
fractionated irradiation. Anticancer Res. 23:3869–3876.
2003.PubMed/NCBI
|
|
43
|
Kaliski A, Maggiorella L, Cengel KA, Mathe
D, Rouffiac V, Opolon P, Lassau N, Bourhis J and Deutsch E:
Angiogenesis and tumor growth inhibition by a matrix
metalloproteinase inhibitor targeting radiation-induced invasion.
Mol Cancer Ther. 4:1717–1728. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dass CR, Tran TM and Choong PF:
Angiogenesis Inhibitors and the need for anti-angiogenic
therapeutics. J Dent Res. 86:927–936. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Luo W, Sharif TR and Sharif M: Substance
P-induced mitogenesis in human astrocytoma cells correlates with
activation of the mitogen-activated protein kinase signaling
pathway. Cancer Res. 56:4983–4991. 1996.PubMed/NCBI
|
|
46
|
Muñoz M, Pérez A, Rosso M, Zamarriego C
and Rosso R: Antitumoral action of the neurokinin-1 receptor
antagonist L-733 060 on human melanoma cell lines. Melanoma Res.
14:183–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Walsh DT, Weg VB, Williams TJ and
Nourshargh S: Substance P induced inflammatory responses in
guinea-pig skin: The effect of specific NK1 receptor antagonists
and the role of endogenous mediators. Br J Pharmacol.
114:1343–1350. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Musumecia G, Coleman R, Imbesi R, Magro G,
Parenti R, Szychlinska MA, Scuderi R, Cinà CS, Castorina S and
Castrogiovanni P: ADAM-10 could mediate cleavage of N-cadherin
promoting apoptosis in human atherosclerotic lesions leading to
vulnerable plaque: A morphological and immunohistochemical study.
Acta Histochem. 116:1148–1158. 2014. View Article : Google Scholar
|
|
49
|
Reiss K and Saftig P: The 'a disintegrin
and metalloprotease' (ADAM) family of sheddases: Physiological and
cellular functions. Semin Cell Dev Biol. 20:126–137. 2009.
View Article : Google Scholar
|
|
50
|
Li B, Yu D and Xu Z: Activated protein C
inhibits amyloid β production via promoting expression of ADAM-10.
Brain Res. 1545:35–44. 2014. View Article : Google Scholar
|
|
51
|
Jones AV, Lambert DW, Speight PM and
Whawell SA: ADAM 10 is over expressed in oral squamous cell
carcinoma and contributes to invasive behaviour through a
functional association with αvβ6 integrin. FEBS Lett.
587:3529–3534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Şimşek E, Aydemir E, Korcum AF and Fişkin
K: Thalidomide combined with irradiation alters the activity of two
proteases. Mol Med Rep. 11:1535–1541. 2015.
|
|
53
|
von Lueder TG, Atar D and Kruma H: Current
role of neprilysin inhibitors in hypertension and heart failure.
Pharmacol Ther. 144:41–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bayés-Genís A, Barallat J, Galán A, de
Antonio M, Domingo M, Zamora E, Urrutia A and Lupón J: Soluble
neprilysin is predictive of cardiovascular death and heart failure
hospitalization in heart failure patients. J Am Coll Cardiol.
65:657–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cohen AJ, Bunn PA, Franklin W, Magill-Solc
C, Hartmann C, Helfrich B, Gilman L, Folkvord J, Helm K and Miller
YE: Neutral endopeptidase: Variable expression in human lung,
inactivation in lung cancer, and modulation of peptide-induced
calcium flux. Cancer Res. 56:831–839. 1996.PubMed/NCBI
|
|
56
|
Göhring B, Holzhausen HJ, Meye A,
Heynemann H, Rebmann U, Langner J and Riemann D: Endopeptidase
24.11/CD10 is down-regulated in renal cell cancer. Int J Mol Med.
2:409–414. 1998.PubMed/NCBI
|
|
57
|
Sato Y, Itoh F, Hinoda Y, Ohe Y, Nakagawa
N, Ueda R, Yachi A and Imai K: Expression of CD10/neutral
endopeptidase in normal and malignant tissues of the human stomach
and colon. J Gastroenterol. 31:12–17. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dragović T, Deddish PA, Tan F, Weber G and
Erdös EG: Increased expression of neprilysin (neutral endopeptidase
24.11) in rat and human hepatocellular carcinomas. Lab Invest.
70:107–113. 1994.
|
|
59
|
Dragovic T, Sekosan M, Becker RP and Erdös
EG: Detection of neutral endopeptidase 24.11 (neprilysin) in human
hepatocellular carcinomas by immunocytochemistry. Anticancer Res.
17:3233–3238. 1997.PubMed/NCBI
|
|
60
|
Esteban F, Muñoz M, González-Moles MA and
Rosso M: A role for substance P in cancer promotion and
progression: A mechanism to counteract intracellular death signals
following oncogene activation or DNA damage. Cancer Metastasis Rev.
25:137–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Langdon S, Sethi T, Ritchie A, Muir M,
Smyth J and Rozengurt E: Broad spectrum neuropeptide antagonists
inhibit the growth of small celllung cancer in vivo. Cancer Res.
52:4554–4557. 1992.PubMed/NCBI
|
|
62
|
Reeve JG and Bleehen NM:
D-Arg1, D-Phe5, D-Trp7,9,
Leu11 substance P induces apoptosis in lung cancer cell
lines in vitro. Biochem Biophys Res Commun. 199:1313–1319. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Seckl MJ, Higgins T, Widmer F and
Rozengurt E: D-Arg1, D-Trp5,7,9,
Leu11 substance P: A novel potent inhibitor of signal
transduction and growth in vitro and in vivo in small cell lung
cancer cells. Cancer Res. 57:51–54. 1997.PubMed/NCBI
|
|
64
|
Woll PJ and Rozengurt E:
D-Arg1, D-Phe5, D-Trp7,9,
Leu11 substance P, a potent bombesin antagonist in
murine Swiss 3T3 cells, inhibits the growth of human small cell
lung cancer cells in vitro. Proc Natl Acad Sci USA. 85:1859–1863.
1988. View Article : Google Scholar
|
|
65
|
Palma C, Bigioni M, Irrissuto C, Nardelli
F, Maggi CA and Manzini S: Anti-tumour activity of tachykinin NK1
receptor antagonists on human glioma U373 MG xenograft. Br J
Cancer. 82:480–487. 2000.PubMed/NCBI
|
|
66
|
Gross K, Karagiannides I, Thomou T, Koon
HW, Bowe C, Kim H, Giorgadze N, Tchkonia T, Pirtskhalava T,
Kirkland JL and Pothoulakis C: Substance P promotes expansion of
human mesenteric preadipocytes through proliferative and
antiapoptotic pathways. Am J Physiol Gastrointest Liver Physiol.
296:G1012–G1019. 2009. View Article : Google Scholar : PubMed/NCBI
|