Introduction
Ischemic brain injury is the underlying
pathophysiology of various common diseases, including traumatic
injury and stroke, which is the second leading cause of mortality
worldwide and a primary cause of disability (1). Rapid revascularization of the
occluded vessels and early reperfusion are recommended to limit
cerebral ischemic damage. However, ischemia-reperfusion (I/R)
injury may occur (2), causing
post-ischemic tissue damage (3).
Efforts have been made to alter the patterns of reperfusion, thus
alleviating I/R injury (3,4). However, to date, effective and safe
methods to reduce ischemic brain injury remain to be
established.
Ischemic postconditioning (IPostC), an emerging
concept for stroke treatment, refers to a series of rapid
intermittent blood flow interruptions early following reperfusion
that alters the blood flow hydrodynamics (4,5).
Previous studies have demonstrated that IPostC reduced infarct
size, diminished necrosis and apoptosis, improved vascular
endothelial dysfunction and restored neurological deficits
following stroke, in numerous human organs and various animal
models (5–7). IPostC compares well with ischemic
preconditioning (4) and has
demonstrated comparable protective effects (5,8).
Previous studies have partially revealed the protective mechanisms
underlying IPostC, which include: i) Attenuation of free radical
generation (2,5); ii) inhibition of neutrophil
infiltration and attenuation of proinflammatory cytokine and
adhesion molecule expression in ischemic brain (5); and iii) promotion of neuronal
survival molecular pathways (9)
and inactivation of apoptotic cell signaling pathways (5,10).
However, the majority of these studies were descriptive and lacked
insight into the underlying molecular mechanisms of IPostC.
MicroRNAs (miRNAs), a novel class of noncoding RNAs,
are important endogenous regulators that post-transcriptionally
modulate the expression of target mRNAs via degradation or
translational inhibition (11).
miRNAs are critical for the maintenance of healthy cellular
function. Quantifying miRNA expression levels and predicting their
function as regulators of single targets and complex networks
requires a combined approach of bioinformatics, molecular and
systems biology. Previous studies have suggested the involvement of
miRNAs in the regulation of I/R injury (12,13).
However, alterations in miRNAs induced by cerebral IPostC in an I/R
mouse model remain to be fully elucidated.
The present study examined alterations in miRNA
expression levels in the cerebral cortex and hippocampus in an I/R
mouse model following IPostC treatment using microarray analyses.
Mice were subjected to I/R in the presence or absence of IPostC.
Subsequently, their neurological functional impairment, and spatial
learning and memory retention abilities were assessed. The cerebral
cortex and hippocampus were then collected and miRNA analysis was
performed. The results of miRNA array were confirmed using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Materials and methods
Animal grouping and experimental
design
Animals were randomly divided into two groups: i)
I/R group (n=16), in which animals underwent 45 min ischemia
followed by 72 h reperfusion; ii) IPostC group (n=17), in which I/R
was followed by three cycles of 15 sec occlusion/30 sec release
started at 2 min subsequent to reperfusion (IP15/30) (14).
Focal cerebral ischemia and IPostC
All investigations conformed to the Guide for the
Care and Use of Laboratory Animals published by the National
Institutes of Health (NIH Publication No. 85-23, revised 1996;
Bethesda, MD, USA). The study was approved by the ethics committee
of the Second Affiliated Hospital of Kunming Medical University
(Kunming, China; permit no. ku-sah-2015004). A total of 36 male
C57BL/6J mice (age, 4–4.5 months; weight, 22–25 g) were purchased
from Kunming Medical University (Kunming, China). Animals were
maintained on a standard diet and water accessed ad libitum,
and housed at 20–25°C under a 12-h light/dark cycle. Efforts were
made to minimize animal numbers and suffering.
Anesthesia was induced by 5% isoflurane
(Sigma-Aldrich China, Inc., Shanghai, China) and maintained with
1–2% isoflurane during surgery. Focal ischemia was generated as
described previously (9,14). Body core temperature was monitored
with a rectal probe and maintained at 37±0.5°C using a heat mat.
Briefly, under the operating microscope, the left common carotid
artery (CCA) and external carotid artery (ECA) were exposed via a
ventral midline neck incision, and were ligated proximally. A 6-0
silicon-coated nylon suture with a 0.23 mm tip diameter (Doccol
Corporation, Sharon, MA, USA) was inserted through the arteriotomy
in the CCA just below the carotid bifurcation 8±0.5 mm until a mild
resistance was felt. The inserted suture was held in place with a
5-0 black silk suture (Beijing Cinontech Co., Ltd., Beijing, China)
at the proximal CCA bifurcation. The suture was removed 45 min
later to allow reperfusion in the ischemic control group. In the
IPostC group, the suture was removed 2–3 mm and reinserted
repeatedly as described previously (14). Following surgery, animals were
returned to their cages. Three mice died following surgery (two in
the I/R group and one in the IPostC group). Therefore, subsequent
analyses were performed on 16 mice in the I/R group and 17 mice in
the IPostC group.
Neurological score evaluation
Neurological scores were assessed at 1, 2 and 3 days
post-operation (dpo). A 28 point scale of focal neurological scores
(FNS) was employed as described previously (15), which comprised the following seven
tests, all of which were scored from 0 to 4: i) Body symmetry, ii)
gait, iii) climbing, iv) circling behavior, v) frontal limb
symmetry, vi) compulsory circling, and vii) whisker response.
Scores for each category were added up, giving a total score for
each animal of 0–28.
Behavioral evaluations
A total of 14 days following surgery, the learning
and memory impairment of mice were assessed. A Morris water maze
(MWM) was used to examine spatial learning by training mice to
locate an underwater platform using visual information, as
described previously (16). The
test was conducted three times per day each day from 15 to 20 dpo.
The time required to find the hidden platform (escape latency; time
limit, 90 sec) was recorded by an observer blinded to the treatment
group and tracked using TopScan software version 3.1 (Clever Sys
Inc., Reston, VA, USA). A 90 sec probe trial was performed 1 day
subsequent to the final learning trial to assess memory. The
platform was removed and the percentage of time spent in the
quadrant where it was previously located was recorded.
miRNA microarray analysis
The miRCURY LNA™ Array (7th generation; version
18.0; Exiqon A/S, Vedbaek, Denmark) contains 3,100 capture probes,
covering all human, mouse and rat miRNAs annotated in miRBase
version 18.0 (www.mirbase.org/), and all viral
miRNAs associated with these species.
Mice (n=3) were randomly selected from each group 20
days following surgery, subsequent to the MWM test, sacrificed by
cervical dislocation and decapitated. Ischemic ipsilateral cortex
and hippocampus were removed within 60 sec and frozen in −70°C
isopentane until further analysis. Following careful rinsing in
chilled phosphate-buffered saline, tissues from the three mice in
each group were pooled and homogenized on ice using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and total RNA was extracted using
TRIzol® and a miRNeasy Mini kit (Qiagen, Inc., Valencia,
CA, USA) according to manufacturer's instructions. RNA quality and
quantity were examined using a Nanodrop spectrophotometer (ND-1000;
NanoDrop Technologies; Thermo Fisher Scientific, Inc.) and RNA
integrity was assessed by gel electrophoresis.
Following RNA isolation from the samples, the
miRCURY Hy3/Hy5™ Power Labeling kit (Exiqon A/S) was used according
to the manufacturer's instructions. The Hy3™-labeled samples were
hybridized on the miRCURY LNA™ Array according to the
manufacturer's instructions. The slides were then washed repeatedly
with the Wash Buffer kit (Exiqon A/S) and centrifuged for 5 min at
80 × g, 20°C). Slides were scanned with the Axon
GenePix® 4000B microarray scanner (Molecular Devices,
LLC, Sunnyvale, CA, USA).
Scanned images were then imported into
GenePix® Pro software version 6.0 (Molecular Devices,
LLC) for grid alignment and data extraction. Replicated miRNAs were
averaged and miRNAs with intensities ≥30 in all samples were chosen
for normalization. Expressed data were normalized using the Median
normalization (17). Subsequent to
normalization, differentially expressed (DE) miRNAs were identified
through Fold Change filtering [only normalized intensity ratios
>2.0 or <0.5 (fold-changes ≥2.0) were defined as
significantly altered miRNAs]. Hierarchical clustering was
performed using MultiExperiment Viewer software, version 4.0
(www.tm4.org/mev.html) for classification
analysis.
Expressional analysis of miRNA
RT-qPCR was performed to measure miRNA expression
levels. The remaining mice (13 in the I/R and 14 in the IPostC
groups) were sacrificed for miRNA analysis immediately following
MWM assessment. Complementary DNA (cDNA) was synthesized from total
RNA, and qPCR performed using gene-specific primers and the
TaqMan® MicroRNA assay kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. The 10 µl PCR reaction contained 0.67
µl cDNA, 4 µl 1X TaqMan Universal PCR master mix and
1 µl primer and probe mix. qPCR was performed using an
Applied Biosystems 7300 Sequence Detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Samples were
normalized to snoRNA202 (18). The
threshold cycle was defined as the fractional cycle number at which
the fluorescence exceeded the fixed threshold (19). The relative expression of genes was
determined using the ΔΔCq method (20).
Statistical analysis
Data are expressed as the mean ± standard error. All
statistical analyses were performed in SPSS version 14.0 (SPSS,
Inc., Chicago, IL, USA). FNS were analyzed using the Kruskal-Wallis
test followed by the Mann-Whitney U-test with Bonferroni
correction. Post-hoc independent samples t-tests were used
to assess significant differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Behavioral evaluation
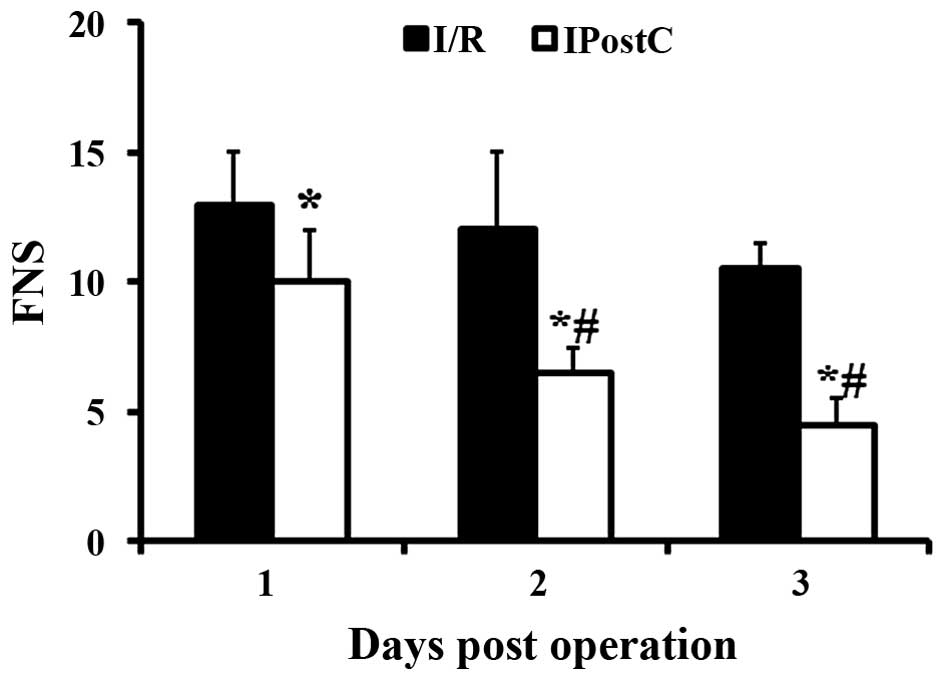
Increased FNS indicated greater impairment (15). IPostC significantly decreased
neurological scores at 1 (P=0.01), 2 (P=0.0024) and 3 (P<0.001)
dpo compared with the I/R only group (Fig. 1). In addition, in the IPostC group
but not in the I/R only group, FNS was significantly decreased at 2
and 3 dpo compared with 1 dpo.
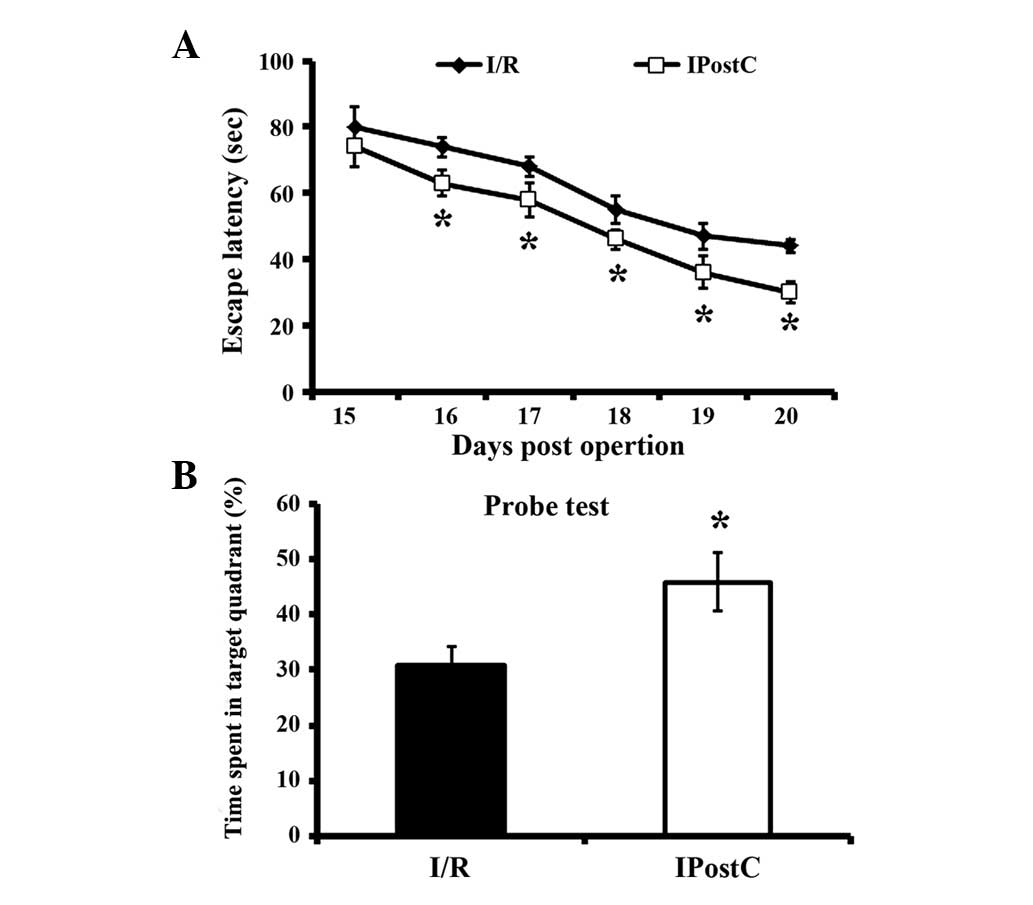
Spatial orientation alteration
All mice subjected to I/R injury demonstrated
significantly decreased escape latency from 16 dpo (Fig. 2). However, in the IPostC group,
mice discovered the platform more rapidly than I/R alone mice, from
16 dpo to 20 dpo. In the probe test, IPostC mice spent a
significantly increased percentage of time in the target quadrant
on 21 dpo compared with I/R group mice, indicating that IPostC
attenuated I/R-induced memory impairment.
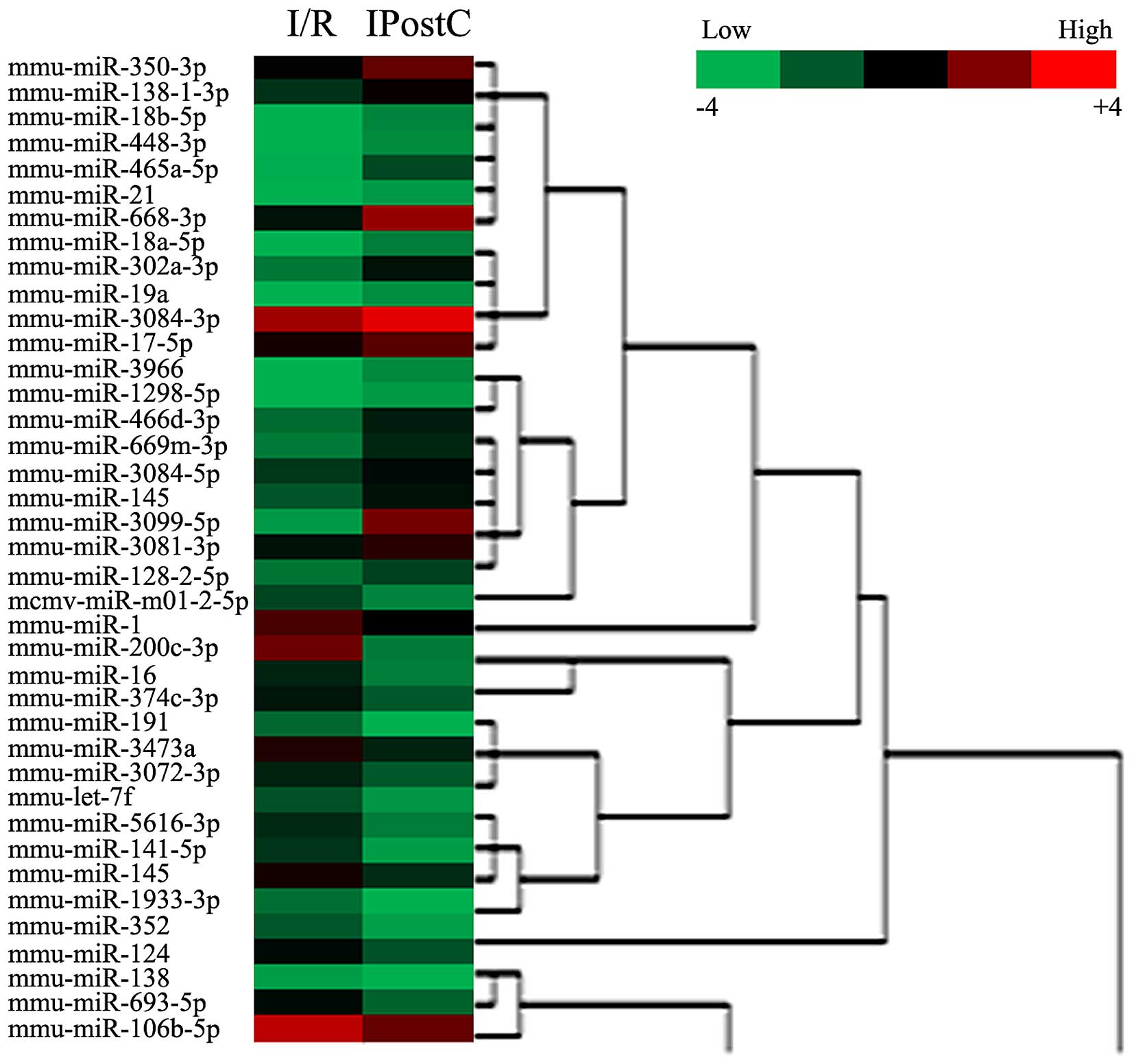
IPostC resulted in DE miRNAs in cerebral
cortex and hippocampus of I/R mice
To identify DE miRNAs induced by IPostC, a Fold
Change filtering was performed. The miRNA expression profile heat
map of these groups was generated by hierarchical clustering. The
color gradient of the heat map represents the log of relative to
mean miRNA expression, with red indicating overexpression and green
underexpression. Hierarchical cluster analysis of these miRNAs
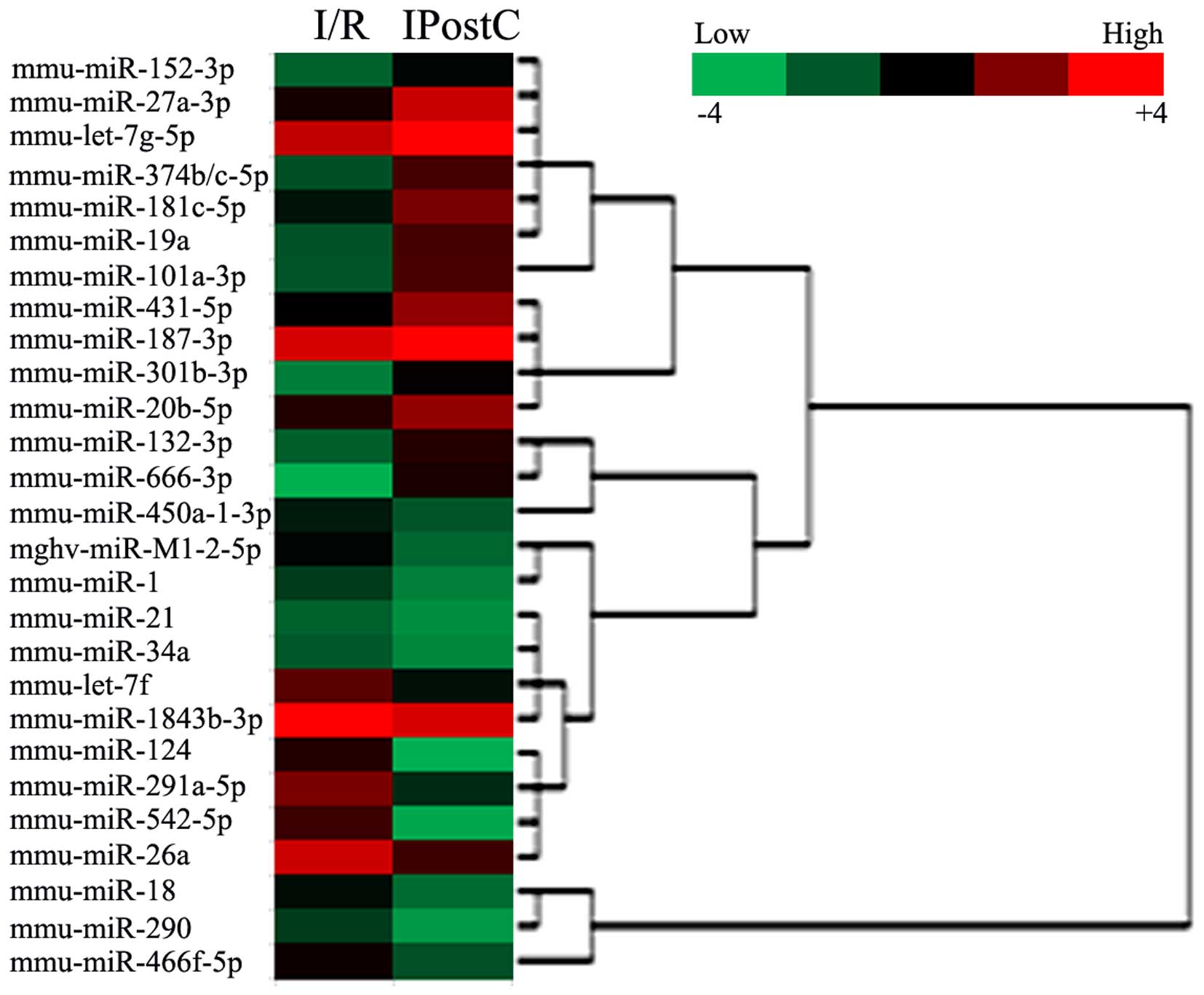
identified DE miRNAs in cerebral cortex (Fig. 3) and hippocampus (Fig. 4) induced by IPostC.
In the cerebral cortex, 39 miRNAs were DE in I/R and
IPostC mice, of which 21 were upregulated, and 18 were
downregulated (Table I and
Fig. 3). In addition, as presented
in Table II and Fig. 4, IPostC induced DE miRNAs in the
hippocampus. A total of 27 miRNAs were DE in I/R and IPostC groups,
of which 13 were upregulated, and 14 were downregulated. The four
miRNAs (miR-1, let-7f, miR-19a and miR-124) that were DE in
cerebral cortex and hippocampus were selected for further
analysis.
 | Table IDifferentially expressed miRNAs in the
cerebral cortex of I/R and IPostC mice. |
Table I
Differentially expressed miRNAs in the
cerebral cortex of I/R and IPostC mice.
| miRNA ID |
Fold-change
(IPostC vs. I/R) | P-value |
|---|
| mmu-miR-350-3p | 2.97 | 0.005271 |
|
mmu-miR-138-1-3p | 2.37 | 0.007454 |
| mmu-miR-18b-5p | 2.57 | 0.005162 |
| mmu-miR-448-3p | 4.68 | 0.000613 |
|
mmu-miR-465a-5p | 4.81 | 0.000516 |
| mmu-miR-21 | 8.15 | 0.000361 |
| mmu-miR-668-3p | 6.71 | 0.000444 |
| mmu-miR-18a-5p | 10.45 | 0.000135 |
|
mmu-miR-302a-3p | 4.98 | 0.000513 |
| mmu-miR-19a | 6.12 | 0.000423 |
|
mmu-miR-3084-3p | 2.11 | 0.008135 |
| mmu-miR-17-5p | 2.04 | 0.008396 |
| mmu-miR-3966 | 8.68 | 0.000323 |
|
mmu-miR-1298-5p | 3.04 | 0.005049 |
|
mmu-miR-466d-3p | 3.37 | 0.004954 |
|
mmu-miR-669m-3p | 3.76 | 0.004239 |
|
mmu-miR-3084-5p | 2.02 | 0.009231 |
| mmu-miR-145 | 2.94 | 0.005365 |
|
mmu-miR-3099-5p | 38.63 | 0.000000 |
|
mmu-miR-3081-3p | 2.03 | 0.008132 |
|
mmu-miR-128-2-5p | 2.14 | 0.007942 |
|
mcmv-miR-m01-2-5p | 0.37 | 0.002235 |
| mmu-miR-1 | 0.45 | 0.007359 |
|
mmu-miR-200c-3p | 0.04 | 0.000321 |
| mmu-miR-16 | 0.24 | 0.001132 |
|
mmu-miR-374c-3p | 0.35 | 0.002924 |
| mmu-miR-191 | 0.13 | 0.000923 |
| mmu-miR-3473a | 0.43 | 0.007549 |
|
mmu-miR-3072-3p | 0.42 | 0.007854 |
| mmu-let-7f | 0.33 | 0.002139 |
|
mmu-miR-5616-3p | 0.28 | 0.001831 |
| mmu-miR-141-5p | 0.19 | 0.000965 |
| mmu-miR-145 | 0.42 | 0.007715 |
|
mmu-miR-1933-3p | 0.19 | 0.000932 |
| mmu-miR-352 | 0.32 | 0.002356 |
| mmu-miR-124 | 0.32 | 0.002646 |
| mmu-miR-138 | 0.35 | 0.002524 |
| mmu-miR-693-5p | 0.25 | 0.001692 |
|
mmu-miR-106b-5p | 0.40 | 0.000364 |
 | Table IIDifferentially expressed miRNAs in
the hippocampus of I/R and IPostC mice. |
Table II
Differentially expressed miRNAs in
the hippocampus of I/R and IPostC mice.
| miRNA ID |
Fold-change
(IPostC vs. I/R) | P-value |
|---|
| mmu-miR-152-3p | 4.25 | 0.000571 |
| mmu-miR-27a-3p | 6.83 | 0.000354 |
| mmu-let-7g-5p | 3.20 | 0.004162 |
|
mmu-miR-374b-5p/mmu-miR-374c-5p | 7.24 | 0.000213 |
|
mmu-miR-181c-5p | 4.89 | 0.000513 |
| mmu-miR-19a | 7.80 | 0.000361 |
|
mmu-miR-101a-3p | 8.28 | 0.000244 |
| mmu-miR-431-5p | 4.76 | 0.000535 |
| mmu-miR-187-3p | 3.82 | 0.001813 |
|
mmu-miR-301b-3p | 7.52 | 0.000123 |
| mmu-miR-20b-5p | 3.42 | 0.002135 |
| mmu-miR-132-3p | 6.37 | 0.003826 |
| mmu-miR-666-3p | 22.44 | 0.000012 |
|
mmu-miR-450a-1-3p | 0.39 | 0.002615 |
|
mghv-miR-M1-2-5p | 0.21 | 0.001259 |
| mmu-miR-1 | 0.34 | 0.002211 |
| mmu-miR-21 | 0.48 | 0.011932 |
| mmu-miR-34a | 0.49 | 0.009924 |
| mmu-let-7f | 0.29 | 0.001023 |
|
mmu-miR-1843b-3p | 0.38 | 0.002549 |
| mmu-miR-124 | 0.04 | 0.000554 |
|
mmu-miR-291a-5p | 0.14 | 0.000939 |
| mmu-miR-542-5p | 0.04 | 0.000431 |
| mmu-miR-26a | 0.21 | 0.001965 |
| mmu-miR-18 | 0.22 | 0.001715 |
| mmu-miR-290 | 0.23 | 0.001332 |
|
mmu-miR-466f-5p | 0.25 | 0.001356 |
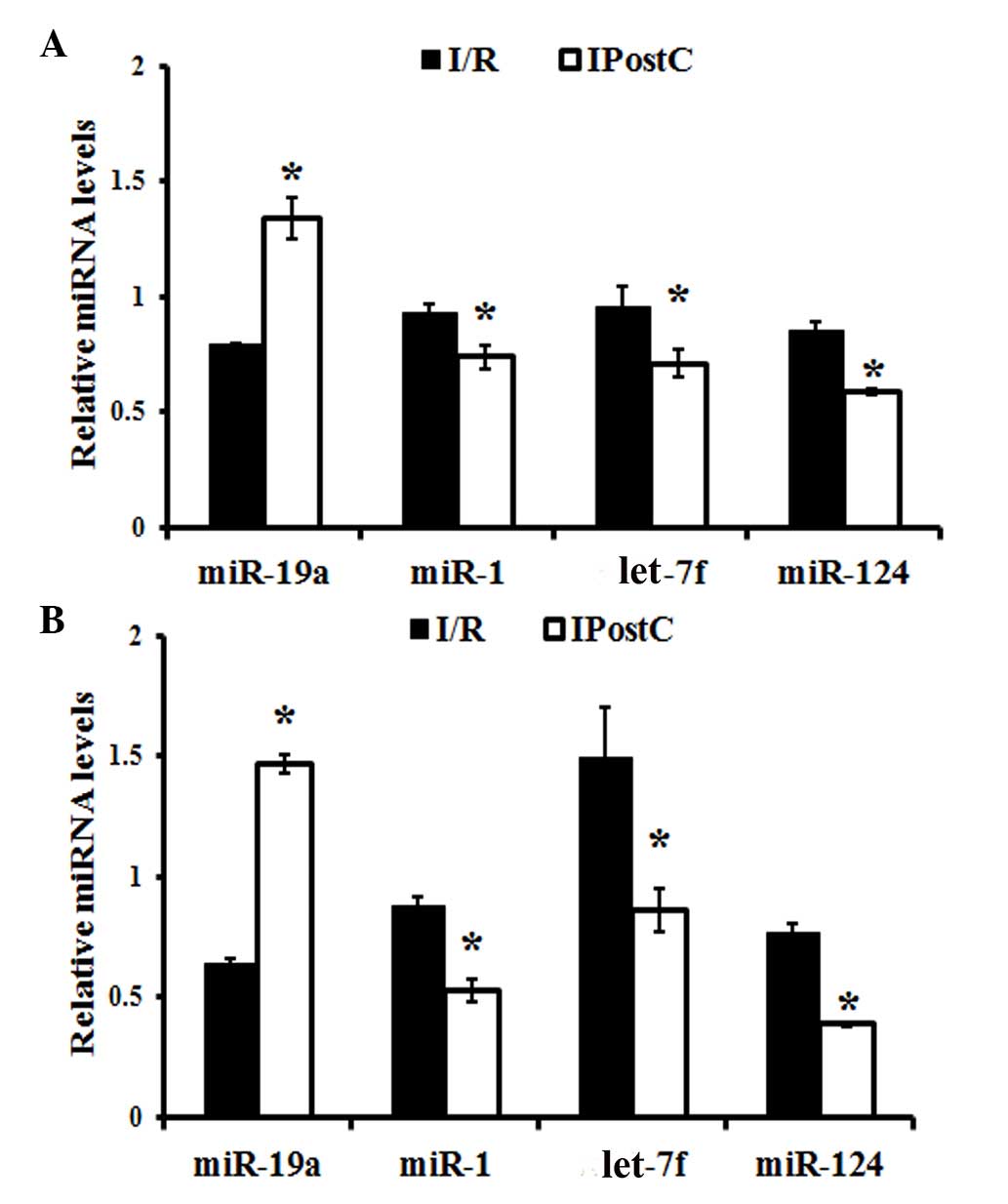
Quantitative analysis of miRNAs
The expression levels of miR-1, let-7f, miR-19a and
miR-124 were evaluated using RT-qPCR. In the cerebral cortex,
miR-19a expression levels were significantly increased
(P<0.001), while miR-1 (P= 0.007), let-7f (P=0.002) and miR-124
(P=0.003) expression levels were significantly decreased, in the
IPostC compared with the I/R group (Fig. 5A). Similar observations were made
in the hippocampus of IPostC-treated mice, which demonstrated an
upregulation of miR-19a expression levels (P<0.001) and
downregulation of miR-1 (P=0.002), let-7f (P=0.001) and miR-124
(P=0.001) expression levels, compared with the I/R group (Fig. 5B).
Discussion
The present study revealed that IPostC, consisting
of three cycles of 15 sec occlusion/30 sec release started 2 min
following reperfusion, attenuated neurological impairment and
hippocampus-associated cognitive deficits induced by I/R injury. In
addition, it was demonstrated that IPostC induced alterations in
miRNAs expression levels in the cerebral cortex and hippocampus
following I/R. In particular, miR-1, let-7f, miR-19a and miR-124
expression levels were significantly altered by IPostC. These
results indicate that modulation of miRNA expression by IPostC may
contribute to the cognitive improvement of these mice following I/R
injury.
FNS evaluation and MWM test results revealed that
I/R produced significant and irreversible neurological deficits and
long-term impairment in the cognitive abilities of mice, consistent
with previous studies (14,21).
However, in the present study, treatment with IPostC attenuated
neurological deficits and cognitive performance in the MWM test
induced by I/R. Previous studies have revealed that IPostC
ameliorates neurological deficits and inhibited brain injury
following stroke (22,23). It has been demonstrated that I/R
destroys up to 85.8% of CA1 hippocampal neurons and 64.1% of
parietal cortical neurons, which contribute to the cognitive
impairment following injury (10).
Evidence demonstrates the results of IPostC: i) Markedly reduced
neuronal loss and delayed neuronal death following reperfusion; ii)
significantly decreased neurological deficit scores, infarct volume
and brain edema; and iii) diminished spatial learning and memory
deficiency associated with cerebral ischemia (10,12,13).
Although it is widely accepted that IPostC increases adult
hippocampal neurogenesis and enhances behavioral performance in
rodents, the mechanisms underlying this process remain to be fully
elucidated. An epigenetic mechanism may be involved.
It has been demonstrated that miRNAs are crucial for
the maintenance of healthy cellular function. They function
primarily by binding to their target mRNAs, resulting in mRNA
degradation or prevention of translation (24,25).
Altered miRNA expression has various consequences for mRNA
transcription and translation. Evidence indicates that miRNAs are
involved in the regulation of I/R injury (26). A previous study demonstrated
distinct expression patterns of miRNAs in stroke etiology,
including atherosclerosis, hyperlipidemia, hypertension and plaque
rupture (27). In addition, it has
been revealed that focal ischemia significantly altered the
temporal expression of numerous miRNAs, which may regulate the mRNA
transcription and translation involved in stroke pathophysiology
(28).
However, limited studies have investigated
alterations in miRNA expression levels in the cerebral cortex and
hippocampus of mice treated with IPostC following I/R injury
(26). The present study revealed
that there were numerous DE miRNAs in the cerebral cortex and
hippocampus of I/R and IPostC-treated mice. Hierarchical cluster
analysis of miRNA profiles suggested an epigenetic mechanism may
contribute to the IPostC-associated improvement in the neurological
and cognitive functions of mice suffering from I/R.
The results of the present study revealed that the
expression levels of miR-1, let-7f and miR-124 were downregulated
in IPostC-treated mice compared with I/R alone. A previous study
has demonstrated the upregulated expression of specific miRNAs in
rodent brains following I/R, suggesting miRNAs may be involved in
the complex response to I/R (24).
miRNA transcripts present in the blood and brain at 24 h following
reperfusion included rno-miR-16, -23a, -191, -292-5p, -320, -451,
-494 and let-7, while miRNAs observed in the blood and brain at 48
h included miR-26a, -26b, -103, -107, -150, -185, -195, -191, -214,
-320, -328, -352, -494 and let-7 (29). Furthermore, the expression levels
of miRNAs in blood have been revealed to be reproducible and
diagnostic for lung cancer, colorectal cancer and diabetes
(30). It has been demonstrated
that following ischemia, anti-miR-1 treatment significantly reduced
cortical infarct volume in adult female rats, while anti-let7
robustly reduced cortical and striatal infarcts, and preserved
sensorimotor function and interhemispheric neural integration
(31). Therefore, the neurological
and cognitive improvement in I/R-injured mice resulting from IPostC
treatment may involve miR-1 and let-7f regulation in the cerebral
cortex and hippocampus. Brain-derived neurotrophic factor (BDNF) is
a neurotrophin family secreted protein that regulates brain
development, synaptogenesis and memory and learning (32). Evidence suggests that endogenous
miR-1 and miR-10 act cooperatively as novel regulators of BDNF long
and short 3′UTR isoforms (33). In
the present study, IPostC reversed the upregulation of miR-1
following I/R, therefore the IPostC-induced cognitive improvement
in I/R may involve miR-1/BDNF. However, further investigations are
required to support this.
miR-124, the brain-specific miRNA involved in neural
tube development, was upregulated in rats subjected to transient
cerebral ischemia (34). The
present study confirmed this finding, demonstrating increased
miR-124 in brains from I/R, compared with IPostC, mice. This
process may be associated with regeneration during the h of
reperfusion in the injured brain cells. Therefore, the functional
improvement induced by IPostC may be associated with expressional
regulation of miR-124.
It was reported that miR-19b is critical for
increasing the number of oligodendroglial cells (35). The overexpression of miR-19b
downregulated phosphatase and tensin homolog protein levels and
induced oligodendrocyte precursor cell proliferation via activation
of downstream targets of the Akt signaling pathway
[phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin
(mTOR)] (35,36). In addition, Xie et al
(22) revealed that IPostC
provided long-term protection by enhancing Akt and mTOR activity
during the acute post-stroke phase, which was abolished by mTOR
inhibitor rapamycin administration. Therefore, the upregulation of
miR-19b observed in the present study may activate the PI3
K/Akt/mTOR signaling pathway, accounting for the neuroprotection
provided by IPostC following I/R.
In conclusion, the results of the present study
demonstrate that IPostC following I/R resulted in an improvement in
neurological and cognitive function, and alterations in miRNA
expression levels in the cerebral cortex and hippocampus of mice of
miR-1, let-7f, miR-19a and miR-124, alone or in combination with
other miRNAs, were associated with this recovery process.
Alterations in miR-1, let-7f, miR-19a and miR-124 expression in the
cerebral cortex and hippocampus of mice following IPostC may be
involved in this improvement. However, further experiments are
required to confirm this involvement and determine the potential
underlying mechanisms.
References
|
1
|
Durukan A and Tatlisumak T:
Preconditioning-induced ischemic tolerance: A window into
endogenous gearing for cerebroprotection. Exp Transl Stroke Med.
2:22010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao H, Sapolsky RM and Steinberg GK:
Interrupting reperfusion as a stroke therapy: Ischemic
postconditioning reduces infarct size after focal ischemia in rats.
J Cereb Blood Flow Metab. 26:1114–1121. 2006.PubMed/NCBI
|
|
3
|
Lin XM, Zhang ZY, Wang LF and Zhang L, Liu
Y, Liu XL, Yang XC, Cui L and Zhang L: Attenuation of tumor
necrosis factor-alpha elevation and improved heart function by
postconditioning for 60 sec in patients with acute myocardial
infarction. Chin Med J (Engl). 123:1833–1839. 2010.
|
|
4
|
Zhao ZQ, Corvera JS, Halkos ME, Kerendi F,
Wang NP, Guyton RA and Vinten-Johansen J: Inhibition of myocardial
injury by ischemic postconditioning during reperfusion: Comparison
with ischemic preconditioning. Am J Physiol Heart Circ Physiol.
285:H579–H588. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao H: Ischemic postconditioning as a
novel avenue to protect against brain injury after stroke. J Cereb
Blood Flow Metab. 29:873–885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhuo C, Wang Y, Wang X, Wang Y and Chen Y:
Cardioprotection by ischemic postconditioning is abolished in
depressed rats: Role of Akt and signal transducer and activator of
transcription-3. Mol Cell Biochem. 346:39–47. 2011. View Article : Google Scholar
|
|
7
|
Ma XJ, Yin HJ, Guo CY, Jiang YR, Wang JS
and Shi DZ: Ischemic postconditioning through percutaneous
transluminal coronary angioplasty in pigs: Roles of PI3K
activation. Coron Artery Dis. 23:245–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao H: The protective effects of ischemic
postconditioning against stroke: From rapid to delayed and remote
postconditioning. Open Drug Discov J. 5:138–147. 2011.PubMed/NCBI
|
|
9
|
Xiong X, Gu L, Zhang H, Xu B, Zhu S and
Zhao H: The protective effects of T cell deficiency against brain
injury are ischemic model-dependent in rats. Neurochem Int.
62:265–270. 2013. View Article : Google Scholar :
|
|
10
|
Wang JY, Shen J, Gao Q, Ye ZG, Yang SY,
Liang HW, Bruce IC, Luo BY and Xia Q: Ischemic postconditioning
protects against global cerebral ischemia/reperfusion-induced
injury in rats. Stroke. 39:983–990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ambros V: MicroRNA pathways in flies and
worms: Growth, death, fat, stress, and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Zhang X, Ren XP, Chen J, Liu H,
Yang J, Medvedovic M, Hu Z and Fan GC: MicroRNA-494 targeting both
proapoptotic and antiapoptotic proteins protects against
ischemia/reperfusion-induced cardiac injury. Circulation.
122:1308–1318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng B, Guo QL, He ZJ, Ye Z, Yuan YJ, Wang
N and Zhou J: Remote ischemic postconditioning protects the brain
from global cerebral ischemia/reperfusion injury by up-regulating
endothelial nitric oxide synthase through the PI3K/Akt pathway.
Brain Res. 1445:92–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Joo SP, Xie W, Xiong X, Xu B and Zhao H:
Ischemic postconditioning protects against focal cerebral ischemia
by inhibiting brain inflammation while attenuating peripheral
lymphopenia in mice. Neuroscience. 243:149–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hill JK, Gunion-Rinker L, Kulhanek D,
Lessov N, Kim S, Clark WM, Dixon MP, Nishi R, Stenzel-Poore MP and
Eckenstein FP: Temporal modulation of cytokine expression following
focal cerebral ischemia in mice. Brain Res. 820:45–54. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Loane DJ, Pocivavsek A, Moussa CE,
Thompson R, Matsuoka Y, Faden AI, Rebeck GW and Burns MP: Amyloid
precursor protein secretases as therapeutic targets for traumatic
brain injury. Nat Med. 15:377–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Min XL, Wang TY, Cao Y, Liu J, Li JT and
Wang TH: MicroRNAs: A novel promising therapeutic target for
cerebral ischemia/reperfusion injury? Neural Regen Res.
10:1799–1808. 2015. View Article : Google Scholar
|
|
18
|
Bhalala OG, Pan L, Sahni V, McGuire TL,
Gruner K, Tourtellotte WG and Kessler JA: MicroRNA-21 regulates
astrocytic response following spinal cord injury. J Neurosci.
32:17935–17947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ouchi Y, Banno Y, Shimizu Y, Ando S,
Hasegawa H, Adachi K and Iwamoto T: Reduced adult hippocampal
neurogenesis and working memory deficits in the
Dgcr8-deficientmouse model of 22q11.2 deletion-associated
schizophrenia can be rescued by IGF2. J Neurosci. 33:9408–9419.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Li H, Yin J, Li L, Deng J, Feng C and Zuo
Z: Isoflurane postconditioning reduces ischemia-induced nuclear
factor-κB activation and interleukin 1β production to provide
neuroprotection in rats and mice. Neurobiol Dis. 54:216–224. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie R, Wang P, Ji X and Zhao H: Ischemic
post-conditioning facilitates brain recovery after stroke by
promoting Akt/mTOR activity in nude rats. J Neurochem. 127:723–732.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rezazadeh H, Hoseini Kahnuee M, Roohbakhsh
A, Shamsizadeh A, Rahmani MR, Bidaki R, Amin F, Kamali B, Bakhshi H
and Allahtavakoli M: Neuroprotective consequences of
postconditioning on embolic model of cerebral ischemia in rat. Iran
J Basic Med Sci. 16:144–149. 2013.PubMed/NCBI
|
|
24
|
Zacharewicz E, Lamon S and Russell AP:
MicroRNAs in skeletal muscle and their regulation with exercise,
ageing, and disease. Front Physiol. 4:2662013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Humphreys DT, Westman BJ, Martin DI and
Preiss T: MicroRNAs control translation initiation by inhibiting
eukaryotic initiation factor 4E/cap and poly(A) tail function. Proc
Natl Acad Sci USA. 102:16961–16966. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weiss JB, Eisenhardt SU, Stark GB, Bode C,
Moser M and Grundmann S: MicroRNAs in ischemia-reperfusion injury.
Am J Cardiovasc Dis. 2:237–247. 2012.PubMed/NCBI
|
|
27
|
Rink C and Khanna S: MicroRNA in ischemic
stroke etiology and pathology. Physiol Genomics. 43:521–528. 2011.
View Article : Google Scholar :
|
|
28
|
Dharap A, Bowen K, Place R, Li LC and
Vemuganti R: Transient focal ischemia induces extensive temporal
changes in rat cerebral MicroRNAome. J Cereb Blood Flow Metab.
29:675–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeyaseelan K, Lim KY and Armugam A:
MicroRNA expression in the blood and brain of rats subjected to
transient focal ischemia by middle cerebral artery occlusion.
Stroke. 39:959–966. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: A novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Selvamani A, Sathyan P, Miranda RC and
Sohrabji F: An antagomir to microRNA Let7f promotes neuroprotection
in an ischemic stroke model. PLoS One. 7:e326622012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cacialli P, Gueguen MM, Coumailleau P,
D'Angelo L, Kah O, Lucini C and Pellegrini E: BDNF expression in
larval and adult Zebrafish brain: Distribution and cell
identification. PLoS One. 11:e01580572016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Varendi K, Kumar A, Härma MA and Andressoo
JO: miR-1, miR-10b, miR-155, and miR-191 are novel regulators of
BDNF. Cell Mol Life Sci. 71:4443–4456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cao X, Pfaff SL and Gage FH: A functional
study of miR-124 in the developing neural tube. Genes Dev.
21:531–536. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Budde H, Schmitt S, Fitzner D, Opitz L,
Salinas-Riester G and Simons M: Control of oligodendroglial cell
number by the miR-17-92 cluster. Development. 137:2127–2132. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Olive V, Bennett MJ, Walker JC, Ma C,
Jiang I, Cordon-Cardo C, Li QJ, Lowe SW, Hannon GJ and He L: miR-19
is a key oncogenic component of mir-17-92. Genes Dev. 23:2839–2849.
2009. View Article : Google Scholar : PubMed/NCBI
|