Introduction
Greater than 1,600,000 new cases of lung cancer
occur each year worldwide. Lung cancer accounts for greater than
1,370,000 deaths annually and has become the leading cause of
cancer-associated mortality. In China, lung cancer is the most
common malignancy, and the incidence and mortality rates, based on
ages, were higher compared with the worldwide average (1). Among the various subtypes of lung
cancer, non-small cell lung cancer (NSCLC) is the most common and
accounts for 80% of lung cancer mortality (2). The 5 year overall survival rate for
patients with NSCLC is less than 15% (3). Nearly 40% of patients with confirmed
NSCLC have stage-III diseases when admitted to the hospital
(4).
With the development of new technology and improved
understanding of the underlying molecular mechanisms of the
disease, the management of NSCLC has been greatly improved over the
recent decades (5,6). However, it is currently difficult to
manage patients with NSCLC. Radiotherapy has been considered to be
the primary treatment for NSCLC. Unfortunately, the effectiveness
of radiotherapy is often limited by resistance (2,7).
Increasing evidence from clinical studies indicates that the
response to radiotherapy varies between patients, ranging from
complete recovery to relapse and distant metastasis due to
resistance to radiotherapy. Additionally, normal tissues are
exposed to inevitable toxic effects as a result from increasing
radiation doses for treatment of radioresistant malignant cells
(8–10). Resistance to radiotherapy is common
in NSCLC cells (11), leading to
induction of the local relapse of NSCLC (12,13).
Radioresistance is most likely the result of tumor heterogeneity
regarding cell of origin, etiology, pathology, and
genetic/molecular pathogenesis (14). Therefore, it is necessary to
develop new therapeutic measures for the treatment of NSCLC,
including targeted gene therapy as a radiosensitizer in order to
treat the disease and increase the survival rate of patients.
MicroRNAs (miRNAs) are small endogenous
single-stranded non-coding RNA molecules. They act as a negative
regulator of gene expression at the post-transcriptional level by
either inhibiting translation of mRNA into functional protein or
accelerating the degradation of the target mRNA (15). According to Sanger miRBase, greater
than 2,000 miRNAs have been identified in the human genome. They
have been reported to be essential in the control of various
features in cancer, including the response to chemotherapy and
radiotherapy by regulating apoptosis or cell cycle (16). Apoptosis-associated tyrosine kinase
1 (AATK1), also known as lemur kinase 1, was first identified as a
protein that was significantly increased during final
differentiation and apoptosis of 32Dcl3, which is a non-tumorigenic
mouse myeloid cell line (17).
AATK is a key regulator in the control of apoptosis, which mediates
the toxicity of radiotherapy towards cells (18). Previous reports determined that
AATK was negatively associated with the activity of SRC
protooncogene, non-receptor tyrosine kinase (Src) in lung cancer
(19), and Src is key in the
occurrence of radiotherapy resistance in NSCLC (20). AATK has been determined to be
subject to the regulation by miRNAs (21). The present study explored potential
miRNAs that regulate the expression of AATK and their importance in
the control of apoptosis induced by radiation exposure.
Materials and methods
Cell culture
The A549 lung cancer cell line was obtained from the
Chinese Academy of Sciences Cell Bank (Shanghai, China), and
incubated in Dulbecco's modified Eagle's medium supplemented with
100 µg/ml streptomycin, 100 U/ml penicillin and 10% fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at 37°C in an atmosphere of 5% CO2 in a
humidified incubator. Cells the logarithmic phase of proliferation
were used for the transfection.
Cell transfection
For the transfection, 1×106 cells were
treated with miR-558 mimics, anti-AATK siRNA or the scramble
sequence (Shanghai GenePharma Co., Ltd., Shanghai, China). The
transfection was performed using 1 µl Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) as specified by the
manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was extracted using a RNeasy Mini kit
(Qiagen, Hilden, Germany). Equal amounts of total RNA (30 ng) from
each treatment group (treated with either miR-558 mimics, anti-AATK
siRNA or scramble) were used for cDNA synthesis using miRNA
specific primers purchased from Applied Biosystems (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The PCR
cycles were as follows: 38°C for 15 min, 85°C for 5 sec for reverse
transcription, followed by 40 cycles of 95°C for 10 sec, 95°C for 5
sec and 61°C for 20 sec. A TaqMan gene expression assay kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) was used to
determine the relative expression levels of miR-558, AATK and U6
(used as an internal control). RT-qPCR was conducted for 40 cycles
in standard mode on the ABI 7900 HT Thermal cycler (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The reaction
conditions were as follows: 40 cycles for 10 min at 50°C, 10 min at
95°C, 15 sec at 95°C and 45 sec at 60°C. The 2−ΔΔCq
method was used to calculate the relative miRNA expression values
(22).
Western blot analysis
Following lysis in lysis buffer [150 mM NaCl, 20 mM
Tris-HCl pH 7.4, 1 mM EGTA, 0.1 mM EDTA, 1% Triton X-100, 2 mM NaF,
Complete Protease Inhibitor Mix and 2 mM sodium orthovanadate
(Roche Diagnostics GmbH, Mannheim, Germany)] for 20 min on ice,
cells were centrifuged at 16,000 × g at 4°C. A 10% SDS-PAGE gel was
used to resolve proteins (30 µg). Next, proteins were
transferred onto nitrocellulose membranes. Subsequently, 5% nonfat
dry milk in Tris-buffered saline Tween-20 (100 mM NaCl, 0.05%
Tween-20 and 10 mM Tris-HCl pH 7.5) was used to block proteins to
avoid non-specific binding. Following incubation with a primary
anti-AATK antibody (1:5,000; cat. no. 87141; Cell Signaling
Technology, Inc., Beverly, MA, USA) and anti-β-actin (1:10,000;
cat. no. 3700; Cell Signaling Technology, Inc.) for 12 h at 4°C,
the blots were washed and cultured with horseradish
peroxidase-conjugated secondary antibodies (1:12,000; cat. no.
sc-53322; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for
1 h at 37°C. The proteins were visualized using an ECL
chemiluminescence kit (GE Healthcare Life Sciences, Chalfont, UK)
according to the manufacturer's protocol and exposed to X-ray film.
The signal was determined using an Odyssey scanner (LI-COR
Biosciences, Lincoln, NE, USA) and signals were normalized against
that of β-actin.
Ionizing radiation
At 48 h after the transfection, γ-ray ionizing
radiation (IR) from a 60Co source was used to radiate
sub-confluent cell monolayers at a rate of 2, 4, 6 and 8
Gy/min.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cells (200 µl) were plated on a 96-well
microtiter plate at a density of 5×104 cells/ml 24 h
prior to IR. Three days following IR, each well was supplemented
with 10 µl MTT reagent (Sigma-Aldrichm St. Louis, MO, USA)
and incubated at 37°C for 4 h. The supernatants were removed and
100 µl dimethyl sulphoxide was added to terminate the
reaction and the plates were mixed for 10 min. The absorbance was
determined at 490 nm. Each experiment was repeated three times and
the mean was calculated.
Plasmid construction
Human genomic DNA was used to amplify the 3′-UTR
region of AATK using a TaqMan gene expression kit (Applied
Biosystems), according to the manufacturer's protocol. Following
amplification, it was inserted into the pmirGLO vector (Promega
Corporation, Madison, WI, USA) next to the luciferase gene to
establish a luciferase reporter plasmid (pmirGLO-WT-AATK-3′UTR). A
QuikChange Site-Directed Mutagenesis kit (Promega Corporation) was
used to perform site-directed mutagenesis of the miR-558 target
site in the AATK 3′-UTR with pmirGLO-WT-AATK-3′UTR as the template,
resulting to a mutant-type reporter plasmid
(pmirGLO-MUT-AATK-3′UTR). In addition, the coding sequence of AATK
was amplified and subcloned into pcDNA3.0 (Invitrogen; Thermo
Fisher Scientific, Inc.) to make the construct (pcDNA3-AATK) for
the purpose of ectopic overexpression.
Dual-luciferase reporter assay
A549 cells were seeded in 96-well plates at a
density of 1×105 cells/well and transfected with 200 ng
luciferase reporter plasmid (pmirGLO-WT-AATK-3′UTR or
pmirGLO-MUT-AATK-3′UTR; Promega Corporation), 50 nM mimics,
scrambled microRNA or miR-558 using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). Cells were collected 48 h
following transfection, and a dual-luciferase reporter assay
(Promega Corporation) was used to determine luciferase
activity.
Apoptosis
Flow cytometry was used to measure apoptosis using
the Apoptest-FITC Kit with annexin V (Dako, Glostrup, Denmark) as
specified in the manufacturer's protocol. Flow cytometry (LSR II,
BD Biosciences, San Jose, CA, USA) was used to assess the
proportion of annexin V-positive cells.
Online miRNA database search
The present study searched the online miRNA
databases, including TargetScan (www.targetscan.org) and miRNA database (www.mirdb.org) to predict the virtual target gene of
the miRNA with the potential binding site (at least 7 consecutive
complementary bases) in the 3′UTR of target mRNA.
Statistical analysis
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) was used to
conduct statistical analysis. The results were presented as the
mean ± standard deviation based on three independent experiments.
One way analysis of variance was used for statistical analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
AATK is a direct target of miR-558
The relationship between the AATK-3′-UTR and the
miRNAs targeting it was predicted through bioinformatics analysis
by miRanda, which indicated that the 3′-UTR of AATK may be targeted
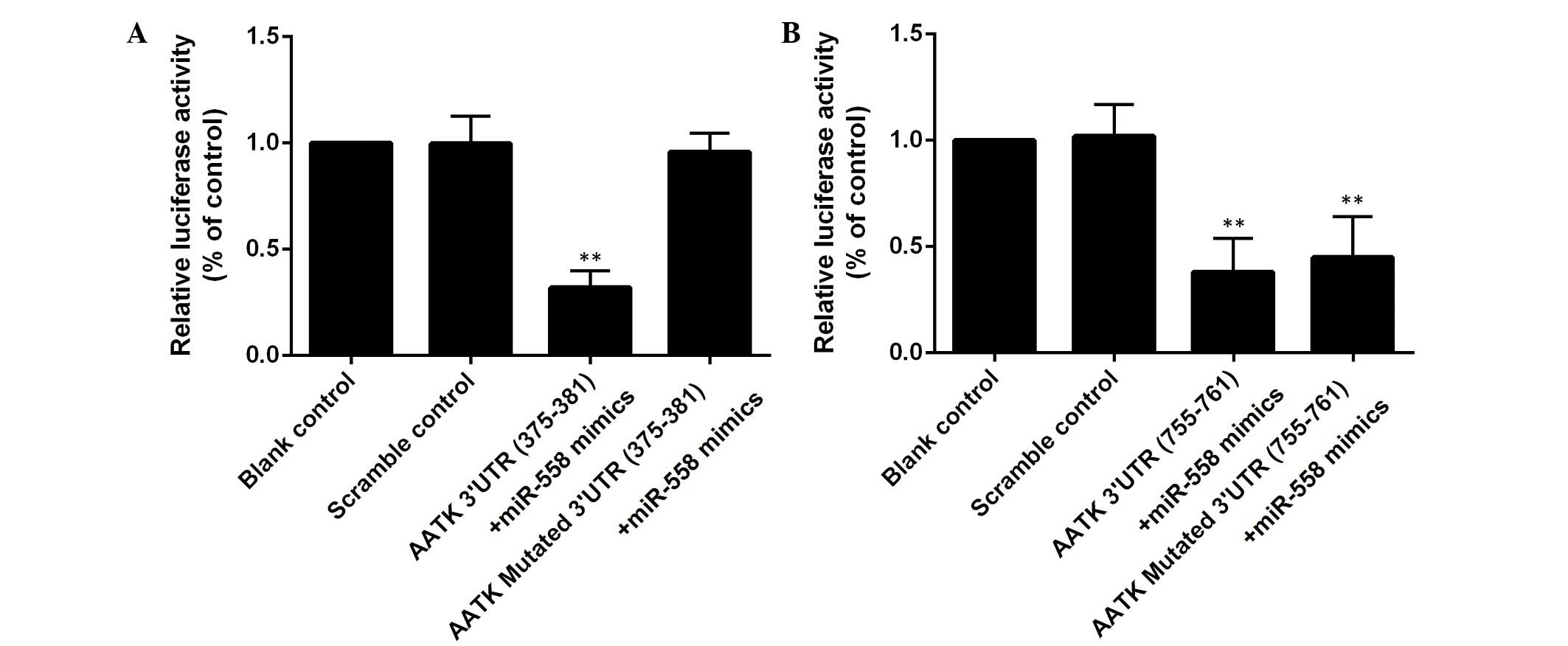
by miR-558 with two potential 'hits' in the 3′UTR of AATK (Fig. 1). To study the interaction between
AATK and miR-558, the full length of the AATK 3′UTR containing
potential miR-558 binding sites or the mutants was selected for
further validation using luciferase reporter assays. miR-558 mimics
significantly reduced the luciferase expression in the A549 cells
co-transfected with the wild type AATK 3′UTR (Fig. 2). When the binding site (375–381
bp) was mutated, the suppression of the luciferase activity was
reversed, while introduction of mutations into another binding site
(755–761 bp) had minimal effects (Fig.
2). These assays demonstrated that AATK is one of the target
genes of miR-558, and that the 375–381 bp region, instead of the
755–761 bp region of the AATK 3′UTR is the binding site of the
miRNA.
miR-558 is a regulator of AATK
To test the hypothesis that miR-558 is a direct
regulator of AATK, western blotting and RT-qPCR were used to
analyze differences in AATK expression in the A549 cells
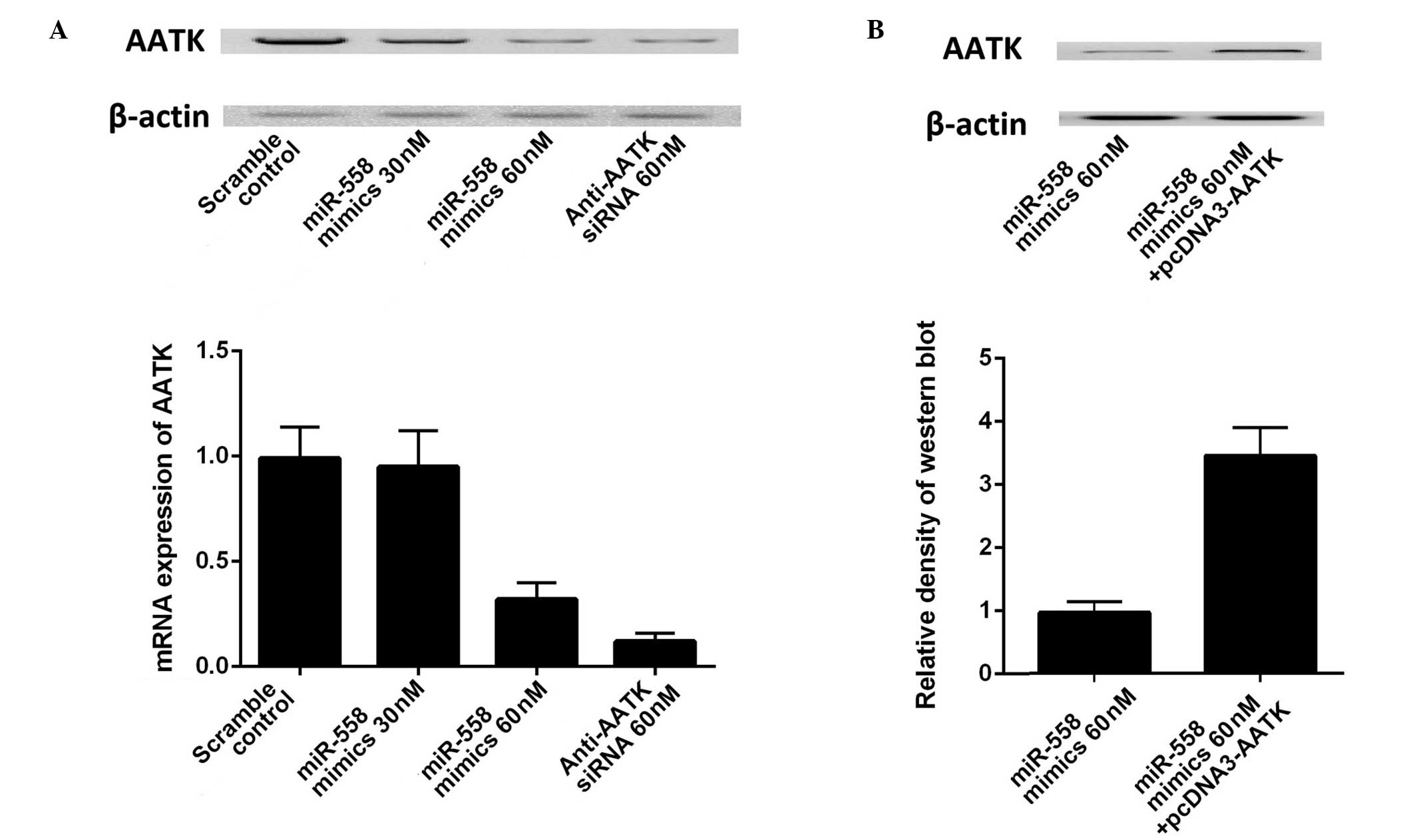
transfected with scrambled control and miR-558 mimics. As presented
in (Fig. 3A), transfection with 30
or 50 nM miR-558 mimics and AATK specific siRNA substantially
suppressed the mRNA and protein expression levels of AATK.
Transfection of the construct containing the coding sequence of
AATK significantly increased the expression of AATK in A549 cells
(Fig. 3B). The results further
confirmed the regulatory relationship between miR-558 and AATK in
lung cancer cells, and the effect of miR-558 was specific as the
scrambled miRNA did not exhibit such an effect.
miR-558 is required for radioresistance
of lung cancer cells
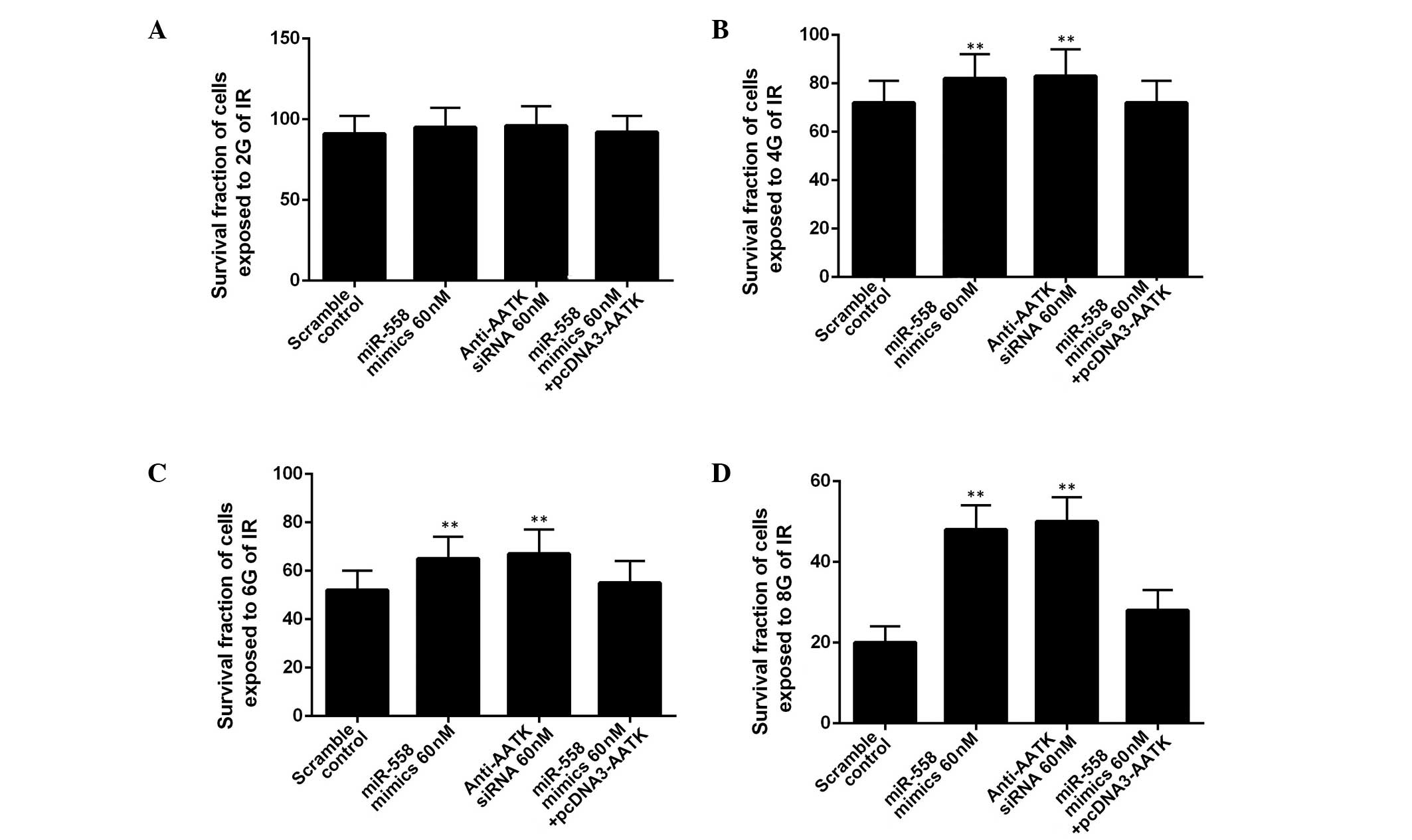
To determine whether miR-558 was required for lung
cancer cell radioresistance, A549 cells were treated with different
doses of ionizing radiation, from 0 to 10 Gy, following
transfection with miR-558 mimics or AATK-specific siRNA. IR
treatment exhibited a dose-dependent inhibitory effect on the
growth of the A549 cells (Fig. 4).
In the A549 cells exposed to 2 Gy, the administration of miR-558
mimics or AATK specific siRNA did not significantly alter the
survival rate of the cells (Fig.
4A). In contrast to the cells exposed to 4, 6 or 8 Gy,
administration of miR-558 mimics or AATK specific siRNA
significantly promoted the survival rate of the cells, with the
overexpression of AATK reversing this effect (Fig. 4B–D). This suggests that the
enhanced radioresistance of the A549 cells transfected with miR-558
may be due to the downregulation of AATK. Additionally, the effect
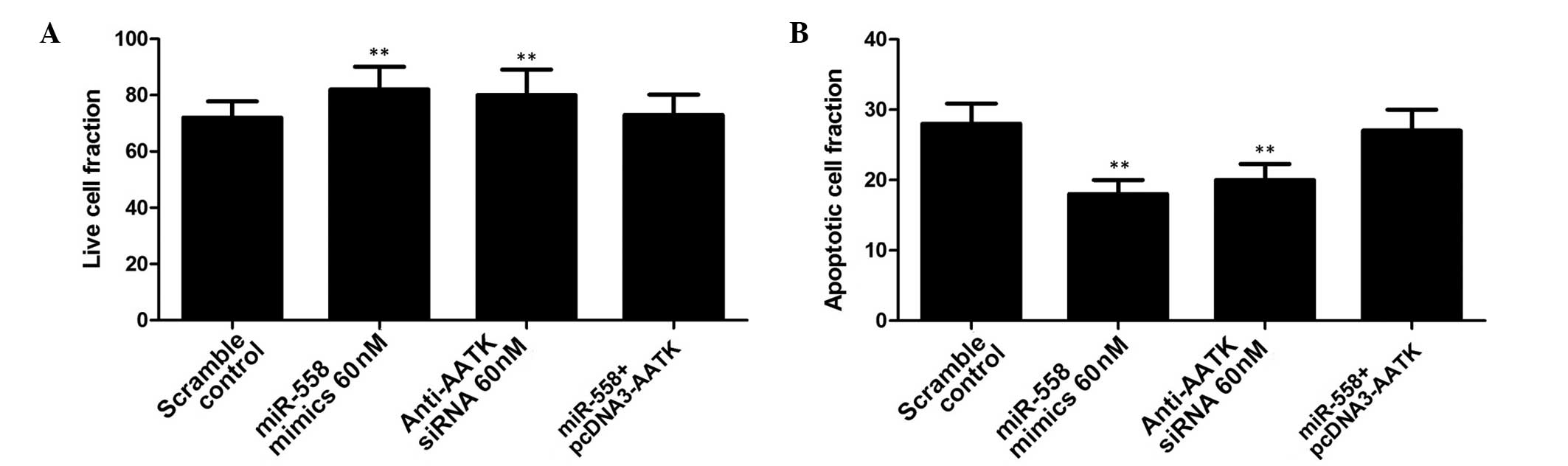
of miR-558 promotion on IR-induced apoptosis in lung cancer cells
was investigated. A549 cells transfected with miR-558 mimics and
AATK siRNA were exposed to IR (4 Gy), and apoptosis measured in
each group. This indicated that the introduction of miR-558 or
anti-AATK siRNA significantly suppressed apoptosis in lung cancer
cells, while ectopic overexpression of AATK partially but
significantly reversed the effect on apoptosis (Fig. 5). This suggests that the increased
radioresistance may have been due to a decreased rate of apoptosis
resulting from downregulation of AATK expression. In addition, the
effect of exposure to radiation on the expression of AATK and
miR-558 was also evaluated, with the results not indicating
alterations of the miRNA and AATK expression levels (data not
shown).
Discussion
It has been established that resistance to
radiotherapy leads to significantly elevated mortality and
morbidity of patients with NSCLC, and it is a key issue in the
treatment of NSCLC. Researchers have been seeking potent approaches
to sensitize cases NSCLC that are often radioresistant.
AATK was identified to be significantly increased
during final differentiation and apoptosis of 32Dcl3 cells, a
non-tumorigenic mouse myeloid cell line (23). High expression of AATK
predominantly occurs in different regions of the brain and it is
detected in other tissues, including the kidney, lung, heart and
skeletal muscle (23,24). The focus of functional studies of
AATK has been placed on the nervous system. A previous study
determined that during retinoic acid-induced neuronal
differentiation of P19 embryonal carcinoma cells, the expression of
AATK was significantly elevated (25). Previous reports have indicated that
AATK is negatively associated with the activity of Src in lung
cancer (19). Src is a member of
the non-receptor tyrosine kinase family, which consists of nine
members, including Fyn, Lyn, Src, Fgr, Yes, Yrk, Hck, Blk and Lck.
Src is functionally associated with numerous cytokine and growth
factor receptors and is important in the regulation of multiple
cellular activities, including cell migration, invasion, adhesion,
proliferation, survival, inflammation and angiogenesis (26). Notably, Src is commonly upregulated
in lung cancer. Immunohistochemical analysis performed in a
previous study indicated that the levels of Src were increased in
50–80% of NSCLC samples tested, and Src overexpression was
significantly associated with poor differentiation (27). Src is key for the occurrence of
radiotherapy resistance in NSCLC (20). Activating mutations of Src have
been reported in colon cancer; however, no activating mutations and
gene amplifications have been identified in lung cancer (28,29).
Therefore, other regulators are potentially involved in the control
of ATKK expression in NSCLC. The present study determined that
AATK, a radiosensitization-related gene, is a target of miR-558 in
lung cancer cells by using in silico analysis and luciferase
reporter system. Additionally, it was identified that transfection
of 30 or 50 nM miR-558 mimics and AATK specific siRNA significantly
suppressed both the mRNA and protein expression levels of AATK.
Various miRNAs have been associated with the
response to radiotherapy in different tumor types, such as
miR-17-92 cluster, which interferes with the response of human
mantle cell lymphoma to radiotherapy (30). miR-181 sensitizes human gliomas to
radiotherapy by acting on Bcl-2 (31). miR-221/miR-222 has been
demonstrated to modulate the response of gastric carcinoma cells to
radiotherapy through phosphatase and tensin homologue (32). It has been demonstrated that
excessive expression of let-7 g and miRNA-9 in H1299 lung cancers
cells leads to sensitization to radiotherapy by impeding nuclear
factor-κB pro-survival cascades (33). miR-558 is a miRNA that has been
recently found in human cells. The upregulation of miR-558 has been
observed in irradiated fibroblasts (34). It also has a regulatory effect on
target genes associated with cell cycle checkpoints and apoptosis
(35). In a previous study,
significant underexpression of miR-558 was observed in breast
cancer tissues (36). It has been
determined that miR-558 in neuroblastoma tissues and cell lines was
upregulated compared with in normal dorsal ganglia, with high
expression of baculoviral IAP repeat containing 6, the host gene of
miR-558, reported in a publicly available neuroblastoma microarray
database (36). It has been
previously determined that miR-558 acts as oncogene to increase
colony formation, in vivo growth and in vitro
proliferation of neuroblastoma cells (37). The current study exposed A549 cells
to different doses of ionizing radiation, from 0 to 10 Gy,
following transfection with miR-558 mimics or AATK specific siRNA,
and determined that the administration of miR-558 mimics or AATK
specific siRNA did not significantly alter the survival rate of the
cells. In contrast to the cells exposed to 4, 6 or 8 Gy,
administration of miR-558 mimics or AATK-specific siRNA
significantly promoted the survival rate of the cells, and
overexpression of AATK reduced this effect.
In conclusion, ATKK was validated as a target gene
of miR-558, and upregulation of miR-558 was observed in
radioresistant lung cancer cells. Additionally, ectopic
overexpression of ATKK partially but significantly reduced the
miR-558-induced radioresistance. The current study provides insight
into miRNA-regulated sensitivity to radiotherapy in NSCLC and
indicates that miR-558 may be a feasible novel target for the
treatment of human NSCLC. Further in vivo experiments in
animal models are required in the future to determine the efficacy
of miR-558.
References
|
1
|
Sun X, Liu W, Wu S, Han H, Lin Y and Dai
X: The morbidity and mortality trend and prediction of lung cancer
in residents of Nangang District of Harbin in China during the past
10 years. Zhongguo Fei Ai Za Zhi. 8:514–517. 2005.PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fidias P and Novello S: Strategies for
prolonged therapy in patients with advanced non-small-cell lung
cancer. J Clin Oncol. 28:5116–5123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Whitehurst AW, Bodemann BO, Cardenas J,
Ferguson D, Girard L, Peyton M, Minna JD, Michnoff C, Hao W, Roth
MG, et al: Synthetic lethal screen identification of
chemosensitizer loci in cancer cells. Nature. 446:815–819. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blackstock AW and Govindan R: Definitive
chemoradiation for the treatment of locally advanced non small-cell
lung cancer. J Clin Oncol. 25:4146–4152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salama JK and Vokes EE: New radiotherapy
and chemoradiotherapy approaches for non-small-cell lung cancer. J
Clin Oncol. 31:1029–1038. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Le Pechoux C: Role of postoperative
radiotherapy in resected non-small cell lung cancer: A reassessment
based on new data. Oncologist. 16:672–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barcellos-Hoff MH, Park C and Wright EG:
Radiation and the microenvironment-tumorigenesis and therapy. Nat
Rev Cancer. 5:867–875. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Madani I, De Neve W and Mareel M: Does
ionizing radiation stimulate cancer invasion and metastasis? Bull
Cancer. 95:292–300. 2008.PubMed/NCBI
|
|
10
|
McBride WH, Chiang CS, Olson JL, Wang CC,
Hong JH, Pajonk F, Dougherty GJ, Iwamoto KS, Pervan M and Liao YP:
A sense of danger from radiation. Radiat Res. 162:1–19. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rödel F, Hoffmann J, Distel L, Herrmann M,
Noisternig T, Papadopoulos T, Sauer R and Rödel C: Survivin as a
radioresistance factor and prognostic and therapeutic target for
radiotherapy in rectal cancer. Cancer Res. 65:4881–4887. 2005.
View Article : Google Scholar
|
|
12
|
Lee S, Lim MJ, Kim MH, Yu CH, Yun YS, Ahn
J and Song JY: An effective strategy for increasing the
radiosensitivity of human lung cancer cells by blocking
Nrf2-dependent antioxidant responses. Free Radic Biol Med.
53:807–816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Provencio M, Sánchez A, Garrido P and
Valcárcel F: New molecular targeted therapies integrated with
radiation therapy in lung cancer. Clin Lung Cancer. 11:91–97. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Danesi R, Pasqualetti G, Giovannetti E,
Crea F, Altavilla G, Del Tacca M and Rosell R: Pharmacogenomics in
non-small-cell lung cancer chemotherapy. Adv Drug Deliv Rev.
61:408–417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hutvagner G and Zamore PD: A microRNA in a
multiple-turnover RNAi enzyme complex. Science. 297:2056–2060.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ketting RF, Fischer SE, Bernstein E, Sijen
T, Hannon GJ and Plasterk RH: Dicer functions in RNA interference
and in synthesis of small RNA involved in developmental timing in
C. elegans. Genes Dev. 15:2654–2659. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Westermann F and Schwab M: Genetic
parameters of neuroblastomas. Cancer Lett. 184:127–147. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dewey WC, Ling CC and Meyn RE:
Radiation-induced apoptosis: Relevance to radiotherapy. Int J
Radiat Oncol Biol Phys. 33:781–796. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma S and Rubin BP: Apoptosis-associated
tyrosine kinase 1 inhibits growth and migration and promotes
apoptosis in melanoma. Lab Invest. 94:430–438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Purnell PR, Mack PC, Tepper CG, Evans CP,
Green TP, Gumerlock PH, Lara PN, Gandara DR, Kung HJ and Gautschi
O: The Src inhibitor AZD0530 blocks invasion and may act as a
radiosensitizer in lung cancer cells. J Thorac Oncol. 4:448–454.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kos A, Olde Loohuis NF, Wieczorek ML,
Glennon JC, Martens GJ, Kolk SM and Aschrafi A: A potential
regulatory role for intronic microRNA-338-3p for its host gene
encoding apoptosis-associated tyrosine kinase. PLoS One.
7:e310222012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Adie EA, Adams RR, Evans KL, Porteous DJ
and Pickard BS: SUSPECTS: Enabling fast and effective
prioritization of positional candidates. Bioinformatics.
22:773–774. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vlodavsky I, Ilan N, Naggi A and Casu B:
Heparanase: Structure, biological functions and inhibition by
heparin-derived mimetics of heparan sulfate. Curr Pharm Des.
13:2057–2073. 2007. View Article : Google Scholar
|
|
25
|
Zetser A, Bashenko Y, Edovitsky E,
Levy-Adam F, Vlodavsky I and Ilan N: Heparanase induces vascular
endothelial growth factor expression: Correlation with p38
phosphorylation levels and Src activation. Cancer Res.
66:1455–1463. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parsons SJ and Parsons JT: Src family
kinases, key regulators of signal transduction. Oncogene.
23:7906–7909. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mazurenko NN, Kogan EA, Zborovskaya IB and
Kisseljov FL: Expression of pp60c-src in human small cell and
non-small cell lung carcinomas. Eur J Cancer. 28:372–377. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Irby RB, Mao W, Coppola D, Kang J, Loubeau
JM, Trudeau W, Karl R, Fujita DJ, Jove R and Yeatman TJ: Activating
SRC mutation in a subset of advanced human colon cancers. Nat
Genet. 21:187–190. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kiefer PE, Bepler G, Kubasch M and
Havemann K: Amplification and expression of protooncogenes in human
small cell lung cancer cell lines. Cancer Res. 47:6236–6242.
1987.PubMed/NCBI
|
|
30
|
Navarro A, Beà S, Fernández V, Prieto M,
Salaverria I, Jares P, Hartmann E, Mozos A, López-Guillermo A,
Villamor N, et al: MicroRNA expression, chromosomal alterations and
immunoglobulin variable heavy chain hypermutations in mantle cell
lymphomas. Cancer Res. 69:7071–7078. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen G, Zhu W, Shi D, Lv L, Zhang C, Liu P
and Hu W: MicroRNA-181a sensitizes human malignant glioma U87MG
cells to radiation by targeting Bcl-2. Oncol Rep. 23:997–1003.
2010.PubMed/NCBI
|
|
32
|
Chun-Zhi Z, Lei H, An-Ling Z, Yan-Chao F,
Xiao Y, Guang-Xiu W, Zhi-Fan J, Pei-Yu P, Qing-Yu Z and Chun-Sheng
K: MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell
proliferation and radioresistance by targeting PTEN. BMC Cancer.
10:3672010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arora H, Qureshi R, Jin S, Park AK and
Park WY: miR-9 and let-7g enhance the sensitivity to ionizing
radiation by suppression of NFκB1. Exp Mol Med. 43:298–304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cummins JM, He Y, Leary RJ, Pagliarini R,
Diaz LA Jr, Sjoblom T, Barad O, Bentwich Z, Szafranska AE,
Labourier E, et al: The colorectal microRNAome. Proc Natl Acad Sci
USA. 103:3687–3692. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maes OC, An J, Sarojini H, Wu H and Wang
E: Changes in MicroRNA expression patterns in human fibroblasts
after low-LET radiation. J Cell Biochem. 105:824–834. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Si H, Sun X, Chen Y, Cao Y, Chen S, Wang H
and Hu C: Circulating microRNA-92a and microRNA-21 as novel
minimally invasive biomarkers for primary breast cancer. J Cancer
Res Clin Oncol. 139:223–229. 2013. View Article : Google Scholar :
|
|
37
|
Shohet JM, Ghosh R, Coarfa C, Ludwig A,
Benham AL, Chen Z, Patterson DM, Barbieri E, Mestdagh P, Sikorski
DN, et al: A genome-wide search for promoters that respond to
increased MYCN reveals both new oncogenic and tumor suppressor
microRNAs associated with aggressive neuroblastoma. Cancer Res.
71:3841–3851. 2011. View Article : Google Scholar : PubMed/NCBI
|