Introduction
Traumatic brain injury (TBI) is a leading
contributor to rates of mortality and permanent disability in
individuals aged <45 years (1,2).
Almost 2,000,000 individuals sustain a TBI annually in the USA,
contributing to one third of all cases of injury-associated
mortality (3). In China, TBI
accounts for between 38.7 and 57.3% of cases of road traffic
accident-associated mortality (4).
The costs of the long-term treatment and rehabilitation following
TBI constitute a considerable burden on society (5), and its therapeutic efficacy remains
unsatisfactory as its pathogenesis is driven by complex and
interactive mechanisms (6).
Following TBI, secondary brain injury is the leading
cause of TBI-associated mortality in hospital inpatients (7). Free radical-induced oxidative damage
occurs rapidly and and is of primary importance during secondary
pathophysiological cascades. During secondary brain injury in TBI,
the brain is vulnerable to oxidative stress due to the high rate of
oxidative metabolic activities, the abundance of polyunsaturated
fatty acids and the relatively low levels of antioxidant enzyme
activity (8). Morphological
responses and neurobehavioral deficits deteriorate under the
effects of oxidative neurodegeneration (9). The dynamic equilibrium between
oxidants and antioxidants is disrupted following cerebral injury by
the excessive consumption of antioxidants or accumulation of
reactive oxygen species (ROS), or the two in combination (10). When the injured areas produce
excess ROS following brain injury, superoxide (O2·) and
nitric oxide (·NO) radicals are produced first, which become more
potent oxidants through a series of reactions and metabolism,
including peroxynitrite (ONOO·), hydroxyl (·OH), carbonate
(CO3·) and nitrogen dioxide (·NO2) radicals
(11). These byproducts further
oxidize proteins, lipids, sugars and nucleotides (12–14).
The above data demonstrate a significant early contribution of
oxidative damage in the secondary injury response in TBI,
reinforcing the requirement of improved antioxidant therapies.
Hydroxysafflor yellow A (HSYA; Fig. 1), a flavonoid compound extracted
from Carthamus tinctorius L. (Asteraceae), has been used as
an active marker compound for controlling the quality of safflower
in the Chinese Pharmacopoeia (15). Previous studies have indicated that
HSYA has cerebral protective effects (16) by reducing protein
oxidation/nitration and lipid peroxides (12,13),
suppressing inflammatory responses (17) and attenuating breakdown of the
blood-brain barrier (BBB) (12).
Previous studies have also reported that HSYA may offer potential
as a therapeutic strategy to improve outcomes following TBI
(18,19). However, no previous investigations
have focused on the mechanism underlying the antioxidant activities
of HSYA in a rat model of TBI. Thus, the present study aimed to
determine the antioxidant effects of HSYA on TBI in rats.
In the present study, to determine the absorption of
HSYA for investigation of the underlying antioxidant effects of
HSYA in TBI, HSYA was identified in the brain tissues of
TBI-induced rats using an ultra performance liquid
chromatography-tandem mass spectrometry (UPLC-MS/MS) method.
Subsequently, the state of oxidative stress in the TBI rat model
following the administration of HSYA was estimated by determining
the levels of superoxide dismutase (SOD), malondialdehyde (MDA) and
catalase (CAT), in addition to the ratio of glutathione
(GSH)/glutathione disulfide (GSSG).
Materials and methods
Plant materials and chemicals
HSYA (purity >98%) was purchased from the
National Institute for the Control of Pharmaceutical and Biological
Products (Beijing, China). Gradient grade methanol for liquid
chromatography was supplied by Merck Millipore (Darmstadt,
Germany). Formic acid was obtained from Sinopharm Chemical Reagent
Company (Shanghai, China) and high purity water was obtained from
Wahaha Co., Ltd. (Hangzhou, China). The assay kits for SOD, MDA,
CAT, GSH and GSSG, and Bradford protein were obtained from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). All other
reagents were of analytical grade.
Animals and surgical procedure
Healthy male Sprague-Dawley (SD) rats (weighing
between 200 and 250 g, age, 8–10 weeks) were supplied by the
Laboratory Animal Research Center of Central South University
(Changsha, China). The rats were housed in an environmentally
controlled breeding room (22–25°C; 12-h light/dark cycle; 50±10%
humidity) with access to a normal standard chow diet and tap water
ad libitum. The animals were maintained under these
conditions for at least 1 week, following which they were fasted
for 12 h with free access to water prior to each experiment. All
animal experiments were approved by the Central South University
Animal Ethics Committee and conformed to the Guidelines for the
Care and Use of Laboratory Animals.
The controlled cortical impact (CCI) model with TBI
was established using an electronic controlled pneumatic impact
device (TBI 0310; Precision Systems and Instrumentation LLC,
Fairfax Station, VA, USA) under 3% pentobarbital anesthesia (50
mg/kg), which was equipped with a hard stop Bimba cylinder (Bimba
Manufacturing, Monee, IL, USA) and an impactor tip (external
diameter, 5 mm); this is an approved instrument for the TBI model.
The parameters of the apparatus were as follows: Depth of impact,
5.00 mm from the cortical surface; impact velocity, 6.00 m/sec;
dwell time, 500 msec. The body temperature of the rats was
monitored throughout surgery, and a heated cage was used to
maintain body temperature at 37.0±0.5°C. Following surgery, the
animals recovered fully in ~20 min, and the survival rate following
surgery was >90%. A total of 96 SD rats were randomly divided
into the following four groups for the efficacy experiment: i)
Vehicle control group, rats with TBI were intragastrically
administered with 4 ml normal saline vehicle (0.9% NaCl); ii) sham
operation group, rats underwent the same surgical procedures, but
without trauma to the cerebral cortex; iii) 10 mg/kg HSYA treatment
group, rats were orally administered with 10 mg/kg HSYA following
trauma; iv) 30 mg/kg HSYA group, rats were orally administered with
30 mg/kg HSYA following trauma. Each group of rats were sacrificed
by decapitation in an ice bath at 6, 12 and 24 h following
intragastric administration. The ipsilateral cortex was immediately
removed, placed in an ice bag and stored at −80°C for biochemical
assays, which were performed within 1 month.
Detection of HSYA in brain tissues using
the UPLC-MS/MS) method
Following the oral administration of HSYA in rats
subjected to TBI, the absorption of the compound in the brain
tissue was determined by comparing the retention times and ion
peaks with the authentic reference using the UPLC-MS/MS method.
The Acquity TQD UPLC-MS/MS system (Waters
Corporation, Milford, MA, USA), consisting of Acquity UPLC online
SPE manager (OSM), Acquity UPLC binary solvent manager, Acquity
UPLC column manager, Acquity UPLC sample manager, Acquity TQ
detector, masstrack online SPE cartridges and Acquity UPLC online
SPE manager software (MassLynx) was used. Analyses were performed
under an electrospray ionization source, which was operated in the
negative mode (ESI−). A Waters Acquity UPLC BEH
C8 (2.1×100 mm; 1.7 µm) column with methanol (A)
and 0.1% formic acid in water (B) was used at a flow rate of 0.3
ml/min to establish the elution gradient (A:B ratios: 0 min, 10:90;
5 min, 60:40; 6 min, 90:10; 7 min, 90:10; 8 min, 10:90). The column
temperature was set at 30°C. The injection volume was 5 µl
using full-loop mode. The detection wavelengths of the photodiode
array detector were set at 200–600 nm. For MS/MS detection, the
following parameters were used: Temperature of source gas
(nitrogen), 110°C; desolvation gas (nitrogen) flow, 650 l/h at
365°C; capillary voltage, 2.5 KV; cone voltage, 26 V; cone gas
flow, 50 l/h; collision gas (argon) flow, 0.2 ml/min. Detection was
performed in the multiple reaction monitoring mode (MRM).
The HSYA (30 mg/kg) was administered to the TBI
rats, and the whole brain was rapidly removed following
decapitation 30 min later. The surface blood products were cleared
with ice-cold ddH2O, and the samples were dissected and
homogenized in 4 ml ice-cold methanol using a TissueLyser LT
homogenizer (Qiagen GmbH, Hilden, Germany). The homogenates were
centrifuged at 1,500 × g at 4°C for 10 min, and the
supernatant was collected separately for evaporation to dryness
under nitrogen at 37°C. Each dry extract was dissolved in 200
µl methanol (20%) and then centrifuged at 7,500 × g
at 4°C for 15 min. The upper layer was filtered through a 0.22
µm nylon filter, and 5 µl was injected automatically
to UPLC-MS/MS for analysis.
Estimation of antioxidant and oxidative
status
The stored cortices were weighed, dissected and
homogenized with nine volumes (1:9, w/v) of ice-cold normal saline
in a homogenizer (TissueLyser LT; Qiagen, GmbH). The homogenates
were centrifuged at 1,500 × g at 4°C for 15 min. The
supernatants were used to measure the oxidative product contents,
antioxidant enzyme activities and redox status, according to the
manufacturer's protocols for the reagent kits (Nanjing Jiancheng
Bioengineering Institute). Tissue protein concentrations were
measured using the Bradford method (20).
The cortical levels of MDA were estimated using the
thiobarbituric acid (TBA) method, as described by Zhao et
al, with minor modifications (21). According to the manufacturer's
protocol, the supernatant and TBA were mixed together and incubated
at 95°C for 40 min. The reaction mixture was cooled to room
temperature under flowing water and centrifuged at 1,750 × g
for 10 min at 4°C. The upper layer was used to determine the change
in absorbance on a spectrophotometer at 532 nm.
In measuring the activities of the antioxidant
enzymes, the supernatant obtained was used to determine the
activities of SOD and CAT. The detailed procedures were in
accordance with the instructions of the assay kits supplied by
Nanjing Jiancheng Bioengineering Institute. The absorbance of the
test solution was measured at a wavelength of 450 nm for SOD
activity and 405 nm for CAT activity. The activities of SOD and CAT
were corrected with protein quantity and expressed as the fold
change relative to the control, as U/mg of protein.
The levels of GSH and GSSG in the cortices were
determined to assess the redox status. The protocol, according to
the specification of the GSH/GSSG assay kit (Nanjing Jiancheng
Bioengineering Institute), was previously reported by Zhao et
al (21). The absorbance was
recorded at 405 nm on a spectrophotometer and then used to
calculate the GSH/GSSG ratio.
Statistical analysis
All data were analyzed using SPSS 15.0 software
(SPSS, Inc. Chicago, IL, USA). One-way analysis of variance and
Dunnett's t-test were used to determine the significant
differences between the four groups. All parameters are expressed
as the mean ± standard deviation. P<0.05 was considered to
indicate a statistically significant difference.
Results
Detection of HSYA using UPLC-MS/MS
The overall intra- and inter-day variations were
<5% for HSYA, and the method was reproducible with good
precision. The accuracy assessments were performed using recovery
assessment, and the recovery of all compounds was >90%. These
results suggested that the UPLC-MS/MS method was suitable for the
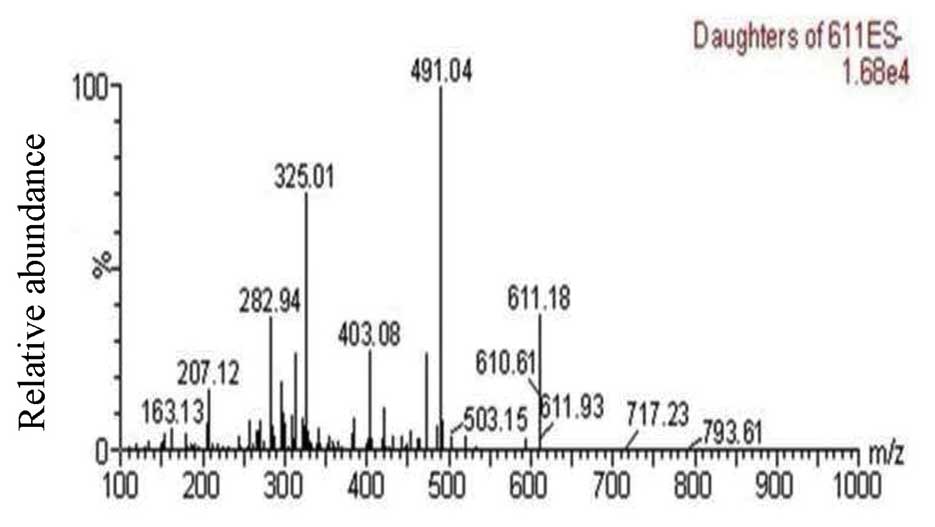
detection of HSYA. As shown in Fig.
2, the predominant mass transitions of HSYA were m/z
611.18→491.04, according to the UPLC-MS/MS method. The precursor
ion was detected at m/z 611 [M–H]−. In the production
mass spectra of [M–H]−, m/z 491 was the prominent
fragment ion. HSYA was identified in the extracts or biopsies based
on its retention time (2.74±0.02 min), and characteristic
[M–H]− ion (m/z 611.18) and fragment ions (m/z 491.04).
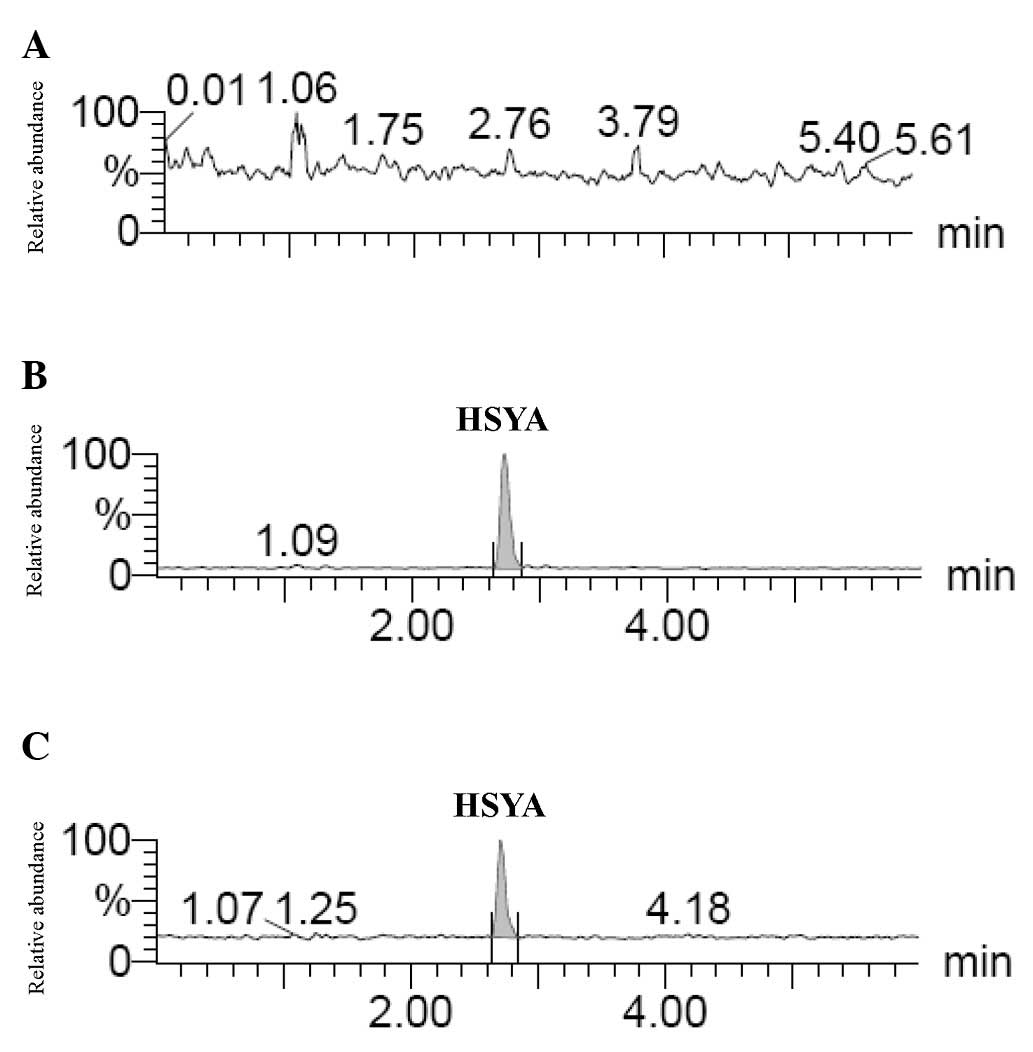
Representative MRM chromatograms of the brain tissues from the
untreated (drug-free), HYSA-treated and TBI+HYSA administration
rats are shown in Fig. 3A–C,
respectively.
Effects of antioxidant assay
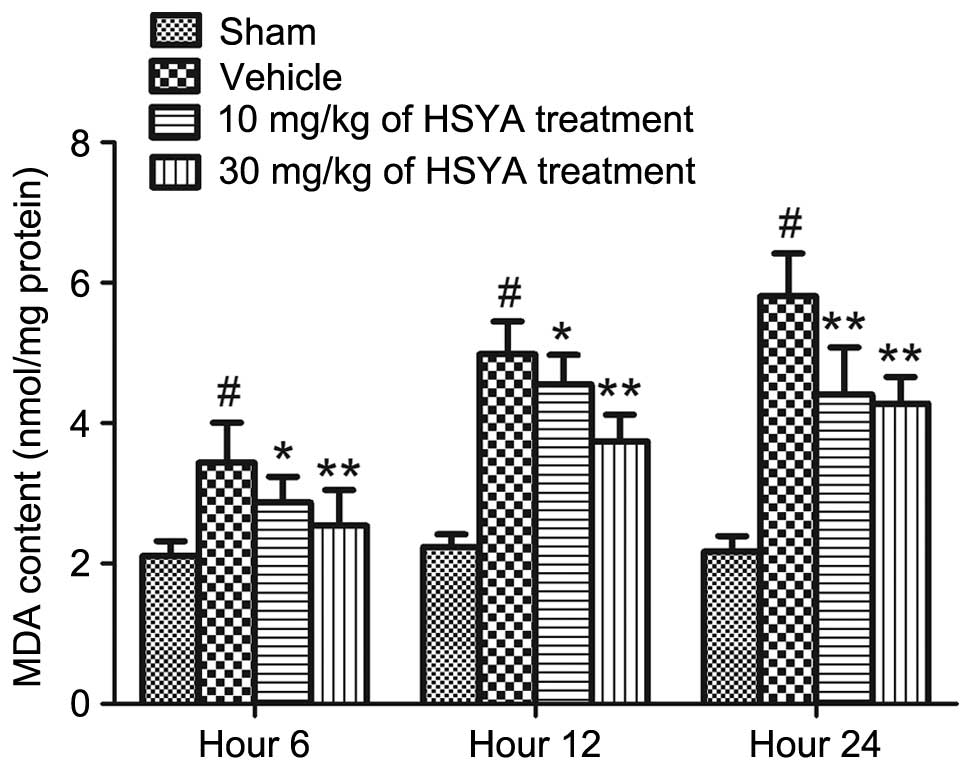
As shown in Fig. 4,
significantly higher levels of MDA were measured in the brains of
the TBI (vehicle) rats, compared with the sham group (P<0.01).
Treatment with HSYA (10 and 30 mg/kg) significantly decreased the
levels of MDA at different time points (6, 12 and 24 h), compared
with the vehicle (P<0.05 and P<0.01). The maximum alteration
was observed at 24 h.
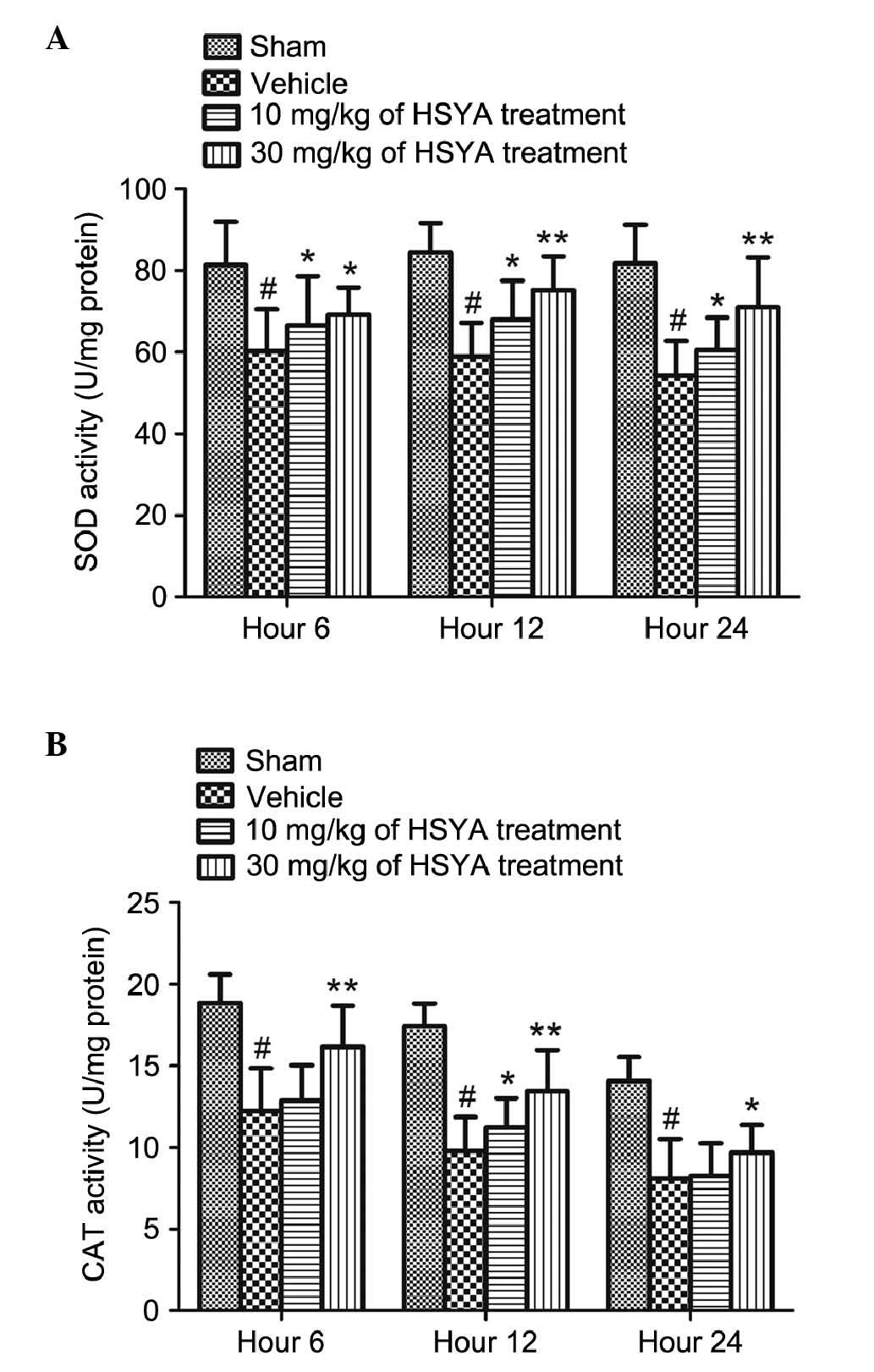
Compared with the sham treatment group, cerebral
injury in the TBI rats led to a significant decline in the
activities of SOD and CAT, as shown in Fig. 5 (P<0.01). The administration of
HSYA (10 and 30 mg/kg) caused a significant increase in the levels
of SOD at different time points (6, 12 and 24 h), compared with the
vehicle-treated group (P<0.05 and P<0.01), particularly
following treatment with the high dose of HSYA (30 mg/kg). The
activity of CAT increased significantly 12 h following treatment
with the low dose of HSYA (10 mg/kg), compared with the vehicle
treatment (P<0.05). Following treatment with a high dose of HSYA
(30 mg/kg), the activities of CAT were significantly increased at
all time points (6, 12 and 2 h), compared with the vehicle
treatment (P<0.05 and P<0.01).
As shown in Table
I, brain injury reduced the levels of GSH and the GSH/GSSG
ratios, and simultaneously increased levels of GSSG (all
P<0.01), compared with the sham group. The treatments with HSYA
(10 and 30 mg/kg) significantly increased the levels of GSH and
GSH/GSSG ratios, and decreased the levels of GSSG in the cerebral
tissues of the TBI rats, compared with the vehicle group.
 | Table IEffect of HSYA on the levels of
reduced and oxidized GSH in brain tissues of rats 6, 12 and 24 h
following traumatic brain injury. |
Table I
Effect of HSYA on the levels of
reduced and oxidized GSH in brain tissues of rats 6, 12 and 24 h
following traumatic brain injury.
| Group | 6 h
| 12 h
| 24 h
|
|---|
| GSH
(µmol/l) | GSSG
(µmol/l) | GSH/GSSG | GSH
(µmol/l) | GSSG
(µmol/l) | GSH/GSSG | GSH
(µmol/l) | GSSG
(µmol/l) | GSH/GSSG |
|---|
| Sham | 91.98±7.09a | 30.61±3.27b | 3.06±0.57a | 92.78±6.49a | 31.44±1.09a | 2.96±0.30a | 93.12±4.97a | 31.61±1.93a | 2.96±0.33a |
| Vehicle | 68.90±6.41 | 41.43±2.72 | 1.75±0.13 | 66.30±7.25 | 40.43±2.93 | 1.64±0.16 | 60.55±6.82 | 42.77±3.22 | 1.42±0.16 |
| 10 mg/kg HSYA | 72.29±3.56c | 31.16±3.68 | 2.18±0.29c | 79.69±6.68c | 31.64±2.54c | 2.53±0.26c | 68.02±6.94b | 36.27±2.79a | 2.21±0.23a |
| 30 mg/kg HSYA | 80.07±6.84c | 34.78±4.62c | 2.66±0.35c | 82.13±7.62c | 32.41±2.28b | 2.86±0.36a | 84.19±7.92a | 33.24±3.08b | 2.68±0.13a |
Discussion
The present study demonstrated that, according to
the UPLC-MS/MS method, HSYA was absorbed in the brain tissues of
TBI rats. Treatment of the TBI rats with HSYA significantly
alleviated the imbalance between oxidants and antioxidants,
resulting in further neuroprotective effects. HSYA increased the
activities of SOD and CAT, the level of GSH and the GSH/GSSG ratio.
In addition, HSYA concomitantly decreased the levels of MDA and
GSSG. The above preliminary data provided evidence suggesting that
HSYA offers potential to be utilized as a neuroprotective drug for
TBI.
To establish a link between the absorbed chemical
compound and its biological activity, HSYA was detected in the
brain tissues of TBI rats using the UPLC-MS/MS method. If no
evidence of absorption is found, any investigation of an
effect-associated component is most likely to be incorrect
(22). Thus, in the present study,
a sensitive and accurate UPLC-MS/MS method was successfully
developed to detect HSYA in the brain tissues of the TBI rats. The
pretreatment of samples makes this method easy to perform in a
short period of time, and the method of analysis conforms to the
criteria for validation of the UPLC-MS/MS method including
calibration curve, intra- and inter-day precisions and relative
standard deviation of recovery (19). According to the mass spectrum of
the HSYA, as shown in Fig. 2, two
mass transitions, which followed were 611→491.04 m/z and 611→325.06
m/z. These two MRM chromatograms were used to record the response.
The HSYA in the biopsies were identified by comparing the retention
time and ion peaks (Fig. 3). The
results provided evidence that HSYA was absorbed in the brains of
the TBI rats. The detection of this bioactive compound may assist
in elucidating the direct pharmacological actions of HSYA for the
treatment of TBI, and enables further investigation of the effects
of HSYA on oxidative stress in the rat model of TBI.
HSYA is a hydrophilic drug with low oral
bioavailability, belonging to the biopharmaceutics classification
system III class of drugs (23,24).
A previous study confirmed that HSYA can cross the BBB (19) and, consequently, HSYA has been
administered via injection in several studies for investigating the
treatment of cerebral diseases (18,25,26).
Of note, following TBI, small molecules (286–10,000 Da) are able to
enter the brain up to a few days following injury due to BBB
disruption (27). Therefore, HSYA,
with a molecular weight of 611 Da, is more readily absorbed into
the brains of TBI rats, compared with normal rat (28). In addition, the period of potential
secondary damage from barrier disruption and the period during
which HSYA has direct access to the injured brain may be longer
than previously suggested (27).
This leads to the accumulation of higher levels of HSYA in the
brain tissues of TBI rats, which may have neuroprotective effects.
Thus, the disruption of the BBB following TBI enables HSYA to cross
the barrier and exert antioxidative effects.
Oxidative stress is important in the pathogenesis of
secondary brain injury following TBI (29). Brain tissue is enriched with fatty
acids, which are readily peroxidized, consumes a substantial
proportion (20%) of total oxygen consumption for its relatively low
weight (2%), and has limited antioxidant defenses (30). In addition, the cerebral metabolic
rate is 7.5-fold higher than the average metabolic rate (31). Due to its higher metabolic rate and
lipid content, the brain is considered to be particularly sensitive
to oxidative damage following TBI. TBI generally results in tardive
neurological dysfunction and death through the processes of
secondary injury (32,33). It is associated with a mechanism
involving excess excitatory amino acids (28), ionic imbalance (34), anomalous proteolytic enzyme
activity (35), a low level of ATP
(36) and oxidative stress. Among
these, continued oxidative stress is a significant contributor to
the deterioration of neurological function (37). Neurological function is vulnerable
to injury by oxidative stress as its rate of oxygen consumption is
high, nerve cells are not readily replenished, and the high levels
of polyunsaturated fatty acids in the brain are targets of the
lipid peroxidation initiated by ROS (38). The accumulated ROS include
superoxide (O2·), hydroxyl radical (·OH), hydrogen
peroxide (H2O2) and hypochlorous acid (HOCl),
which induces protein oxidation, inhibits the mitochondrial
electron transport chain, cleaves DNA and leads to the peroxidation
of cellular and vascular structures (39). The above processes lead to
hypoperfusion, disruption of axonal guidance, disordered metabolism
and brain edema (40).
MDA is the end product of membrane lipid
peroxidation, which is generated from cellular membrane damage in
oxidative stress (41). Therefore,
it is widely considered to be a valuable index for measuring the
extent of oxidative stress. SOD and CAT enzymes are crucial
mediators in scavenging rapidly generated oxygen free radicals
following TBI. SOD is a first line anti-oxidative enzyme, which is
responsible for the defense mechanism against ROS and other
superoxide anion-free radicals (42). SOD first catalyzes the
transformation of superoxide radicals to H2O2
(43), following which CAT
transforms H2O2 into H2O and
O2 (44). The GSH
system is well known to protect cells against oxidative damage in
the pivotal role of redox homeostasis. It consists of reduced and
oxidized forms of GSH. GSH is a predominant intracellular
nonenzymatic antioxidant in tissues. During the processes of
oxidative damage, GSH binds directly to oxygen free radicals to
facilitate the conversion of H2O2, to then
become GSSG (45,46). The measurement of GSH/GSSG is a
useful indicator of oxidative stress and can be used to monitor
antioxidant effects (46).
Accordingly, the present study determined the levels
of MDA, SOD and CAT, and the GSH/GSSG ratio to investigate the
antioxidant effects. The results of the present study showed that
HSYA treatment significantly prevented the increases in the levels
of MDA, SOD and CAT. Furthermore, the levels of GSH were increased
and the levels of GSSG were decreased following treatment with
HSYA, compared with those in the vehicle group. The elevation of
the GSH/GSSG ratio in the present study also reflects the
anti-oxidative effect of HSYA. These results suggested that HSYA
attenuated the excessive formation of free radicals secondary to
TBI. Further investigations are required to investigate the
detailed mechanisms of the signaling pathway underlying the effects
of HSYA on brain cells against oxidative stress in vivo and
in vitro.
In conclusion, treatment with the absorbed bioactive
compound HSY attenuated oxidative injury to markedly inhibit
neuronal injury following TBI, indicating that HSYA offers
potential in TBI treatment as a neuroprotective therapy for the
prevention of oxidative stress. The present study presented a
canonical method to elucidate the mechanism of action in herbal
chemicals, and suggested that HSYA may be a promising therapeutical
compound for the treatment of TBI.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant nos. 81303074, 81403259,
81202781 and 81303098) and the Administration of Traditional
Chinese Medicine of Hunan Province, China (grant no. 201455).
Abbreviations:
|
TBI
|
traumatic brain injury
|
|
HSYA
|
hydroxysafflor yellow A
|
|
SOD
|
superoxide dismutase
|
|
MDA
|
malondialdehyde
|
|
CAT
|
catalase
|
|
GSH
|
glutathione
|
|
GSSG
|
glutathione disulfide
|
|
ROS
|
reactive oxygen species
|
|
BBB
|
blood-brain barrier
|
|
UPLC-MS/MS
|
ultra performance liquid
chromatography-tandem mass spectrometry
|
|
SD
|
Sprague-Dawley
|
|
CCI
|
controlled cortical impact
|
|
ESI−
|
electrospray ionization source
operated in negative mode
|
|
MRM
|
multiple reaction monitoring mode
|
|
TBA
|
thiobarbituric acid
|
|
ANOVA
|
one-way analysis of variance
|
References
|
1
|
Harrison-Felix C, Kolakowsky-Hayner SA,
Hammond FM, Wang R, Englander J, Dams-O'Connor K, Kreider SE,
Novack TA and Diaz-Arrastia R: Mortality after surviving traumatic
brain injury: Risks based on age groups. J Head Trauma Rehabil.
27:E45–E56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roozenbeek B, Maas AI and Menon DK:
Changing patterns in the epidemiology of traumatic brain injury.
Nat Rev Neurol. 9:231–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Corps KN, Roth TL and McGavern DB:
Inflammation and neuroprotection in traumatic brain injury. JAMA
Neurol. 72:355–362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu X, Hu J, Zhuo L, Fu C, Hui G, Wang Y,
Yang W, Teng L, Lu S and Xu G: Epidemiology of traumatic brain
injury in eastern China, 2004: A prospective large case study. J
Trauma. 64:1313–1319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tuominen R, Joelsson P and Tenovuo O:
Treatment costs and productivity losses caused by traumatic brain
injuries. Brain Inj. 26:1697–1701. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lei J, Gao G and Jiang J: Acute traumatic
brain injury: Is current management evidence based? An empirical
analysis of systematic reviews. J Neurotrauma. 30:529–537. 2013.
View Article : Google Scholar
|
|
7
|
Kou K, Hou XY, Sun JD and Chu K: Current
pre-hospital traumatic brain injury management in China. World J
Emerg Med. 5:245–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jin W, Wang H, Yan W, Zhu L, Hu Z, Ding Y
and Tang K: Role of Nrf2 in protection against traumatic brain
injury in mice. J Neurotrauma. 26:131–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith JA, Park S, Krause JS and Banik NL:
Oxidative stress, DNA damage and the telomeric complex as
therapeutic targets in acute neurodegeneration. Neurochem Int.
62:764–775. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adibhatla RM and Hatcher JF: Lipid
oxidation and peroxidation in CNS health and disease: From
molecular mechanisms to therapeutic opportunities. Antioxid Redox
Sign. 12:125–169. 2010. View Article : Google Scholar
|
|
11
|
Hall ED, Detloff MR, Johnson K and Kupina
NC: Peroxynitrite-mediated protein nitration and lipid peroxidation
in a mouse model of traumatic brain injury. J Neurotrauma. 21:9–20.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun L, Yang L, Xu YW, Liang H, Han J, Zhao
RJ and Cheng Y: Neuroprotection of hydroxysafflor yellow A in the
transient focal ischemia: Inhibition of protein
oxidation/nitration, 12/15-lipoxy-genase and blood-brain barrier
disruption. Brain Res. 1473:227–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan L, Dang X, Shi Z, Zhang C and Wang K:
Hydroxysafflor yellow A protects PC12 cells against the apoptosis
induced by oxygen and glucose deprivation. Cell Mol Neurobiol.
31:1187–1194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gracy RW, Talent JM, Kong Y and Conrad CC:
Reactive oxygen species: The unavoidable environmental insult?
Mutat Res. 428:17–22. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pharmacopoeia Commission of PRC:
Pharmacopoeia of the People's Republic of China. Chemical Industry
Press; Beijing: pp. 1412010
|
|
16
|
Qi Z, Yan F, Shi W, Dong W, Zhao Y, Shen
J, Ji X, Liu KJ and Luo Y: AKT-related autophagy contributes to the
neuroprotective efficacy of hydroxysafflor yellow A against
ischemic stroke in rats. Transl Stroke Res. 5:501–509. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Zhang S, Lu M, Chen Z, Chen C, Han
L, Zhang M and Xu Y: Hydroxysafflor yellow A suppresses
inflammatory responses of BV2 microglia after oxygen-glucose
deprivation. Neurosci Lett. 535:51–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bie XD, Han J and Dai HB: Effects of
hydroxysafflor yellow A on the experimental traumatic brain injury
in rats. J Asian Nat Prod Res. 12:239–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo Y, Wang Y, Huang X, Lv H, Fan R, Huang
W, Gan P, Liu W, Yan K, Xia Z and Liu J: Determination of
hydroxysafflor yellow A in biological fluids of patients with
traumatic brain injury by UPLC-ESI-MS/MS after injection of
Xuebijing. Biomed Chromatogr. 28:1090–1095. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharma SK and Babitch JA: Application of
Bradford's protein assay to chick brain subcellular fractions. J
Biochem Biophys Methods. 2:247–250. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao H, Chai W, Gao W, Xu L, Zhang H and
Yang Y: Hyperoxygenated solution: Effects on acute hypobaric
hypoxia-induced oxidative damage in rabbits. High Alt Med Biol.
10:283–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Huang X, Liang Q, Fan R, Qin F,
Guo Y, Yan KP, Liu W, Luo JK, Li YH, et al: A strategy for
detecting absorbed bioactive compounds for quality control in water
extract of rhubarb by ultra performance liquid chromatography with
photodiode array detector. Chin J Integr Med. 18:690–698. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qi J, Zhuang J, Wu W, Lu Y, Song Y, Zhang
Z, Jia J and Ping Q: Enhanced effect and mechanism of water-in-oil
microemulsion as an oral delivery system of hydroxysafflor yellow
A. Int J Nanomedicine. 6:985–991. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lv LZ, Tong CQ, Yu J, Han M and Gao JQ:
Mechanism of enhanced oral absorption of hydrophilic drug
incorporated in hydrophobic nanoparticles. Int J Nanomedicine.
8:2709–2717. 2013.PubMed/NCBI
|
|
25
|
Wei X, Liu H, Sun X, Fu F, Zhang X, Wang
J, An J and Ding H: Hydroxysafflor yellow A protects rat brains
against ischemia-reperfusion injury by antioxidant action. Neurosci
Lett. 386:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu H, Wang Z, Ma C, Tian J, Fu F, Li C,
Guo D, Roeder E and Liu K: Neuroprotective effects of
hydroxysafflor yellow A: In vivo and in vitro studies. Planta Med.
69:429–433. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Habgood MD, Bye N, Dziegielewska KM, Ek
CJ, Lane MA, Potter A, Morganti-Kossmann C and Saunders NR: Changes
in blood-brain barrier permeability to large and small molecules
following traumatic brain injury in mice. Eur J Neurosci.
25:231–238. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang CY, Liu Q, Huang QX, Liu JT, He YH,
Lu JJ and Bai XY: Activation of PPARγ is required for
hydroxysafflor yellow A of Carthamus tinctorius to attenuate
hepatic fibrosis induced by oxidative stress. Phytomedicine.
20:592–599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mojtahedzadeh M, Ahmadi A, Mahmoodpoor A,
Beigmohammadi MT, Abdollahi M, Khazaeipour Z, Shaki F, Kuochaki B
and Hendouei N: Hypertonic saline solution reduces the oxidative
stress responses in traumatic brain injury patients. J Res Med Sci.
19:867–874. 2014.PubMed/NCBI
|
|
30
|
Gan L, Wang ZH, Zhang H, Zhou R, Sun C,
Liu Y, Si J, Liu YY and Wang ZG: Protective effects of shikonin on
brain injury induced by carbon ion beam irradiation in mice. Biomed
Environ Sci. 28:148–151. 2015.PubMed/NCBI
|
|
31
|
Li J and Wang Y: Effect of different
methods of hypoxic exercise training on free radical oxidation and
antioxidant enzyme activity in the rat brain. Biomed Rep.
1:925–929. 2013.
|
|
32
|
Xie Z, Lei B, Huang Q, Deng J, Wu M, Shen
W and Cheng Y: Neuroprotective effect of Cyclosporin A on the
development of early brain injury in a subarachnoid hemorrhage
model: A pilot study. Brain Res. 1472:113–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yakovlev AG, Knoblach SM, Fan L, Fox GB,
Goodnight R and Faden AI: Activation of CPP32-like caspases
contributes to neuronal apoptosis and neurological dysfunction
after traumatic brain injury. J Neurosci. 17:7415–7424.
1997.PubMed/NCBI
|
|
34
|
Kim JY, Kim N, Yenari MA and Chang W:
Hypothermia and pharmacological regimens that prevent
overexpression and overactivity of the extracellular
calcium-sensing receptor protect neurons against traumatic brain
injury. J Neurotrauma. 30:1170–1176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schoch KM, Evans HN, Brelsfoard JM,
Madathil SK, Takano J, Saido TC and Saatman KE: Calpastatin
overexpression limits calpain-mediated proteolysis and behavioral
deficits following traumatic brain injury. Exp Neurol. 236:371–382.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sullivan PG, Rabchevsky AG, Waldmeier PC
and Springer JE: Mitochondrial permeability transition in CNS
trauma: Cause or effect of neuronal cell death? J Neurosci Res.
79:231–239. 2005. View Article : Google Scholar
|
|
37
|
Alexi T, Borlongan CV, Faull R, Williams
CE, Clark RG, Gluckman PD and Hughes PE: Neuroprotective strategies
for basal ganglia degeneration: Parkinson's and Huntington's
diseases. Prog Neurobiol. 60:409–470. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bar-Or D, Bar-Or R, Rael LT and Brody EN:
Oxidative stress in severe acute illness. Redox Biol. 4:340–345.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ehsaei M, Khajavi M, Arjmand MH, Abuee MA,
Ghayour-Mobarhan M and Hamidi Alamdari D: Prooxidant-antioxidant
balance in patients with traumatic brain injury. Acta Neurol Belg.
115:69–73. 2015. View Article : Google Scholar
|
|
40
|
Freire MA: Pathophysiology of
neurodegeneration following traumatic brain injury. West Indian Med
J. 61:751–755. 2012.
|
|
41
|
Xue T, Luo P, Zhu H, Zhao Y, Wu H, Gai R,
Wu Y, Yang B, Yang X and He Q: Oxidative stress is involved in
Dasatinib-induced apoptosis in rat primary hepatocytes. Toxicol
Appl Pharmacol. 261:280–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lau WK, Mak JC, Chan KH and Law AC:
Cigarette smoke-induced cerebral cortical interleukin-6 elevation
is not mediated through oxidative stress. Neurotox Res. 22:170–176.
2012. View Article : Google Scholar :
|
|
43
|
Zelko IN, Mariani TJ and Folz RJ:
Superoxide dismutase multigene family: A comparison of the CuZn-SOD
(SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures,
evolution, and expression. Free Radic Biol Med. 33:337–349. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chelikani P, Fita I and Loewen PC:
Diversity of structures and properties among catalases. Cell Mol
Life Sci. 61:192–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ansari MA, Roberts KN and Scheff SW:
Oxidative stress and modification of synaptic proteins in
hippocampus after traumatic brain injury. Free Radic Biol Med.
45:443–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang D, Yuan X, Liu T, Liu L, Hu Y, Wang Z
and Zheng Q: Neuroprotective activity of lavender oil on transient
focal cerebral ischemia in mice. Molecules. 17:9803–9817. 2012.
View Article : Google Scholar : PubMed/NCBI
|