Introduction
Influenza A virus (IAV) causes significant epidemics
of respiratory disease and deaths in humans (1). The rapidly increasing prevalence of
drug resistance and frequent mutations in strain (2), leads to failure of flu vaccine and
neuraminidase inhibitor, which demonstrated that there is need for
development of novel treatments (3–5).
Since IAV infection is a threat to public health, it is critical to
develop anti-IAV drug.
Atractylodis Rhizoma is rootstock of
Atractylodes lancea (Thunb.) DC. or Atractylodes
chinensis (DC.) Koidz., which belongs to the Asteraceae
family. This herb is mainly distributed in the region of East China
such as Jiangsu, Henan, Shanxi and Shanxi provinces and is used as
crude extracts/decoctions or a component in various herbal
formulations for centuries (6). It
is described as a basic component of antiviral, anti-inflammatory,
hepatoprotective, and anticancer agents. It aids digestion
medicines in the authoritative medical book of Ancient China
'Compendium of Materia Medica' and 2015 version of 'Chinese
Pharmacopoeia', with daily dose of 3–9 g. In folk medicine, it has
been used as a remedy for the treatment of pulmonary and digestive
system diseases for several centuries. Especially in Jiangsu,
Zhejiang, and other coastal areas, it was always compatible and
decocted with other natural medicinal plants (such as
Saposhnikoviae Radix and Notopterygii Rhizoma et
Radix) to prevent cold and flu in popular medicine (7).
Previously, biological studies reported that it has
various pharmacological actions, such as antibacterial, antiviral,
anti-inflammatory and anti-allergic, anticancer, gastroprotective
and neuroprotective activities (8–11).
Results from the toxicity studies in animal models suggested safety
profiles of A. lancea and its active constituents. Wang
et al in 2015 reported that codonolactone, a sesquiterpene
lactone from A. lancea, impaired the development of tumor
angiogenesis by downregulating Runx2 activation to inhibit matrix
metalloproteinases expression and vascular endothelial growth
factor secretion in endothelial cells (12). Hinesol isolated from essential oils
of A. lancea rhizome inhibited human leukemia HL-60 cell
growth and induced apoptosis through the JNK signaling pathway
(13). Atractylenolide I, the
major sesquiterpenoid from A. lancea, has significant
antitumor activity in lung carcinoma cells via a
mitochondria-mediated apoptosis pathway (14). Furthermore, atractylenolide III
significantly inhibited IgE/Ag-mediated degranulation in RBL-2H3
cells without affecting cell viability (15). The anti-allergic activity of it may
depend on the inhibition of Akt, p38, and JNK kinase
phosphorylation and IgE/Ag-mediated [Ca2+]i elevation
(16). However, antiviral
properties and possible molecular mechanisms of A. lancea
extracts and active constituents against IAV-induced lung injury
remain unknown. In the present study, protective effects of A.
lancea extracts and active constituents were investigated in
vitro and in vivo.
Materials and methods
Virus and reagents
The influenza A/PR/8/34 virus (H1N1 subtype) and
A/shenzhen/203/2001 (H3N2 subtype) were donated by Professor Yi-Yu
Lu from the Zhejiang Center for Disease Control and Prevention
(Zhejiang, China). The infection was induced under ether anesthesia
by intranasal inoculation of IAV, which was adapted to mouse lungs
with tissue culture infective dose (TCID50) 10−3.5. This
virus caused pneumonia in mice.
Extraction and isolation
The herb was purchased from Huadong Pharmacy
(Shanghai, China), and identified by Dr Jiaqi He, Zhejiang Chinese
Medical University (Zhejiang, China). The voucher specimen
(reference no. Y20141022) has been deposited at Pharmaceutical
Department, Zhejiang Medical College (Shanghai, China).
Dried powders of Atractylodis Rhizoma (100 g)
was extracted with 600 ml of 95% (v/v) ethanol for 2 h at 90°C to
give an extract (9.6 g) which was suspended in 100 ml of deionized
water as the test sample 'ethanolic extract'. Another 100 g of
powder was extracted with 600 ml of deionized water for 2 h at
90°C, then the supernatant was concentrated to 100 ml as the test
sample 'aqueous extract'.
Supercritical carbon dioxide (CO2)
extraction (SCE) was performed at pressure of 350 bar, temperature
of 50°C for a duration of 60 min dynamic extraction time using
HA121 supercritical fluid extractor (Huaan instrumental factory,
Jiangsu, China). Ethanol (99.9%) was used as modifier with a flow
rate of 5 ml/min. The supercritical CO2 flow rate was
set at 15 g/min and the duration of static extraction time was
fixed to 30 min. Solvent was removed and the yield obtained was
5.8%. The concentration of SCE prepared for in vitro studies
was 1 mg/ml in phosphate buffer saline.
The supercritical CO2 extract (50 g) was
subjected to a silica gel column using petroleum ether (boiling
point 60–90°C) and EtOAc (30:1→1:1, v/v) to obtain compound 4 (6
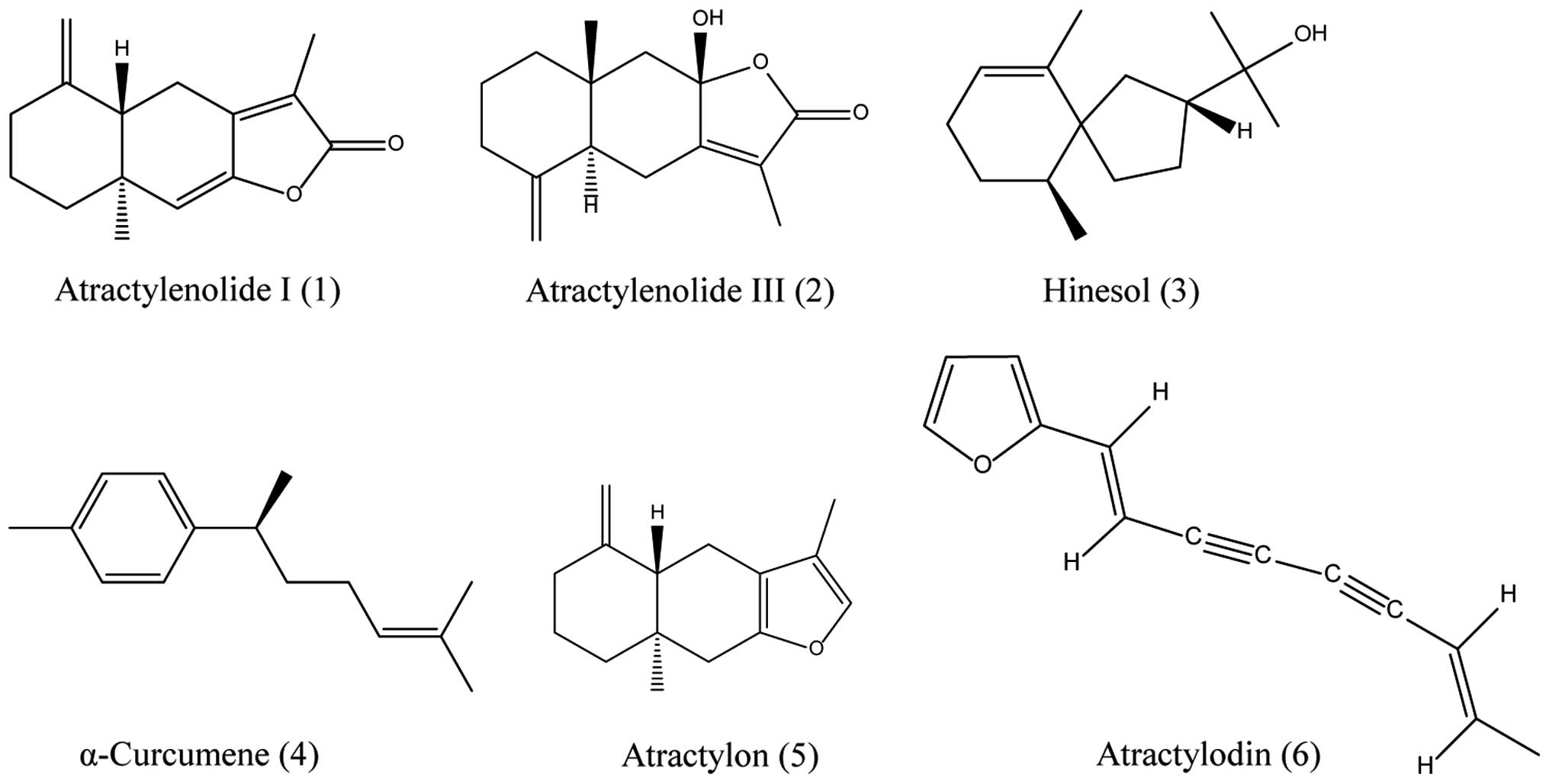
mg), 3 (2 mg), 5 (50 mg), 6 (4 mg), 1 (10 mg) and 2 (13 mg),
respectively. The structures of the compounds were determined using
spectroscopic techniques including ultraviolet (UV), mass
spectrometry, 1H and 13C NMR spectrometry (Fig. 1).
Antiviral activity and cytotoxicity
assay
The anti-viral activities of the extracts and
compounds were measured by the cytopathic effect (CPE) inhibition
assay (17). Briefly, 100
μl of virus suspension (200TCID50) was added to the
Madin-Darby canine kidney (MDCK) cells in 96-well plates cultured
for 60 min, followed by washing virus with a medium without serum.
Then, the medium containing the desired concentration of test
compounds was added. After incubation at 37°C in a 5%
CO2 incubator for 3 days, the culture was fixed and
counted for the plaque numbers. The IC50 of the CPE with
respect to virus control was estimated using the Reed-Muench
method.
To test for cytotoxicity, 100 μl of
maintenance medium containing serial 2-fold dilutions of the test
compounds was added in 96-well culture plate in which each well
contained a concentration of 5,000 cells/100 μl. Control
cells were incubated without test compounds but with DMSO (0.2%).
After cells were cultured at 37°C in a 5% CO2 incubator
for 3 days, 20 μl of MTT (5 mg/ml in cell culture medium)
was added to each well and cells were incubated for an additional 3
h at 37°C. The reaction product was determined at 570 nm with a
microplate reader. Cytotoxicity was expressed as the 50%
cell-inhibitory concentration (CC50). The selective
index (SI) is determined as CC50/IC50.
Infection model and drug treatment
Male ICR mice of 22±2 g weight were anesthetized
with Et2O. Subsequently, animals were intranasally
challenged with 20 μl of mouse-adapted 10LD50 IAV
and then divided into normal control group, virus control group,
ribavirin treatment group and drug treatment groups (24 mice each
group). Atractylon or ribavirin was intragastrically administered
to experimental groups once daily for 5 days, beginning 2 h after
infection. For the model control group, the mice were only given
saline at the same intervals. The mice were observed daily for
changes in weight, and survivability (18). To monitor the histological changes
and challenge dose of the virus in the lungs of IAV-infected
animal, 10 mice per group were sacrificed to calculate lung index
and viral load after day 6 post-infection.
Serum cytokine analysis
After 5 days of treatment, the body weight of the
animals were measured on sacrifice. Blood samples were collected
and centrifuged at 3,000 × g for 20 min to obtain the serum, which
was stored at 4°C until use. The serum levels of interferon-β
(IFN-β), interleukin (IL)-6, tumor necrosis factor (TNF)-α, and
IL-1β were determined by enzyme linked immunosorbent assay (ELISA).
The ELISA kits used in this study were obtained from Bioval
Technologies (Shanghai, China).
Histopathology
The whole lungs of 10 mice in each group were
removed. A portion of each lung was dissected and fixed in a 10%
neutral buffered formalin solution. The remaining lung tissue was
quickly frozen and kept at −80°C for biochemical analysis. The
fixed tissues were routinely processed, embedded in paraffin,
sectioned (4 μm), deparaffinized, and rehydrated according
to standard techniques. The tissue sections were stained with
hematoxylin and eosin.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
The mouse lung samples were homogenized and
extracted with TRIzol reagents to obtain the total RNAs
(Biotechnology Co., Ltd., Shanghai, China). The cDNA was
synthesized according to the kit protocol (Takara Bio, Dalian,
China). The specific primer pairs [i.e., Toll-like receptor 7
(TLR-7), MyD88, tumor necrosis factor receptor-associated factor 6
(TRAF6) and IFN-β and 18sRNA] were designed and used for the
analysis as shown in Table I.
These primers were synthesized by the Biotechnology Co., Ltd. The
eukaryotic 18S rRNA was primarily selected as the housekeeping gene
in this study because of the limited change in its expression under
different experimental conditions.
 | Table ISequences of primers used for the
RT-qPCR assays. |
Table I
Sequences of primers used for the
RT-qPCR assays.
| Gene | Primer sequences
(5′-3′) | Size (bp) | Annealing (°C) |
|---|
| TLR7 | F:
GGCATTCCCACTAACACCACCAA | 143 | 63 |
| R:
GCTTTGGACCCCAGTAGAACAGG | | |
| TRAF6 | F:
GAATCACTTGGCACGACACTT | 227 | 63 |
| R:
GAGTTTCCATTTTGGCAGTCA | | |
| MyD88 | F:
GGATGGTAGTGGTTGTCTCTGA | 145 | 63 |
| R:
CTGGGGAACTCTTTCTTCATTG | | |
| IFN-β | F:
ATCCTCCAAACAACTCTCCTGT | 105 | 63 |
| R:
CTCCTGACACTCCAAACTGCT | | |
| 18S (internal
reference) | F:
CGGACACGGACAGGATTGACA | 94 | 63 |
| R:
CCAGACAAATCGCTCCACCAACTA | | |
The amplification reaction was conducted in a 25
μl glass capillary containing 2 μl of cDNA, 0.5
μl of each of the forward and reverse primers (i.e., final
concentration of each, 0.5 μM), 12.5 μl 2X
SYBR-Green, and nuclease-free water for a final volume of 25
μl for each reaction. The following thermal profile for the
RT-qPCR assays was used in all the primer sets: 95°C for 1 min,
followed by 40 cycles of denaturation at 95°C for 10 sec, annealing
at 55°C for 25 sec, and elongation at 64°C for 25 sec with the
acquisition of fluorescent data. The relative expression levels of
the genes were calculated using the 2−ΔΔCt ×
106 method.
Statistical analysis
All the values were expressed as mean values ±
standard deviation. All statistical analyses were performed using
one-way ANOVA followed by the Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results and Discussion
Atractylodis Rhizoma has been used for
treatment of infections in the upper respiratory tract for
thousands of years in Asia (19).
To elucidate its mechanism and chemical basis, the extracts of the
herb for antiviral activities in MDCK cells were assessed by CPE
reduction assay. To obtain the bioactive component related to its
antiviral activity, the herb was extracted with water, ethanol and
supercritical CO2 to obtain different polar extraction.
Among these polar extractions, SCE showed the highest antiviral
activity by CPE assay (Table II).
To clarify the chemical compositions of SCE, the mobile phase
consisted of petroleum ether and EtOAc was used as gradient elution
for separation with the silica gel column. Six compounds were
isolated and then identified by using spectroscopic techniques
including UV, mass spectrometry, 1H and 13C NMR spectrometry.
Compared with the spectral data reported in previous literature
(20–22), their chemical structures were
characterized to be atractylenolide I (1), atractylenolide III (2), hinesol, α-curcumene (4), atractylon (5) and atractylodin (6), respectively, as shown in Fig. 1.
 | Table IIAntiviral activities of
Atractylodis Rhizoma extracts and compounds against
influenza virus in MDCK cells by the CPE assay. |
Table II
Antiviral activities of
Atractylodis Rhizoma extracts and compounds against
influenza virus in MDCK cells by the CPE assay.
| Samples | H1N1
| H3N2
| SI |
|---|
|
CC50 |
IC50 | SI |
IC50 |
|---|
| Ethanolic
extract | 885.0 | 348.7 | 2.5 | 350.4 | 2.5 |
| Supercritical
CO2 extract | 1407.5 | 294.4 | 4.8 | 310.3 | 4.5 |
| Aqueous
extract | 1335.0 | ND | ND | ND | ND |
| Atractylenolide
I | 203.5 | 24.1 | 8.4 | 35.1 | 5.8 |
| Atractylenolide
III | 191.2 | 24.4 | 7.8 | 28.6 | 6.7 |
| Hinesol | 181.3 | 54.2 | 3.3 | 51.2 | 3.5 |
|
(+)-α-curcumene | 137.7 | 18.4 | 7.5 | 22.1 | 6.2 |
| Atractylodin | 162.4 | ND | ND | ND | ND |
| Atractylon | 261.4 | 8.9 | 29.4 | 9.4 | 27.8 |
| Ribavirin | 116.30 | 14.2 | 8.2 | 10.7 | 10.9 |
The CPE assay showed that except atractylodin,
another 5 compounds had obvious antiviral activities. In
particular, the sesquiterpene lactone atractylon had most potent
bioactivity with values of SI (29.4 for H1N1 and 27.8 for H3N2),
which were approximately 3-fold higher than those of ribavirin.
Recently, many reports have shown that sesquiterpene lactones as a
large class of polyphenolic compounds in our daily food and plants
have been paid more attention due to their various pharmacological
activities such as anti-cancer, anti-inflammatory, antimicrobial,
antioxidant and antiviral activities (23–27).
Previous studies also proved that the spatial arrangement of the
terpenoid skeleton fused with an α-methylene-γ-lactone moiety
produces maximal antiviral activity (28). Our findings showed that the
sesquiterpene lactones (atractylenolide I, atractylenolide III and
atractylon) inhibited the formation of agglutination by IAV
suggested the mechanism of the compounds against influenza virus
might be related to blocking the adsorption of the virus to host
cells or inhibiting the replication of virus, but further
investigations have to be done.
To evaluate pharmacodynamic action of atractylon
thoroughly, protective effects of atractylon on IAV-induced lung
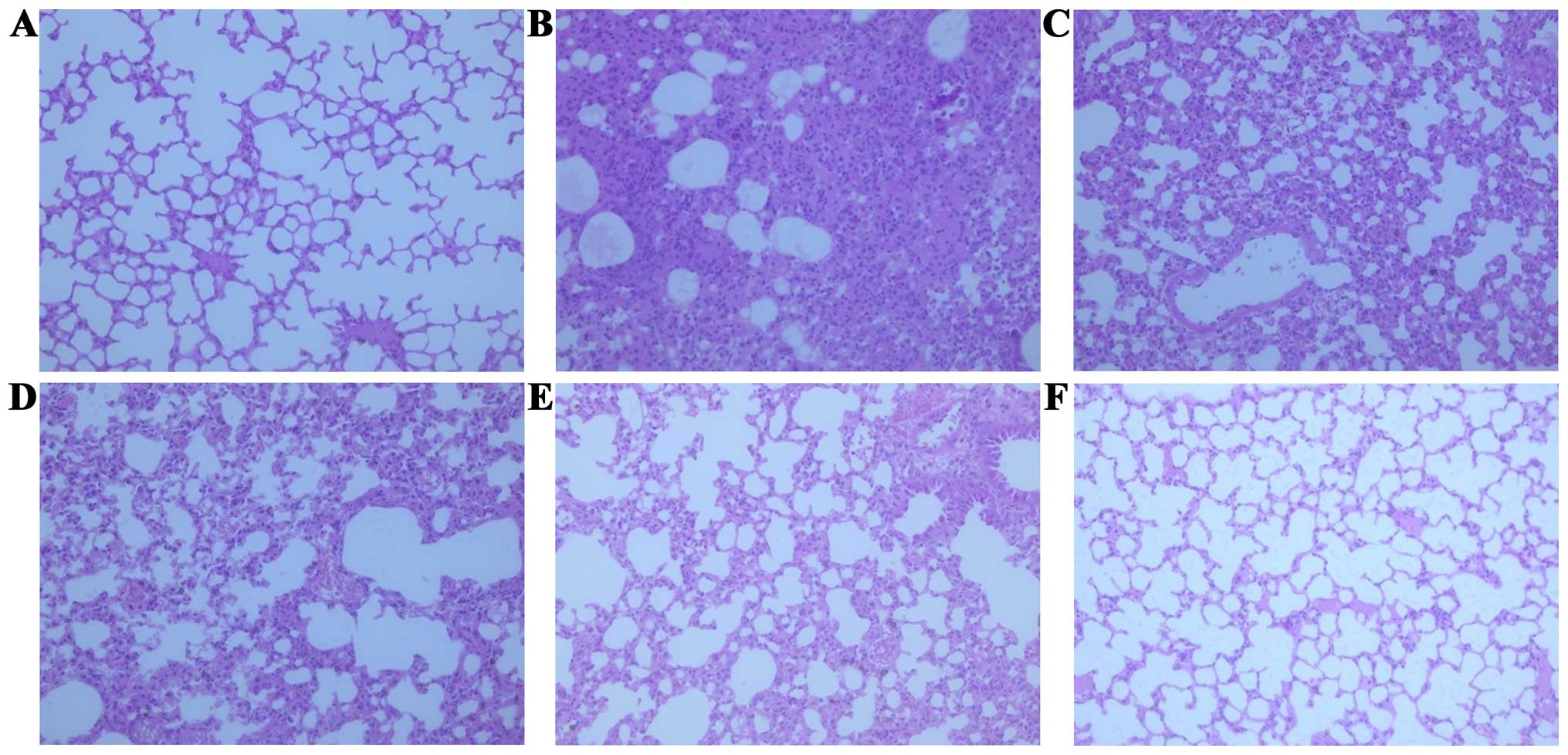
injury and death were observed in vivo. The histological
changes in the lungs of the mice were noted with the aggravation of
the infection. After IAV challenge, lung index was obviously
increased compared to the normal control group (Table III) since IAV causes red blood
cell agglutination and monocytic infiltration, resulting in
interstitial pneumonia and progressive lung weight (29,30).
The IAV-induced lung injury characterized by the presence of
interstitial edema, hemorrhage and infiltration of inflammatory
cells could also be observed in the tissues (Fig. 2). However, oral administration with
atractylon or ribavirin daily for 5 days could significantly
inhibit lung indexes (P<0.05). Moreover, the inhibition of
atractylon was found to proportionally increase in a dose-dependent
manner. The histological damage was improved by the atractylon
treatment (10–40 mg/kg). To monitor the infectivity and challenge
dose of the virus in mouse lung, we also determined the viral loads
in the lung by RFQ-PCR analysis (Table IV). Viral loads in the
atractylon-treated groups were drastically reduced compared with
that in the model group (P<0.01). These results were in
accordance with the CPE assay, indicating that atractylon could
directly inhibit viral replication.
 | Table IIIInhibition of atractylon and
ribavirin on IVA-induced acute lung injury. |
Table III
Inhibition of atractylon and
ribavirin on IVA-induced acute lung injury.
| Groups | Dose (mg/kg) | Lung index
(mg/g) | Inhibitory rate
(%) | Viral load
(×106 copies) |
|---|
| Normal control | – | 7.35±0.51b | – | – |
| Model control | – | 21.26±4.75 | – | 6.69±0.74 |
| Ribavirin | 50 | 12.68±3.30b | 61.7 | 1.38±0.56b |
| Atractylon | 40 | 10.90±3.76b | 74.5 | 0.36±0.15b |
| 20 | 13.08±4.54b | 58.8 | 2.56±0.72b |
| 10 | 17.43±4.16a | 27.5 | 4.79±0.84b |
 | Table IVProtective effects of atractylon and
ribavirin in IVA-infected mice. |
Table IV
Protective effects of atractylon and
ribavirin in IVA-infected mice.
| Groups | Dose (mg/kg) | Survived/total | Survival rate
(%) | MDD ± SD | Life prolong rate
(%) |
|---|
| Normal control | – | 20/20 | 100.0 | 14.0±0.0a | – |
| Model control | – | 3/20 | 15.0 | 7.9±2.8 | – |
| Ribavirin | 50 | 10/20 | 50.0 | 11.1±3.1a | 40.5 |
| Atractylon | 40 | 14/20 | 70.0 | 12.4±2.6a | 57.0 |
| 20 | 11/20 | 55.0 | 11.2±3.3a | 41.8 |
| 10 | 6/20 | 30.0 | 9.3±3.6a | 17.7 |
Innate immunity is the primary line of antiviral
defense (31). This type of
immunity allows the infected and neighboring cells to establish an
early antiviral state in the host after virus exposure. Innate
immunity also prepares the adaptive immune response and recognizes
viral components through pattern recognition receptors (PRRs)
during infection. IAV infection is recognized through two classic
adaptors of PRRs to induce robust type I IFN (IFN-α/β) responses;
these PRR pathways involve TLR7 in the endosome, which converge on
TRAF6, interferon regulatory factor 3 (IRF3) and IRF7 to induce the
production of proinflammatory cytokines and type I IFNs (32–35).
The induction of pro-inflammatory cytokines and IFNs is important
to induce antiviral gene expression, which interfere with viral
replication, recruit leukocytes and lymphocytes to the infection
part, and provide the requisite signals, thereby activating the
adaptive immune response for efficient viral clearance (36,37).
The serum levels of IFN-α, IL-1β, IL-6 and TNF-α
were measured to observe the inflammatory cytokine levels of
peripheral blood during IAV infection. In this study, it was found
that the administration of atractylon significantly increased the
serum levels of IFN-β but decrease the levels of other
pro-inflammatory cytokines (IL-6, TNF-α and IL-1β) as shown in
Table V. Previous studies have
shown that IFN-β has an important role in the control of influenza
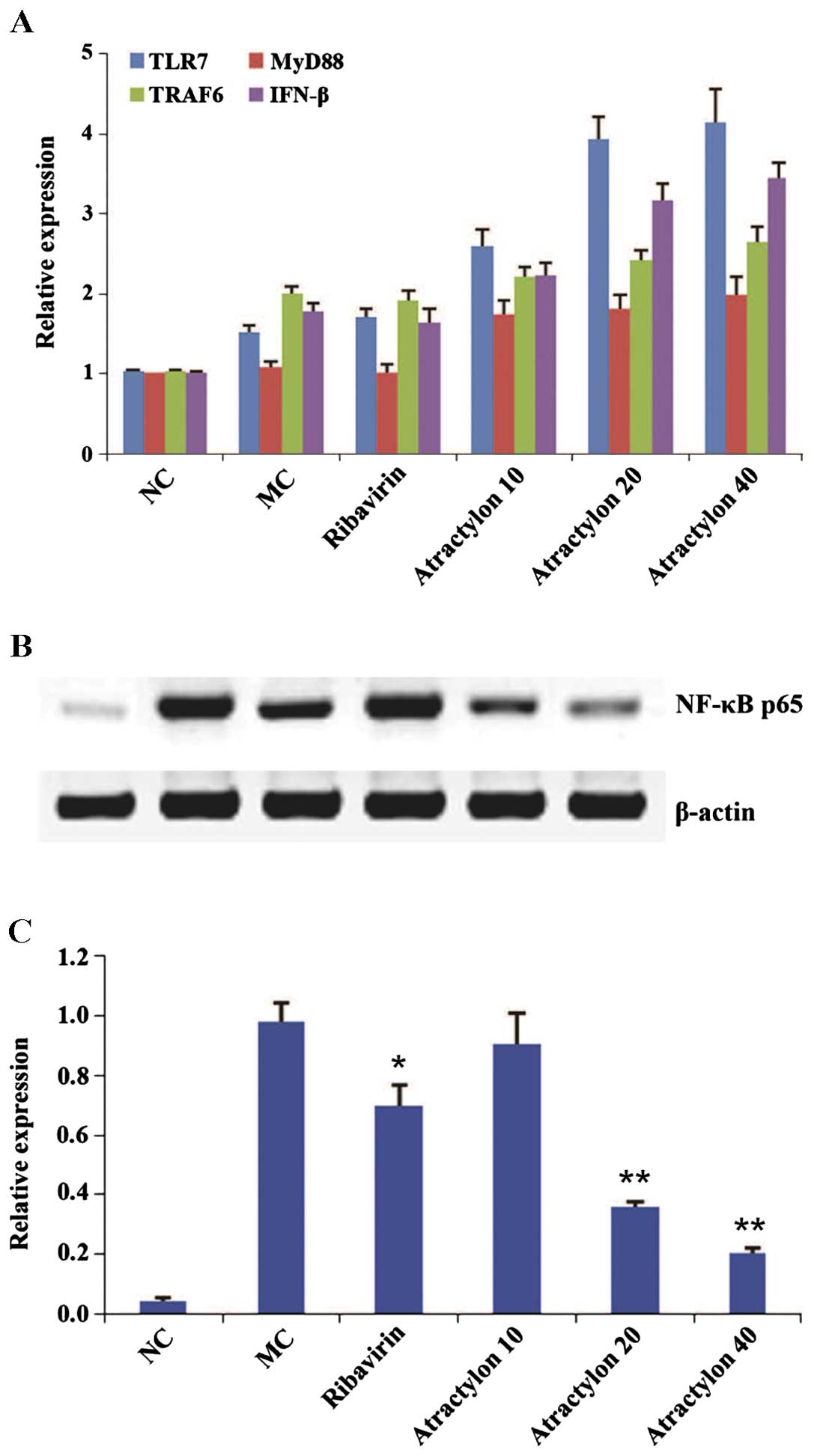
infection. By contrast, the mRNA expressions of TLR7 and its
downstream genes MyD88, TRAF6 and IFN-β were obviously upregulated
(P<0.01) compared with the model control group (Fig. 3), which indicated that the
above-mentioned pathway was activated. Furthermore, the results of
the western blot analysis showed that the expression levels of
nuclear factor-κB (NF-κB) p65 proteins in the model control group
were significantly increased compared with the normal control group
(P<0.01). However, the protein expressions in the
atractylon-treatment groups were significantly increased
(P<0.01). It is well known that NF-κB p65 is the most crucial
inflammatory protein of TLR7 downstream factors, which can transfer
to the cell nucleus and cause proinflammatory cytokine release, had
a lower expression (38–40). These findings were also consistent
with above analysis of serum pro-inflammatory cytokines. Therefore,
these combined results suggested that the effects of atractylon
during IAV infection were partially-dependent on the activation of
TLR7 pathway to induce the type I IFN production and NF-κB p65
inhibition.
 | Table VEffects of atractylon on serum
cytokines in IVA-infected mice. |
Table V
Effects of atractylon on serum
cytokines in IVA-infected mice.
| Groups | Dose (mg/kg) | IFN-β (pg/ml) | IL-6 (pg/ml) | TNF-α (pg/ml) | IL-1β (pg/ml) |
|---|
| Normal control | – | 22.9±2.75 | 13.6±1.21b | 8.64±1.09b | 9.15±0.38b |
| Model control | – | 24.5±2.52 | 29.8±2.66 | 18.9±1.37 | 21.3±2.06 |
| Ribavirin | 50 | 23.3±2.79 | 18.5±5.99b | 13.4±1.54b | 21.6±3.36 |
| Atractylon | 40 | 30.8±3.31b | 12.8±1.16b | 9.20±0.77b | 13.4±0.74b |
| 20 | 29.4±3.27a | 17.4±1.11b | 11.8±1.94b | 16.5±1.17b |
| 10 | 25.8±2.87 | 19.5±2.76b | 16.2±2.27a | 17.2±1.35b |
In summary, a SCE prepared from Atractylodis
Rhizoma showed potential inhibition against IAV. Six compounds
isolated from SCE were characterized by spectral analysis to be
atractylenolide I, atractylenolide III, hinesol, α-curcumene,
atractylon and atractylodin. Among these, atractylon had the most
significant anti-IAV activities and highest SI values in
vitro. Treatment of atractylon also significantly alleviated
pulmonary inflammation in IAV-infected mice, and protective effects
of atractylon might be related to its activation of TLR7 to induce
the production of type I IFNs, but to inhibit NF-κB activation.
These findings suggested that atractylon may warrant further
evaluation as a possible agent for influenza treatment.
Acknowledgments
This study was supported by 3-year plan of action of
traditional Chinese medicine in Shanghai (nos. ZY3-JSFC-1-1011 and
ZY3-RCPY-1-1001), Shanghai Pudong New Area Commission of Health and
Family Control (no. PDYNZJ2014-08), and Shanghai Legendary Medical
Practitioner of TCM CHEN Jian-jie Studio (no.
ZYSNXD-CC-MZY003).
References
|
1
|
Richard M and Fouchier RA: Influenza A
virus transmission via respiratory aerosols or droplets as it
relates to pandemic potential. FEMS Microbiol Rev. 40:68–85. 2016.
View Article : Google Scholar
|
|
2
|
Li TC, Chan MC and Lee N: Clinical
implications of antiviral resistance in influenza. Viruses.
7:4929–4944. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen Z, Lou K and Wang W: New
small-molecule drug design strategies for fighting resistant
influenza A. Acta Pharm Sin B. 5:419–430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watanabe T and Kawaoka Y: Influenza
virus-host interactomes as a basis for antiviral drug development.
Curr Opin Virol. 14:71–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ganjhu RK, Mudgal PP, Maity H, Dowarha D,
Devadiga S, Nag S and Arunkumar G: Herbal plants and plant
preparations as remedial approach for viral diseases. Virusdisease.
26:225–236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koonrungsesomboon N, Na-Bangchang K and
Karbwang J: Therapeutic potential and pharmacological activities of
Atractylodes lancea (Thunb.) DC. Asian Pac J Trop Med. 7:421–428.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhong Y, Wang X, Xu G, Mao B, Zhou W, Min
J, Jiang H, Diao X and Fu J: Modified Yupingfeng formula for the
treatment of stable chronic obstructive pulmonary disease: A
systematic review of randomized controlled trials. Afr J Tradit
Complement Altern Med. 11:1–14. 2013. View Article : Google Scholar
|
|
8
|
Xu J, Chen D, Liu C, Wu XZ, Dong CX and
Zhou J: Structural characterization and anti-tumor effects of an
insulin-type fructan from Atractylodes chinensis. Int J Biol
Macromol. 82:765–771. 2016. View Article : Google Scholar
|
|
9
|
Wang C, He L, Wang N and Liu F: Screening
anti-inflammatory components from Chinese traditional medicines
using a peritoneal macrophage/cell membrane
chromatography-offline-GC/MS method. J Chromatogr B Analyt Technol
Biomed Life Sci. 877:3019–3024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang JL, Huang WM and Zeng QY:
Atractylenolide I protects mice from lipopolysaccharide-induced
acute lung injury. Eur J Pharmacol. 765:94–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji ZH, Liu C, Zhao H and Yu XY:
Neuroprotective effect of biatractylenolide against memory
impairment in D-galactose-induced aging mice. J Mol Neurosci.
55:678–683. 2015. View Article : Google Scholar
|
|
12
|
Wang S, Cai R, Ma J, Liu T, Ke X, Lu H and
Fu J: The natural compound codonolactone impairs tumor induced
angiogenesis by downregulating BMP signaling in endothelial cells.
Phytomedicine. 22:1017–1026. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Masuda Y, Kadokura T, Ishii M, Takada K
and Kitajima J: Hinesol, a compound isolated from the essential
oils of Atractylodes lancea rhizome, inhibits cell growth and
induces apoptosis in human leukemia HL-60 cells. J Nat Med.
69:332–339. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu H, Zhu Y, Zhang T, Zhao Z, Zhao Y,
Cheng P, Li H, Gao H and Su X: Anti-tumor effects of
atractylenolide I isolated from Atractylodes macrocephala in human
lung carcinoma cell lines. Molecules. 18:13357–13368. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ji GQ, Chen RQ and Wang L:
Anti-inflammatory activity of atractylenolide III through
inhibition of nuclear factor-κB and mitogen-activated protein
kinase pathways in mouse macrophages. Immunopharmacol
Immunotoxicol. 15:1–5. 2015.
|
|
16
|
Zhang NN, Park DK and Park HJ: The
inhibitory activity of atractylenolide III, a sesquiterpenoid, on
IgE-mediated mast cell activation and passive cutaneous anaphylaxis
(PCA). J Ethnopharmacol. 145:278–285. 2013. View Article : Google Scholar
|
|
17
|
Wu Q, Yu C, Yan Y, Chen J, Zhang C and Wen
X: Antiviral flavonoids from Mosla scabra. Fitoterapia. 81:429–433.
2010. View Article : Google Scholar
|
|
18
|
Yu CH, Yu WY, Fang J, Zhang HH, Ma Y, Yu
B, Wu F and Wu XN: Mosla scabra flavonoids ameliorate the influenza
A virus-induced lung injury and water transport abnormality via the
inhibition of PRR and AQP signaling pathways in mice. J
Ethnopharmacol. 179:146–155. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen ZB: Study and application of herbal
disinfectants in China. Biomed Environ Sci. 17:492–498. 2004.
|
|
20
|
Zhao C and He C: Preparative isolation and
purification of atractylon and atractylenolide III from the Chinese
medicinal plant atractylodes macrocephala by high-speed
counter-current chromatography. J Sep Sci. 29:1630–1636. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li CQ, He LC, Dong HY and Jin JQ:
Screening for the anti-inflammatory activity of fractions and
compounds from Atractylodes macrocephala koidz. J Ethnopharmacol.
114:212–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Li F, Xu Y, Yang W, Qu L, Xiang Q,
Liu C and Li D: Chemical composition and synergistic antioxidant
activities of essential oils from Atractylodes macrocephala and
Astragalus membranaceus. Nat Prod Commun. 8:1321–1324.
2013.PubMed/NCBI
|
|
23
|
Ozçelik B, Gürbüz I, Karaoglu T and
Yeşilada E: Antiviral and antimicrobial activities of three
sesquiterpene lactones from Centaurea solstitialis L. ssp.
solstitialis. Microbiol Res. 164:545–552. 2009. View Article : Google Scholar
|
|
24
|
Zhang HJ, Nguyen VH, Nguyen MC, Soejarto
DD, Pezzuto JM, Fong HH and Tan GT: Sesquiterpenes and butenolides,
natural anti-HIV constituents from Litsea verticillata. Planta Med.
71:452–457. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harmatha J, Vokáč K, Buděšínský M, Zídek Z
and Kmoníčková E: Immunobiological properties of sesquiterpene
lactones obtained by chemically transformed structural
modifications of trilobolide. Fitoterapia. 107:90–99. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Ford C, Ulloa JL, Catalán CA, Grau A,
Martino VS, Muschietti LV and Merfort I: The sesquiterpene lactone
polymatin B from Smallanthus sonchifolius induces different cell
death mechanisms in three cancer cell lines. Phytochemistry.
117:332–339. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Felix S, Sandjo LP, Opatz T and Erkel G:
Anti-inflammatory drimane sesquiterpene lactones from an
Aspergillus species. Bioorg Med Chem. 22:2912–2918. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hwang DR, Wu YS, Chang CW, Lien TW, Chen
WC, Tan UK, Hsu JT and Hsieh HP: Synthesis and anti-viral activity
of a series of sesquiterpene lactones and analogues in the
subgenomic HCV replicon system. Bioorg Med Chem. 14:83–91. 2006.
View Article : Google Scholar
|
|
29
|
Guo J, Cao Y, Qin K, Zhao X, Wang D, Li Z,
Xin L, Shu Y and Zhou J: Limited effect of recombinant human
mannose-binding lectin on the infection of novel influenza A (H7N9)
virus in vitro. Biochem Biophys Res Commun. 458:77–81. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sawai-Kuroda R, Kikuchi S, Shimizu YK,
Sasaki Y, Kuroda K, Tanaka T, Yamamoto T, Sakurai K and Shimizu K:
A polyphenol-rich extract from Chaenomeles sinensis (Chinese
quince) inhibits influenza A virus infection by preventing primary
transcription in vitro. J Ethnopharmacol. 146:866–872. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen S, Cheng A and Wang M: Innate sensing
of viruses by pattern recognition receptors in birds. Vet Res.
44:822013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Raj RS, Bonney EA and Phillippe M:
Influenza, immune system, and pregnancy. Reprod Sci. 21:1434–1451.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoo JK, Kim TS, Hufford MM and Braciale
TJ: Viral infection of the lung: Host response and sequelae. J
Allergy Clin Immunol. 132:1263–1276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ichinohe T: Respective roles of TLR, RIG-I
and NLRP3 in influenza virus infection and immunity: Impact on
vaccine design. Expert Rev Vaccines. 9:1315–1324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dash P and Thomas PG: Host detection and
the stealthy phenotype in influenza virus infection. Curr Top
Microbiol Immunol. 386:121–147. 2015.
|
|
36
|
Ramirez-Ortiz ZG, Prasad A, Griffith JW,
Pendergraft WF III, Cowley GS, Root DE, Tai M, Luster AD, El Khoury
J, Hacohen N, et al: The receptor TREML4 amplifies TLR7-mediated
signaling during antiviral responses and autoimmunity. Nat Immunol.
16:495–504. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Goff PH, Hayashi T, Martínez-Gil L, Corr
M, Crain B, Yao S, Cottam HB, Chan M, Ramos I, Eggink D, et al:
Synthetic Toll-like receptor 4 (TLR4) and TLR7 ligands as influenza
virus vaccine adjuvants induce rapid, sustained, and broadly
protective responses. J Virol. 89:3221–3235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ramos I and Fernandez-Sesma A: Modulating
the innate immune response to influenza A virus: Potential
therapeutic use of anti-inflammatory drugs. Front Immunol.
6:3612015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kell AM and Gale M Jr: RIG-I in RNA virus
recognition. Virology. 479–480:110–121. 2015. View Article : Google Scholar
|
|
40
|
Gambhir S, Vyas D, Hollis M, Aekka A and
Vyas A: Nuclear factor kappa B role in inflammation associated
gastrointestinal malignancies. World J Gastroenterol. 21:3174–3183.
2015.PubMed/NCBI
|