Introduction
Psoriasis is a potentially debilitating disease,
which due to chronic inflammation, may have effects on other organ
systems in addition to the skin, resulting in increased risk of
cardiovascular, liver and renal disease (1–4).
Analyses of immune cells circulating in the blood of patients with
psoriasis have revealed unique gene signatures compared with
healthy controls, providing evidence of the systemic nature of the
disease (5). Abnormal immune
responses in the skin are involved in the pathophysiology of
psoriasis, resulting in inflammation and increased epidermal
proliferation. Therapies that block T-cell proliferation or
specific therapies that block cytokines, including tumor necrosis
factor (TNF), interleukin (IL)-17, IL-12 and IL-23, have been
demonstrated to be effective in the treatment of psoriasis
patients. The various cytokines involved in this aberrant
immune-mediated disease are produced by T cells, myeloid cells
including dendritic cells and macrophages, and epidermal
keratinocytes. Although it may appear counterintuitive to have
immunoregulatory cells in autoimmune and inflammatory diseases,
chronic inflammatory responses initiate an increase of regulatory
immune cells to attempt to downregulate the response. These
immunoregulatory cells may produce various molecules that alter the
inflammatory milieu (6).
Myeloid-derived suppressor cells (MDSCs), are a
population of immature myeloid cells with an immune regulatory
role. Myeloid-derived suppressor cells originate from common
myeloid progenitors in the bone marrow; however, under inflammatory
conditions an aberrant sustained myelopoiesis may result in the
accumulation of immature myeloid cells. These myeloid cells then
deviate from the standard path of differentiation depending on the
inflammatory milieu. This deviation may lead to the activation of
immunoregulatory pathways, including activation of the enzymes
arginase 1 and nitric oxide synthase 2, and production of reactive
oxygen species. These metabolic pathways have been demonstrated to
affect every arm of the immune system (7,8).
MDSC suppress T-cell proliferation (9), inhibit natural killer (NK) cell
cytotoxicity (10), modulate
macrophage polarization (11,12)
and induce the development of regulatory T cells (13). MDSCs have been demonstrated to
modulate the immune response to various diseases, including
numerous types of cancer (14–19),
inflammatory bowel disease (IBD) (20), traumatic stress (21), burns (22), infections (23,24)
and transplantation (25). In
addition, a recent study has indicated that MDSCs are elevated in
patients with psoriasis (26).
MDSCs under certain conditions may differentiate into endothelial
cells, macrophages, dendritic cells or neutrophils (27,28).
In various human disease states, including psoriasis, MDSCs have
been identified as human leukocyte antigen
(HLA)-DRlo/−cluster of differentiation
(CD)15+ (granulocytic MDSC) and
HLA-DRlo/−CD14+ (monocytic MDSC) (26,29).
The aim of the present study was to assess
circulating MDSC levels in the peripheral blood of patients with
psoriasis relative to healthy controls. Furthermore, the production
of cytokines, chemokines and matrix metalloproteinases (MMPs) by
MDSCs, which may contribute to the inflammatory state, was
investigated.
Materials and methods
Blood donors and sample preparation
A protocol for informed consent was developed with
the support of the Clinical & Translational Science Institute
at the University of Pittsburgh (Pittsburgh, PA, USA), and the
present study was approved by the Internal Review Board of the
University of Pittsburgh. Healthy controls or patients with
psoriasis covering >10% body surface area, and who did not have
viral hepatitis, human immunodeficiency virus or an autoimmune
disease, and were not on any immune-modulating systemic medications
(for example, prednisone, biologics, methotrexate, cyclosporine or
retinoids) were enrolled in the present study following the receipt
of signed informed consent (n=11 healthy controls; n=15 patients
with psoriasis). The average age of patients was 38 years for
healthy controls and 48 years for psoriasis patients, which was not
significantly different (P=0.080). The average body mass index of
patients was 25 for healthy controls and 26 for psoriasis patients,
which was not significantly different (P=0.890). The healthy
control group consisted of 5 males and 6 females, and the psoriasis
group consisted of 5 males and 10 females. Blood (up to 20 ml per
draw) was collected in heparinized tubes (BD Biosciences, San Jose,
CA, USA) and processed within 4 h by diluting blood with
phosphate-buffered saline (PBS) to 40 ml. Diluted blood was
underlaid with 10 ml Ficoll-Paque Premium 1.084 density gradient
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Samples
were centrifuged at 300 × g, at room temperature and without
brake for 20 min. The peripheral blood mononuclear cell
(PBMC)-containing interphase layer was removed, washed with PBS,
and resuspended in Recovery™ cell culture freezing medium
(Invitrogen; Thermo Fisher Scientific, Inc.) and aliquoted into
cryovials. Samples were placed into a −80°C freezer in a Mr. Frosty
container (Thermo Fisher Scientific, Inc.) to ensure controlled
cooling at 1°C/minute. Samples were transferred to liquid nitrogen
for storage after 1 week. Prior to analysis, stored samples were
thawed rapidly in a 37°C water bath.
Flow cytometry
Cells (1×106 per tube) were Fc blocked
for 5 min, using 2 μl anti-CD32 (catalog no. 555447; BD
Biosciences), and the following antibodies were added in
magnetic-activated cell sorting (MACS) separation buffer (Miltenyi
Biotec, Inc., Auburn, CA, USA): Anti-HLA-DR-phycoerythrin (PE;
G46-6; catalog no. 555812), anti-CD14-PacificBlue (M5E2; catalog
no. 558121), anti-CD15-allophycocyanin (APC; HI98; catalog no.
551376), anti-CD3-fluorescein isothiocyanate (FITC; SK7; catalog
no. 349201), anti-CD56-FITC (NCAM16.2; catalog no. 340410),
anti-CD20-FITC (L27; catalog no. 347673), anti-CD19-FITC (SJ25C1;
catalog no. 340719), anti-CD4-Paci-ficBlue (RPA-T4; catalog no.
561844) and anti-CD8-BV786 (RPA-T8; catalog no. 563823), purchased
from BD Biosciences. Antibodies were incubated for 30 min at 4°C at
concentrations indicated by the manufacturer. Cells were
subsequently fixed with 1% paraformaldehyde in MACS separation
buffer, and samples were analyzed on an LSR II (BD Biosciences).
One aliquoted and cryopreserved healthy control blood sample served
as an interexperimental control in serial flow cytometric analyses.
Granulocytic MDSCs were defined as
HLA-DRlo/−CD15+, and monocytic MDSCs as
HLA-DRlo/−CD14+, as described previously
(29).
Suppression assay and MDSC
purification
Patient samples were thawed and 3×106
PBMCs were removed and labeled with 1 μmol/l 5-(and 6)
carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen;
Thermo Fisher Scientific, Inc.) for 15 min at 37°C, according to
the manufacturer's protocol. The remaining thawed unlabeled PBMCs
were resuspended in MACS separation buffer and labeled with
anti-HLA-DR microbeads (Miltenyi Biotec, Inc.). MACS was then
performed on LS columns (Miltenyi Biotec, Inc.) according to the
manufacturer's protocol. HLA-DR depleted cells were then labeled
with anti-CD14 microbeads and separated on columns to obtain
HLA-DRlo/−CD14+ cells. Flow through cells
(HLA-DRlo/−CD14− cells) were then labeled
with anti-CD3 microbeads (Miltenyi Biotec, Inc.) and separated to
obtain CD3+ cells. Cells were resus-pended in AIM-V
medium (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented
with 2 mM Glutamax (Invitrogen; Thermo Fisher Scientific, Inc.), 50
U/ml penicillin/50 μg/ml streptomycin (Invitrogen; Thermo
Fisher Scientific, Inc.) and 500 U/ml IL-2 (BD Biosciences).
Culture plates (96-well) were precoated overnight with 50
μl/well 1 μg/ml anti-CD3 (BD Biosciences).
CFSE-labeled PBMCs were plated at 2×105 cells/well and
MDSCs were added at various ratios. For stimulation, 2.5
μg/ml anti-CD28 (BD Biosciences) was added to culture wells
in addition to the plate bound anti-CD3. Final volume was 300
μl/well. On day 6 of culture, cells were harvested, labeled
with anti-CD4 and anti-CD8 antibodies as aforementioned, and CFSE
dilution was analyzed by flow cytometry.
Cytokine analysis
Cells were purified and cultured as aforementioned
(2×105/well in 96-well plates) for 2 days, and then
supernatants were collected and stored until analysis. Supernatants
were analyzed by Luminex bead-based technology at the Luminex Core
Facility at the University of Pittsburgh Cancer Institute
(Pittsburgh, PA, USA).
Statistical analysis
Data are expressed as mean ± standard error. Groups
were compared using two-tailed t-tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
MDSC levels in the circulation of
patients with psoriasis
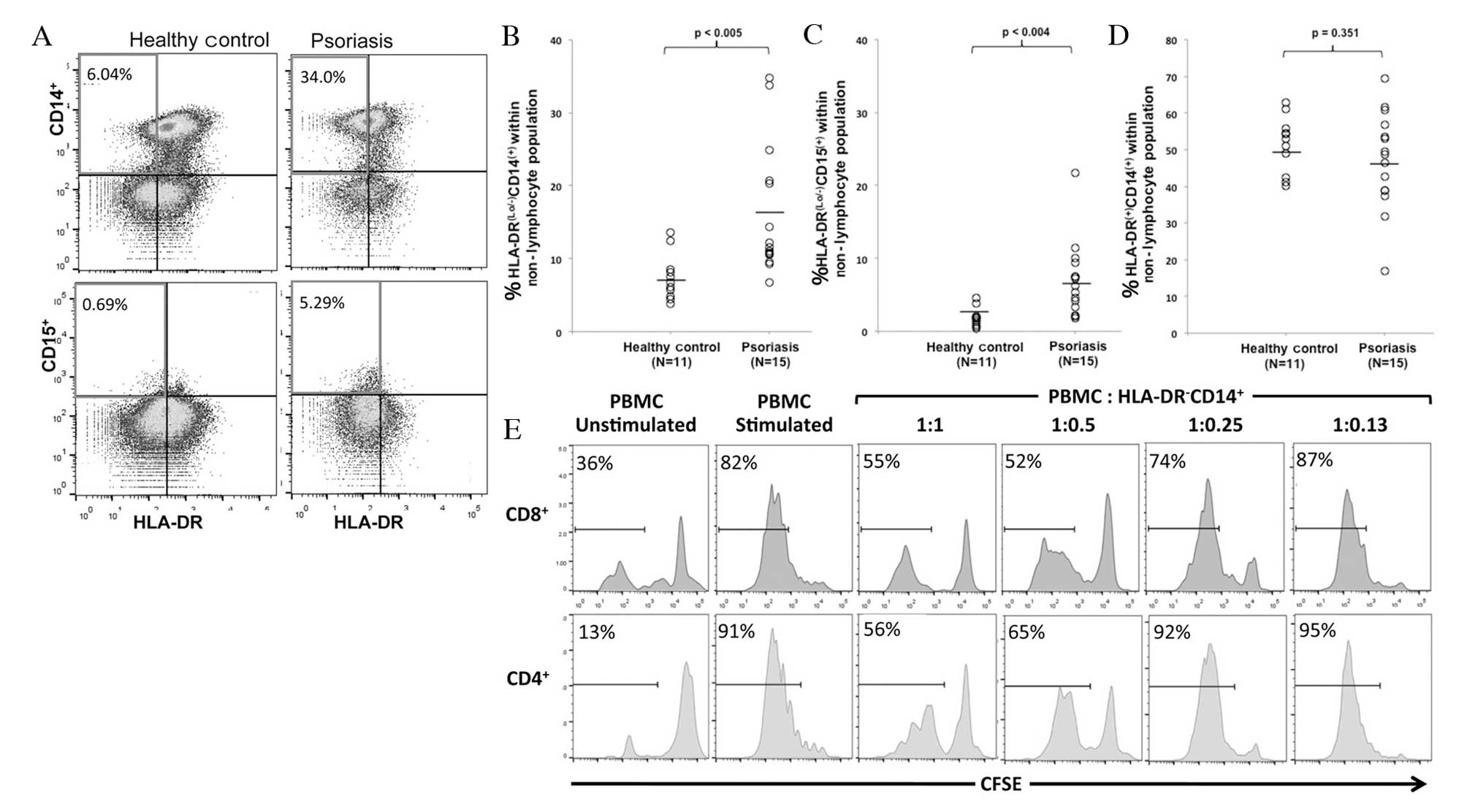
PBMCs were isolated from patients with psoriasis and
healthy controls. There were no statistically significant
differences in age or body mass index between the two groups. Flow
cytometry was performed to analyze MDSC levels within the PBMCs by
gating on non-lymphocytes, cells negative for T, B, and NK cell
markers
(CD3−CD19−CD20−CD56−),
and analyzing CD14, CD15 and HLA-DR (Fig. 1A). A significant increase was
observed in HLA-DRlo/−CD14+ cells (P=0.005),
considered to be monocytic MDSCs, and
HLA-DRlo/−CD15+ cells (P=0.004),
considered to be granulocytic MDSCs, in patients with psoriasis
compared with healthy controls (Fig.
1B and C). There was no significant difference in the
percentage of mature monocytes (HLA-DR+CD14+)
between the two groups (P=0.351) (Fig.
1D).
Circulating MDSCs are properly identified
by phenotype and function
MDSCs are defined by their suppression of
T-lymphocyte proliferation in response to various stimuli in
vitro, in addition to phenotypic markers. As granulocytic MDSCs
exist in relatively low numbers, are difficult to isolate and have
been demonstrated to lose their regulatory function following
cryopreservation (30), the
regulatory function of HLA-DRlo/−CD14+ cells
was analyzed. MDSCs from the PBMCs of patients with psoriasis were
isolated, and co-cultured at various ratios with autologous
CFSE-labeled PBMCs activated with anti-CD3 and -CD28. CFSE dilution
in activated CD4+ and CD8+ T cells was
analyzed following 6 days of culture, and a representative
experiment revealed that CD4+ and CD8+ T-cell
proliferation was suppressed by MDSCs in a dose-dependent manner
(Fig. 1E). At a 1:1 PBMC to MDSC
ratio, CD8+ and CD4+ T-cell proliferation is
greatly reduced compared with T cell proliferation in the absence
of MDSC or in the presence of low numbers of MDSC (ratio 1:0.13).
This in vitro regulatory function indicates that the
population identified in the present study is that described
previously as MDSCs (29).
Analysis of molecules produced by
MDSCs
MDSCs were isolated from patients with psoriasis and
were cultured in vitro in the absence of polyclonal
stimulation. Culture supernatants were then analyzed. The following
molecules were produced by unstimulated MDSCs: MMP9, MMP1, IL-8,
growth-related oncogene (GRO) and monocyte chemoattractant protein
(MCP)-1 (Fig. 2). The following
molecules were not produced in significant quantities by
unstimulated MDSCs: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-9,
IL-10, IL-12p70, IL-13, IL-15, IL-17A, IL-17E, IL-17F, IL-21,
IL-22, IL-23, IL-27, IL-28A, IL-31, IL-33, interferon (IFN)α, IFNγ,
IFNγ-induced protein 10, FMS-like tyrosine kinase 3 ligand,
fractalkine, granulocyte colony-stimulating factor (CSF),
granulocyte-macrophage CSF, MCP-3, macrophage-derived chemokine,
macrophage inflammatory protein (MIP)-1α, MIP-1β, MIP-3α,
platelet-derived growth factor (PDGF)-AA, PDGF-BB, regulated on
activation, normal T cell expressed and secreted, TNFα, TNFβ,
transforming growth factor α, vascular endothelial growth factor,
sCD40L, MMP2, MMP3, MMP7, MMP8, MMP10, MMP12 and MMP13 (Fig. 2 and data not shown). MDSCs cultured
under various in vitro conditions may produce these
molecules; however, the aim of the present study was to determine
which molecules are produced at baseline by circulating MDSCs.
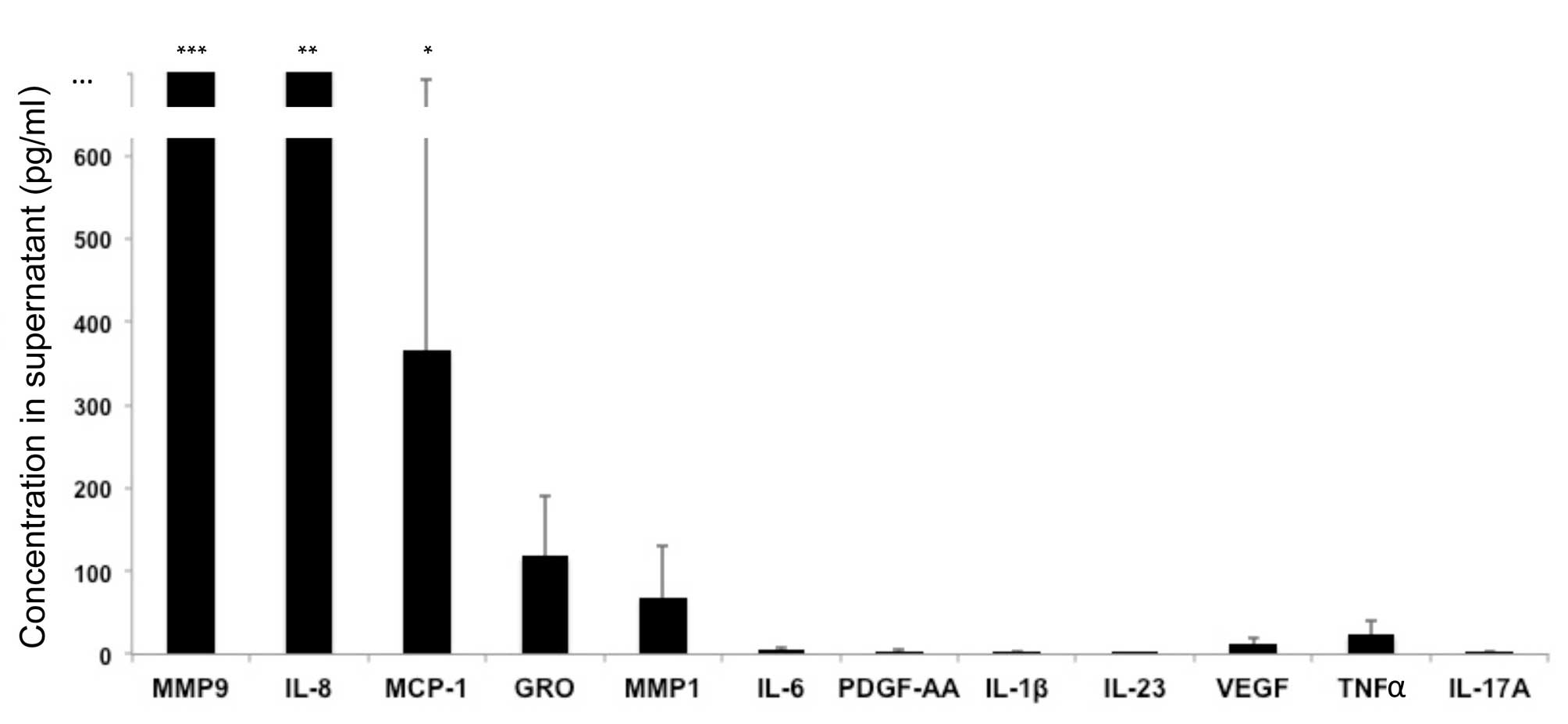 | Figure 2MDSCs produce various molecules.
MDSCs were cultured in vitro in the absence of polyclonal
activation, and culture supernatants were analyzed. Data are
expressed as the mean ± standard deviation (n=7 patients). MMP9,
MMP1, IL-8, GRO and MCP-1 were produced by unstimulated MDSCs.
***2509±1685 pg/ml; **2094±1219 pg/ml;
*367±324 pg/ml. MDSCs, myeloid-derived suppressor cells;
MMP, matrix metalloproteinase; IL, interleukin; GRO, growth-related
oncogene; MCP, monocyte chemoattractant protein; PDGF,
platelet-derived growth factor; VEGF, vascular endothelial growth
factor; TNF, tumor necrosis factor. |
Discussion
MDSCs have been identified in various skin diseases,
including melanoma (15), squamous
cell carcinoma (31), radiation
and burn injury (32), contact
dermatitis (33), atopic
dermatitis (6,34), and a recent study reported elevated
levels of MDSCs in patients with psoriasis (26). The aim of the present study was to
determine if MDSCs are elevated in the circulation of patients with
psoriasis. The results demonstrated that granulocytic and monocytic
MDSC subtypes were significantly increased in patients with
psoriasis compared with healthy controls. MDSCs were identified by
phenotype (HLA-DRlo/−CD15+ for granulocytic
MDSC and HLA-DRlo/−CD14+ for monocytic MDSC)
and function (suppression of CD4+ and CD8+ T
cells). This suppression of T cells may initially appear
counterintuitive, as psoriasis is associated with overactive T
cells, and therapeutic strategies for patients with psoriasis may
involve suppressing T cell activity. However, in IBD, a disease
that may similarly be treated through T-cell suppression, MDSCs
were demonstrated to be elevated, but markedly increased levels
were required in vivo to successfully suppress the disease
(20,35,36).
In contrast to in vitro studies, in vivo circulating
MDSCs may not successfully interact with pathogenic T cells, they
may differentiate into other cell types, or they may suppress only
certain T-cell subtypes. A recent study, which demonstrated
elevated levels of MDSCs in patients with psoriasis, also
demonstrated that MDSCs from healthy controls and psoriatic
patients induced conversion of T effector cells to T regulatory
cells (26). However, MDSCs from
patients with psoriasis induced T regulatory cells that were
markedly less suppressive, and thus less able to regulate the
inflammatory response.
The present study hypothesized that MDSCs in
patients with psoriasis may contribute to the inflammatory state,
and have additional roles to the regulation of T-cell activity.
Previous reports have revealed that myeloid cells from the
peripheral blood of patients with psoriasis produce increased
levels of IL-1β, IL-8, TNF-α and IL-6 compared with healthy
controls (37,38). In addition, blood monocytes from
patients with psoriasis have unique gene signatures that contribute
to the chronic inflammatory state (5). In the present study, MDSCs from
patients with psoriasis were demonstrated to produce MMP9, MMP1,
IL-8, GRO, and MCP-1. These molecules may contribute to recruitment
of other inflammatory cells, including neutrophils and monocytes.
Various cytokines and molecules are produced by myeloid lineage
cells; this is consistent with the production by MDSC of molecules
that may be involved in sustaining MDSC function and accumulation
in a feedback mechanism, and in addition may contribute to the
recruitment of other cell types, and skewing immune responses
(8). MMPs, including MMP9 and
MMP1, aid the migration of immune cells through tissue, and have
been demonstrated to promote the accumulation of MDSC (39). IL-8 is a chemokine involved in
recruitment and migration of neutrophils, T cells and basophils,
and may act as an angiogenic factor (40). Similarly, MCP-1 is highly
chemotactic for monocytes, T cells, basophils and NK cells
(40). GRO may be involved in
recruitment of neutrophils, may block differentiation of myeloid
cells, and may induce production of immune suppressive cytokines by
myeloid cells (41). These
molecules may thus contribute to further accumulation of
inflammatory cells, may provide a feedback mechanism that leads to
accumulation of further regulatory or suppressive cells, and may
have far reaching effects in other organ systems when produced in
circulation by elevated levels of MDSC. The chronic inflammatory
state in patients with psoriasis may contribute to the increased
risk of comorbidities. Notably, molecules including MMP9, MMP1 and
IL-8 have been implicated in the pathogenesis of atheromas and
cardiovascular disease (42). As
MDSCs accumulate in patients with acute coronary syndrome, this may
indicate a potential association between these molecules, MDSCs,
inflammation and cardiovascular disease (43).
MDSCs have been recognized in patients with various
types of cancer and autoimmune diseases. The results of the present
study demonstrated that patients with psoriasis have significantly
elevated levels of MDSCs in circulation. These cells are a
heterogeneous population of immature myeloid cells that have
immunoregulatory function, and may produce various factors that
contribute to the chronic inflammatory state in these patients. As
novel therapeutics and targets are discovered for the treatment of
psoriasis, the present study identified an additional potential
immune network that may be important in the pathogenesis of the
disease.
Acknowledgments
Funding for the present study was awarded to D.I.
from the Ostrow Graff Family Discovery Grant at the National
Psoriasis Foundation. The authors would like to thank Dr Louis Falo
and lab manager Miss Cara Carey, at the University of Pittsburgh
Medical Center, for allowing the use of laboratory space and
equipment to perform the experiments. This project used the
University of Pittsburgh Cancer Institute Cancer Biomarkers
Facility: Luminex Core Laboratory that is supported in part by the
National Institutes of Health (grant no. P30CA047904), and the
University of Pittsburgh Clinical and Translational Science
Institute.
References
|
1
|
Ogdie A, Yu Y, Haynes K, Love TJ, Maliha
S, Jiang Y, Troxel AB, Hennessy S, Kimmel SE, Margolis DJ, et al:
Risk of major cardiovascular events in patients with psoriatic
arthritis, psoriasis and rheumatoid arthritis: A population-based
cohort study. Ann Rheum Dis. 74:326–332. 2015. View Article : Google Scholar :
|
|
2
|
Chi CC, Wang J, Chen YF, Wang SH, Chen FL
and Tung TH: Risk of incident chronic kidney disease and end-stage
renal disease in patients with psoriasis: A nationwide
population-based cohort study. J Dermatol Sci. 78:232–238. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wan J, Wang S, Haynes K, Denburg MR, Shin
DB and Gelfand JM: Risk of moderate to advanced kidney disease in
patients with psoriasis: Population based cohort study. BMJ.
347:f59612013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ganzetti G, Campanati A and Offidani A:
Non-alcoholic fatty liver disease and psoriasis: So far, so near.
World J Hepatol. 7:315–326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang CQ, Suarez-Farinas M, Nograles KE,
Mimoso CA, Shrom D, Dow ER, Heffernan MP, Hoffman RW and Krueger
JG: IL-17 induces inflammation-associated gene products in blood
monocytes, and treatment with ixekizumab reduces their expression
in psoriasis patient blood. J Invest Dermatol. 134:2990–2993. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ilkovitch D: Role of immune-regulatory
cells in skin pathology. J Leukoc Biol. 89:41–49. 2011. View Article : Google Scholar :
|
|
7
|
Ilkovitch D and Lopez DM:
Urokinase-mediated recruitment of myeloid-derived suppressor cells
and their suppressive mechanisms are blocked by MUC1/sec. Blood.
113:4729–4739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bronte V, Brandau S, Chen SH, Colombo MP,
Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A,
Ostrand-Rosenberg S, et al: Recommendations for myeloid-derived
suppressor cell nomenclature and characterization standards. Nat
Commun. 7:121502016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gabrilovich DI, Bronte V, Chen SH, Colombo
MP, Ochoa A, Ostrand-Rosenberg S and Schreiber H: The terminology
issue for myeloid-derived suppressor cells. Cancer Res. 67:425–426.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu C, Yu S, Kappes J, Wang J, Grizzle WE,
Zinn KR and Zhang HG: Expansion of spleen myeloid suppressor cells
represses NK cell cytotoxicity in tumor-bearing host. Blood.
109:4336–4342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sinha P, Clements VK, Bunt SK, Albelda SM
and Ostrand-Rosenberg S: Cross-talk between myeloid-derived
suppressor cells and macrophages subverts tumor immunity toward a
type 2 response. J Immunol. 179:977–983. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ilkovitch D and Lopez DM: The liver is a
site for tumor-induced myeloid-derived suppressor cell accumulation
and immunosuppression. Cancer Res. 69:5514–5521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang B, Pan PY, Li Q, Sato AI, Levy DE,
Bromberg J, Divino CM and Chen SH: Gr-1+CD115+ immature myeloid
suppressor cells mediate the development of tumor-induced T
regulatory cells and T-cell anergy in tumor-bearing host. Cancer
Res. 66:1123–1131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Youn JI, Nagaraj S, Collazo M and
Gabrilovich DI: Subsets of myeloid-derived suppressor cells in
tumor-bearing mice. J Immunol. 181:5791–5802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Filipazzi P, Valenti R, Huber V, Canese P,
Iero M, Castelli C, Mariani L, Parmiani G and Rivoltini L:
Identification of a new subset of myeloid suppressor cells in
peripheral blood of melanoma patients with modulation by a
granulocyte-macrophage colony-stimulation factor-based antitumor
vaccine. J Clin Oncol. 25:2546–2553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Diaz-Montero CM, Salem ML, Nishimura MI,
Garrett-Mayer E, Cole DJ and Montero AJ: Increased circulating
myeloid-derived suppressor cells correlate with clinical cancer
stage, metastatic tumor burden, and doxorubicin-cyclophosphamide
chemotherapy. Cancer Immunol Immunother. 58:49–59. 2009. View Article : Google Scholar
|
|
17
|
Almand B, Clark JI, Nikitina E, van Beynen
J, English NR, Knight SC, Carbone DP and Gabrilovich DI: Increased
production of immature myeloid cells in cancer patients: A
mechanism of immunosuppression in cancer. J Immunol. 166:678–689.
2001. View Article : Google Scholar
|
|
18
|
Kusmartsev S, Su Z, Heiser A, Dannull J,
Eruslanov E, Kübler H, Yancey D, Dahm P and Vieweg J: Reversal of
myeloid cell-mediated immunosuppression in patients with metastatic
renal cell carcinoma. Clin Cancer Res. 14:8270–8278. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoechst B, Ormandy LA, Ballmaier M, Lehner
F, Krüger C, Manns MP, Greten TF and Korangy F: A new population of
myeloid-derived suppressor cells in hepatocellular carcinoma
patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology.
135:234–243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haile LA, von Wasielewski R,
Gamrekelashvili J, Krüger C, Bachmann O, Westendorf AM, Buer J,
Liblau R, Manns MP, Korangy F and Greten TF: Myeloid-derived
suppressor cells in inflammatory bowel disease: A new
immunoregulatory pathway. Gastroenterology. 135:871–881. e1–5.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Makarenkova VP, Bansal V, Matta BM, Perez
LA and Ochoa JB: CD11b+/Gr-1+ myeloid suppressor cells cause T cell
dysfunction after traumatic stress. J Immunol. 176:2085–2094. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Noel JG, Osterburg A, Wang Q, Guo X, Byrum
D, Schwemberger S, Goetzman H, Caldwell CC and Ogle CK: Thermal
injury elevates the inflammatory monocyte subpopulation in multiple
compartments. Shock. 28:684–693. 2007.PubMed/NCBI
|
|
23
|
Delano MJ, Scumpia PO, Weinstein JS, Coco
D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S,
Al-Quran SZ, et al: MyD88-dependent expansion of an immature
GR-1(+)CD11b(+) population induces T cell suppression and Th2
polarization in sepsis. J Exp Med. 204:1463–1474. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
De Santo C, Salio M, Masri SH, Lee LY,
Dong T, Speak AO, Porubsky S, Booth S, Veerapen N, Besra GS, et al:
Invariant NKT cells reduce the immunosuppressive activity of
influenza A virus-induced myeloid-derived suppressor cells in mice
and humans. J Clin Invest. 118:4036–4048. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dugast AS, Haudebourg T, Coulon F, Heslan
M, Haspot F, Poirier N, Vuillefroy de Silly R, Usal C, Smit H,
Martinet B, et al: Myeloid-derived suppressor cells accumulate in
kidney allograft tolerance and specifically suppress effector T
cell expansion. J Immunol. 180:7898–7906. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Soler DC, Young AB, Fiessinger L,
Galimberti F, Debanne S, Groft S, McCormick TS and Cooper KD:
Increased, but functionally impaired, CD14(+) HLA-DR(-/low)
myeloid-derived suppressor cells in psoriasis: A mechanism of
dysregulated T cells. J Invest Dermatol. 136:798–808. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang L, DeBusk LM, Fukuda K, Fingleton B,
Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP and Lin PC:
Expansion of myeloid immune suppressor Gr+CD11b+ cells in
tumor-bearing host directly promotes tumor angiogenesis. Cancer
Cell. 6:409–421. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nefedova Y, Fishman M, Sherman S, Wang X,
Beg AA and Gabrilovich DI: Mechanism of all-trans retinoic acid
effect on tumor-associated myeloid-derived suppressor cells. Cancer
Res. 67:11021–11028. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Talmadge JE and Gabrilovich DI: History of
myeloid-derived suppressor cells. Nat Rev Cancer. 13:739–752. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kotsakis A, Harasymczuk M, Schilling B,
Georgoulias V, Argiris A and Whiteside TL: Myeloid-derived
suppressor cell measurements in fresh and cryopreserved blood
samples. J Immunol Methods. 381:14–22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gehad AE, Lichtman MK, Schmults CD, Teague
JE, Calarese AW, Jiang Y, Watanabe R and Clark RA: Nitric
oxide-producing myeloid-derived suppressor cells inhibit vascular
E-selectin expression in human squamous cell carcinomas. J Invest
Dermatol. 132:2642–2651. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mendoza AE, Neely CJ, Charles AG,
Kartchner LB, Brickey WJ, Khoury AL, Sempowski GD, Ting JP, Cairns
BA and Maile R: Radiation combined with thermal injury induces
immature myeloid cells. Shock. 38:532–542. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Marhaba R, Vitacolonna M, Hildebrand D,
Baniyash M, Freyschmidt-Paul P and Zöller M: The importance of
myeloid-derived suppressor cells in the regulation of autoimmune
effector cells by a chronic contact eczema. J Immunol.
179:5071–5081. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Skabytska Y, Wolbing F, Günther C, Köberle
M, Kaesler S, Chen KM, Guenova E, Demircioglu D, Kempf WE, Volz T,
et al: Cutaneous innate immune sensing of Toll-like receptor 2–6
ligands suppresses T cell immunity by inducing myeloid-derived
suppressor cells. Immunity. 41:762–775. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guan Q, Moreno S, Qing G, Weiss CR, Lu L,
Bernstein CN, Warrington RJ, Ma Y and Peng Z: The role and
potential therapeutic application of myeloid-derived suppressor
cells in TNBS-induced colitis. J Leukoc Biol. 94:803–811. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smith AR and Reynolds JM: Editorial: The
contribution of myeloid-derived suppression to inflammatory
disease. J Leukoc Biol. 96:361–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mizutani H, Ohmoto Y, Mizutani T, Murata M
and Shimizu M: Role of increased production of monocytes TNF-alpha,
IL-1beta and IL-6 in psoriasis: Relation to focal infection,
disease activity and responses to treatments. J Dermatol Sci.
14:145–153. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Okubo Y and Koga M: Peripheral blood
monocytes in psoriatic patients overproduce cytokines. J Dermatol
Sci. 17:223–232. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Melani C, Sangaletti S, Barazzetta FM,
Werb Z and Colombo MP: Amino-biphosphonate-mediated MMP-9
inhibition breaks the tumor-bone marrow axis responsible for
myeloid-derived suppressor cell expansion and macrophage
infiltration in tumor stroma. Cancer Res. 67:11438–11446. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ilkovitch D and Lopez DM: Immune
modulation by melanoma-derived factors. Exp Dermatol. 17:977–985.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen HW, Chen HY, Wang LT, Wang FH, Fang
LW, Lai HY, Chen HH, Lu J, Hung MS, Cheng Y, et al: Mesenchymal
stem cells tune the development of monocyte-derived dendritic cells
toward a myeloid-derived suppressive phenotype through
growth-regulated oncogene chemokines. J Immunol. 190:5065–5077.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mehra VC, Ramgolam VS and Bender JR:
Cytokines and cardiovascular disease. J Leukoc Biol. 78:805–818.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang YG, Xiong X, Chen ZY, Liu KL, Yang
JH, Wen Q, Wu FQ, Hu XF, Peng YD, Wu JJ, et al: Expansion of
myeloid-derived suppressor cells in patients with acute coronary
syndrome. Cell Physiol Biochem. 35:292–304. 2015. View Article : Google Scholar : PubMed/NCBI
|