Introduction
Various types of DNA lesions may result in genomic
instability. To rectify this situation, cells activate the DNA
damage response to recognize and repair DNA lesions (1). However, when complete repair of the
damage is not achieved, the accumulation of unrepaired DNA damage
may result in various diseases, including cancer (2), immunodeficiency (3) and cardiovascular disease (4). The primary causes of DNA damage may
be divided into exogenous and endogenous factors, the latter of
which primarily arise due to intracellular oxidative stress. Free
radicals are a class of substances containing unpaired electrons,
of which oxygen free radicals account for ~95%. Free radicals are
generated in three ways: i) Cracking of covalent bonds, ii)
single-electron loss and iii) single-electron gain. On entering the
body, a large proportion of oxygen is utilized by the respiratory
chain, whereas 2–3% is catalyzed by oxidase and further converted
to reactive oxygen species (ROS), the majority of which is
eliminated by antioxidant enzymes and molecules (1,5,6).
However, excessive ROS, which may occur following the breakdown of
the body homeostasis of oxidation and antioxidation, may attack
double-stranded DNA and impair DNA repair mechanisms, resulting in
DNA breakage and nucleotide modifications (7).
Atherosclerosis (AS) is a primary cause of mortality
and morbidity in high-income countries (8). It is predicted that the mortality
rate of atherosclerosis will surpass that of infectious diseases by
2020 (9). In recent years, studies
on signal transduction and gene regulation have revealed that
oxidative stress and inflammation are two key elements in AS
occurrence. ROS and oxidized low density lipoprotein are the
primary causes of endothelial injury and excessive production of
inflammatory cytokines. In addition, by increasing growth factors
such as platelet derived growth factor, ROS indirectly transform
vascular smooth muscle cells (VSMCs) from a contractile to a
synthetic phenotype. Synthetic phenotype VSMCs synthesize collagen
and elastic fibers, producing a fibrous cap surrounding the lipid
pool and forming a typical atherosclerotic plaque. As the fibrous
cap ages, it becomes thinner and more prone to rupture (10). Matthews et al (11) revealed that oxidative stress may
lead to DNA damage in VSMCs by the breakage of DNA strands,
oxidative modification of guanine and regulation of associated
enzyme activity, and ultimately affect the occurrence and
progression of AS.
Numerous therapeutic agents have been demonstrated
to stimulate DNA damage (12–14).
Isoproterenol (Iso), a synthetic catecholamine and potent
vasodilator, is widely administered in cardiac arrest and shock
(15,16). However, it has been demonstrated to
induce DNA damage in cardiomyocytes and results in cardiac wall
hypertrophy (17,18). Kim et al (19) demonstrated that Iso elevated levels
of intracellular ROS and increased cerebrovascular damage.
The present study aimed to assess the prevention of
Iso-induced vascular damage via treatment with a compound present
in various herbs. Chlorogenic acid (CGA) is a naturally occurring
polyphenol, which is the main active ingredient in traditional
Chinese medicine honeysuckle and eucommia leaves and is present in
sunflower seeds, fruits, vegetables, soybeans, wheat and coffee
beans. CGA has anticancer, anti-inflammatory and anti-oxidative
activities (20–22). CGA is formed by the condensation of
caffeic acid and quinic acid, two phenolic compounds. As it has
phenolic hydroxide groups, CGA is highly reductive and extremely
vulnerable to oxidation, enabling it to scavenge free radicals and
affect anti-lipid peroxidation.
In the present study, Iso-induced DNA damage in
VSMCs and the levels of intracellular ROS were analyzed, and the
protective effect of CGA pretreatment on this damage was
investigated.
Materials and methods
Reagents
Iso, CGA, collagenase II and trypsin were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Primary rabbit antibodies
against phosphorylated (p) ataxia telangiectasia mutated (ATM;
catalog no. 13050), p-Rad3-related protein (ATR; catalog no. 2853),
p-breast cancer 1 (BRCA1; catalog no. 9009), p-C-terminal Src
homologous kinase 2 (Chk2; catalog no. 2197), γ-H2A histone family
member X (γ-H2AX; catalog no. 2595) and TATA binding protein (TBP;
catalog no. 8515) were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). Mouse anti-α-smooth muscle actin (α-SMA;
catalog no. A5228), which is a vascular-specific capture antibody,
was obtained from Sigma-Aldrich. Rabbit anti-glyceraldehyde
3-phosphate dehydrogenase (GAPDH; catalog no. 5632-1) was purchased
from Epitomics (Burlingame, CA, USA). Fluorescein isothiocyanate
(FITC) -conjugated goat anti-mouse IgG (catalog no. sc-2010) was
obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
HRP-labeled goat anti-rabbit IgG (catalog no. A0208) and cell lysis
buffer were purchased from Beyotime Institute of Biotechnology
(Haimen, China). The bicinchoninic acid (BCA) protein quantitation
kit was obtained from Pierce; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). The SuperSignal chemiluminescence substrate kit
was purchased from EMD Millipore (Billerica, MA, USA). All other
chemicals used were of analytical grade.
Isolation and culture of VSMCs
A total of 22 C57BL/6 female mice was obtained from
Shanghai Slac Laboratory Animal Co. Ltd. (Shanghai, China) and
maintained in specific pathogen-free conditions at 22–26°C under a
12-h light/dark cycle, with ad libitum access to food and
water, at Tongji University (Shanghai, China). The protocol was
approved by the Institutional Animal Care and Use Committee of
Tongji University (Shanghai, China). The thoracic aorta was removed
from 4- to 5-week-old mice following sacrifice by cervical
dislocation. The aorta was digested with 1 mg/ml collagenase II and
100 µg/ml trypsin at 37°C for 40 min. Following digestion, cells
were pelleted at 3,000 × g for 6 min at room temperature,
and plated in Dulbecco's modified Eagle's medium (DMEM; Wisent,
Inc., St. Bruno, QC, Canada) containing 1 g/l glucose, 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
Cells were cultured in a humidified incubator with 5%
CO2 at 37°C. Every 48 h, cells were washed with
phosphate-buffered saline (PBS) three times and placed in fresh
media. Primary cells following the second passage were used for
experiments.
Immunofluorescence identification of
isolated cardiomyocytes
Cultured VSMCs were washed twice with PBS and fixed
in 4% paraformaldehyde solution (Sangon Biotech Co., Ltd.,
Shanghai, China) at room temperature for 20 min. Following
fixation, VSMCs were washed with PBS to remove the fixative and
immersed in 0.5% (v/v) Triton-X-100 (Sigma-Aldrich) in PBS for 5
min to achieve permeability. VSMCs were then washed with 0.5%
Tween® 20 in PBS (PBST) three times and immersed in 5%
bovine serum albumin (BSA; Sigma-Aldrich) in PBS for 1 h at 37°C.
VSMCs were washed twice with PBST and incubated with the anti-α-SMA
antibody diluted 1:100 in 2% BSA in PBS and covered with Parafilm
overnight at 4°C. The primary antibody was removed via three washes
with PBST. Subsequently, a FITC-conjugated secondary antibody at a
dilution of 1:200 in 2% BSA was added and incubated at room
temperature for 1 h in the dark. The secondary antibody was removed
via washing three times in PBST for 5 min, and cells were stained
with 4′,6-diamidino-2-phenylindole (DAPI) solution at room
temperature in the dark for 20 min. The staining solution was
removed and the cells washed with PBST. Anti-fade mounting medium
was added and cell imaging was performed under a fluorescence
microscope (Olympus Corporation, Tokyo, Japan). Identification of
VSMCs was confirmed by morphological observation. The majority of
the VSMCs cultured in vitro exhibited a fusiform shape with
a large nucleus and defined fiber filaments.
Cytotoxicity of Iso and CGA on
VSMCs
Cell viability was measured by a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Iso (0, 1, 5, 10, 15, 20 or 25 µM) and CGA (0, 10, 50, 100,
150 or 200 µM) were added to a 96-well plate containing adhesive
VSMCs (5,000 cells/well), with 6 wells for each concentration.
Following incubation in a 5% CO2 incubator at 37°C for
12 h, the medium in each well was removed and the cells were washed
twice with PBS. MTT solution (20 in 100 µl DMEM) was added to each
well and the plate was incubated in a 5% CO2 incubator
at 37°C for 4 h. The medium was removed and 150 µl dimethyl
sulfoxide (Sangon Biotech Co., Ltd.) was added to each well on a
micro-oscillator (Kylin-Bell Lab Instruments Co., Ltd., Haimen,
China); the plate was shaken for 10 min to dissolve the crystals.
The absorbance in each well was read using a Multiskan FC
microplate reader (Thermo Fisher Scientific, Inc.) at a wavelength
of 570 nm.
Western blot analysis
Following treatment, medium was removed and the
cells were washed three times with PBS. Cells were lysed in cell
lysis buffer containing 1% phenylmethylsulfonyl fluoride (PMSF;
Beyotime Institute of Biotechnology) for 30 min on ice and
centrifuged at 14,000 × g for 10 min at 4°C. Nuclear
proteins were extracted from Iso-treated cells using a nuclear and
cytoplasmic protein extraction kit (Beyotime Institute of
Biotechnology) containing 1% (v/v) PMSF. The total protein contents
were quantified with a BCA protein assay kit to ensure equal
protein loading (~50 µg protein) in each well prior to gel
electrophoresis. The prepared samples were boiled in 5X sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
sample loading buffer (Beyotime Institute of Biotechnology) for 5
min. The samples were separated on 10% SDS-PAGE gels and
transferred to polyvinylidene difluoride membranes. Membranes were
blocked for 1 h in 5% non-fat milk in Tris/HCl-buffered saline
containing 1% Tween 20 and 0.8% NaCl (TBST) at room temperature and
then incubated overnight with appropriate primary antibodies at
4°C. The dilution of all primary antibodies was 1:1,000, except the
anti-GAPDH antibody, which was diluted to 1:5,000. Following
washing with TBST, the membranes were incubated with the
HRP-conjugated secondary antibody (1:3,000) for 1 h at room
temperature. The membranes were rinsed and soaked in SuperSignal
chemiluminescence substrate kit. Immunoreactive bands were
visualized with a chemiluminescence imaging analyzer and Quantity
One® software version 4.6.2 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Measurement of ROS
The level of intracellular ROS following Iso and CGA
treatment was measured using a fluorescent probe,
2′,7′-dichloro-dihydro-fluorescein diacetate (DCFH-DA; Reactive
Oxygen Species assay kit; Beyotime Institute of Biotechnology), as
described previously (23). In
brief, cells were loaded with serum-free medium containing 10 µM
DCFH-DA (v/v, 1:1,000) and incubated at 37°C for 30 min. The
residual DCFH-DA solution was removed and cells were washed three
times with serum-free medium for 5 min, and then with PBS. Cells
were observed under a fluorescence microscope (Olympus Corporation)
equipped with a charge-coupled device camera (Hamamatsu Photonics
K.K., Hamamatsu, Japan). Quantification of the fluorescence
intensity was performed using ImageJ software version 1.42
(National Institutes of Health, Bethesda, MD, USA). The data are
expressed as percentages compared with the control intensity.
Statistical analysis
Statistical analyses were performed using SPSS
software version 14.0 (SPSS, Inc., Chicago, IL, USA). Groups were
compared using one-way analyses of variance and Fisher's protected
least significant difference test. All data are expressed as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference. The experiments were repeated
at least three times independently.
Results
High-purity isolation of VSMCs
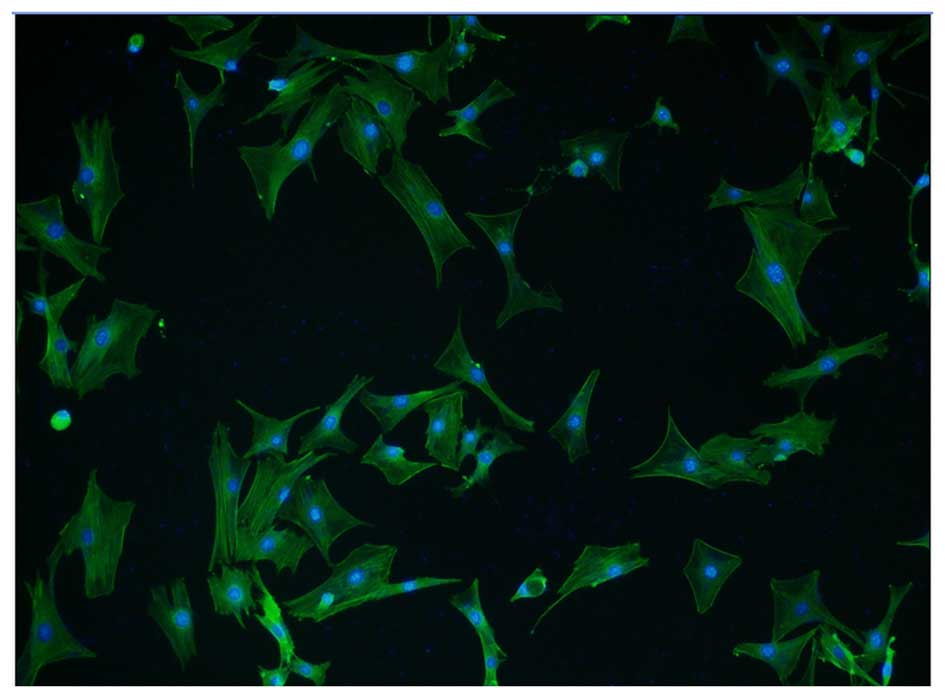
Immunofluorescence staining using an α-SMA antibody
and a FITC-conjugated secondary antibody was performed to determine
whether the isolated cells were high-purity VSMCs. Cells were
counterstained with DAPI, which penetrates the cell membrane and
binds to double-stranded DNA, fluorescing blue. α-SMA positive
cells accounted for >95% of total cells from at least five
fields of view, indicating the high purity of VSMCs (Fig. 1). In addition, VSMCs isolated
following 48 h in culture exhibited discernible fiber filaments and
a well-formed spindle shape.
Effects of Iso and CGA on cell
viability
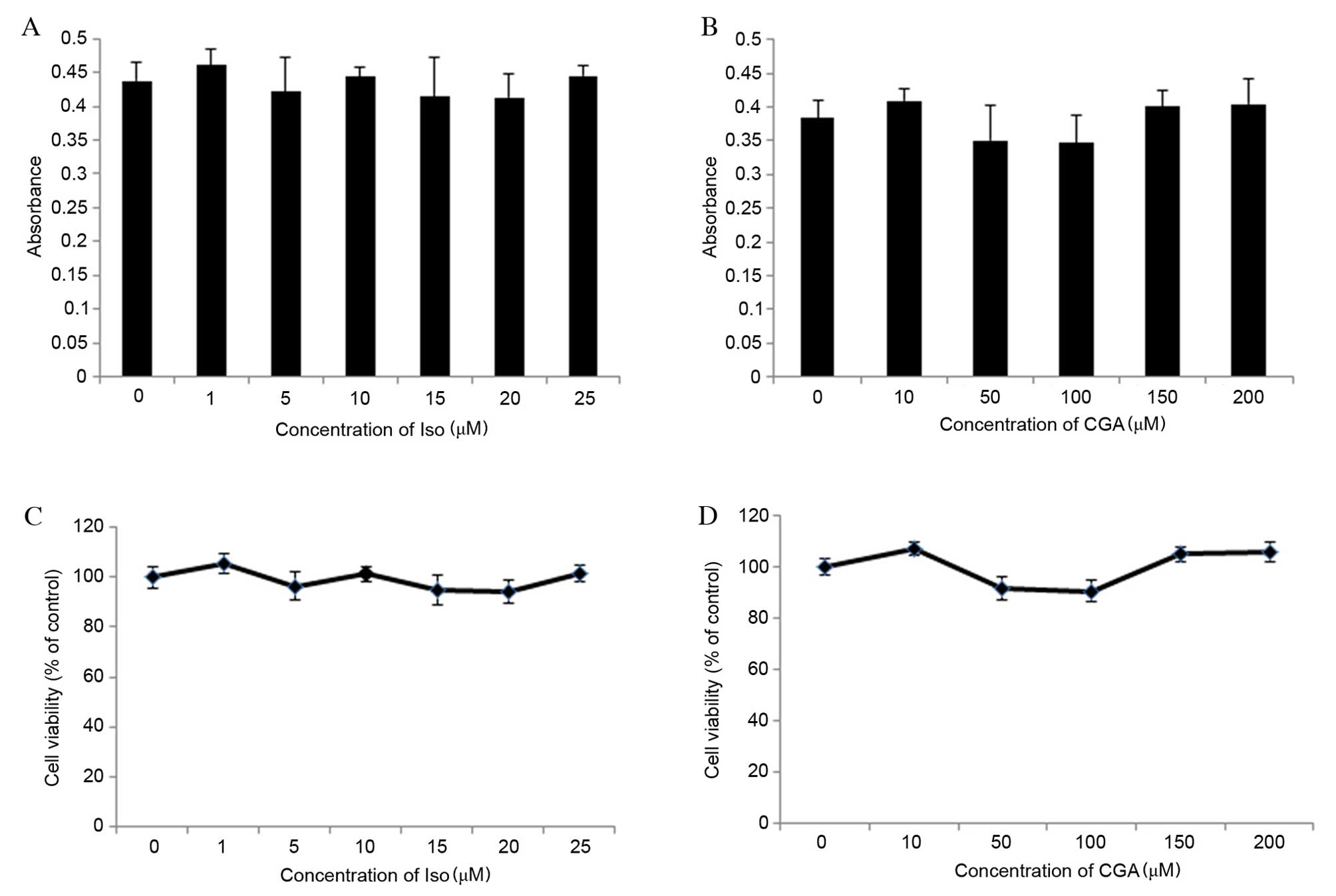
Cell viability was determined using an MTT assay,
which determined the optical density (OD) values of Iso-and
CGA-treated cells to be between 0.3 and 0.5 (Fig. 2A and B, respectively). OD values
between 0 and 0.7 are consistent with the linear association of
cell viability. The percentage of cell viability was calculated
relative to control wells designated as 100% viable. The results
revealed that concentrations of Iso ranging from 1 to 25 µM and CGA
ranging from 10 to 200 µM did not significantly affect cell
viability compared with the control (Fig. 2C and D, respectively). Therefore,
the effects of Iso and CGA observed in subsequent experiments were
not due to cell toxicity.
CGA protects VSMCs from Iso-induced
DNA damage
γ-H2AX, a double-stranded DNA break marker of DNA
damage, is expressed in the nuclei of cells. TBP served as the
loading control for nuclear proteins. Following treatment of VSMCs
with 10 µM Iso for 48 h, the protein expression level of γ-H2AX
increased significantly by 2.1-fold (P=0.0012; Fig. 3A). However, pretreatment with 50 µM
CGA significantly inhibited Iso-induced γ-H2AX upregulation by 50%
compared with the Iso group without CGA pretreatment (P=0.0081).
Furthermore, almost no change in γ-H2AX was visible compared to the
control group following 100 µM CGA pretreatment of the Iso-induced
VSMCs (P=0.1050). These results indicated that CGA may inhibit
Iso-induced DNA strand breaks.
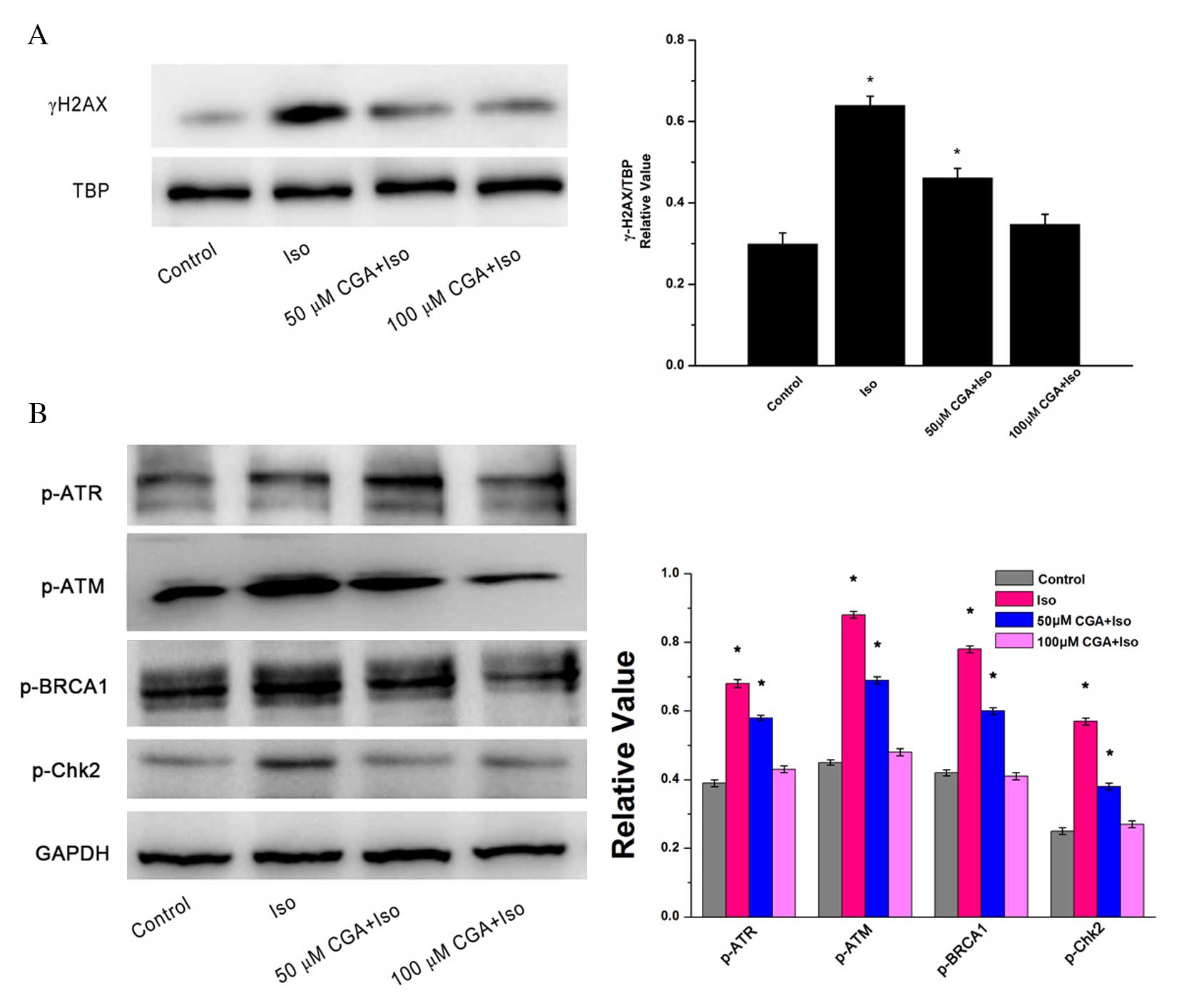 | Figure 3.DNA damage following treatment of
vascular smooth muscle cells with Iso, in the presence or absence
of CGA pretreatment, as determined by western blot analysis. (A)
Alterations in the protein expression levels of the nuclear protein
γ-H2AX. TBP served as a loading control. (B) Alterations in the
protein expression levels of p-ATR, p-ATM, BRCA-1 and Chk2. GAPDH
served as a loading control. The western blot represents one of at
least three independent experiments that demonstrated similar
results. *P<0.05 vs. control. Iso, isoproterenol; CGA,
chlorogenic acid; γ-H2AX, γ-H2A histone family member X; TBP, TATA
binding protein; p, phosphorylated; ATR, Rad3-related protein; ATM,
ataxia telangiectasia mutated; BRCA1, breast cancer 1; Chk2,
C-terminal Src homologous kinase 2; GAPDH, glyceraldehyde
3-phosphate dehydrogenase. |
To investigate the signaling pathway of Iso-induced
DNA damage, alterations in the protein expression levels of DNA
damage-associated proteins were observed. There were similar trends
in the protein expression levels of p-ATM, p-ATR, p-BRCA1 and
p-Chk2, which reflected changes in γ-H2AX expression. Iso treatment
induced significant upregulation of these DNA damage associated
proteins relative to the control (p-ATM, P=0.0023; p-ATR, P=0.0015;
p-BRCA1, P=0.0013; p-Chk2, P=0.0020). Following CGA pretreatment,
Iso-induced DNA damage was notably reduced in VSMCs (Fig. 3B). The inhibition of DNA
damage-induced upregulation of p-ATM, p-ATR, p-BRCA1 and p-Chk2
expression levels was CGA dose-dependent. These results suggested
that CGA may inhibit Iso-induced DNA damage checkpoint arrest.
CGA reduces Iso-induced ROS in
VSMCs
It has previously been reported that there is an
association between inflammation and ROS (24,25),
and that CGA scavenges oxygen free radicals in cells (26,27).
Therefore, ROS levels were examined in VSMCs treated with Iso in
the absence or presence of CGA treatment. Following Iso treatment,
the level of ROS significantly increased in VSMCs after 48 h
(P=0.0008). Pretreatment with CGA significantly decreased the level
of ROS compared with Iso treatment alone (50 µM, P=0.0327; 100 µM,
0.0938; Fig. 4). At 10 µM CGA, the
ROS level was reduced from an increase of 2.3-fold to an increase
of 1.6-fold relative to untreated cells. Furthermore, 50 µM CGA
resulted in a further decrease of ROS levels. At 100 µM CGA, the
level of ROS in VSMCs returned almost to the basal level of control
cells (Fig. 4). These results
indicated that the level of ROS increased during Iso-induced DNA
damage and that CGA may inhibit DNA damage by suppressing ROS.
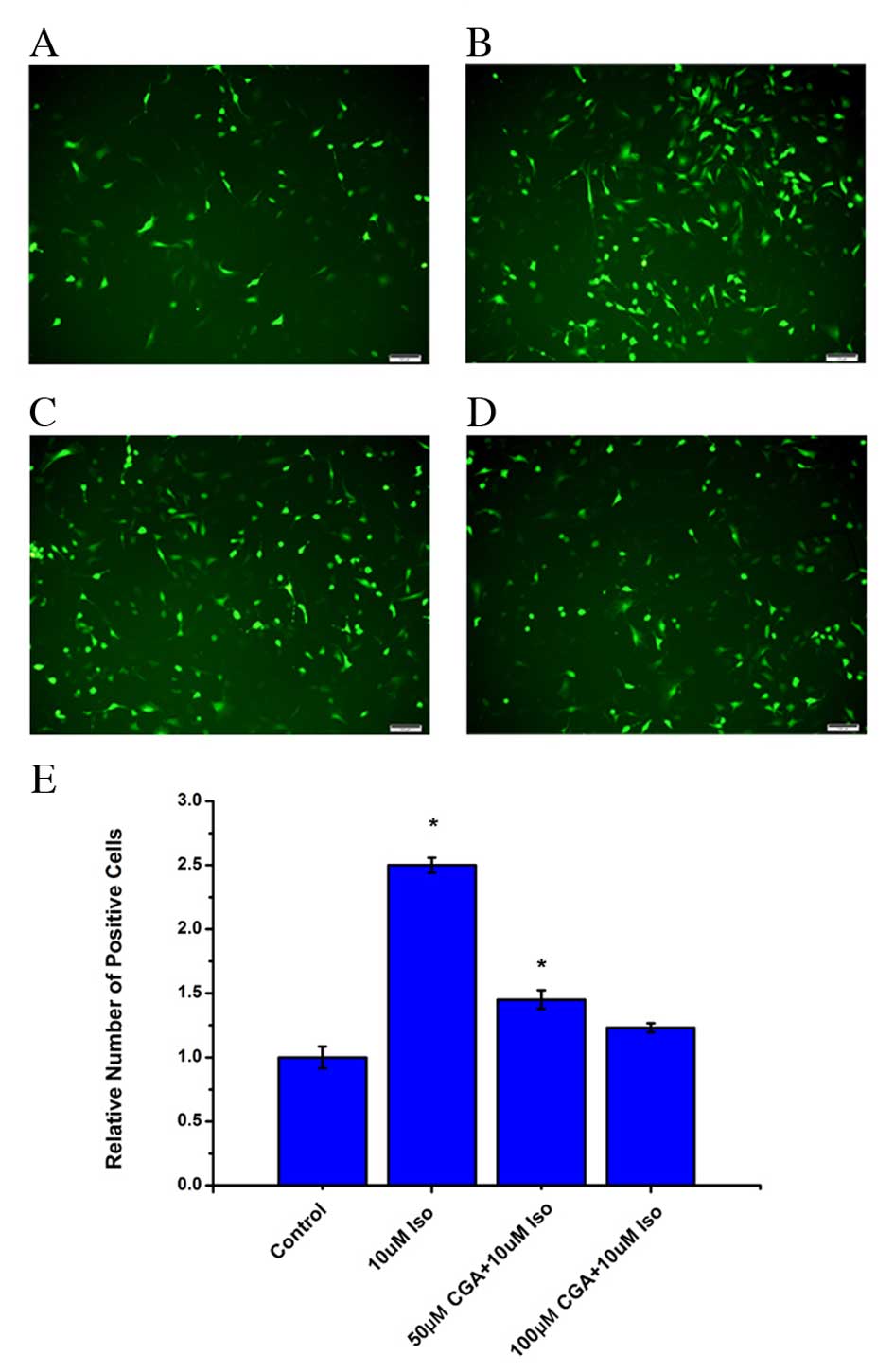 | Figure 4.Levels of ROS in vascular smooth
muscle cells following treatment with Iso, in the absence or
presence of CGA pretreatment, as measured using a fluorescent
probe, 2′,7′-dichloro-dihydro-fluorescein diacetate. ROS levels
were measured in (A) control cells, (B) 10 µM Iso-treated cells,
(C) 50 µM CGA-and 10 µM Iso-treated cells, and (D) 100 µM CGA- and
10 µM Iso-treated cells. (E) Quantification ROS levels. Scale
bars=100 µM. *P<0.05 vs. control. ROS, reactive oxygen species;
Iso, isoproterenol; CGA, chlorogenic acid. |
Discussion
VSMCs are crucial for maintaining the physiological
function and remodeling of blood vessels (28,29).
Oxidative stress leading to cell DNA damage can accelerate the
aging process in VSMCs, known as stress-induced premature
senescence, which plays a crucial role in the process of the
formation of AS (30). Therefore,
investigation into the effects of Iso on VSMCs and the prevention
of DNA damage induced by this therapeutic agent is key. CGA, as an
active component of certain Chinese herbal medicines, has been
investigated due to its various effects, including anticancer and
anti-inflammatory effects. Pang et al (31) revealed that CGA may prevent
acetaminophen-induced liver oxidative stress injury via the
regulation of cytochrome P450 metabolism enzymes and certain
important anti-oxidant signal molecules, including proteins from
the peroxiredoxins family. Cha et al (32) demonstrated that CGA reduced
UVB-mediated oxidative stress in human HaCaT keratinocytes. The
preventive effect of CGA with regards to VSMC damage and inhibition
of Iso-induced DNA damage may have important implications in the
clinical setting.
The present study revealed that 10 µM Iso may induce
DNA damage in VSMCs and increase intracellular ROS. Pretreatment
with CGA, particularly at the 100 µM concentration, may effectively
block damage to VSMCs induced by Iso through abrogating the
increase in protein expression levels of γ-H2AX, p-ATM, p-ATR,
p-BRCA1 and p-Chk2 and further preventing ROS formation. In
addition, the increase in levels of intracellular reactive oxygen
species (ROS) induced by Iso was inhibited by CGA pretreatment in a
dose-dependent manner. These results support the findings of
previous studies (22,33,34),
suggesting that CGA may be a promising drug for protection against
vascular diseases. Furthermore, CGA is widely present in plant
leaves, flowers and fruits (35,36),
which are used as herbal ingredients in certain traditional Chinese
medicines. Therefore, CGA may have potential applications for the
prevention of DNA damage.
Acknowledgements
The authors would like to thank the Laboratory
Animal Center of Tongji University (Shanghai, China) for their care
of the mice.
References
|
1
|
Zhang C, Luo T, Cui S, Gu Y, Bian C, Chen
Y, Yu X and Wang Z: Poly(ADP-ribose) protects vascular smooth
muscle cells from oxidative DNA damage. BMB Rep. 48:354–359. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lord CJ and Ashworth A: The DNA damage
response and cancer therapy. Nature. 481:287–294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gasser S and Raulet D: The DNA damage
response, immunity and cancer. Semin Cancer Biol. 16:344–347. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee SH and Blair IA: Oxidative DNA damage
and cardiovascular disease. Trends Cardiovasc Med. 11:148–155.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Narayanaswamy PB, Hodjat M, Haller H,
Dumler I and Kiyan Y: Loss of urokinase receptor sensitizes cells
to DNA damage and delays DNA repair. PLoS One. 9:e1015292014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valko M, Jomova K, Rhodes CJ, Kuča K and
Musílek K: Redox- and non-redox-metal-induced formation of free
radicals and their role in human disease. Arch Toxicol. 90:1–37.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gray K, Kumar S, Figg N, Harrison J, Baker
L, Mercer J, Littlewood T and Bennett M: Effects of DNA damage in
smooth muscle cells in atherosclerosis. Circ Res. 116:816–826.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weber C and Noels H: Atherosclerosis:
Current pathogenesis and therapeutic options. Nat Med.
17:1410–1422. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fryburg DA and Vassileva MT:
Atherosclerosis drug development in jeopardy: The need for
predictive biomarkers of treatment response. Science Transl.
3:72cm62011.
|
|
10
|
Kunieda T, Minamino T, Nishi J, Tateno K,
Oyama T, Katsuno T, Miyauchi H, Orimo M, Okada S, Takamura M, et
al: Angiotensin II induces premature senescence of vascular smooth
muscle cells and accelerates the development of atherosclerosis via
a p21-dependent pathway. Circulation. 114:953–960. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matthews C, Gorenne I, Scott S, Figg N,
Kirkpatrick P, Ritchie A, Goddard M and Bennett M: Vascular smooth
muscle cells undergo telomere-based senescence in human
atherosclerosis: Effects of telomerase and oxidative stress. Circ
Res. 99:156–164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lebwohl M, Ting PT and Koo JY: Psoriasis
treatment: Traditional therapy. Ann Rheum Dis. 64:Suppl 2.
ii83–ii86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Havelka AM, Berndtsson M, Olofsson MH,
Shoshan MC and Linder S: Mechanisms of action of DNA-damaging
anticancer drugs in treatment of carcinomas: Is acute apoptosis an
‘off-target’ effect? Mini Rev Med Chem. 7:1035–1039. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheung-Ong K, Giaever G and Nislow C:
DNA-damaging agents in cancer chemotherapy: Serendipity and
chemical biology. Chem Biol. 20:648–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leone M, Boyadjiev I, Boulos E, Antonini
F, Visintini P, Albanèse J and Martin C: A reappraisal of
isoproterenol in goal-directed therapy of septic shock. Shock.
26:353–357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wiramus S, Textoris J, Bardin R, Vigne C,
Kelway C, Martin C and Leone M: Isoproterenol infusion and
microcirculation in septic shock. Heart Lung Vessel. 6:274–279.
2014.PubMed/NCBI
|
|
17
|
Hara MR, Kovacs JJ, Whalen EJ, Rajagopal
S, Strachan RT, Grant W, Towers AJ, Williams B, Lam CM, Xiao K, et
al: A stress response pathway regulates DNA damage through
β2-adrenoreceptors and β-arrestin-1. Nature. 477:349–353. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Parthasarathy A, Gopi V, Devi KMS, Balaji
N and Vellaichamy E: Aminoguanidine inhibits ventricular fibrosis
and remodeling process in isoproterenol-induced hypertrophied rat
hearts by suppressing ROS and MMPs. Life Sci. 118:15–26. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HK, Park WS, Warda M, Park SY, Ko EA,
Kim MH, Jeong SH, Heo HJ, Choi TH, Hwang YW, et al: Beta adrenergic
overstimulation impaired vascular contractility via
actin-cytoskeleton disorganization in rabbit cerebral artery. PLoS
One. 7:e438842012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng R, Lu Y, Bowman LL, Qian Y,
Castranova V and Ding M: Inhibition of activator protein-1,
NF-kappaB, and MAPKs and induction of phase 2 detoxifying enzyme
activity by chlorogenic acid. J Biol Chem. 280:27888–27895. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yun N, Kang JW and Lee SM: Protective
effects of chlorogenic acid against ischemia/reperfusion injury in
rat liver: Molecular evidence of its antioxidant and
anti-inflammatory properties. J Nutr Biochem. 23:1249–1255. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Shen D, Tang X, Li X, Wo D, Yan H,
Song R, Feng J, Li P, Zhang J and Li J: Chlorogenic acid prevents
isoproterenol-induced hypertrophy in neonatal rat myocytes. Toxicol
Lett. 226:257–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Esposti M Degli: Measuring mitochondrial
reactive oxygen species. Methods. 26:335–340. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choudhury S, Ghosh S, Gupta P, Mukherjee S
and Chattopadhyay S: Inflammation-induced ROS generation causes
pancreatic cell death through modulation of Nrf2/NF-κB and SAPK/JNK
pathway. Free Radic Res. 49:1371–1383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim W, Youn H, Kang C and Youn B:
Inflammation-induced radioresistance is mediated by ROS-dependent
inactivation of protein phosphatase 1 in non-small cell lung cancer
cells. Apoptosis. 20:1242–1252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Shen D, Tang X, Li X, Wo D, Yan H,
Song R, Feng J, Li P, Zhang J and Li J: Chlorogenic acid prevents
isoproterenol-induced hypertrophy in neonatal rat myocytes. Toxicol
Lett. 226:257–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi H, Shi A, Dong L, Lu X, Wang Y, Zhao
J, Dai F and Guo X: Chlorogenic acid protects against liver
fibrosis in vivo and in vitro through inhibition of oxidative
stress. Clin Nutr S0261-5614. 00093–00095. 2016.
|
|
28
|
Pruett ND, Hajdu Z, Zhang J, Visconti RP,
Kern MJ, Wellik DM, Majesky MW and Awgulewitsch A: Changing
topographic Hox expression in blood vessels results in regionally
distinct vessel wall remodeling. Biol Open. 1:430–435. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen J, Xu L and Huang C: DHEA inhibits
vascular remodeling following arterial injury: A possible role in
suppression of inflammation and oxidative stress derived from
vascular smooth muscle cells. Mol Cell Biochem. 388:75–84. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kurz DJ, Decary S, Hong Y, Trivier E,
Akhmedov A and Erusalimsky JD: Chronic oxidative stress compromises
telomere integrity and accelerates the onset of senescence in
humanendothelial cells. J Cell Sci. 117:2417–2426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pang C, Sheng YC, Jiang P, Wei H and Ji
LL: Chlorogenic acid prevents acetaminophen-induced liver injury:
The involvement of CYP450 metabolic enzymes and some antioxidant
signals. J Zhejiang Univ Sci B. 16:602–610. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cha JW, Piao MJ, Kim KC, Yao CW, Zheng J,
Kim SM, Hyun CL, Ahn YS and Hyun JW: The polyphenol chlorogenic
acid attenuates UVB-mediated oxidative stress in human HaCaT
keratinocytes. Biomol Ther. 22:136–142. 2014. View Article : Google Scholar
|
|
33
|
Cinkilic N, Cetintas SK, Zorlu T, Vatan O,
Yilmaz D, Cavas T, Tunc S, Ozkan L and Bilaloglu R: Radioprotection
by two phenolic compounds: Chlorogenic and quinic acid, on X-ray
induced DNA damage in human blood lymphocytes in vitro. Food Chem
Toxicol. 53:359–363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Zhang X, Zhang Q and Yang Z:
Oxidative damage to DNA by 1,10-phenanthroline/L: -Threonine copper
(II) complexes with chlorogenic acid. Biometals. 23:265–273. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen X, Sang X, Li S, Zhang S and Bai L:
Studies on a chlorogenic acid-producing endophytic fungi isolated
from Eucommia ulmoides Oliver. J Ind Microbiol Biotechnol.
37:447–454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuan Y, Wang Z, Jiang C, Wang X and Huang
L: Exploiting genes and functional diversity of chlorogenic acid
and luteolin biosyntheses in Lonicera japonica and their
substitutes. Gene. 534:408–416. 2014. View Article : Google Scholar : PubMed/NCBI
|