Introduction
Bone regeneration is an important step in treating
bone defects, fractured nonunion and osteoporosis. Previously, stem
cells, including bone marrow-derived mesenchymal stem cells (MSCs)
and others, have been used to solve the problem of obtaining seed
cells for artificial bone tissue engineering (1,2). An
effective way to promote the osteogenic differentiation of seed
cells was to use gene therapy techniques to transfect
osteoinductive regulatory factors into the seed cells, and to
effectively express the regulatory factors in the seed cells, thus
contributing to the osteogenic differentiation of seed cells
(3,4).
Currently, there are various osteoinductive
regulator types, among which the bone morphogenetic proteins (BMPs)
have previously been heavily investigated. BMPs are part of the
TGF-β superfamily, which includes 43 members. It was previously
demonstrated that BMPs could induce mesenchymal cells to
irreversibly differentiate into cartilages and bone cells in
vivo (5–7). Additionally, the wingless-type mouse
mammary tumor virus integration site family is a class of Wnt
gene-encoded secretory glycoproteins. The highly conserved Wnt
signaling pathway is important in numerous species, it controls
early embryonic development and regulates cell proliferation
(8,9). Selecting the ideal carrier, so that
the target gene could be specifically, controllably and efficiently
expressed, is crucial for successful gene therapy. The lentiviral
vector system produces effectively expressed osteoinductive factors
in the seed cells of artificial bone of bone tissue engineering,
leading to high and prolonged expression of osteoinductive factors
(10).
The current study was approved by was approved by
the Ethics Committee of the General Hospital of Tianjin Medical
University and aimed to construct a third-generation of autologous
inactivated BMP2, BMP4, BMP6, BMP7, BMP9 and Wnt3a lentiviral
vectors, and integrate them into the genome of MC3T3-E1 murine MSCs
(MMSCs) to produce osteoinductive factor gene-modified MMSCs, and
thus, determine effective ways to further enhance the osteogenic
differentiation ability of the cells.
Materials and methods
Construction of pELNS-BMPs and
pELNS-Wnt3a vectors
A cDNA library (Wuhan Genesil Biotechnology Co.,
Ltd., Wuhan, China) was used as the template, and polymerase chain
reaction (PCR) was performed to amplify the coding sequences of
BMP2, BMP4, BMP6, BMP7, BMP9 and Wnt3a, according to the method of
the previously reported method (11). The gene sequencing results were:
Mouse BMP2 (NM_007553.2), BMP4 (NM_007554.2), BMP6 (NM_007556.2),
BMP7 (NM_007557.2), BMP9 (NM_019506.4), Wnt3a (NM_009522.2). The
upstream PCR primer introduced an Nhe I restriction enzyme
site and the downstream introduced a Sal I restriction
enzyme site to the amplified sequences. The primer sequences used
are presented in Table I.
 | Table I.Primer sequences of BMPs and
Wnt3a. |
Table I.
Primer sequences of BMPs and
Wnt3a.
| Primer | Sequence | Fragment size
(bp) |
|---|
| M-BMP2-P | F
5′-CTAGCTAGCATGGTGGCCGGGACCCGCTGTCTTC-3′ | 1185 |
|
| R
5′-ACGCGTCGACCTAACGACACCCGCAGCCCTCCAC-3′ |
|
| M-BMP4-P | F
5′-CTAGCTAGCATGATTCCTGGTAACCGAATGCTG-3′ | 1228 |
|
| R
5′-ACGCGTCGACTCAGCGGCATCCACACCCCTCTAC-3′ |
|
| M-BMP6-P | F
5′-CTAGCTAGCATGCCCGGGCTGGGGCGGAG-3′ | 1543 |
|
| R
5′-ACGCGTCGACTTAATGGCAACCACAAGCTCTCACGACC-3′ |
|
| M-BMP7-P | F
5′-CTAGCTAGCATGCACGTGCGCTCGCTGCG-3′ | 1293 |
|
| R
5′-ACGCGTCGACCTAGTGGCAGCCACAGGCCCGGAC-3′ |
|
| M-BMP9-P | F
5′-CTAGCTAGCATGTCCCCTGGGGCCTTCCG-3′ | 1287 |
|
| R
5′-ACGCGTCGACCTACCTACACCCACACTCAGCC-3′ |
|
| M-Wnt3a-P | F
5′-CTAGCTAGCATGGCTCCTCTCGGATACCTCTTAG-3′ | 1059 |
|
| R
5′-ACGCGTCGACCTACTTGCAGGTGTGCACGTCATAG-3′ |
|
Bi-enzyme cleavage with Nhe I and Sal
I (New England BioLabs, Inc., Ipswich, MA, USA) was performed on
the amplified fragments at 37°C for 3 h, which were then ligated
with purified fragments from the pELNS A recombinant lentivirus
(Wuhan Genesil Biotechnology Co., Ltd., Wuhan, China) that had been
bi-enzyme-digested, the recombinant was than enzyme-digested and
sent for the further sequencing and confirmation.
Construction of lentiviral
plasmid
According to the the method of a previous study
(12), the alkaline lysis method
was used prepare the target gene-constructed plasmids, pELNS-BMP2,
pELNS-BMP4, pELNS-BMP6, pELNS-BMP7, pELNS-BMP9 and pELNS-Wnt3a, and
the envelope protein plasmid pLP/vesicular stomatitis virus G
glycoprotein (VSVG; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and the packaging protein plasmid pLP1 and pLP2
(Invitrogen; Thermo Fisher Scientific, Inc.). This vector system
used enhanced green fluorescent protein (EGFP) as the reporter
gene.
The Ca3(PO4)2
coprecipitation method was used to transfect the 4 types of plasmid
(pELNS target gene plasmid, 3 µg; pLP/VSVG plasmid, 2.8 µg; pLP1
plasmid, 4.2 µg; and pLP2 plasmid, 2 µg) into 293FT cells (American
Type Culture Collection, Manassas, VA, USA), which were incubated
with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA), at
37°C and 5% CO2. Transduction was conducted using
Lipofectamine 2000 transfection reagent (Gibco; Thermo Fisher
Scientific, Inc.). Following, cotransfection for 72 h, the
supernatant was collected, filtered with 0.22 µm filter and stored
at −80°C. Counting of the number of green fluorescent protein
(GFP)-positive cells was used to detect the viral titer, with the
specific method described by Sugiyama et al (11).
Transfection of recombinant lentivirus
into MMSCs MC3T3-E1
MC3T3-E1 MMSCs (American Type Culture Collection)
were cultured in low-glucose Dulbecco's modified Eagle's complete
medium (Gibco; Thermo Fisher Scientific, Inc.), supplemented with
10% fetal bovine serum at 37°C and 5% CO2 for culture to
the third or fourth passage. A preliminary experiment was performed
to determine the optimal multiplicity of infection (MOI) of each
virus when infecting MC3T3-E1 cells. MC3T3-E1 cells were infected
according to the viral titer and best MOI for each virus using
polybrene at a final concentration of 8 µg/ml, and were observed
and identified under a BX61 fluorescent microscope (Olympus
Corporation, Tokyo, Japan) 72 h later. The single-gene transfection
groups (6 groups) were as follows: pELNS-BMP2, pELNS-BMP4,
pELNS-BMP6, pELNS-BMP7, pELNS-BMP9 and pELNS-Wnt3a. The dual-gene
cotransfection groups (8 groups) were as follows: T1, BMP2,4; T2,
BMP4,7; T3, BMP2,9; T4, BMP7,9; T5, BMP2,7; T6, BMP4,9; T7, BMP2,6;
and T8, BMP2 and Wnt3a.
Detection of runt related
transcription factor 2 (Runx2) mRNA by reverse
transcription-quantitative PCR (RT-qPCR)
Runx2 is a crucial gene for osteogenesis. The Runx2
mRNA expression level in each group was detected. TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract
the total cellular RNA. The RT reaction with 2 µg total RNA was
performed using the PrimeScript First Strand cDNA Synthesis kit
(Takara Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's instructions. Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was used as the internal reference gene. The
primers used in the PCR were as follows: Runx2, F
5′-ACAGAACCACAAGTGCGGTGC-3′ and R 5′-ACTGTGGTTAGAGAGCACTCACTGA-3′;
GAPDH, F 5′-AGGCGCTCACTGTTCTCTCCC-3′ and R
5′-GCAAGGCTCGTAGACGCGGTT-3′. The PCR cycling conditions were as
follows: 95°C for 1 min; 40 cycles at 94°C for 5 sec, 60°C for 20
sec and 72°C for 10 sec; then final extension at 72°C for 5 min.
The relative mRNA levels were calculated using the
2−ΔΔCq method (13).
Expression level detection of bone
γ-carboxyglutamate protein (BGP) and alkaline phosphatase (ALP) by
enzyme-linked immunosorbent assay (ELISA)
After four generations of cell passage followed by
transfection, the supernatant of each group was collected and the
absorbance was measured using a microplate reader (R&D Systems,
Inc., Minneapolis, MN, USA) according to the specifications of the
BGP ELISA kit (cat. no. 682556) and ALP ELISA kit (cat. no. 687242)
(Antibody Research Corp., St. Charles, MO, USA). Based on the
measurement results of standards, the contents of BGP and ALP in
the culture supernatant were calculated.
Protein level detection of BMPs and
Wnt3a in BMP2 and 7 cotransfected MC3T3-E1 cells by western
blotting
The protein expression levels of BMPs and Wnt3a in
BMP2- and BMP7-cotransfected MC3T3-E1 cells were detected by
western blotting according to a previously reported method
(14). In brief, primary
antibodies as goat polyclonal BMP2, BMP4, BMP6 and BMP7 antibodies,
and rabbit polyclonal BMP9, Wnt3a and β-actin antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
The target stripes were then observed.
Statistical analysis
All data are expressed as the mean ± standard
deviation (x ± s). SPSS statistical software (version 17.0; SPSS,
Inc., Chicago, IL, USA) was used to perform statistical analysis.
The multiple-sample comparisons were performed using one way
analysis of variance and the intergroup comparison using the least
significant difference test.
Results
Identification of target
gene-expressed plasmid
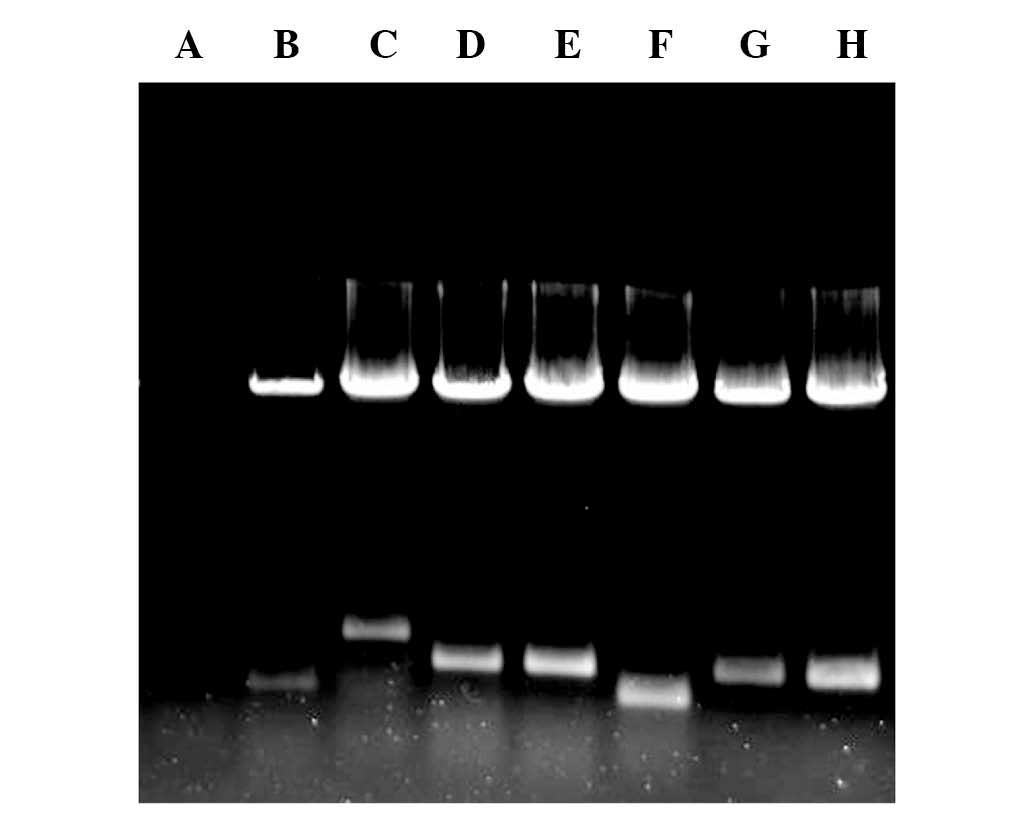
All the target gene-expressed vectors were digested
by Nhe I and Sal I. The electrophoresis separation
resulted in two bands; one was the linearized plasmid DNA, while
the other was the target gene band. The size of each target gene
fragment was consistent with the design (Fig. 1), and the sequencing results were
consistent with those in Genbank (www.ncbi.nlm.nih.gov/genbank), indicating that the
expression vectors were successfully constructed.
Transfection of single-gene
recombinant lentivirus into MC3T3-E1 cells
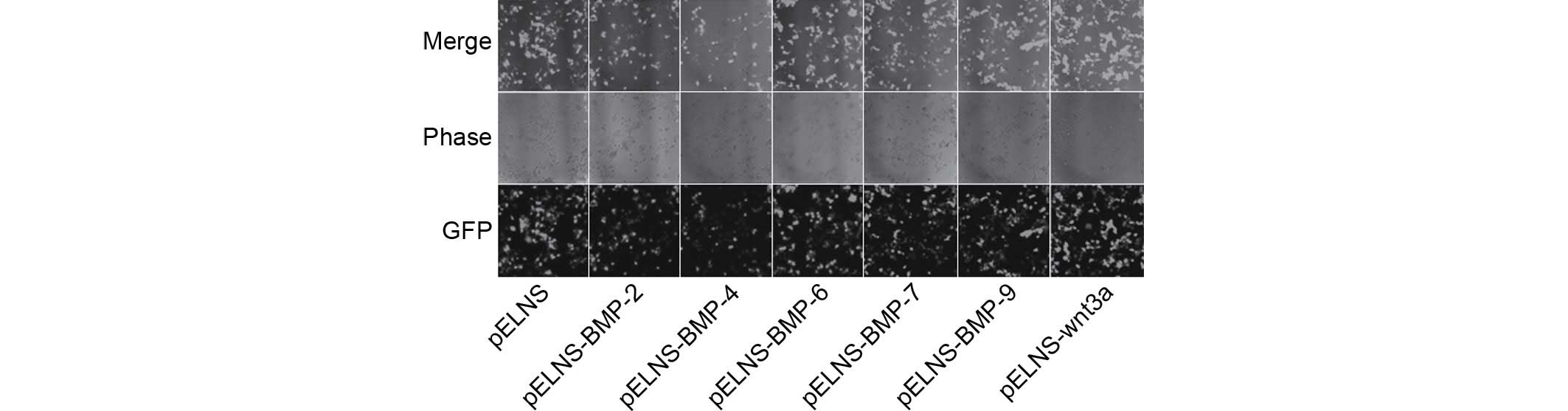
Fluorescence microscopy demonstrated that the
infected cells exhibited green fluorescence, indicating the
recombinant lentivirus had been successfully infected into MC3T3-E1
cells (Fig. 2).
Detection of Runx2 mRNA by
RT-qPCR
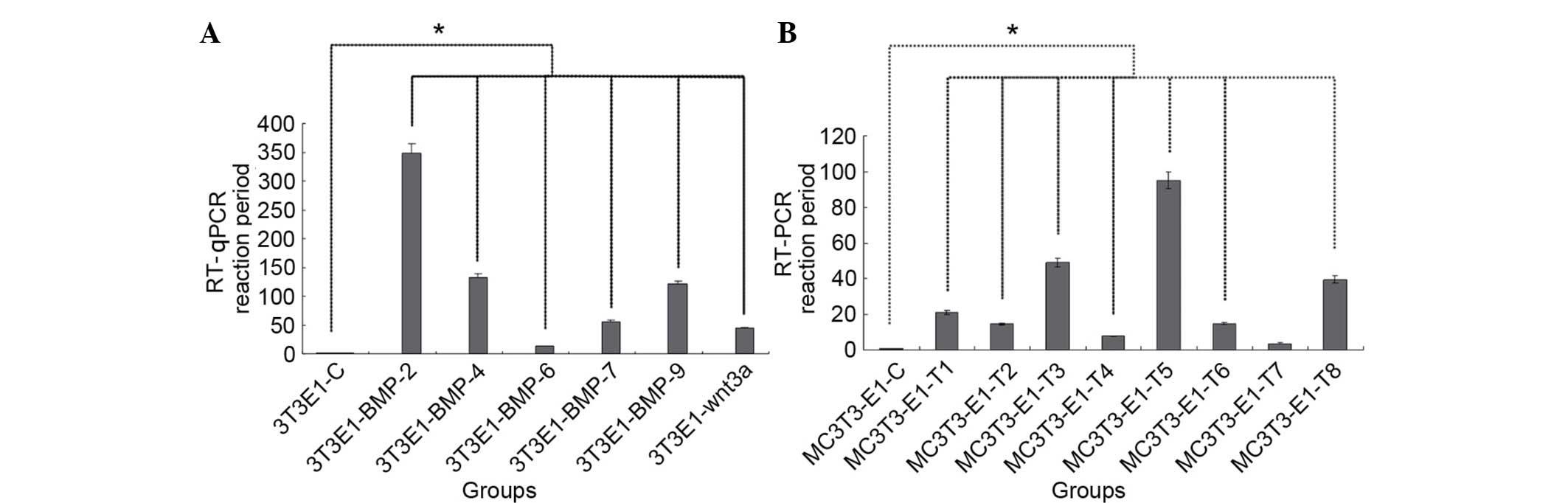
In the single transfection cells, the results of
RT-qPCR demonstrated that the relative expression level of Runx2
mRNA in the BMP2 group was the highest, sequentially followed by
the BMP4 group, BMP9 group, BMP7 group, Wnt3a group and BMP6 group
(Fig. 3A), and the differences
between the test groups and the control were statistically
significant as indicated in Fig.
3A (P<0.05).
In the bi-gene combined transfection cells, the
results demonstrated that the expression level of Runx2 mRNA was
highest in the T5 (BMP2,7) group, followed by T3 (BMP2,9) group, T8
(BMP2 and Wnt3a) group, T1 (BMP2,4) group, T2 (BMP4,7) group, T6
(BMP4,9) group, T4 (BMP7,9) group and T7 (BMP2,6) group (Fig. 3B), and the differences between the
test groups (excluding T7) and the control were statistically
significant as indicated in Fig.
3B (P<0.05).
BGP and ALP ELISA
The results demonstrated that the expression level
of BGP in the T5 (BMP2,7) group was the highest, sequentially
followed by the T3 group, T8 group, T4 group, T2 group, T6 group,
T1 group and T7 group (Table II).
The expression level of ALP in T5 (BMP2,7) group was the highest,
followed by the T3 group, T1 group, T8 group, T2 group, T4 group,
T6 group and T7 group (Table II),
the differences between values in the T1, T2, T3, T4, T5, T6, T7
and T8 groups with the control group were statistically significant
(P<0.05).
 | Table II.Absorbance values of BGP and ALP in 8
groups of cotransfected MC3T3-E1 cells by enzyme-linked
immunosorbent assay. |
Table II.
Absorbance values of BGP and ALP in 8
groups of cotransfected MC3T3-E1 cells by enzyme-linked
immunosorbent assay.
|
| BGP | ALP |
|---|
|
|
|
|
|---|
| Group | 1st | 2nd | 1st | 2nd |
|---|
| MSCs control | 0.026±0.005 | 0.035±0.006 | 0.004±0.004 | 0.037±0.003 |
| Positive
control | 1.05±0.02 | 1.72±0.04 | 1.02±0.36 | 1.64±0.38 |
| T1 |
0.21±0.01a |
0.34±0.01a |
0.44±0.04a |
0.63±0.08a |
| T2 |
0.25±0.003a |
0.42±0.01a |
0.33±0.09a |
0.59±0.04a |
| T3 |
0.43±0.01a |
0.71±0.02a |
0.61±0.13a |
0.78±0.09a |
| T4 |
0.28±0.01a |
0.46±0.01a |
0.37±0.07a |
0.49±0.05a |
| T5 |
0.60±0.01a |
0.97±0.02a |
0.62±0.17a |
0.90±0.17a |
| T6 |
0.25±0.01a |
0.41±0.01a |
0.33±0.06a |
0.44±0.06a |
| T7 |
0.23±0.004a |
0.18±0.006a |
0.14±0.05a |
0.16±0.04a |
| T8 |
0.31±0.007a |
0.50±0.006a |
0.42±0.09a |
0.56±0.17a |
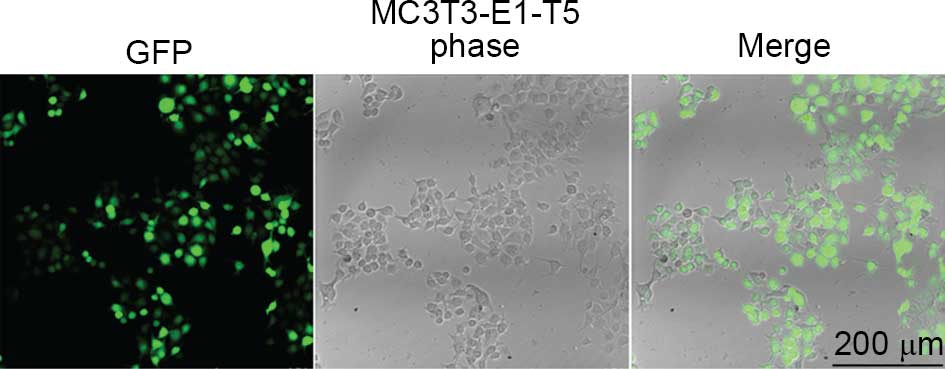
Fluorescence detection
The T5 (BMP2,7) group cells exhibited green
fluorescence, indicating the lentivirus was successfully infected
into MC3T3-E1 cells (Fig. 4).
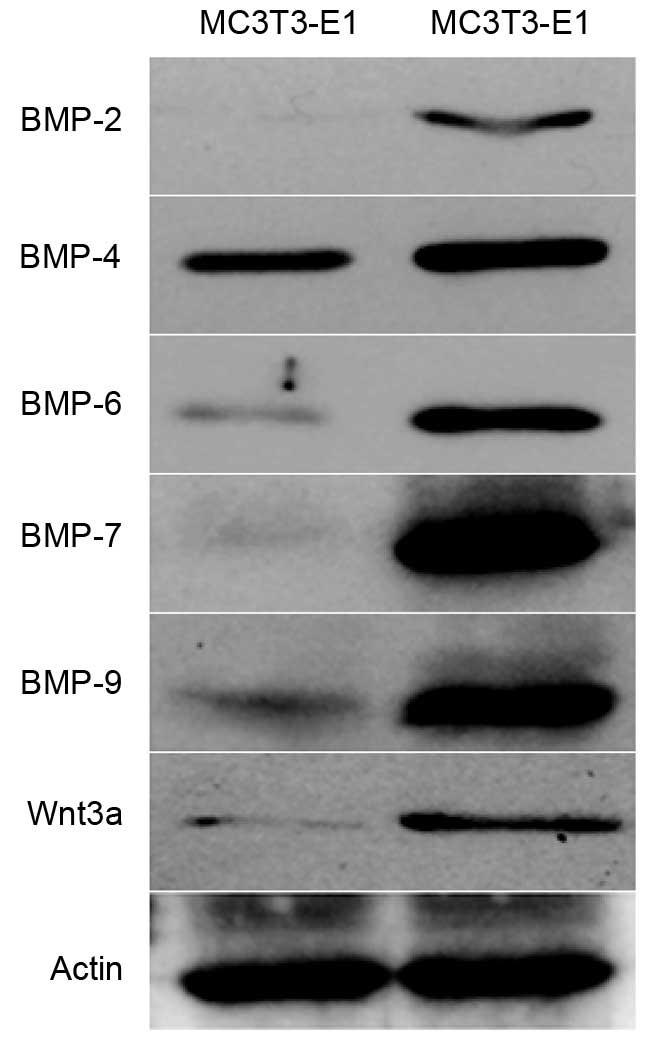
Western blot analysis
The results demonstrated that BMP2 and BMP7 (T5
group) cotransfected MC3T3-E1 cells exhibited higher expression
levels of BMP-2, BMP-4, BMP-6, BMP-7, BMP-9 and Wnt3a protein
compared with untransfected cells (Fig. 5).
Discussion
Genetically modified stem cells
The crucial element and research focus of bone
tissue engineering technology has been how to promote cell
proliferation and osteogenic differentiation. The transplantation
therapy of in vitro gene-modified stem cells was a
combination of cell therapy and gene therapy, compared with adult
cells, the stem cells exhibited a higher proliferation ability and
increased survival time. Furthermore, a previous study demonstrated
that gene-modified MSCs exhibited long-term expression of exogenous
genes, and maintained this ability following transplantation into
the body (15). Thus,
gene-modified MSCs may overcome the loss of its therapeutic
effects. Additionally, they could be used as a vector to express
osteogenic differentiation regulators for gene therapy, thus
compared with simple cell transplantation or gene therapy, may
provide improved treatment effects (16).
Selection of viral vectors
The ideal carrier for gene therapy should allow the
efficient transduction and long-term stable expression of the
transgene, and result in minimal side effects. The most commonly
used viral vectors are predominantly retrovirus, adenovirus,
adeno-associated virus and lentivirus (17–20).
Lentivirus is a subfamily of retrovirus. Compared
with adenoviruses, adeno-associated viruses and retroviruses, the
greatest advantages of using a lentivirus are the longer gene
expression time, reduced host immune responses, lower cytotoxicity
and easier use. In a previous study, a lentivirus-mediated
transgene remained expressed at 6 months after transduction, and
the concomitant inflammation caused was lower compared with that of
adenoviruses (21). Additionally,
lentiviruses have strong packaging capacities, which can hold up to
8–10 kb of exogenous target gene fragments, while unlikely to cause
insertional mutagenesis. Thus, lentiviruses have broad prospects
for gene therapy (22). The
third-generation lentiviral vector system induces genes to be
inserted into the lentiviral vector, thus it artificially controls
the expression of transferred genes, and retains its inactivated
properties, thus the system is also known as the regulable
lentiviral vector (23).
In order to improve the biological safety of viral
vectors, the current study used improved four-plasmid packaging
system, thus, separated the encoding genes of packaging plasmid,
and the packaging system was composed of packaging plasmid pLP1
(encoding gag/Pol gene) and pLP2 (encoding Rev gene), envelope
protein plasmid pLP/VSVG and vector plasmid pELNS. This vector
system only retained the gag, Pol and Rev genes of human
immunodeficiency virus-1, and there was no autoploidy within the
four plasmids of this four-plasmid cotransfection system. This
ensured the reduced possibility of replication competent virus
generation. This vector system replaced the cytomegalovirus (CMV)
internal promoter with the eukaryotic elongation factor-1α (EF-1α)
promoter, based on the pRRL-SIN-CMV-EGFP-WPRE plasmid, and the
promoter effects EF-1α were stronger. This carrier system used EGFP
as the reporter gene, thus, the advantages were: i) EGFP does not
interfere with cell growth and functions, the EGFP-carrying fusion
gene protein not exhibited the EGFP green fluorescence, it also
maintained the physiological functions of target proteins and
exhibited no cytotoxicity (24),
and allowed detection of the gene expression levels in living
cells; ii) the observation was simple, EGFP was used as a reporter
gene that could be directly observed in living cells with no need
of a substrate and internal control, when the EGFP gene was
transfected into eukaryotic cells and expressed, it could easily
observe the green fluorescent distribution of EGFP fusion protein
inside the living cells to analyze the distribution and
localization of unknown gene-expressed proteins within the cells;
and iii) the EGFP-labeling method exhibited higher sensitivity and
resolution than immunohistochemistry and conventional labeling
methods. Thus, as a very promising marker, EGFP provided great
convenience for the study of gene function and expression
regulation. The expression levels of EGFP green fluorescence
confirmed the effects of plasmid transfection, and the conditions
of packaging, infection and exogenous gene expression of the
recombinant virus inside cells. The present study demonstrated that
the third-generation lentiviral vector pELNS successfully
introduced BMP2, BMP4, BMP6, BMP7, BMP9 and Wnt3a into MMSCs
MC3T3-E1, and the expression was stable (25,26).
Osteogenic differentiation-inducing
factor
Different members of BMP family have different
distributions and osteogenic activity. Studies have previously
suggested that BMP2 predominantly stimulates recruitment and
differentiation of the undifferentiated mesenchymal cells and bone
cells, thus participating the bone reconstruction. In 1996, Iwasaki
demonstrated that the specific receptors for BMP2 are expressed on
the surface of bone cells and on the surface of all
non-hematopoietic stem cells (27).
A previous study has demonstrated that BMP4
inhibited the proliferation of lung fibroblasts through the classic
Smad I and c-Jun-N-terminal kinase pathway, and promoted their
differentiation into muscle cells (28). In vitro experiments
demonstrated that BMP4 promoted osteoblastic differentiation and
exerted a catalytic effect on bone collagen production (29). BMP6 was demonstrated to be
important at an earlier stage of bone formation and induced the
differentiation of precursor cells of chondrocytes, promoted the
formation of cartilage and osteoblastic phenotype, thus, involved
in cartilage and bone formation. Compared with other BMP family
members, BMP6 generated stronger osteoinductive effects on
intracartilage and intramembranous ossification (30). BMP7 have been demonstrated to bind
with BMP type I and II receptors stimulating Smad5 signaling to the
nucleus and influencing the gene transcription, thus, promoting the
formation of bone and cartilage, and repairing defects (31). BMP9 (also termed growth
differentiation factor 2) was previously demonstrated to be a
potential synergic promoting factor involved in the proliferation
and colony formation of hematopoietic stem progenitor cells, and
associated with the differentiation maintenance of cholinergic
nerve cells in the central nervous system. BMP9 was also
demonstrated to be involved in regulating glucose metabolism and a
variety of other cellular functions. Among numerous biological
effects of BMP9, its effect on bone and cartilage formation remains
the most important (32).
The Wnt family, and its various homologs, are
secreted glycoproteins. Their functions vary with different tissues
or developmental stages. The Wnt genes are proto-oncogenes, and the
Wnt signaling pathway involves the transduction of a signal from
the cell surface to the nucleus via a secreted signaling protein, a
transmembrane receptor protein and various complex intracellular
proteins (33).
Runx2 is part of the Runx-domain gene family, and
among various osteogenic differentiation regulatory factors, Runx2
has been considered to be the master regulator of osteoblast
differentiation. Runx2 is expressed within the mesenchyme of
mineralized tissues, including bones, and in the early development
stage of epithelial tissues (34).
The present study detected the expression of Runx2 mRNA levels,
thus, evaluated its involvement in osteogenic differentiation.
The current study successfully constructed the
recombinant lentiviruses, pELNS-BMP-2, pELNS-BMP-4, pELNS-BMP-6,
pELNS-BMP-7, pELNS-BMP-9 and pELNS-Wnt3a, and transfected them into
MC3T3-E1 cells. By detecting the expression level changes of Runx2
mRNA, it was demonstrated that the osteogenic effects of BMP2 were
the strongest, and those of BMP6 were the weakest compared with
controls. The effect of combined transfection of BMP2 with BMP4,
BMP6, BMP7, BMP9 and Wnt3a, and the combination of BMP4 with BMP7,
BMP4 with BMP9, and BMP7 with BMP9, were determined in MC3T3-E1
cells. The results demonstrated that the osteogenic effects of BMP7
with BMP2 were the strongest. ELISA results demonstrated that the
expression level of BGP in the T5 (BMP2,7) group was the highest,
followed by the T3, T8, T4, T2, T6, T1 and T7 groups. The
expression level of ALP in the T5 (BMP2,7) group was the highest,
sequentially followed by the T3, T1, T8, T2, T4, T6 and T7 groups.
BMP2 and BMP7 have previously been considered to be the most
effective and promising clinical osteogenic factors, with
recombinant human BMP2 and BMP7 used as bone regeneration therapies
for bone delayed union, bone nonunion, bone defects and spinal
fusion surgery (35). However
recent research and clinical practice has indicated that the use of
recombinant BMP2 and BMP7 protein could not solve the medical
problem of bone regeneration (36). Thus, how to improve the
osteoinductive abilities of cells and to provide efficient,
inexpensive and convenient bone regeneration materials has become a
common topic of concern, and urgently required in the clinical.
BMP2 combined with BMP7 may improve the osteoinductive abilities.
The current study established that cotransfection with the BMP2 and
BMP7 lentiviruses effectively induced the osteogenic
differentiation of MC3T3-E1 cells, thus laying the foundation for
future in vivo studies. In conclusion, the third-generation
lentiviral vectors effectively improved the osteogenic efficiencies
of MC3T3-E1 cells, which provided an important theoretical basis
and therapeutic strategy for bone reconstruction and tissue
engineering.
References
|
1
|
Grässel S and Lorenz J: Tissue-engineering
strategies to repair chondral and osteochondral tissue in
osteoarthritis: Use of mesenchymal stem cells. Curr Rheumatol Rep.
16:4522014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ochi M, Nakasa T, Kamei G, Usman MA and
Mahmoud E: Regenerative medicine in orthopedics using cells,
scaffold and microRNA. J Orthop Sci. 19:521–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bayrak ES, Mehdizadeh H, Akar B, Somo SI,
Brey EM and Cinar A: Agent-based modeling of osteogenic
differentiation of mesenchymal stem cells in porous biomaterials.
Conf Proc IEEE Eng Med Biol Soc. 2014:2924–2927. 2014.PubMed/NCBI
|
|
4
|
Oryan A, Alidadi S, Moshiri A and Maffulli
N: Bone regenerative medicine: Classic options, novel strategies
and future directions. J Orthop Surg Res. 9:182014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwon TK, Song JM, Kim IR, Park BS, Kim CH,
Cheong IK and Shin SH: Effect of recombinant human bone
morphogenetic protein-2 on bisphosphonate-treated osteoblasts. J
Korean Assoc Oral Maxillofac Surg. 40:291–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan F, Luo S, Jiao Y, Deng Y, Du X, Huang
R, Wang Q and Chen W: Molecular characterization of the BMP7 gene
and its potential role in shell formation in Pinctada martensii.
Int J Mol Sci. 15:21215–21228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang RN, Green J, Wang Z, Deng Y, Qiao M,
Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S, et al: Bone
morphogenetic protein (BMP) signaling in development and human
diseases. Genes Dis. 1:87–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Li YP, Paulson C, Shao JZ, Zhang
X, Wu M and Chen W: Wnt and the Wnt signaling pathway in bone
development and disease. Front Biosci (Landmark Ed). 19:379–407.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zahoor M, Cha PH, Min do S and Choi KY:
Indirubin-3′-oxime reverses bone loss in ovariectomized and
hindlimb-unloaded mice via activation of the Wnt/β-catenin
signaling. J Bone Miner Res. 29:1196–1205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heilbronn R and Weger S: Viral vectors for
gene transfer: Current status of gene therapeutics. Handb Exp
Pharmacol. 197:143–170. 2010. View Article : Google Scholar
|
|
11
|
Sugiyama O, An DS, Kung SP, Feeley BT,
Gamradt S, Liu NQ, Chen IS and Lieberman JR: Lentivirus-mediated
gene transfer induces long-term transgene expression of BMP-2 in
vitro and new bone formation in vivo. Mol Ther. 11:390–398. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Benskey MJ and Manfredsson FP: Lentivirus
production and purification. Methods Mol Biol. 1382:107–114. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krause C, Korchynskyi O, de Rooij K,
Weidauer SE, de Gorter DJ, van Bezooijen RL, Hatsell S, Economides
AN, Mueller TD, Löwik CW and ten Dijke P: Distinct modes of
inhibition by sclerostin on bone morphogenetic protein and Wnt
signaling pathways. J Biol Chem. 285:41614–41626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou S, Zilberman Y, Wassermann K, Bain
SD, Sadovsky Y and Gazit D: Estrogen modulates estrogen receptor
alpha and beta expression, osteogenic activity, and apoptosis in
mesenchymal stem cells (MSCs) of osteoporotic mice. J Cell Biochem
Suppl. 36:144–155. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hernigou P, Flouzat-Lachaniette CH,
Delambre J, Poignard A, Allain J, Chevallier N and Rouard H:
Osteonecrosis repair with bone marrow cell therapies: State of the
clinical art. Bone. 70:102–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Virk MS, Conduah A, Park SH, Liu N,
Sugiyama O, Cuomo A, Kang C and Lieberman JR: Influence of
short-term adenoviral vector and prolonged lentiviral vector
mediated bone morphogenetic protein-2 expression on the quality of
bone repair in a rat femoral defect model. Bone. 42:921–931. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kotterman MA and Schaffer DV: Engineering
adeno-associated viruses for clinical gene therapy. Nat Rev Genet.
15:445–451. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deichmann A and Schmidt M: Biosafety
considerations using gamma-retroviral vectors in gene therapy. Curr
Gene Ther. 13:469–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maier P, von Kalle C and Laufs S:
Retroviral vectors for gene therapy. Future Microbiol. 5:1507–1523.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pauwels K, Gijsbers R, Toelen J, Schambach
A, Willard-Gallo K, Verheust C, Debyser Z and Herman P:
State-of-the-art lentiviral vectors for research use: Risk
assessment and biosafety recommendations. Curr Gene Ther.
9:459–474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rothe M, Modlich U and Schambach A:
Biosafety challenges for use of lentiviral vectors in gene therapy.
Curr Gene Ther. 13:453–468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dull T, Zufferey R, Kelly M, Mandel RJ,
Nguyen M, Trono D and Naldini L: A third-generation lentivirus
vector with a conditional packaging system. J Virol. 72:8463–8471.
1998.PubMed/NCBI
|
|
24
|
Miller F, Hinze U, Chichkov B, Leibold W,
Lenarz T and Paasche G: Validation of eGFP fluorescence intensity
for testing in vitro cytotoxicity according to ISO 10993-5. J
Biomed Mater Res B Appl Biomater. Dec 24–2015.Epub ahead of print.
View Article : Google Scholar
|
|
25
|
Witting SR, Vallanda P and Gamble AL:
Characterization of a third generation lentiviral vector
pseudotyped with Nipah virus envelope proteins for endothelial cell
transduction. Gene Ther. 20:997–1005. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mátrai J, Chuah MK and VandenDriessche T:
Recent advances in lentiviral vector development and applications.
Mol Ther. 18:477–490. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iwasaki S, Hattori A, Sato M, Tsujimoto M
and Kohno M: Characterization of the bone morphogenetic protein-2
as a neurotrophic factor. Induction of neuronal differentiation of
PC12 cells in the absence of mitogen-activated protein kinase
activation. J Biol Chem. 271:17360–17365. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Usas A, Ho AM, Cooper GM, Olshanski A,
Peng H and Huard J: Bone regeneration mediated by BMP4-expressing
muscle-derived stem cells is affected by delivery system. Tissue
Eng Part A. 15:285–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeffery TK, Upton PD, Trembath RC and
Morrell NW: BMP4 inhibits proliferation and promotes myocyte
differentiation of lung fibroblasts via Smad1 and JNK pathways. Am
J Physiol Lung Cell Mol Physiol. 288:L370–L378. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gitelman SE, Kirk M, Ye JQ, Filvaroff EH,
Kahn AJ and Derynck R: Vgr-1/BMP-6 induces osteoblastic
differentiation of pluripotential mesenchymal cells. Cell Growth
Differ. 6:827–836. 1995.PubMed/NCBI
|
|
31
|
Desmyter S, Goubau Y, Benahmed N, de Wever
A and Verdonk R: The role of bone morphogenetic protein-7
(Osteogenic Protein-1) in the treatment of tibial fracture
non-unions. An overview of the use in Belgium. Acta Orthop Belg.
74:534–537. 2008.PubMed/NCBI
|
|
32
|
Bibbo C, Nelson J, Ehrlich D and Rougeux
B: Bone morphogenetic proteins: Indications and uses. Clin Podiatr
Med Surg. 32:35–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou S, Mizuno S and Glowacki J: Wnt
pathway regulation by demineralized bone is approximated by both
BMP-2 and TGF-β1 signaling. J Orthop Res. 31:554–560. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wagner ER, Zhu G, Zhang BQ, Luo Q, Shi Q,
Huang E, Gao Y, Gao JL, Kim SH, Rastegar F, et al: The therapeutic
potential of the Wnt signaling pathway in bone disorders. Curr Mol
Pharmacol. 4:14–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Conway JD, Shabtai L, Bauernschub A and
Specht SC: BMP-7 versus BMP-2 for the treatment of long bone
nonunion. Orthopedics. 37:e1049–e1057. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cirano FR, Togashi AY, Marques MM,
Pustiglioni FE and Lima LA: Role of rhBMP-2 and rhBMP-7 in the
metabolism and differentiation of osteoblast-like cells cultured on
chemically modified titanium surfaces. J Oral Implantol.
40:655–659. 2014. View Article : Google Scholar : PubMed/NCBI
|