Introduction
Tremella aurantialba, a wood-inhabiting
host-specific fungus, has been commonly used in traditional Chinese
medicine (1). Numerous studies
have analyzed polysaccharides extracted from its mycelium and fruit
body as well as the crude extract of the fermentation broth
(2–4). These extracted polysaccharides have
been demonstrated to have pharmacological properties, including
antidiabetic, antitumor and antihyperlipidemic activities (5–7), as
well as immunostimulatory effects (8). Kiho et al (9) revealed that a polysaccharide isolated
from T. aurantialba produced significant antidiabetic
effects, and decreased serum cholesterol, free fatty acid and
triglyceride levels in diabetic mice. Furthermore, polysaccharides
extracted using hot water and ethanol (70%) from T.
aurantialba exerted potent inhibitory effects on the growth of
prostate cancer cell lines, including LNCaP and PC-3 (10). Therefore, T. aurantialba
polysaccharides may be valuable sources of food and pharmaceutical
agents.
To date, the extraction of polysaccharides from
T. aurantialba has not been commercially feasible. This is
largely due to a long cultivation cycle and low production, as well
as sensitivity to seasons and insects. To address these issues,
liquid fermentation technology has been adopted to increase the
yield of T. aurantialba and its polysaccharide content.
Numerous studies have investigated the biological
functions of mycelium polysaccharides from T. aurantialba in
cardiovascular and cerebral diseases, diabetes mellitus, and in
blood fat and pressure control (2,11,12).
However, little is known about the antioxidant and
immunostimulatory activities of mycelium polysaccharides. In the
present study, crude mycelium polysaccharide (CMCP) and purified
mycelium polysaccharide (MCP) were isolated from the mycelia of
T. aurantialba using liquid fermentation technology. The
antioxidant activities of these polysaccharides were evaluated by
determining their reducing power, and 2,2-diphenyl-1-picrylhydrazyl
(DPPH) and hydroxyl radical scavenging ability. Furthermore,
immunostimulatory effects were analyzed by investigating cellular
proliferation, and the release of nitric oxide (NO), tumor necrosis
factor-α (TNF-α), interleukin (IL)-1 and IL-6 by RAW264.7
macrophages.
Materials and methods
Materials
T. aurantialba was purchased from The
Agricultural Culture Collection of China (Beijing, China). The
RAW264.7 mouse macrophage cell line was obtained from the cell bank
of The Chinese Academy of Sciences (Shanghai, China). Dimethyl
sulfoxide (DMSO),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
DPPH, and lipopolysaccharide (LPS) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Dulbecco's modified Eagle's
medium (DMEM) and fetal bovine serum (FBS) were obtained from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Standard
monosaccharides including glucose, xylose, rhamnose, arabinose,
mannose and galactose were obtained from Sangon Biotech Co., Ltd.
(Shanghai, China). Sephadex G-100 was purchased from GE Healthcare
Life Sciences (Chalfont, UK).
Incubation and fermentation of T
aurantialba. T. aurantialba was initially
cultured on potato dextrose agar (PDA) slant tube medium containing
20% potato, 2% glucose and 2% agar, and then transferred to PDA
plates. Following incubation at 28°C for 14 days, sections of T.
aurantialba mycelium from PDA plates were transferred to liquid
medium containing 20% potato, 2% glucose, 0.3% KH2PO4, 0.15%
MgSO4·7H2O and 0.01–0.02 mg/ml vitamin B1. Fermentation was
performed in a flask shaken at 220 rpm for 14 days at 28°C.
Preparation and purification of
polysaccharides from T. aurantialba mycelia
Mycelia were obtained from culture broths using a
Buchner funnel and air-dried. Extraction was performed by
incubating mycelia with 1.25 mol/l NaOH solution containing 0.05%
(w/v) NaBH4 at room temperature for 4–6 h. Following pumping
filtration to remove mycelia fragments, the residues were
neutralized with 2 mol/l acetic acid and centrifuged at 4,000 ×
g for 30 min at room temperature. Supernatants were
collected, concentrated to 1/5 of the original volume and
precipitated with four volumes of 95% (v/v) ethanol solution. The
precipitate was collected and washed with deionized water.
Subsequently, the solution was deproteinated by mixing with an
equal volume of Sevage reagent (chloroform:n-butanol [4:1 (v/v)])
and centrifuging at 4,000 × g for 30 min at 4°C. This
solution was then precipitated with four volumes of 95% (v/v)
ethanol solution. The precipitate was freeze-dried and designated
as CMCP.
CMCP was dissolved in deionized water (5 mg/ml) and
purified by gel permeation chromatography using a Sephadex G-100
column (1×30 cm). Aliquots (1 ml) were applied to the column, which
was eluted with 0.1 mol/l NaCl at a flow rate of 0.75 ml/min, with
each tube collecting 0.75 ml effluent. The polysaccharide content
of effluent was determined by the phenol-sulfuric acid method
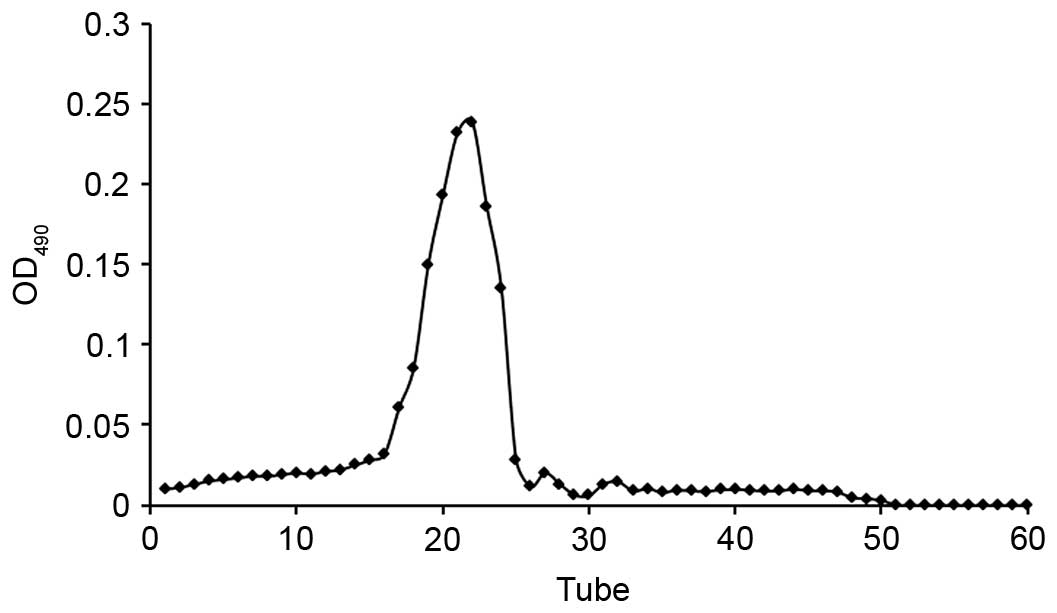
(13). The elution curve was
obtained indirectly using the number of collecting tubes as the
abscissa and the absorbance of the effluent-phenol-sulfuric acid
reaction system as the ordinate (Fig.
1). Collected liquid from the single peak was merged and
dialyzed against deionized water in a cellulose dialysis tube. The
solution was freeze-dried in a vacuum to obtain the purified
polysaccharide (MCP).
Chemical analysis of
polysaccharides
Determination of total sugar, uronic acid and
protein content
The total sugar content of CMCP and MCP was analyzed
using the phenol-sulfuric acid method (13), while the uronic acid and protein
content were determined by the carbazole-sulfuric acid and
Coomassie brilliant blue methods (14), respectively.
Monosaccharide analysis of MCP
MCP (20 mg) was hydrolyzed with 1 M H2SO4 at 100°C
for 5 h in a thermostatic water bath (HH-8; Jintan Xinxin
Experimental Instrument Co., Ltd., Changzhou, China). The
hydrolysate was neutralized with excess BaCO3. The obtained
solution was centrifuged at 2,600 × g for 15 min at room
temperature. Subsequently, the free monosaccharide was obtained
from the supernatant by drying in a vacuum oven at 45°C. The dried
monosaccharide (10 mg) was added to hydroxylamine hydrochloride (10
mg), inositol (2 mg) and pyridine (0.5 ml) and incubated at 90°C
for 30 min. The solution was then mixed with acetic anhydride (0.5
ml) in a constant temperature water bath at 90°C for 30 min. The
monosaccharide composition was determined by gas chromatography
analysis using the methods described previously (15).
Determination of molecular weight of MCP
The molecular weight (MW) of samples was determined
by gel permeation chromatography (GPC) using a method described in
our previous study (16). In
brief, the samples were separated on an Agilent 1200 Liquid
Chromatography system equipped with a G1310A pump, a PL aquagel-OH
column (7.5×300 mm; Agilent Technologies, Inc., Santa Clara, CA,
USA.) and a differential refractive index detector (RID; G1362A).
The column and RID detector temperature was maintained at 25°C, the
flow rate of the mobile phase (0.1 M NaNO3) was 0.8 ml/min, and all
solutions were filtered with 0.45 µm syringe filter. The column was
calibrated with dextran standards of varying molecular weights
(10,000, 41,100, 84,400, 133,800, 275,900 and 606,200 Da).
Infrared spectroscopy of MCP
Fourier transform infrared (FTIR) spectroscopy of
MCP (2 mg) mixed with dry KBr (200 mg) was performed at room
temperature in the 4000 cm-1 to 500 cm-1 region (Thermo Fisher
Scientific, Inc.).
Antioxidant activity assay of
polysaccharides
Determination of reducing power
The reducing power of CMCP and MCP was determined
according to a previously described method (17) with slight modifications. Briefly,
various concentrations of polysaccharides (50, 100, 200, 400 or
1,000 µg/ml) in 1 ml distilled water were mixed with phosphate
buffer [2.5 ml, 2 M (pH 6.6)] and potassium ferricyanide [K3Fe
(CN)6; 0.25 ml, 1% (w/v)]. Following an incubation at 50°C for 20
min, 0.5 ml trichloroacetic acid [10% (w/v)] was added to the
mixture to terminate the reaction. The solution was centrifuged at
3,000 × g for 10 min at room temperature. An aliquot of 1.5
ml supernatant was collected, mixed with 0.1 ml FeCl3 [0.1%, (w/v)]
and 3 ml deionized water, and incubated at room temperature for 5
min. The absorbance of polysaccharides was measured at a wavelength
of 700 nm using an ultraviolet visible spectrophotometer
(UVmini-1240; Shimadzu International Trading Co., Ltd, Shanghai,
China).
DPPH scavenging activity
DPPH radical scavenging activity was determined
according to a previously described method (18) with slight modifications. A total of
1 ml polysaccharide (50, 100, 200, 400 or 1,000 µg/ml) was added to
a 0.004% ethanol solution of DPPH (3.0 ml), and incubated at room
temperature for 30 min in the dark. In the control group, 95%
ethanol replaced the DPPH solution, while distilled water was used
as the blank. The absorbance of each reaction mixture was measured
at a wavelength of 517 nm using an ultraviolet visible
spectrophotometer (Shimadzu International Trading Co., Ltd.). The
DPPH scavenging activity was calculated as follows:
Scavenging ability
(%)=[1-(Asample517-Acontrol517)/Ablank517]
×100
Hydroxyl radical scavenging activity
The hydroxyl radical scavenging activity of the
polysaccharide was determined using Fenton's reaction, as
previously described (19). The
reaction mixture consisted of 1 ml polysaccharide (50, 100, 200,
400 or 1,000 ug/ml), 0.9 ml EDTA-FeSO4 (0.15 mM), 0.5 ml H2O2 (8.8
mM) and 0.5 ml salicylic acid (9 mM). In the control group, water
replaced the sample and sodium phosphate replaced the H2O2, while
water was used as the blank. Following incubation at 37°C for 60
min, the absorbance of samples was measured at a wavelength of 510
nm. The hydroxyl radical scavenging activity was calculated as
follows:
Scavenging ability
(%)=[1-(Asample510-Acontrol510)/Ablank510]
×100
RAW264.7 cell culture
RAW264.7 cells were cultured in DMEM supplemented
with 10% FBS, in a water-jacketed incubator (Thermo Fisher
Scientific, Inc.) at 37°C with 5% CO2 in a humidified atmosphere.
The medium was replaced every day, and the cells were passaged
every second day. Cells were used in subsequent experiments when
80% confluency was reached.
MTT assay
An MTT assay was performed as previously described
(20) to determine the effect of
polysaccharides on the proliferation of RAW264.7 cells. Briefly,
RAW264.7 cells, at a density of 5×104/ml, were seeded in
96-well plates and incubated with 100 µl test samples at various
concentrations (50, 100 or 200 µg/ml) for 48 h. Cells treated with
LPS (1 µg/ml) served as a positive control, while cells treated
with medium alone were used as a negative control. Subsequently, 10
µl of MTT (5 mg/ml) was added to each well and incubated for a
further 4 h at 37°C. The plates were centrifuged at 1,000 ×
g for 5 min at room temperature. Following removal of the
supernatant, 100 µl of DMSO was added to each well. Plates were
agitated for 10 min to dissolve the produced formazan crystals, and
the optical densities were measured at a wavelength of 570 nm using
a microplate reader.
Influence of polysaccharide on NO secretion of
RAW264.7
Nitrite accumulation served as a marker of NO
production in culture medium, and was measured using the Griess
reaction (21). RAW264.7 cells
were seeded in 96-well plates at a density of 5×104 cells/well and
incubated at 37°C and 5% CO2 in a humidified atmosphere for 6 h.
Cells were treated with polysaccharides (50, 100 or 200 µg/ml), LPS
(positive control) or culture media alone (negative control).
Nitrite production was determined using a Griess kit (Beyotime
Institute of Biotechnology, Shanghai, China) according to the
manufacturer's instructions.
Influence of polysaccharide on cytokine secretion
by RAW264.7 cells
The production of IL-1, IL-6 and TNF-α by RAW264.7
cells was detected using enzyme-linked immunosorbent assay kits
(cat. nos. MLB00C, M6000B and MTA00B, respectively; R&D
Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's instructions. Cells (5×104 cells/well) were seeded
in 96-well plates and treated as for the experimental, positive
control and negative control groups described above.
Statistical analysis
Data analyses were performed using SPSS software
version 19.0 (IBM SPSS, Armonk, NY, USA). Data are presented as the
mean ± standard deviation. The results were analyzed using one-way
analysis of variance followed by the least significant difference
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Fermentation of T. aurantialba was
successful
Fig. 2 presents the
growth curve of T. aurantialba in culture plates (Fig. 2A) and the growth curve of mycelium
in shake-flasks (Fig. 2B). The
colony of T. aurantialba was roundish, white and opaque with
a matte surface. The diameter of the colony in the plate increased
gradually over time, and covered the plate by day 14. In addition,
the biomass of mycelium in shake-flasks increased over time,
reaching 0.34 g/50 ml on the day 10, following which the biomass
plateaued. Mycelium consisted of golden yellow spherical particles,
with good dispersion in the liquid medium. The fermentation broth
was clear, with a color that darkened gradually over time.
Mycelia polysaccharide were isolated
and the composition analyzed
The yield of CMCP from T. aurantialba was
1.53%, the total carbohydrate content was 11.92% and the residual
protein content was 21.7%. The total carbohydrate and residual
protein content of MCP were 86.59 and 3.5%, respectively, following
deproteinization using the Sevage method and purification on a
Sephadex G-100 column. The uronic acid content was 16.4% and the
molecular weight of MCP as determined by GPC was 4.3×104 g/mol
(Table I).
 | Table I.Molecular weight of MCP. |
Table I.
Molecular weight of MCP.
|
| Molecular weight
(×104 g/mol) |
|
|---|
|
|
|
|
|---|
| Sample |
Mw/Mn |
Mw |
Mn |
|---|
| MCP | 4.30 | 2.95 | 1.46 |
Table II presents
the monosaccharide composition and content of MCP. MCP was
comprised of D-glucose, D-galactose and D-mannose, with traces of
D- rhamnose, D-arabinose and D-xylose.
 | Table II.Monosaccharide composition of
purified mycelium polysaccharides. |
Table II.
Monosaccharide composition of
purified mycelium polysaccharides.
| Monosaccharide
composition | Content (%) |
|---|
| Rhamnose |
1.31 |
| Arabinose |
0.74 |
| Xylose |
1.36 |
| Mannose |
4.53 |
| Glucose | 82.17 |
| Galactose |
9.89 |
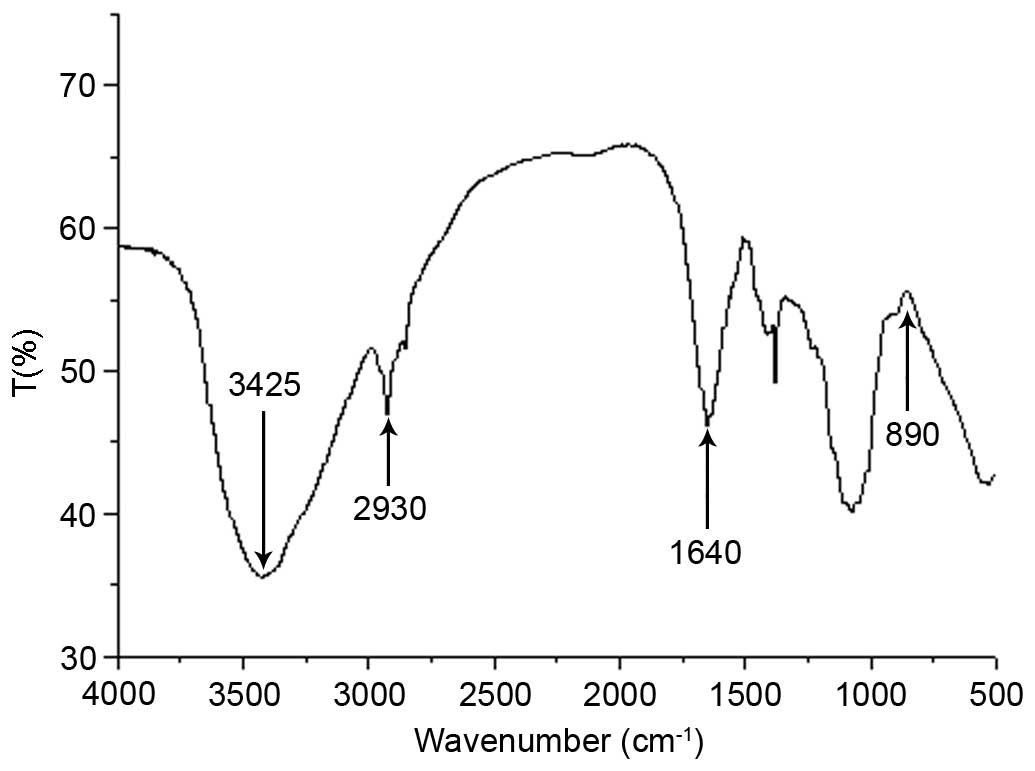
The FTIR spectrum of MCP is presented in Fig. 3. The FTIR spectrum revealed a
strong broad absorption peak at 3,425 cm−1 due to the
O-H stretching vibration of the polysaccharide and a peak at 2,930
cm−1 due to the C-H stretching vibration. FTIR spectrum
of MCP exhibited an absorption peak at 1,640 cm−1, which
was a characteristic of the C=O stretching vibration. No notable
C=O vibration was observed at 3,000–2,500 cm−1,
suggesting that the content of uronic acid was low. In addition, an
absorption peak at 890 cm−1 indicated that the
polysaccharide was connected by a β-glycosidic bond.
Antioxidant activity of CMCP and
MCP
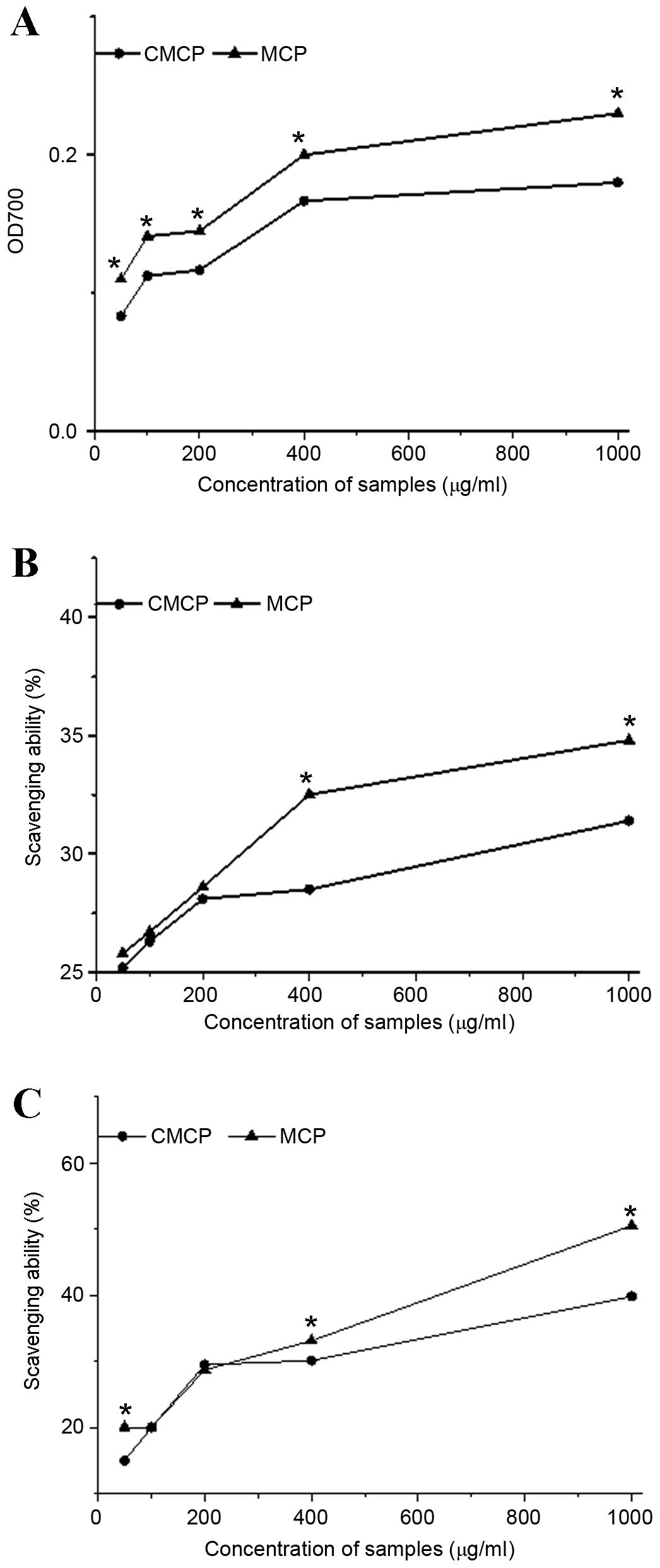
The reducing power of MCP was greater than
CMCP
As presented in Fig.
4A, the reducing power of CMCP and MCP was increased in a
dose-dependent manner in the range of 50–1,000 µg/ml. The reducing
power of MCP was 1.26-fold greater than that of CMCP. At 1,000
µg/ml, the maximum reducing power of CMCP and MCP was 0.18 and
0.23, respectively.
DPPH scavenging activity was increased with MCP
treatment compared to CMCP
As presented in Fig.
4B, a rapid increase was observed in the DPPH scavenging
activity with increasing MCP concentrations (50–400 µg/ml), while
this rapid increase occurred in the DPPH scavenging activity of
CMCP between 50 and 200 µg/ml. At a concentration of 1,000 µg/ml,
the scavenging effect of MCP and CMCP reached maximums of 35.02 and
31.84%, respectively. In addition, no significant difference was
observed between MCP and CMCP at concentrations of 50–200 µg/ml.
The DPPH scavenging activity of MCP was significantly greater than
that of CMCP at concentrations >200 µg/ml (400 µg/ml, P=0.002;
1,000 µg/ml, P=0.008; Fig.
4B).
Scavenging activity of hydroxyl radicals was
increased with MCP compared with CMCP
The hydroxyl radical scavenging activity of MCP and
CMCP is presented in Fig. 4C. The
scavenging activity of the hydroxyl radicals increased with
increasing concentrations of MCP and CMCP, with the greatest
scavenging ability at 1,000 µg/ml. The scavenging ability of MCP
was significantly greater than that of CMCP at 50 and 400–1,000
µg/ml (50 µg/ml, P=0.003; 400 µg/ml, P=0.006; 1,000 µg/ml,
P<0.001). No statistical difference was observed at
concentrations of 100–200 µg/ml.
The difference between MCP and CMCP antioxidant
activities may be due to differences in total carbohydrate
content.
Immunostimulatory activity of CMCP and MCP
MCP induced proliferation of RAW264.7 cells
Fig. 5 presents the
effects of polysaccharides on the proliferation of RAW264.7 cells.
MCP stimulated the proliferation of RAW264.7 cells at
concentrations of 50–200 µg/ml (P<0.05), in a dose-dependent
manner. At 200 µg/ml, MCP was more effective than the positive
control. However, no stimulative effects of CMCP on RAW264.7 cell
proliferation were observed at concentrations of 50–200 µg/ml
(P>0.05). These results indicate that MCP, but not CMCP,
significantly induced proliferation of RAW264.7 cells.
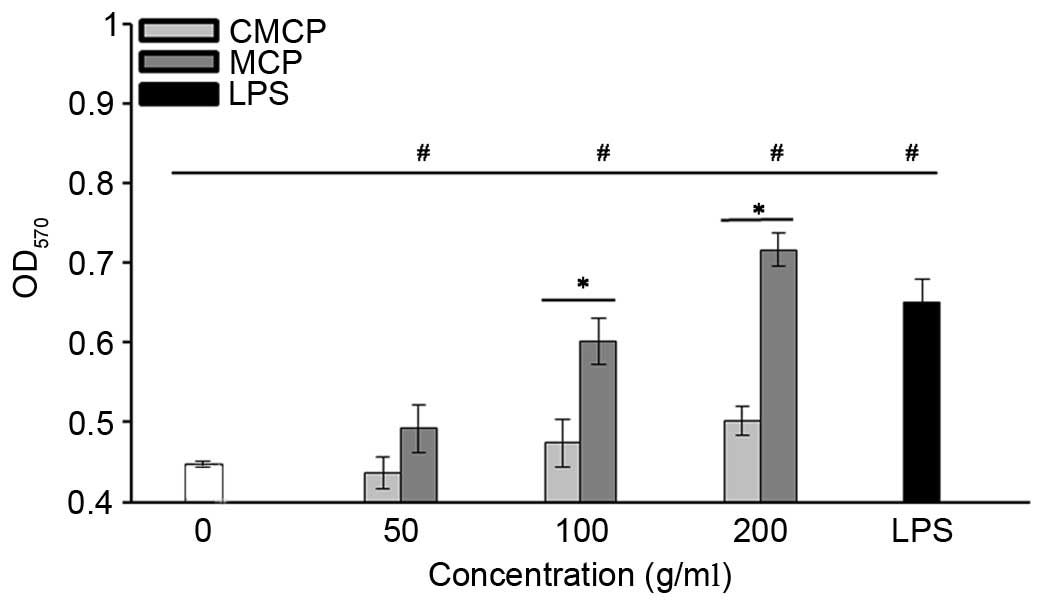
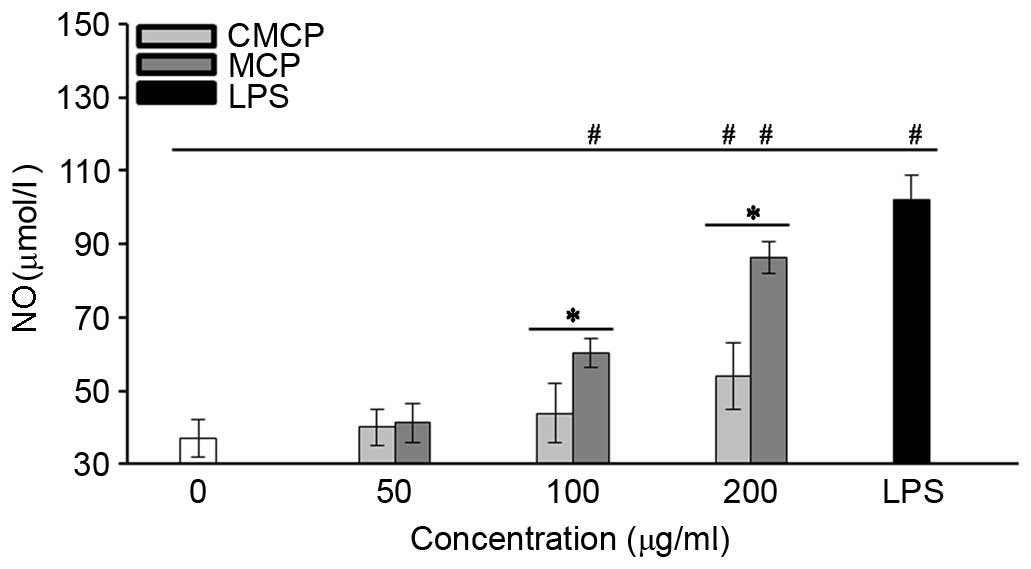
High concentration of MCP and CMCP increased NO
secretion by RAW264.7 cells
The effects of CMCP and MCP on NO production by
RAW264.7 cells were evaluated by measuring the release of nitrite.
As presented in Fig. 6, no
significant increase in NO production was observed with 50 µg/ml
MCP or CMCP (P>0.05). Concentrations of MCP >100 µg/ml
significantly increased NO production compared with the negative
control (P<0.05). Increased NO production was observed in the
CMCP-treated cells only at a concentration of 200 µg/ml, and
remained significantly reduced compared with MCP at the same
concentration (P<0.05).
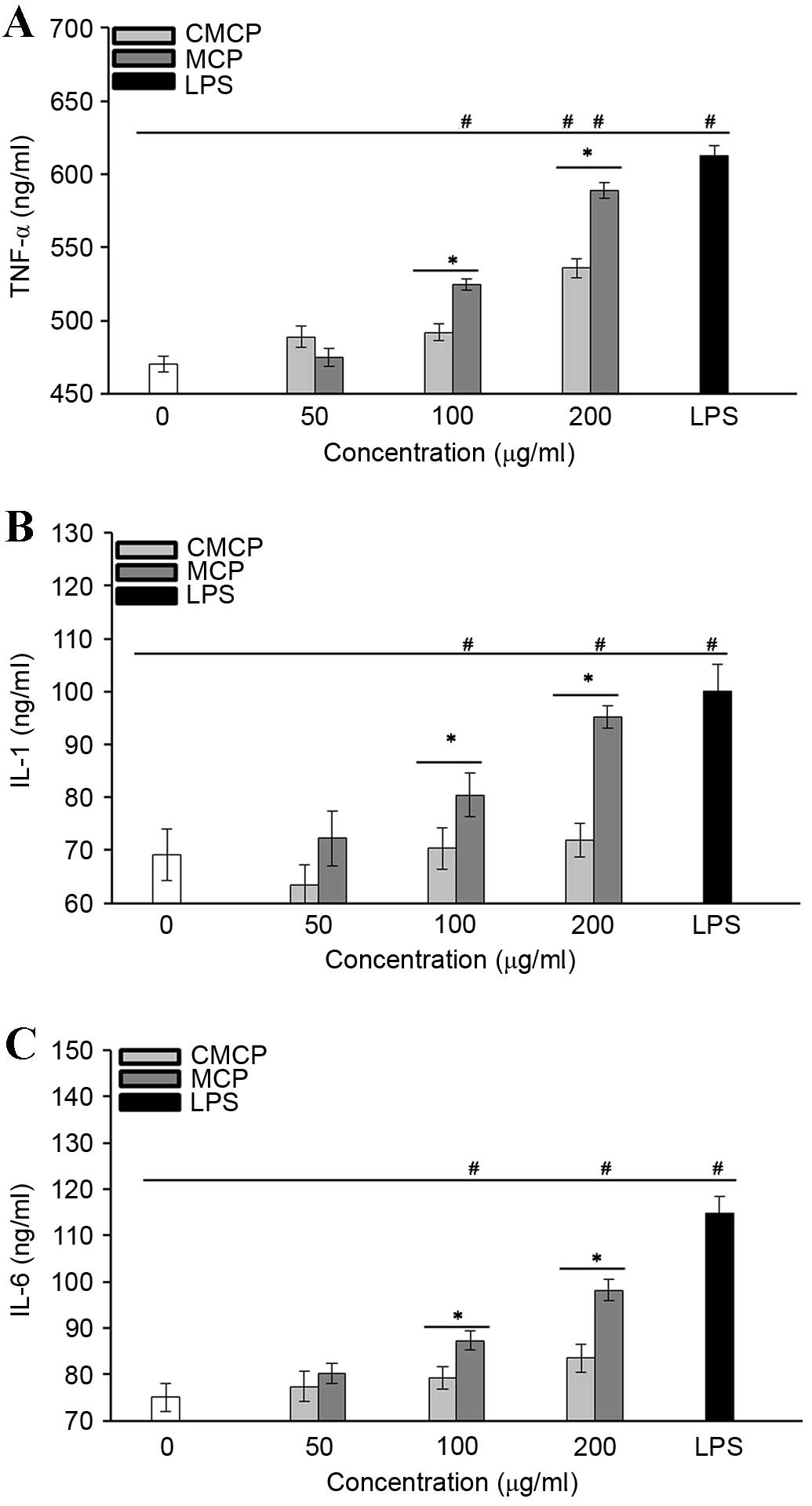
MCP greatly increased cytokine secretion by
RAW264.7 cells
The effects of MCP and CMCP on the production of
TNF-α (Fig. 7A), IL-1 (Fig. 7B) and IL-6 (Fig. 7C) by RAW264.7 cells were
investigated. Cytokine levels were significantly upregulated in
cells treated with 100 and 200 µg/ml MCP compared with those in the
negative control group (P<0.05). CMCP did not induce production
of IL-1 or −6 at the concentrations tested (P>0.05); however, it
did stimulate TNF-α secretion at 200 µg/ml (P<0.05), indicating
that CMCP has a reduced potency compared with MCP. These results
indicate that the effects of polysaccharides on macrophage
activation may be associated with the total carbohydrate content in
MCP and CMCP.
Discussion
The composition and structure of polysaccharides
from T. aurantialba have been reported to be closely
associated with their biological properties. Biological activities
may be affected by numerous factors, including diverse
monosaccharide composition, glycosidic bond type and molecular
weight, as well as molecular conformation. Kiho et al
(22) reported that the
polysaccharide TAP from T. aurantialba comprised mannose,
xylose, glucuronic acid and glucose, and exhibited potent
hypoglycemic activity, which was the result of non-reducing
terminal α-D-mannopyranosyl residues. Furthermore, the specific
structure of TAP contributed to its effect on a key hepatic enzyme
and plasma cholesterol levels in healthy and diabetic mice
(22,23). In the present study, chemical
analysis indicated that MCP obtained by fermentation was composed
primarily of D-glucose, D-galactose and D-mannose, and was
connected by a β-glycosidic bond.
Accumulating evidence indicates antioxidant
properties for numerous edible mushrooms, including D.
indusiata, T. giganteum and P. cystidiosus (24,25).
Kasuga et al (26)
demonstrated that methanolic extracts from ear mushrooms exhibited
marked reducing power in chelating ferrous ions and scavenging of
DPPH and hydroxyl radicals. In addition, it has been reported that
various extracts from H. marmoreus exerted antioxidant
activities of 38.6–65.2% and a reducing power of 0.99 at a
concentration of 5 mg/ml (27). Du
et al (28) revealed that
the chloroform extract derived from T. aurantialba fruiting
bodies exhibited satisfactory antioxidant activity, and all
chloroform, ethyl acetate and ethanol extracts exerted a greater
scavenging activity on hydroxyl compared with superoxide anion
radicals. However, less is known about the antioxidant activity of
polysaccharides from T. aurantialba mycelium. In the present
study, MCP obtained following fermentation exerted a greater
reducing power compared with CMCP in the concentration range
evaluated. Furthermore, MCP exhibited superior scavenging
activities of DPPH and hydroxyl radicals compared with CMCP. These
results indicate that the difference in antioxidant activities
between MCP and CMCP may be associated with the total carbohydrate
content.
Immunomodulatory effects have been associated with
polysaccharides (29). The
mechanism underlying immunoregulation primarily involves the
induction of proliferation of various immune cells, including
macrophages, lymphocytes and natural killer cells, and the
stimulation of inflammatory mediator production by these cells
(30). Therefore, proliferation
assays are an appropriate method to rapidly screen the
immunostimulatory activity of polysaccharides. Numerous reports
have demonstrated the immunostimulatory activity of polysaccharides
isolated from T. aurantialba. Lee et al (6) revealed that methanol soluble
substances extracted from the fruiting body of T.
aurantialba improved the activity of B lymphocytes, in which
the alkaline phosphatase activity was increased 1.16-fold at the
concentration of 200 µg/ml. Du et al (31) indicated that the acidic
polysaccharide TAPA1 from T. aurantialba markedly stimulated
the proliferation of murine lymphocytes in vitro in a
dose-dependent manner. In the present study, 50–200 µg/ml MCP
significantly increased proliferation of RAW264.7 macrophages. In
addition, MCP exhibited a greater effect than LPS, while only 200
µg/ml CMCP promoted RAW264.7 cell proliferation.
Macrophages are important components of the immune
system, which are crucial in host defense and acute inflammatory
responses (32). NO, which is
produced by macrophages, is an inorganic molecule that is critical
in injury, inflammation and defense. Previous studies have
indicated that polysaccharides stimulate NO production by
macrophages, accompanied an improvement in immune function
(33,34). Du et al (35) suggested that all polysaccharides
(TAPA1, TAPA1-deac and TAPA1-ac) isolated from T.
aurantialba fruiting bodies stimulated RAW264.7 macrophages to
produce NO. In the present study, a significant increase in NO
production was observed following treatment with >100 µg/ml MCP,
compared with the negative control group (P<0.05). CMCP promoted
NO production at the concentration of 200 µg/ml; however, this
remained significantly reduced compared with MCP.
Following stimulation by various external factors,
activated macrophages generate a variety of other mediators
responsible for numerous homeostatic, immunologic and inflammatory
processes, including IL-1, IL-6 and TNF-α. Therefore, cytokine
production may be reflective of the inflammatory process and may
provide a method to assess the effects of polysaccharide on
macrophage activation. In the present study, 100 and 200 µg/ml MCP
significantly upregulated the levels of IL-1, IL-6 and TNF-α
produced by RAW264.7 macrophages, compared with the negative
control group (P<0.05). CMCP induced only TNF-α production at a
concentration of 200 µg/ml, with a reduced potency compared with
MCP. Taken together, these results suggest that the total
carbohydrate content in MCP and CMCP may contribute to differences
in immunostimulatory activities.
In conclusion, CMCP was extracted from T.
aurantialba mycelia following liquid fermentation. Purification
by gel chromatography produced purified polysaccharide MCP.
Compared with CMCP, MCP demonstrated significantly increased
antioxidant and immunostimulatory activities. Due to the large
difference in total carbohydrate content of MCP and CMCP, the
findings of the present study suggest that increased total
carbohydrate content may contribute to the increase in antioxidant
and immunostimulatory activities. However, further studies are
required to identify the mechanisms underlying the antioxidant and
immunostimulatory activities of MCP, and to investigate the
biological properties of MCP in vivo, to validate its
potential clinical applications.
Acknowledgements
The present study was supported by the Research Fund
for the Doctoral Program of Higher Education of China (grant no.
20110093110008) and the College Students' Innovative Training
Program of Jiangnan University (grant no. 2015324Y).
References
|
1
|
Bandoni RJ and Boekhout T: Tremelloid
genera with yeast phases Fibulobasidium Bandoni, Holtermannia
Saccardo & Traverso, Sirobasidium de Lagerheim &
Patouillard, Tremella Persoon, Trimorphomyces Bandoni &
OberwinklerThe Yeasts: A Taxonomic Study. Kurtzman CP and Fell JW:
4th. Elsevier B.V.; Amsterdam: pp. 705–717. 1998, View Article : Google Scholar
|
|
2
|
Zhang Z, Li Y and Zhang K: Application of
statistical analysis for the optimization of mycelia and
polysaccharide production by Tremella aurantialba. Food Technol
Biotechnol. 45:45–50. 2007.
|
|
3
|
Zhang ZC, Lian B, Huang DM and Cui FJ:
Compare activities on regulating lipid-metabolism and reducing
oxidative stress of diabetic rats of Tremella aurantialba broth's
extract (TBE) with its mycelia polysaccharides (TMP). J Food Sci.
74:H15–H21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ding Z, Li J, Liu J, Lu Y, Wang C and
Zheng Q: Tremellin, a novel symmetrical compound, from the
basidiomycete Tremella aurantialba. Helv Chim Acta. 85:882–884.
2002. View Article : Google Scholar
|
|
5
|
Kiho T, Kochi M, Usui S, Hirano K, Aizawa
K and Inakuma T: Antidiabetic effect of an acidic polysaccharide
(TAP) from Tremella aurantia and its degradation product (TAP-H).
Biol Pharm Bull. 24:1400–1403. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee GW, Kim HY, Hur H, Lee MW, Shim MJ,
Lee UY and Lee TS: Antitumor and immuno-modulatory effect of crude
polysaccharides from fruiting body of Tremella aurantialba against
mouse sarcoma 180. Korean J Mycol. 36:66–74. 2008. View Article : Google Scholar
|
|
7
|
Wang H, Qu W, Chu S, Li M and Tian C:
Studies on the preventive and therapeutic effects of the
polysaccharide of Tremella aurantialba mycelia on diet-induced
hyperlipidemia in mice. Acta Nutr Sin. 24:431–432. 2002.(In
Chinese).
|
|
8
|
Du XJ, Zhang JS, Yang Y, Tang QJ, Jia W
and Pan YJ: Purification, chemical modification and
immunostimulating activity of polysaccharides from Tremella
aurantialba fruit bodies. J Zhejiang Univ Sci B. 11:437–442. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kiho T, Kochi M, Usui S, Hirano K, Aizawa
K and Inakuma T: Antidiabetic Effect of an Acidic Polysaccharide
(TAP) from Tremella aurantia Schw.: Fr.(Heterobasidiomycetes) in
Genetically Diabetic КК-Аy Mice. Int J Med Mushrooms. 4:115–123.
2002. View Article : Google Scholar
|
|
10
|
Kiho T, Iguchi K, Usui S and Hirano K:
Effect of Polysaccharides and 70% ethanol extracts from medicinal
mushrooms on growth of human prostate cancer LNCaP and PC-3 Cells.
Int J Med Mushrooms. 12:205–211. 2010. View Article : Google Scholar
|
|
11
|
Du X, Zhang Y, Mu H, Lv Z, Yang Y and
Zhang J: Structural elucidation and antioxidant activity of a novel
polysaccharide (TAPB1) from Tremella aurantialba. Food
Hydrocolloid. 43:459–464. 2015. View Article : Google Scholar
|
|
12
|
Zhang W, Qu W, Zhang X, Deng Y and Zhu S:
The anti-hyperglycemic activity of polysaccharides from Tremella
aurantialba mycelium. Acta Nutr Sin. 26:300–303. 2003.(In
Chinese).
|
|
13
|
Masuko T, Minami A, Iwasaki N, Majima T,
Nishimura S and Lee YC: Carbohydrate analysis by a phenol-sulfuric
acid method in microplate format. Anal Biochem. 339:69–72. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun Y, Wang H, Guo G, Pu Y and Yan B: The
isolation and antioxidant activity of polysaccharides from the
marine microalgae Isochrysis galbana. Carbohydr Polym. 113:22–31.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Xie M, Nie S, Li C and Wang Y:
Purification, composition analysis and antioxidant activity of a
polysaccharide from the fruiting bodies of Ganoderma atrum. Food
Chem. 107:231–241. 2008. View Article : Google Scholar
|
|
16
|
Deng C, Fu H, Teng L, Hu Z, Xu X, Chen J
and Ren T: Anti-tumor activity of the regenerated triple-helical
polysaccharide from Dictyophora indusiata. Int J Biol Macromol.
61:453–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oyaizu M: Studies on products of browning
reaction-antioxidative activities of products of browning reaction
prepared from glucosamine. Jpn J Nutr Diet. 44:307–315. 1986.
View Article : Google Scholar
|
|
18
|
Rumbaoa R, Cornago D and Geronimo I:
Phenolic content and antioxidant capacity of Philippine potato
(Solanum tuberosum) tubers. J Food Compos Anal. 22:546–550. 2009.
View Article : Google Scholar
|
|
19
|
Thomas C, Mackey MM, Diaz AA and Cox DP:
Hydroxyl radical is produced via the Fenton reaction in
submitochondrial particles under oxidative stress: Implications for
diseases associated with iron accumulation. Redox Rep. 14:102–108.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tiffen JC, Bailey CG, Ng C, Rasko JE and
Holst J: Luciferase expression and bioluminescence does not affect
tumor cell growth in vitro or in vivo. Mol Cancer. 9:2992010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ji Z, Tang Q, Zhang J, Yang Y, Jia W and
Pan Y: Immunomodulation of RAW264. 7 macrophages by GLIS, a
proteopolysaccharide from Ganoderma lucidum. J Ethnopharmacol.
112:445–450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kiho T, Kobayashi T, Morimoto H, Usui S,
Ukai S, Hirano K, Aizawa K and Inakuma T: Structural features of an
anti-diabetic polysaccharide (TAP) from Tremella aurantia. Chem
Pharm Bull (Tokyo). 48:1793–1795. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kiho T, Morimoto H, Kobayashi T, Usui S,
Ukai S, Aizawa K and Inakuma T: Effect of a polysaccharide (TAP)
from the fruiting bodies of Tremella aurantia on glucose metabolism
in mouse liver. Biosci Biotechnol Biochem. 64:417–419. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mau JL, Chao GR and Wu KT: Antioxidant
properties of methanolic extracts from several ear mushrooms. J
Agri Food Chem. 49:5461–5467. 2001. View Article : Google Scholar
|
|
25
|
Yang J, Lin H and Mau J: Antioxidant
properties of several commercial mushrooms. Food Chem. 77:229–235.
2002. View Article : Google Scholar
|
|
26
|
Kasuga A, Aoyagi Y and Sugahara T:
Antioxidative activities of several mushroom extracts. Jpn Soc Food
Sci Technol. 40:56–63. 1993. View Article : Google Scholar
|
|
27
|
Lee Yu, Jian Shao, Lian Pei and Mau Jeng:
Antioxidant properties of extracts from a white mutant of the
mushroom Hypsizigus marmoreus. J Food Compos Anal. 21:116–124.
2008. View Article : Google Scholar
|
|
28
|
Du XJ, Zhang JS, Liu YF, Tang QJ, Jia Z,
Yang Z and Pan YJ: The antioxidant activity of various extracts
from Tremella aurantialba fruiting bodies and their protective
effects on PC 12 cells injured by oxidation. Acta Agri Shanghai.
26:49–52. 2010.
|
|
29
|
Su Z, Dai Z and Yang J: Research progress
on immune mechanism of polysaccharide. J Yunnan Agri Univ.
21:205–209. 2006.
|
|
30
|
He Q and Zhang S: Advances in the studies
on the mechanism of immuno-potentiation effect of polysaccharides
from edible-medicinal fungi. Acta Edulis Fungi. 11:52–58. 2004.(In
Chinese).
|
|
31
|
Du X, Zhang J, Yang Y, Ye L, Tang Q, Jia
W, Liu Y, Zhou S, Hao R, Gong C and Pan Y: Structural elucidation
and immuno-stimulating activity of an acidic heteropolysaccharide
(TAPA1) from Tremella aurantialba. Carbohyd Res. 344:672–678. 2009.
View Article : Google Scholar
|
|
32
|
Lee MY, Lee JA, Seo CS, Ha H, Lee H, Son
JK and Shin HK: Anti-inflammatory activity of Angelica dahurica
ethanolic extract on RAW264. 7 cells via upregulation of heme
oxygenase-1. Food Chem Toxicol. 49:1047–1055. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shin JY, Song JY, Yun YS, Yang HO, Rhee DK
and Pyo S: Immunostimulating effects of acidic polysaccharides
extract of Panax ginseng on macrophage function. Immunopharm
immunot. 24:469–482. 2002. View Article : Google Scholar
|
|
34
|
Lee KY and Jeon YJ: Macrophage activation
by polysaccharide isolated from Astragalus membranaceus. Int
Immunopharmacol. 5:1225–1233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Du X, Zhang J, Lv Z, Ye L, Yang Y and Tang
Q: Chemical modification of an acidic polysaccharide (TAPA1) from
Tremella aurantialba and potential biological activities. Food
Chem. 143:336–340. 2014. View Article : Google Scholar : PubMed/NCBI
|