|
1
|
Fukao T, Mitchell G, Sass JO, Hori T, Orii
K and Aoyama Y: Ketone body metabolism and its defects. J Inherit
Metab Dis. 37:541–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Daum RS, Lamm PH, Mamer OA and Scriver CR:
A ‘new’ disorder of isoleucine catabolism. Lancet. 2:1289–1290.
1971. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fukao T, Scriver CR and Kondo N: t2
Collaborative Working Group: The clinical phenotype and outcome of
mitochondrial acetoacetyl-CoA thiolase deficiency
(beta-ketothiolase or T2 deficiency) in 26 enzymatically proved and
mutation-defined patients. Mol Genet Metab. 72:109–114. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sass JO: Inborn errors of ketogenesis and
ketone body utilization. J Inherit Metab Dis. 35:23–28. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hori T, Yamaguchi S, Shinkaku H, Horikawa
R, Shigematsu Y, Takayanagi M and Fukao T: Inborn errors of ketone
body utilization. Pediatr Int. 57:41–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abdelkreem E, Otsuka H, Sasai H, Aoyama Y,
Hori T, Abd El Aal M, Mahmoud S and Fukao T: Beta-ketothiolase
deficiency: Resolving challenges in diagnosis. Journal of Inborn
Errors of Metabolism & Screening. 4:2016. View Article : Google Scholar
|
|
7
|
Kano M, Fukao T, Yamaguchi S, Orii T,
Osumi T and Hashimoto T: Structure and expression of the human
mitochondrial acetoacetyl-CoA thiolase-encoding gene. Gene.
109:285–290. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Masuno M, Kano M, Fukao T, Yamaguchi S,
Osumi T, Hashimoto T, Takahashi E, Hori T and Orii T: Chromosome
mapping of the human mitochondrial acetoacetyl-coenzyme A thiolase
gene to 11q22.3-q23.1 by fluorescence in situ hybridization.
Cytogenet Cell Genet. 60:121–122. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukao T, Yamaguchi S, Kano M, Orii T,
Fujiki Y, Osumi T and Hashimoto T: Molecular cloning and sequence
of the complementary DNA encoding human mitochondrial
acetoacetyl-coenzyme A thiolase and study of the variant enzymes in
cultured fibroblasts from patients with 3-ketothiolase deficiency.
J Clin Invest. 86:2086–2092. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukao T, Maruyama S, Ohura T, Hasegawa Y,
Toyoshima M, Haapalainen AM, Kuwada N, Imamura M, Yuasa I, Wierenga
RK, et al: Three Japanese patients with beta-ketothiolase
deficiency who share a mutation, c.431A>C (H144P) in ACAT1:
Subtle abnormality in urinary organic acid analysis and blood
acylcarnitine analysis using tandem mass spectrometry. JIMD Rep.
3:107–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fukao T, Yamaguchi S, Orii T, Osumi T and
Hashimoto T: Molecular basis of 3-ketothiolase deficiency:
Identification of an AG to AC substitution at the splice acceptor
site of intron 10 causing exon 11 skipping. Biochim Biophys Acta.
1139:184–188. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fukao T, Yamaguchi S, Orii T, Schutgens
RB, Osumi T and Hashimoto T: Identification of three mutant alleles
of the gene for mitochondrial acetoacetyl-coenzyme A thiolase. A
complete analysis of two generations of a family with
3-ketothiolase deficiency. J Clin Invest. 89:474–479. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fukao T, Song XQ, Yamaguchi S, Kondo N,
Orii T, Matthieu JM, Bachmann C and Hashimoto T: Identification of
three novel frameshift mutations (83delAT, 754insCT, and 435 + 1G
to A) of mitochondrial acetoacetyl-coenzyme A thiolase gene in two
Swiss patients with CRM-negative beta-ketothiolase deficiency. Hum
Mutat. 9:277–279. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thummler S, Dupont D, Acquaviva C, Fukao T
and de Ricaud D: Different clinical presentation in siblings with
mitochondrial acetoacetyl-CoA thiolase deficiency and
identification of two novel mutations. Tohoku J Exp Med. 220:27–31.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Law CY, Lam CW, Ching CK, Yau KC, Ho TW,
Lai CK and Mak CM: NMR-based urinalysis for beta-ketothiolase
deficiency. Clin Chim Acta. 438:222–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fukao T, Yamaguchi S, Wakazono A, Orii T,
Hoganson G and Hashimoto T: Identification of a novel exonic
mutation at −13 from 5′ splice site causing exon skipping in a girl
with mitochondrial acetoacetyl-coenzyme A thiolase deficiency. J
Clin Invest. 93:1035–1041. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
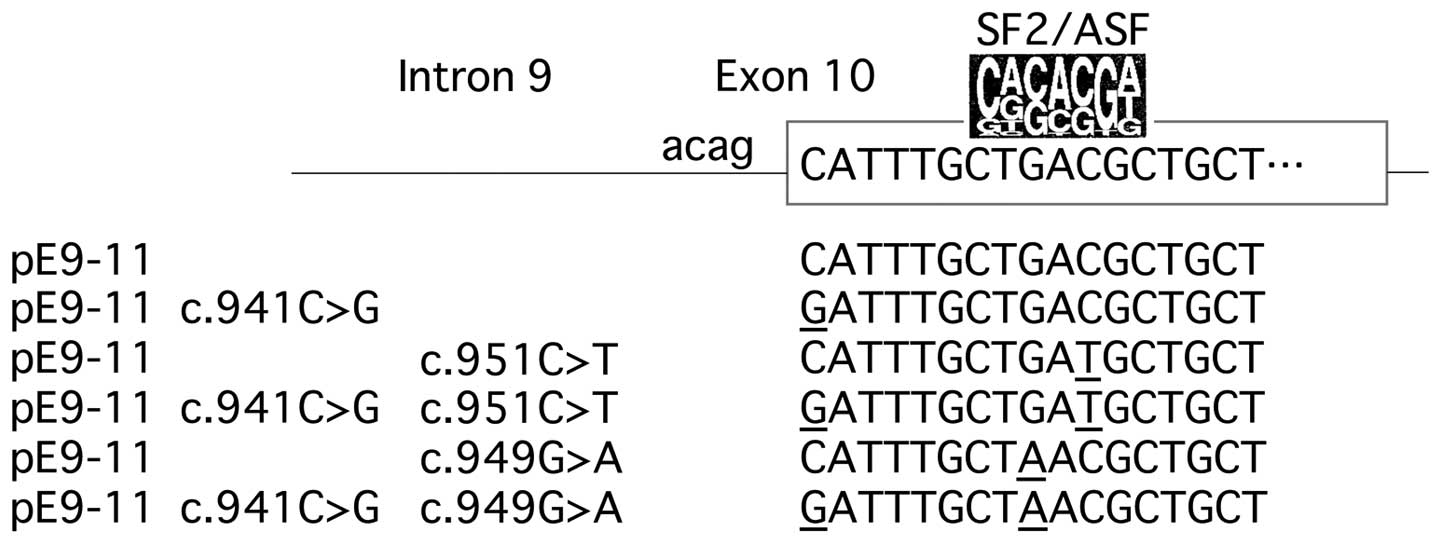
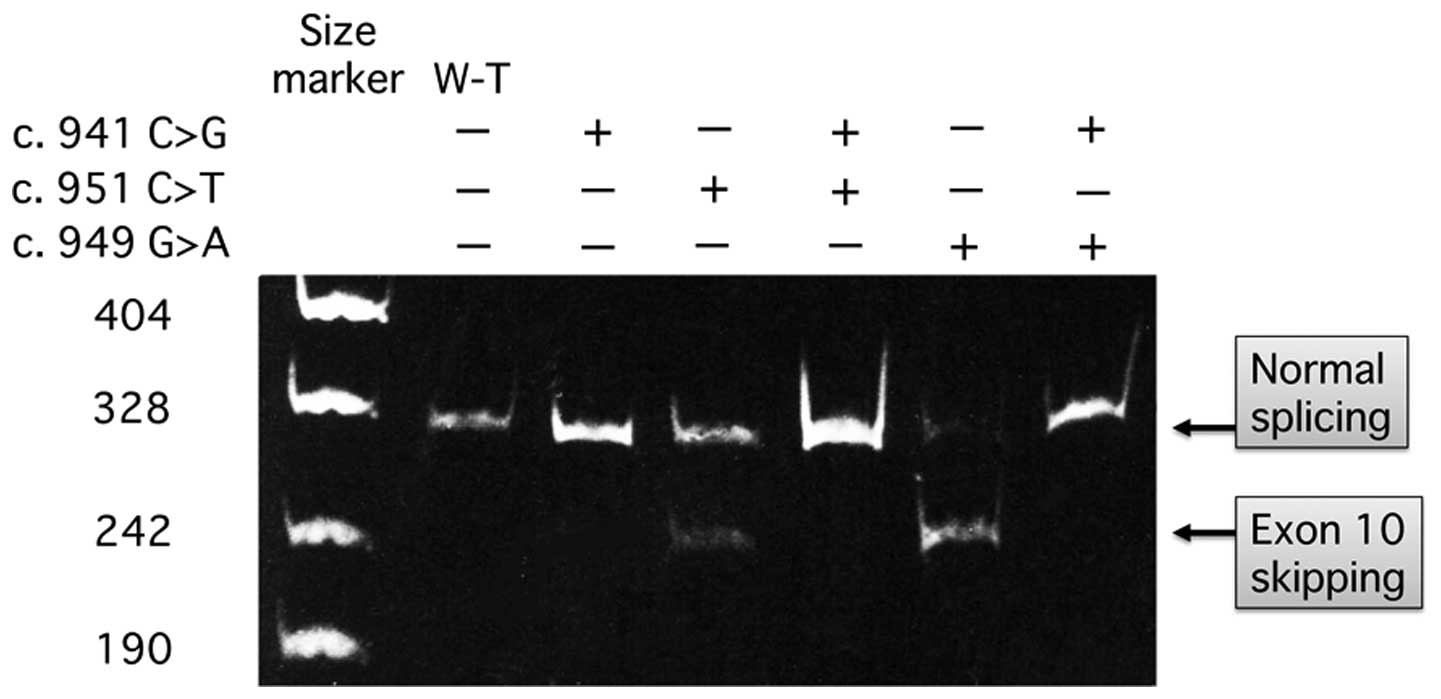
Fukao T, Horikawa R, Naiki Y, Tanaka T,
Takayanagi M, Yamaguchi S and Kondo N: A novel mutation
(c.951C>T) in an exonic splicing enhancer results in exon 10
skipping in the human mitochondrial acetoacetyl-CoA thiolase gene.
Mol Genet Metab. 100:339–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fukao T, Nakamura H, Song XQ, Nakamura K,
Orii KE, Kohno Y, Kano M, Yamaguchi S, Hashimoto T, Orii T and
Kondo N: Characterization of N93S, I312T, and A333P missense
mutations in two Japanese families with mitochondrial
acetoacetyl-CoA thiolase deficiency. Hum Mutat. 12:245–254. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fukao T, Boneh A, Aoki Y and Kondo N: A
novel single-base substitution (c.1124A>G) that activates a
5-base upstream cryptic splice donor site within exon 11 in the
human mitochondrial acetoacetyl-CoA thiolase gene. Mol Genet Metab.
94:417–421. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Watanabe H, Orii KE, Fukao T, Song XQ,
Aoyama T, IJlst L, Ruiter J, Wanders RJ and Kondo N: Molecular
basis of very long chain acyl-CoA dehydrogenase deficiency in three
Israeli patients: Identification of a complex mutant allele with
P65L and K247Q mutations, the former being an exonic mutation
causing exon 3 skipping. Hum Mutat. 15:430–438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niwa H, Yamamura K and Miyazaki J:
Efficient selection for high-expression transfectants with a novel
eukaryotic vector. Gene. 108:193–199. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang GX, Fukao T, Rolland MO, Zabot MT,
Renom G, Touma E, Kondo M, Matsuo N and Kondo N: Mitochondrial
acetoacetyl-CoA thiolase (T2) deficiency: T2-deficient patients
with ‘mild’ mutation were previously misinterpreted as normal by
the coupled assay with tiglyl-CoA. Pediatr Res. 56:60–64. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goldstrohm AC, Greenleaf AL and
Garcia-Blanco MA: Co-transcriptional splicing of pre-messenger
RNAs: Considerations for the mechanism of alternative splicing.
Gene. 277:31–47. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cooper TA and Mattox W: The regulation of
splice-site selection, and its role in human disease. Am J Hum
Genet. 61:259–266. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Robberson BL, Cote GJ and Berget SM: Exon
definition may facilitate splice site selection in RNAs with
multiple exons. Mol Cell Biol. 10:84–94. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shapiro MB and Senapathy P: RNA splice
junctions of different classes of eukaryotes: Sequence statistics
and functional implications in gene expression. Nucleic Acids Res.
15:7155–7174. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin S and Fu XD: SR proteins and related
factors in alternative splicing. Adv Exp Med Biol. 623:107–122.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Caceres EF and Hurst LD: The evolution,
impact and properties of exonic splice enhancers. Genome Biol.
14:R1432013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cartegni L, Hastings ML, Calarco JA, de
Stanchina E and Krainer AR: Determinants of exon 7 splicing in the
spinal muscular atrophy genes, SMN1 and SMN2. Am J Hum Genet.
78:63–77. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singh NN, Androphy EJ and Singh RN: An
extended inhibitory context causes skipping of exon 7 of SMN2 in
spinal muscular atrophy. Biochem Biophys Res Commun. 315:381–388.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakamura K, Fukao T, Perez-Cerda C, Luque
C, Song XQ, Naiki Y, Kohno Y, Ugarte M and Kondo N: A novel
single-base substitution (380C>T) that activates a 5-base
downstream cryptic splice-acceptor site within exon 5 in almost all
transcripts in the human mitochondrial acetoacetyl-CoA thiolase
gene. Mol Genet Metab. 72:115–121. 2001. View Article : Google Scholar : PubMed/NCBI
|