Introduction
Vulnerable atherosclerotic plaques are composed of a
lipid-rich necrotic core covered by a thin fibrous cap
predominantly comprised of smooth muscle cells (SMCs) and
structural collagen, and such plaques are infiltrated with
inflammatory cells (1).
Inflammation has an important function in lesion destabilization
and rupture, and macrophages are the major cellular components of
vulnerable plaques, which are responsible for releasing numerous
inflammatory cytokines, thus contributing to the progression of
plaque vulnerability (2).
Macrophages that infiltrate the plaques synthesize several
proteolytic enzymes that are responsible for degrading lesion
structure constituents. One such family of enzymes, matrix
metalloproteinases (MMPs), efficiently degrade extracellular matrix
(ECM) proteins (3). MMP-2, −3, and
−9, in particular, degrade basement collagens, including elastin,
laminin and gelatin, and have been implicated in the weakening of
the fibrous cap (4,5). Macrophages can weaken advanced
plaques by secreting proteases, primarily MMPs, which digest the
ECM and collagen that provide the architectural structure and
physical strength of the cap. Furthermore, studies have
demonstrated that MMPs are distributed throughout the vulnerable
regions and that their activities are significantly higher in
unstable carotid plaques compared with the stable lesions (6–8).
Thus, control of MMP activation is a potential therapeutic approach
for targeting advanced plaques.
In the advanced stage of atherosclerosis, the
receptor of advanced glycation end products (RAGE)-dependent
signaling pathway is critical for chemokine and adhesion molecule
generation, and also acts as an amplified step/amplifier for
inflammatory processes (9).
Studies have suggested that the ligation of RAGE and its ligands,
including oxidized low-density lipoprotein and advanced glycation
end products, may activate mitogen-activated protein kinases
(MAPKs) and nuclear factor-κB (NF-κB) to result in MMP
upregulation, which accelerates erosion and thinning of the fibrous
caps, and is responsible for plaque instability (10,11).
Additionally, in macrophages, the important consequences of
RAGE-dependent signaling include activation of NADPH-oxidase and
MAPKs, which elevates the generation of reactive oxygen species
(ROS), pro-inflammatory factors and RAGE itself, which further
promotes this signal transduction, thus creating a positive
feedback loop (12,13).
Furthermore, RAGE can induce the sustained
activation of NF-κB, the fundamental transcriptional factor of the
inflammatory response, by stimulating the degradation of NF-κB
inhibitor α (IκBα) and synthesis of NF-κB p65, thus, ensuring a
prolonged inflammatory response (14). Therefore, RAGE is considered to be
a link between pro-inflammatory cytokine release and
atherosclerosis initiation. Furthermore, oxidative stress leads to
endothelial cell injury and vascular inflammation expansion by
direct activation of the redox-sensitive transcription nuclear
factor NF-κB via several mechanisms (15). Thus, the vicious circle between ROS
and sustained activation of the RAGE axis perpetuates the damaging
effects caused by chronic inflammation within the dysfunctional
vascular wall. Therefore, suppression of ROS generation and RAGE
expression may be a useful anti-atherosclerotic target.
Tanshinone II A (TSIIA) is the main lipid soluble
active ingredient of Salvia miltiorrhiza, a traditional
Chinese herb, commonly used for the prevention and treatment of
cardiovascular diseases, including atherosclerosis. Numerous
studies in animal models and human patients have been demonstrated
that TSIIA is an effective free-radical scavenger with anti-oxidant
and anti-inflammatory properties, and is able to suppress the
expression of adhesion molecules and chemokines. However, the
precise mechanism of action of TSIIA on vulnerable atherosclerotic
plaque stability has not been elucidated (16,17).
The present study presents evidence that TSIIA inhibits the
progression of advanced lesions via anti-oxidant/anti-inflammatory
biological properties. TSIIA inhibits the expression RAGE and MAPK
signaling pathway proteins, and NF-κB activation, leading to
reduced expression of pro-inflammatory factors, including vascular
cellular adhesion molecule-1 (VCAM-1), intercellular adhesion
molecule-1 (ICAM-1) and MMPs in the advanced lesions of
apolipoprotein E (apoE)−/− mice, which are the most used
model mice for atherosclerosis due to their plasma cholesterol,
triglycerides, chylomicron and very-low-density lipoprotein levels
that are ~5–10times than that of control mice. In addition,
apoE−/− mice rapidly and easily develop the formation of
advanced plaques (18,19).
Materials and methods
Preparation of animals
Eight-week-old male apoE−/− mice with a
C57BL/6 J background were obtained from Peking University Health
Science Center [purchased from Jackson Laboratory (Ben Harbor, ME,
USA)]. The mice (n=16) were fed a high-fat,
cholesterol-rich/atherogenic diet containing 21% fat, 19.5% casein,
and 1.25% cholesterol for 8 weeks, and housed at 20–24°C and 45–55%
humidity with a 12-h light-dark cycle. The mice were divided into a
TSIIA group (n=8) and a control group (n=8). In the TSIIA group,
TSIIA was dissolved in distilled water and administered daily by
oral garage at a dose of 30 mg/kg for 8 weeks, and in the control
group equal volumes of distilled water were used as described
previously (17). TSIIA extracted
from the roots of Salvia miltiorrhiza was purchased from
Guangxi Wuzhou Pharmaceutical Group Co., Ltd. (Wuzhou, China). The
chemical purity of TSIIA was ~97%. All mice were anesthetized by
intraperitoneal injection with sodium pentobarbital (0.055
mg·kg−1) and dissected longitudinally, removing the
heart, brachiocephalic arteries (BCA), descending arteries and
blood. Blood samples were collected from the mice for the
measurement of plasma glucose and lipid levels. All animal
protocols were approved by the animal ethics committee of the
China-Japan Friendship Hospital (Beijing, China).
En face analysis of the descending
aortas
Six descending aortas of each group were subjected
to en face lipid staining. The aortas were dissected from
the left subclavian artery to the iliac bifurcation and
subsequently opened longitudinally and stained with Oil Red O for
10 min to visualize the extent of the lipid deposition.
Quantitative analysis of lesion size was performed by capturing
images of the aorta using a digital camera (DXC-960MD; Sony
Corporation, Tokyo, Japan), and then the data were analyzed using
Image Pro Plus 6 software. Firstly, the bar was calibrated and the
positive areas of immunohistochemical and Sirius Red stains were
selected and counted precisely, then the data were outputted.
Quantification of atherosclerotic
lesions in the brachiocephalic artery
The BCA were dissected and fixed overnight in 4%
polymerized formaldehyde, embedded in paraffin, and sectioned to
5-µm thick as described previously (20). Every sixth section was stained with
the modified Movat pentachrome stain (21) and Sirius Red (22). The atherosclerotic lesions were
analyzed using Image Pro Plus 6 software (Media Cybernetics, Inc.,
Rockville, MD, USA).
Immunohistochemical staining
Every sixth section from the BCA and aortic root was
subjected to immunohistochemical analysis to identify macrophages
and SMCs, and to measure MAC-3 (CD107b) and α-actin expression.
Briefly, the sections were blocked by normal goat serum for 30 min
at room temperature and then incubated with polyclonal antibodies
at 37°C for 60 min or at 4°C overnight and then with horseradish
peroxidase (HRP)-conjugated anti-rabbit immunoglobulin G (IgG) at
37°C for 60 min. Finally, the coverslips were mounted with
1,4-diazabicyclo[2.2.2]octane and analyzed using an upright
fluorescent microscope (Carl Zeiss AG, Oberkochen, Germany).
Antibodies against α-actin (sc-32763; 1:100 dilution) was purchased
from Santa Cruz Biotechnology Inc. (Dallas, TX, USA) and MAC-3, a
macrophage marker (550292; 1:60 dilution), was purchased from BD
Bioscience (Franklin Lakes, NJ, USA) and the HRP-conjugated
antibody (PV6000) was purchased from Beijing OriGene Technologies,
Inc. (Beijing, China). The bar was calibrated and the positive
areas of immunohistochemical and Sirius Red stains were selected
and counted precisely, then the data were outputted.
Polarization microscopy
Subsequent to staining with picrosirius red, the
sections were imaged by using standard bright-field imaging in
addition to polarized light microscopy according to the certified
protocols. Firstly, a white light source and two polarizing filters
were added (Carl Zeiss AG). The light passed through a polarizer,
the sample and an analyzer. The birefringent material, particularly
collagen, changes the polarization status of the light, resulting
in increases or reductions of the light intensity observed,
depending on the relative orientation through the sample and the
filters (23).
Western blotting
The descending arteries were dissected and subjected
to western blotting for protein level analysis. The arteries mixed
with RIPA were ground in a homogenizer on ice to form homogeneous
lysates, then were centrifuged at 14,000 × g for 10 min at
4°C. The supernatants were removed from the cellular debris and
protein concentration was determined using the Bicinchoninic Acid
Protein Assay kit (Beyotime Institute of Biotechnology, Inc.,
Nanjing, China) (24). The lysates
(10–30 µg of protein) were separated by using 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, transferred to
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA), blocked with 5% nonfat dry milk for 60 min, and probed with
antibodies at 4°C overnight. The blots were incubated with
HRP-conjugated anti-IgG for 1 h at 37°C, followed by detection with
electrochemiluminescence reagents (Santa Cruz Biotechnology, Inc.).
Antibodies against IκBα (sc-847; 1:1,000 dilution), phospho-IκBα
(sc-7977; Ser32; 1:500 dilution), MMP-2 (sc-10736; 1:500 dilution),
MMP-3 (sc-31074; 1:500 dilution), VCAM-1 (sc-8304; 1:1,000
dilution), β-actin (sc-130656; 1:1,000 dilution), galectin-3
(sc-20157; 1:3,000 dilution) and MCP-1 (sc-28879; 1:1,000 dilution)
were purchased from Santa Cruz Biotechnology, Inc. The antibodies
against RAGE (SAB1401326; 1:500 dilution) and MMP-9 (AV33090;
1:1,000 dilution) were purchased from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany). Antibodies against phospho-JNK
(#4668; Thr183/Tyr185; 1:1,000 dilution), JNK (#9252; 1:1,000
dilution), phospho-p38 (#4511; Thr180/Tyr182; 1:1,000 dilution),
p38 (#8690; 1:1,000 dilution), phospho-ERK1/2 (#4370;
Thr202/Tyr204; 1:1,000 dilution), ERK1/2 (#4695; 1:1,000 dilution),
NF-κB p65 (#8242; 1:1,000 dilution), and phospho-NF-κB (#3033;
Ser536; 1:1,000 dilution) were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA), while antibody to CD68 (FA-11;
1:1,000 dilution) was purchased from AbD Serotec (Raleigh, NC,
USA). In order to quantify the arterial protein levels,
densitometry analysis data were obtained using ImageJ software,
version 1.49 (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
All values are represented as the mean ± standard
error of the indicated number of measurements. Unpaired Student's
t-tests and analysis of variance for repeated measures were
conducted in SigmaPlot software, version 13.0 (Systat Software,
Inc. San Jose, CA, USA), and P<0.05 was considered to indicate a
statistically significant difference.
Results
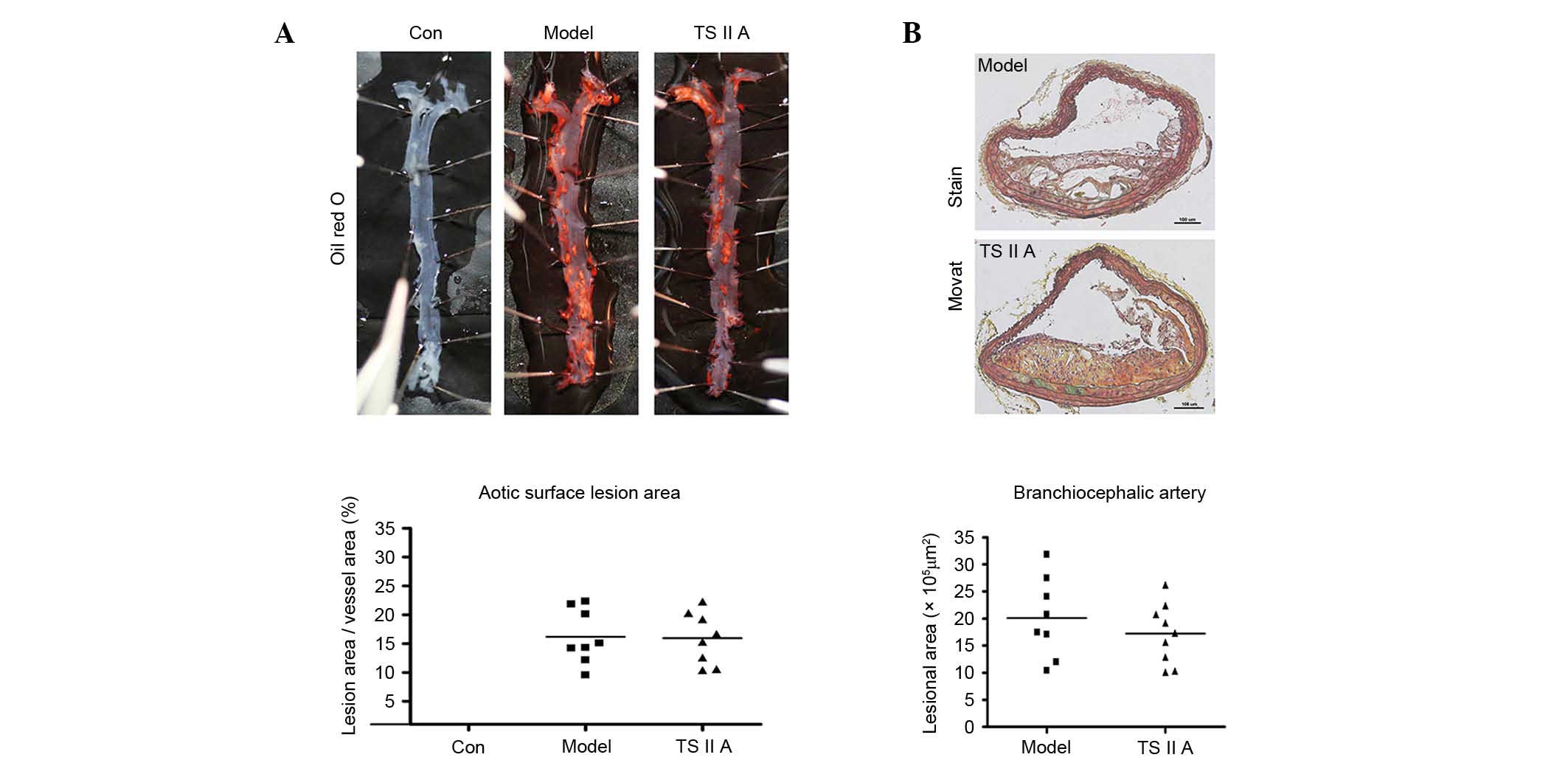
TSIIA exhibits no effect on
atherosclerotic lesion size in apoE−/− mice
The effect of TSIIA on atherosclerotic lesion size
was examined in apoE−/− mice. The descending aortic
lesion areas were measured according to quantitative
histomorphology of Oil Red O-stained en face specimens.
However, no significant decrease in the percentage of aortic area
following TSIIA treatment was detected (Fig. 1A). Similarly, no statistical
difference in BCA lesion area was observed between the TSIIA and
model groups by Movat staining (Fig.
1B). These results indicate that TSIIA failed to reduce
vulnerable lesion size.
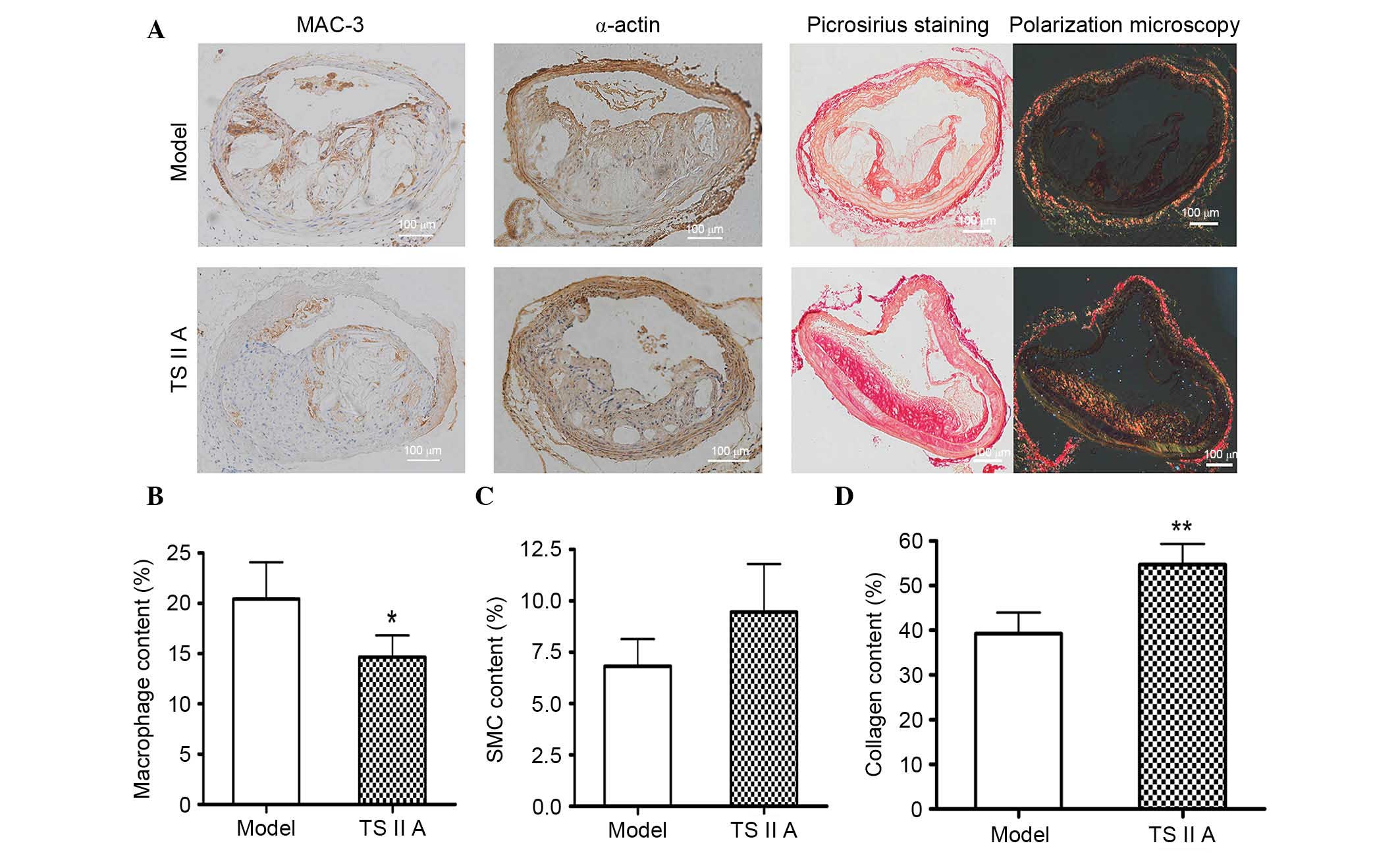
TSIIA alters the vulnerable lesion
composition within the BCA of apoE−/− mice
Lesion composition, rather than size, predominantly
determines the stability of vulnerable plaques; therefore, the
architectural composition, including macrophages, SMCs, and
collagen, was evaluated in the plaques by histological staining.
Staining for MAC-3, macrophage marker, demonstrated that the number
of macrophages was decreased following TSIIA administration
compared with the model control group (P<0.05; Fig. 2A and B). Compared with the model
group, SMC (Fig. 2C) and collagen
contents (P<0.01; Fig. 2D) were
higher in plaques of the BCA following TSIIA treatment, as
demonstrated by immunohistochemical and Sirius Red staining.
Furthermore, SMCs were predominantly parallel with the collagen and
distributed in the fibrous cap (Fig.
2A). All of these factors facilitate lesion stability.
TSIIA suppresses NF-κB activation and
downregulates inflammatory factor expression
TSIIA has been previously demonstrated to exert both
anti-oxidant and anti-inflammatory activities. Inflammatory
factors, particularly VCAM-1, ICAM-1 and monocyte chemotactic
protein-1 (MCP-1), are required for inflammatory responses, which
are responsible for the accumulation of macrophages into
atheroma-prone areas. Thus, the expression of several inflammatory
factors, and CD68 and NF-κB accumulation were measured in the
descending arteries and sections of the aortic root using western
blotting.
As demonstrated in Fig.
3, TSIIA administration led to a significant decrease in the
macrophage markers galectin-3 (P<0.05) and CD68 (P<0.05)
compared with the control group (Fig.
3A). VCAM-1 (P<0.05), ICAM-1 (P<0.05) and MCP-1
(P<0.01) expression levels were also decreased by TSIIA compared
with the control group, demonstrating the anti-inflammatory
activity of TSIIA (Fig. 3B).
Subsequently, the activation of NF-κB, the major transcriptional
factor responsible for inflammatory factor expression, and IκB, its
main inhibitor, were examined. NF-κB binds to IκB to form a
complex, which restricts NF-κB to the cytoplasm. Phosphorylation of
IκB results in its ubiquitination and degradation, and the
subsequent release of NF-κB to the nucleus (25,26).
The results of the present study demonstrated that, compared with
the model group, the phosphorylation of IкB (P<0.05) and NF-κB
(P<0.05) were decreased, and the total level of IкB was
increased (P<0.05) in the descending arteries of
apoE−/− mice treated with TSIIA (Fig. 3C), indicating that the
anti-inflammatory abilities of TSIIA, at least partially, depend on
the inhibition of NF-κB activation.
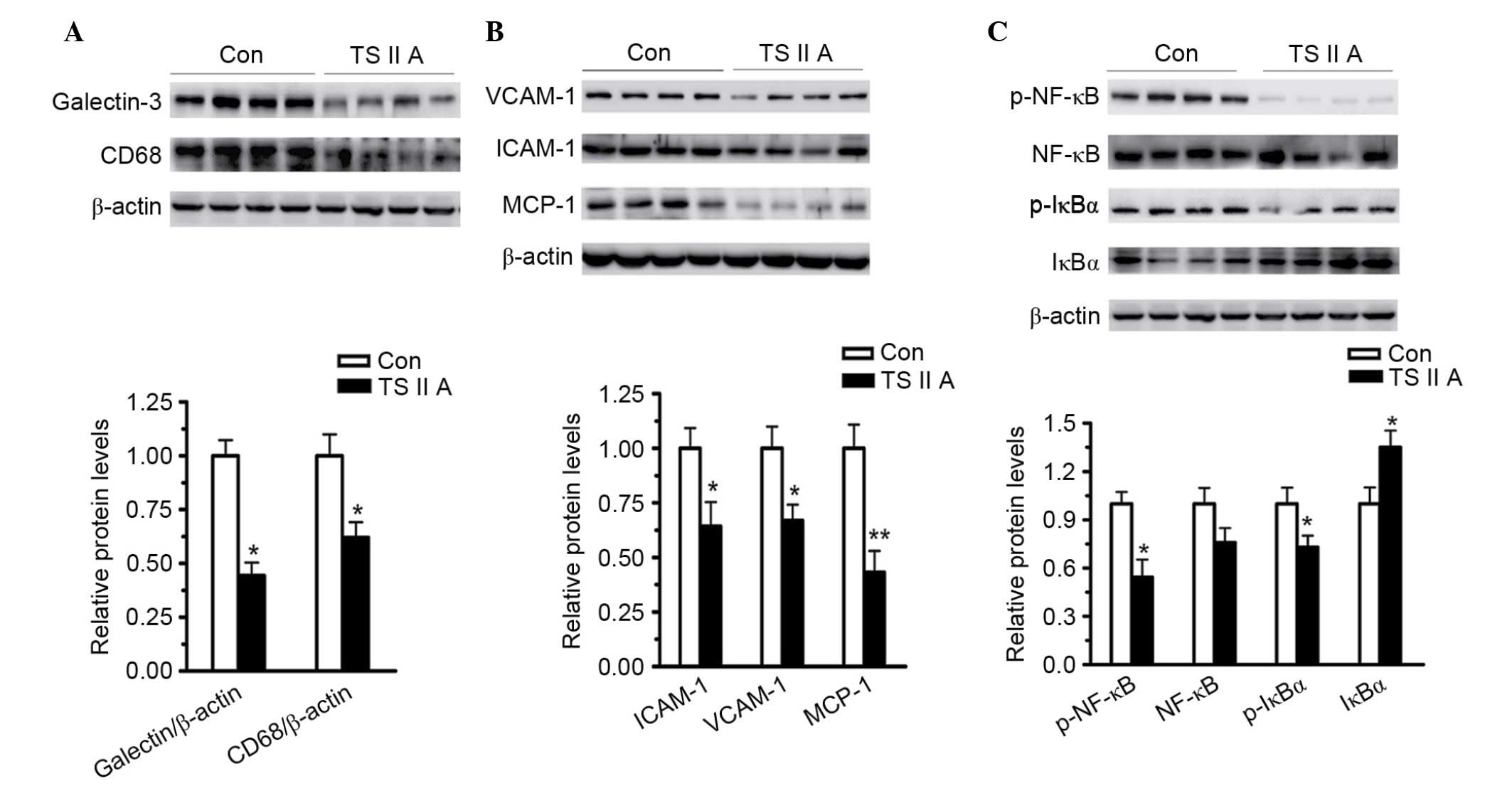 | Figure 3.TSIIA suppresses NF-κB activation and
downregulates inflammatory factors, adhesion molecules and MCP-1.
(A) Expression levels of macrophage markers, galectin-3 and CD68,
in descending arteries of apoE−/− mice was detected by
western blotting (P=0.0157 and P=0.0274). (B) Expression levels of
VCAM-1, ICAM-1 and MCP-1 in the descending arteries were detected
using western blotting (P=0.0218, 0.0223 and 0.0018). (C) NF-κB
activation in the descending arteries was analyzed by measuring
NF-κB, p-NF-κB, IκBα, and p-IκBα levels (P=0.2743, 0.0234, 0.00428
and 0.034). Data represent the mean ± standard error (n=4).
*P<0.05, **P<0.01 vs. con. Con, control; TSIIA, Tanshinone II
A; VCAM-1, vascular cell adhesion molecule; ICAM-1, intercellular
adhesion molecule; MCP-1, monocyte chemoattractant protein-1; p-,
phosphorylated; NF-κB, nuclear factor-κB; IκBα, NF-κB inhibitor
α. |
TSIIA decreases MMP-2, −3 and −9
expression by suppressing RAGE and activating the MAPK signaling
pathway
The present study also investigated the mechanisms
underlying elevated collagen content in the plaques from BCA in
apoE−/− mice administered with TSIIA. MMP-2, −3 and −9
are critical enzymes for collagen degradation. Western blotting
revealed that TSIIA treatment reduced MMP-2, −3 and −9 expression
in the descending arteries compared with control levels (P<0.05;
Fig. 4A). These results indicate
that TSIIA-elevated collagen content be caused by downregulation of
MMPs in the vulnerable plaques. Furthermore, previous studies have
indicated that RAGE and the MAPK signaling pathway are responsible
for NF-κB activation and MMP expression. The expression levels of
MMPs, RAGE and phospho-MAPK protein in the descending arteries were
determined using western blotting. Phosphorylation activates JNK,
ERK1/2 and p38, and western blotting revealed that TSIIA
administration reduced, but did not abolish, the phosphorylation of
JNK, ERK1/2 and p38 compared with control levels (Fig. 4B). Furthermore, the expression
levels of RAGE were assayed using western blotting, and the results
demonstrated that TSIIA administration reduced the RAGE expression
levels compared with control levels (P<0.01; Fig. 4C). Taken together, these results
suggest that TSIIA decreased MMP and RAGE expression and suppressed
MAPK signaling pathway activation, which may have resulted in
vulnerable plaque stabilization.
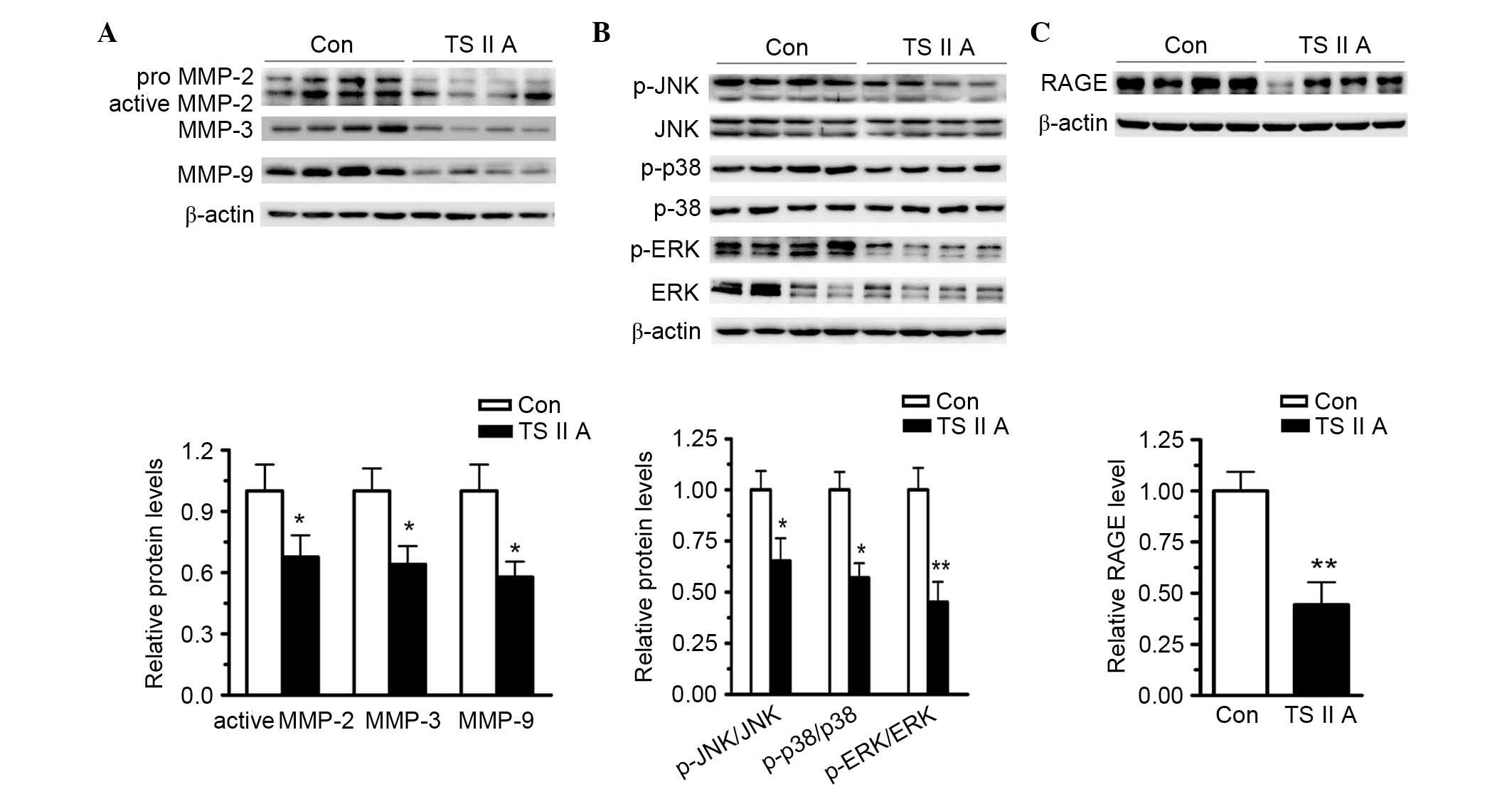 | Figure 4.TSIIA decreases the expression of
MMP-2, −3, and −9, and RAGE, and inhibits the activation of
proteins involved in the MAPK signaling pathways. (A) TSIIA
suppressed active MMP-2, −3, and −9, and RAGE expression in the
descending arteries (P=0.0351, 0.0292 and 0.0127). (B) Activations
of ERK1/2, JNK and p38 were determined by the analysis of ERK1/2,
p-ERK1/2, JNK, p-JNK, p38, and p-p38 protein levels (P=0.0022,
0.0233 and 0.0199). (C) TSIIA inhibited RAGE expression (P=0.0051).
Data represent the mean ± standard error (n=4). *P<0.05,
**P<0.01 vs. con. Con, control; TSIIA, Tanshinone II A; MMP,
matrix metalloproteinase; p-, phosphorylated; JNK, c-Jun N-terminal
kinase; ERK, extracellular signal-regulated kinase; RAGE, receptor
for advanced glycation endproducts. |
Discussion
The present study presented experimental evidence
that TSIIA stabilizes vulnerable atherosclerotic lesions in
apoE−/− mice. In particular, the results demonstrated:
i) The inhibitory effects of TSIIA on MMP expression and
activation; ii) the association between TSIIA-induced suppression
of NF-κB, VCAM-1, ICAM-1 and MCP-1, and reduced atherosclerotic
plaque inflammation; and iii) the TSIIA-induced suppression of RAGE
and downstream MAPK signaling pathways.
Morphological analysis has indicated that vulnerable
plaque stability is more heavily dependent on plaque composition
than the degree of stenosis. Unstable lesions are characterized by
a distinct lipid necrotic core under the weakened fibrous cap,
which protects the necrotic core from physical stress and
inflammatory cell invasion (1,2). ECM
components, collagen and elastin in particular, constitute an
architecture that maintains fibrous cap integrity; thus, the
balance between the synthesis and degradation of these matrix
components is responsible for plaque vulnerability (27). Macrophages provide the major
inflammatory factor source in vulnerable lesions and are thought to
control this balance due to their secretion of enzymes that digest
the ECM, particularly the MMP family (28).
How do macrophages contribute to remodeling unstable
lesions? A series of studies revealed the tight correlation between
macrophage infiltration in rupture-prone atheroma, such as the
vulnerable shoulder areas, and the thinning of the fibrous cap and
local MMP activation and accumulation, particularly MMP-2, 3 and 9
and collagenases that degrade collagen (5,29).
The MMP-induced digestion of collagen and other ECM components
weakens the protective caps and provides a path for the migration
of inflamed cells from the circulation, and furthermore,
facilitates the release of matrix-bound bioactive molecules,
including fibroblast growth factor-2 and transforming growth
factor-β (30). Therefore,
suppressing MMP activity may be an effective treatment for
atherosclerosis. In human and animal models of atherosclerosis,
levels of various MMPs, including MMP-2 −3, −7, −9 and −12, are
increased in advanced plaques. In fact, abundant MMPs have been
identified in vulnerable regions of human plaques (28,31).
Evidence from human epidemiological and genetic experiments
revealed that MMP-9 is most closely associated with plaque
instability and clinical manifestations. Only activated
macrophage-secreted MMP-9 is sufficient to induce the rupture of
unstable lesions atherosclerotic mice, and MMP-9-induced
proteolysis is central to the rupture of human plaques (4,30).
Macrophages also secret MMP-2, which degrades
collagen within the fibrous caps of human advanced plaques
(32,33). Silence et al (5) demonstrated that MMP-3 destabilizes
plaques by degrading matrix components, and also enhances
macrophage accumulation and migration in the lesions, potentially
by secreting urokinase and plasmin, critical activators of
macrophage-secreted pro-MMPs, and causes the digestion of elastin
and collagen. Furthermore, MMP-3 has similar proteolytic abilities
to those of MMP-9, but also may directly determine MMP-9 and MMP-2
activities. The current study demonstrated that MMP-2, −3 and −9
were reduced and collagen was increased in vulnerable lesions in
apoE−/− mice administered with TSIIA, indicating that
increased collagen content following TSIIA treatment is associated
with resistance to degradation by proteolytic enzymes, particularly
the MMPs, major enzymes of the ECM digestion process. However,
TSIIA decreased MMP levels (Fig.
4A) were accompanied by reduced macrophage accumulation
(Fig. 2B) using its inflammatory
inhibition abilities. ECM is primarily synthesized by SMCs within
the plaques. The present study demonstrated that the number of SMCs
in the plaques was increased by TSIIA treatment, although the
difference between the treatment and control groups was not
significant (Fig. 2C). As SMCs are
the only cellular source of collagen, TSIIA stabilizes the advanced
plaques, at least partially, by increasing the number of SMCs
within the plaques.
Adhesion molecules and chemokines, including ICAM-1,
VCMA-1 and MCP-1, are important cytokines that determine macrophage
infiltration and accumulation (34). The activation of the
redox-sensitive transcription, NF-κB, leads to directly increased
expression pro-inflammatory cytokines and adhesion molecules. In
atherosclerotic conditions, NF-κB is a well-known transcription
factor with an important role in regulating the expression of
inflammatory factors, including cytokines, chemokines, adhesion
molecules and MMPs, in macrophages (35). In resting cells, the NF-κB dimer is
located in the cytoplasm, bound to its inhibitory protein, IκB.
Activation of IκB kinase, which induces IκB phosphorylation and
facilitates its ubiquitination and degradation, results in NF-κB
activation. Activated NF-κB then translocates to the nucleus, which
results in its binding with target DNA (particularly genes that
encoded pro-inflammatory factors) or cooperation with other
transcription regulatory factors (25,26).
Previous studies have demonstrated that various atherogenic
stimuli, including oxidant stress, inflammatory responses and
smoking, activate the NF-κB dimer; therefore, controlling NF-κB
activation is considered to be important for estimating the
therapeutic abilities of anti-atherogenic drugs. In the present
study, NF-κB and IκB activations were evaluated following TSIIA
treatment. TSIIA decreased NF-κB activation, increased IκB levels,
and reduced the expression of pro-inflammatory factors (Fig. 3). Thus, the inhibitory effects of
TSIIA on NF-κB activity may partially benefit from its effective
radical clearance abilities. The antioxidant abilities of TSIIA
were identified in earlier studies (36,37).
RAGE and downstream MAPK signaling is significantly
elevated in atherosclerosis, and this pathway is associated with
NF-κB activation, inflammatory mediator generation and oxidative
stress in macrophages. Furthermore, RAGE has the unique ability to
sustain NF-κB activation through de novo synthesis of NF-κB
p65 mRNA and provide a constantly growing pool of transcriptionally
active NF-κB (38). Studies have
demonstrated that RAGE overexpression is associated with enhanced
inflammatory reactions within lesion macrophages and that this
effect may contribute to plaque destabilization by inducing MMP
expression (39). Furthermore,
RAGE may destabilize the atherosclerotic plaques by inducing MAPK-
and prostaglandin E2-dependent MMP-2 and MMP-9 production (40,41).
In a previous report, RAGE knockout or blockade in
apoE−/− mice resulted in decreased expression of MMP and
other inflammatory cytokines (10,42).
MAPKs are conserved serine/threonine kinases that are part of a
coordinated and integrated signal pathway that responds to diverse
atherosclerotic stimuli, oxidative damage and inflammatory
mediators/responses. Furthermore, numerous studies identified that
the action of ERK and other members of the MAPK family, including
p38 and JNK, subsequently activates activator protein-1 and NF-κB,
and are thus pivotal for the induction of MMPs and other
inflammatory factors in macrophages (43–45).
Findings of several studies have indicated that the activation of
ERK1/2, JNK and p38 are important signaling mechanisms responsible
for the expression of inflammatory factors, including ICAM-1,
VCAM-1, and MCP-1, within macrophages when RAGE is activated in
vivo or in vitro (46,47).
The present study identified the inhibitory effects of TSIIA on the
activation of MAPK and RAGE signals, and the suppression of MMP2, 3
and 9 (Fig. 4) and inflammatory
factors, including ICAM-1, VCAM-1 and MCP-1 (Fig. 3) following TSIIA treatment.
In conclusion, the current study provides novel data
to support the hypothesis that TSIIA administration may stabilize
vulnerable plaques, primarily due to its anti-inflammatory effects,
suppression of NF-κB, MAPK proteins phosphorylation and the RAGE
axis, subsequently leading to decreased MMPs-induced matrix
degradation and inflammatory factor expression in
apoE−/− mice.
References
|
1
|
Burke AP, Kolodgie FD, Farb A, Weber D and
Virmani R: Morphological predictors of arterial remodeling in
coronary atherosclerosis. Circulation. 105:297–303. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stoll G and Bendszus M: Inflammation and
atherosclerosis: Novel insights into plaque formation and
destabilization. Stroke. 37:1923–1932. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Newby AC, George SJ, Ismail Y, Johnson JL,
Sala-Newby GB and Thomas AC: Vulnerable atherosclerotic plaque
metalloproteinases and foam cell phenotypes. Thromb Haemost.
101:1006–1011. 2009.PubMed/NCBI
|
|
4
|
Gough PJ, Gomez IG, Wille PT and Raines
EW: Macrophage expression of active MMP-9 induces acute plaque
disruption in apoE-deficient mice. J Clin Invest. 116:59–69. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Silence J, Lupu F, Collen D and Lijnen HR:
Persistence of atherosclerotic plaque but reduced aneurysm
formation in mice with stromelysin-1 (MMP-3) gene inactivation.
Arterioscler Thromb Vasc Biol. 21:1440–1445. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bäck M, Ketelhuth DF and Agewall S: Matrix
metalloproteinases in atherothrombosis. Prog Cardiovasc Dis.
52:410–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Toutouzas K, Synetos A, Nikolaou C,
Tsiamis E, Tousoulis D and Stefanadis C: Matrix metalloproteinases
and vulnerable atheromatous plaque. Curr Top Med Chem.
12:1166–1180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berg G, Miksztowicz V and Schreier L:
Metalloproteinases in metabolic syndrome. Clin Chim Acta.
412:1731–1739. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun L, Ishida T, Yasuda T, Kojima Y, Honjo
T, Yamamoto Y, Yamamoto H, Ishibashi S, Hirata K and Hayashi Y:
RAGE mediates oxidized LDL-induced pro-inflammatory effects and
atherosclerosis in non-diabetic LDL receptor-deficient mice.
Cardiovasc Res. 82:371–381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harja E, Bu DX, Hudson BI, Chang JS, Shen
X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, et al:
Vascular and inflammatory stresses mediate atherosclerosis via RAGE
and its ligands in apoE−/− mice. J Clin Invest.
118:183–194. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang F, Banker G, Liu X, Suwanabol PA,
Lengfeld J, Yamanouchi D, Kent KC and Liu B: The novel function of
advanced glycation end products in regulation of MMP-9 production.
J Surg Res. 171:871–876. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goldin A, Beckman JA, Schmidt AM and
Creager MA: Advanced glycation end products: Sparking the
development of diabetic vascular injury. Circulation. 114:597–605.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoefen RJ and Berk BC: The role of MAP
kinases in endothelial activation. Vascul Pharmacol. 38:271–273.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bierhaus A, Schiekofer S, Schwaninger M,
Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Klöting
I, et al: Diabetes-associated sustained activation of the
transcription factor nuclear factor-kappaB. Diabetes. 50:2792–2808.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kutuk O and Basaga H: Inflammation meets
oxidation: NF-kappaB as a mediator of initial lesion development in
atherosclerosis. Trends Mol Med. 9:549–557. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang FT, Cao Y, Wang TQ, Wang LJ, Guo J,
Zhou XS, Xu SW, Liu WH, Liu PQ and Huang HQ: Tanshinone IIA
attenuates atherosclerosis in ApoE (−/−) mice through
down-regulation of scavenger receptor expression. Eur J Pharmacol.
650:275–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu S, Little PJ, Lan T, Huang Y, Le K, Wu
X, Shen X, Huang H, Cai Y, Tang F, et al: Tanshinone II-A
attenuates and stabilizes atherosclerotic plaques in
apolipoprotein-E knockout mice fed a high cholesterol diet. Arch
Biochem Biophys. 515:72–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith JD and Breslow JL: The emergence of
mouse models of atherosclerosis and their relevance to clinical
research. J Intern Med. 242:99–109. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Neill TP: Apolipoprotein E-deficient
mouse model of human atherosclerosis. Toxicol Pathol. 25:20–1.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Basta G, Schmidt AM and De Caterina R:
Advanced glycation end products and vascular inflammation:
Implications for accelerated atherosclerosis in diabetes.
Cardiovasc Res. 63:582–592. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garvey W, Fathi A, Bigelow F, Carpenter B
and Jimenez C: Improved Movat pentachrome stain. Stain Technol.
61:60–62. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Llewellyn BD: An improved Sirius red
method for amyloid. J Med Lab Technol. 27:308–309. 1970.PubMed/NCBI
|
|
23
|
Jan NJ, Grimm JL, Tran H, Lathrop KL,
Wollstein G, Bilonick RA, Ishikawa H, Kagemann L, Schuman JS and
Sigal IA: Polarization microscopy for characterizing fiber
orientation of ocular tissues. Biomed Opt Express. 6:4705–4718.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong X, Meng Y and Kalkanis SN: Serum
proteins are extracted along with monolayer cells in plasticware
and interfere with protein analysis. J Biol Methods. 3:e512016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ahn KS and Aggarwal BB: Transcription
factor NF-kappaB: A sensor for smoke and stress signals. Ann N Y
Acad Sci. 1056:218–233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamamoto Y and Gaynor RB: IkappaB kinases:
Key regulators of the NF-kappaB pathway. Trends Biochem Sci.
29:72–79. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chistiakov DA, Sobenin IA and Orekhov AN:
Vascular extracellular matrix in atherosclerosis. Cardiol Rev.
21:270–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Q, Jin M, Yang F, Zhu J, Xiao Q and
Zhang L: Matrix metalloproteinases: Inflammatory regulators of cell
behaviors in vascular formation and remodeling. Mediators Inflamm.
2013:9283152013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Berg G, Miksztowicz V and Schreier L:
Metalloproteinases in metabolic syndrome. Clin Chim Acta.
412:1731–1739. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
De Nooijer R, Verkleij CJ, von der Thüsen
JH, Jukema JW, van der Wall EE, van Berkel TJ, Baker AH and Biessen
EA: Lesional overexpression of matrix metalloproteinase-9 promotes
intraplaque hemorrhage in advanced lesionsbut not at earlier stages
of atherogenesis. Arterioscler Thromb Vasc Biol. 26:340–346. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Razansky D, Harlaar NJ, Hillebrands JL,
Taruttis A, Herzog E, Zeebregts CJ, van Dam GM and Ntziachristos V:
Multispectral optoacoustic tomography of matrix metalloproteinase
activity in vulnerable human carotid plaques. Mol Imaging Biol.
14:277–285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Murillo CA, Woodside KJ, Guo Q, Zhang S,
O'Connor KL and Hunter GC: Integrin and matrix metalloproteinase
expression in human carotid plaque. J Surg Res. 155:157–164. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang F, Jin XP, Zhu M, Lin XF, Hu XF, Wang
WF, Han Z and Huang LZ: Genotype association of C(−735)T
Polymorphism of the MMP-2 gene with the risk of carotid
atherosclerosis-vulnerable plaque in the Han Chinese population.
Vasc Med. 16:13–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reiner Z and Tedeschi-Reiner E: New
information on the pathophysiology of atherosclerosis. Lijec Vjesn.
123:26–31, (In Croatian). PubMed/NCBI
|
|
35
|
Boyle JJ: Macrophage activation in
atherosclerosis: Pathogenesis and pharmacology of plaque rupture.
Curr Vasc Pharmacol. 3:63–68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen W, Tang F, Xie B, Chen S, Huang H and
Liu P: Amelioration of atherosclerosis by tanshinone IIA in
hyperlipidemic rabbits through attenuation of oxidative stress. Eur
J Pharmacol. 674:359–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wei B, You MG, Ling JJ, Wei LL, Wang K, Li
WW, Chen T, Du QM and Ji H: Regulation of antioxidant system,
lipids and fatty acid β-oxidation contributes to the
cardioprotective effect of sodium tanshinone IIA sulphonate in
isoproterenol-induced myocardial infarction in rats.
Atherosclerosis. 230:148–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fukami K, Yamagishi S and Okuda S: Role of
AGEs-RAGE system in cardiovascular disease. Curr Pharm Des.
20:2395–2402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cipollone F, Iezzi A, Fazia M, Zucchelli
M, Pini B, Cuccurullo C, De Cesare D, De Blasis G, Muraro R, Bei R,
et al: The receptor RAGE as a progression factor amplifying
arachidonate-dependent inflammatory and proteolytic response in
human atherosclerotic plaques: Role of glycemic control.
Circulation. 108:1070–1077. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang F, Banker G, Liu X, Suwanabol PA,
Lengfeld J, Yamanouchi D, Kent KC and Liu B: The novel function of
advanced glycation end products in regulation of MMP-9 production.
J Surg Res. 171:871–876. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cipollone F, Iezzi A, Fazia M, Zucchelli
M, Pini B, Cuccurullo C, De Cesare D, De Blasis G, Muraro R, Bei R,
et al: The receptor RAGE as a progression factor amplifying
arachidonate-dependent inflammatory and proteolytic response in
human atherosclerotic plaques: Role of glycemic control.
Circulation. 108:1070–1077. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla
E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, et al: RAGE
blockade stabilizes established atherosclerosis in diabetic
apolipoprotein E-null mice. Circulation. 106:2827–2835. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Saraswathi V and Hasty AH: The role of
lipolysis in mediating the proinflammatory effects of very low
density lipoproteins in mouseperitoneal macrophages. J Lipid Res.
47:1406–1415. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim JY, Kim WJ, Kim H, Suk K and Lee WH:
The Stimulation of CD147 Induces MMP-9 expression through ERK and
NF-kappaB in macrophages: Implicationfor atherosclerosis. Immune
Netw. 9:90–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu X, Lin SG, Huang XR, Bacher M, Leng L,
Bucala R and Lan HY: Macrophage migration inhibitory factor induces
MMP-9 expression in macrophages via the MEK-ERK MAPkinase pathway.
J Interferon Cytokine Res. 27:103–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lin SJ, Shyue SK, Hung YY, Chen YH, Ku HH,
Chen JW, Tam KB and Chen YL: Superoxide dismutase inhibits the
expression of vascular cell adhesion molecule-1 and intracellular
cell adhesion molecule-1 induced by tumor necrosis factor-alpha in
human endothelial cells through the JNK/p38 pathways. Arterioscler
Thromb Vasc Biol. 25:334–340. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guo ZJ, Niu HX, Hou FF, Zhang L, Fu N,
Nagai R, Lu X, Chen BH, Shan YX, Tian JW, et al: Advanced oxidation
protein products activate vascular endothelial cells via a
RAGE-mediated signaling pathway. Antioxid Redox Signal.
10:1699–1712. 2008. View Article : Google Scholar : PubMed/NCBI
|