Introduction
Atropine sulfate is an anticholinergic drug with a
wide spectrum of activity (1),
exerting diverse effects on numerous systems. Rapid administration
of atropine during resuscitation may be life-saving (2). Atropine has also been used for the
treatment of anticholinesterase pesticide poisoning (3), bradycardia and associated hypotension
(4). In addition, atropine may
significantly slow the progression of myopia in children (5). Furthermore, atropine has been
demonstrated to have a significant anti-emetic effect (6).
Although the importance of atropine in the treatment
of organophosphate poisoning is generally recognized, numerous side
effects of atropine have been reported, suggesting potential
toxicity (7). Atropine used in
dobutamine stress echocardiograms has been reported to cause
morbidity (8). Atropine has been
shown to be cytotoxic to human corneal epithelial cells via the
induction of cell cycle arrest and death receptor-mediated
mitochondrion-dependent apoptosis (9). In the heart, atropine toxicity
resulted in altered expression levels of E-cadherin and serotonin
(10), and in the lung, atropine
decreased pulmonary gas exchange in a dose-dependent manner
(11). In addition, atropine
alters pulse rate, pupil diameter and salivary flow (12). The use of atropine eye drops has
been reported to cause significant toxicity (13), and a dose of atropine 1% may result
in pupillary mydriasis and accommodative paralysis (14). Previous studies have demonstrated
that atropine is primarily involved in decreasing male fertility by
inhibiting the transport of sperm and semen in rats (15). In addition, the
angiotensin-converting enzyme (ACE) and adenosine 5′-triphosphate
binding cassette sub-family G member 2 (ABCG2) were observed to be
altered in the testes in some conditions, such as selenium-induced
toxicity (16,17). However, the alterations in ACE and
ABCG2 expression levels in the testes following atropine-induced
toxicity remain to be elucidated.
The present study performed immunohistochemistry and
in situ hybridization (ISH) to evaluate the expression
levels of ACE and ABCG2 in the testes, and determine whether
protein and gene expression levels were altered by atropine-induced
toxicity.
Materials and methods
Animals and study design
A total of 16 healthy adult male Wistar rats, (age,
2 months; weight, 210–250 g; Sun Yat-sen University, Guangzhou,
China), were used for the purposes of the present study. All
animals were housed individually in stainless-steel wire-bottom
cages in an air-conditioned room at a temperature of 25°C, 50%
relative humidity and a 12-h light/dark cycle. Rats had free access
to standard pellet chow and water throughout the experimental
period. All procedures described in the present study were approved
by the ethics committee of Dali University (Dali, China).
Animals were randomly assigned to one of two groups
(n=8 rats/group): The atropine group, which received
intraperitoneal injections of a physiological dose of 15 mg/kg/day
atropine for seven days (one injection per day) and the control
group, which received identical volumes of normal saline for seven
days (10).
On day eight, the control and experimental animals
were deeply anesthetized with 1% sodium pentobarbital, (Harbin
Pharmaceutical Group, Co., Ltd., Harbin, China) and the testes were
removed. The testes were harvested for histopathology,
immunohistochemistry and ISH.
Histopathology
Testicular tissues were fixed in phosphate-buffered
4% formalin (pH 7.4) for 24 h and embedded in paraffin. Testes were
sectioned (4-µm) on a microtome and stained with hematoxylin and
eosin. The slides were coded, and semiquantitative analysis of the
sections was performed in a blinded manner by a pathologist using a
light microscope. Histopathological alterations were evaluated as
described previously (18,19).
Immunohistochemistry
Testes were immersed in 4% formaldehyde in
phosphate-buffered saline (PBS; pH 7.2), embedded in paraffin and
sectioned coronally (4-µm) on a microtome. Sections were
deparaffinized, and immersed in 0.3% H2O2 in
PBS for 10 min followed by 1% normal goat serum in PBS for 3 min to
reduce nonspecific reactions. Primary mouse anti-ACE (dilution,
1:400; cat. no. sc-23908; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) or rabbit anti-ABCG2 (dilution, 1:400; cat. no. sc-130933;
Santa Cruz Biotechnology, Inc.) antibodies were added to sections
and incubated overnight at 4°C. Subsequently, sections were washed
three times in PBS and incubated with biotin-conjugated goat
anti-mouse and goat anti-rabbit IgG secondary antibodies (cat. nos.
sc-23908 and sc-130933, respectively; dilution, 1:400; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. Following five
washes with PBS, tissue sections were incubated for 10 min in
streptavidin-peroxidase (horseradish peroxidase; Santa Cruz
Biotechnology, Inc.) and then washed three further times with PBS.
Bound antibody was visualized with diaminobenzidine (DAB), and
sections were counterstained with hematoxylin according to the
methods described previously (20–22).
PBS was substituted for primary antibody as the negative
control.
ISH
ACE and ABCG2 genes were detected using ISH kits
purchased from Wuhan Boster Biological Technology, Ltd., Wuhan,
China (catalog nos. MK-2335 and MK-2675, respectively). ISH was
performed according to the manufacturer's instructions, with slight
modifications. Briefly, slides were denatured with 70% formamide in
2X saline sodium citrate buffer at 65°C for 10 min. The probe
mixture was denatured at 65°C, incubated at 37°C for 10 min and
subsequently applied to the slides in a moist chamber. Following
overnight hybridization, slides were washed with PBS for 5 min.
Positive signals were visualized with DAB and sections were
counterstained with hematoxylin. The slides were dried at room
temperature (23).
Image processing
Total integrated optical density (IOD), a parameter
representing ACE and ABCG2 expression levels in testicular tissue,
was determined using a microscope (BX41; Olympus Corporation,
Tokyo, Japan), digital camera (DP-10; Olympus Corporation) and
image-analysis program (MetaMorph software version 4.65; Molecular
Devices, LLC, Sunnyvale, CA, USA). A total of five images were
captured of each immuno- and ISH-stained section (magnification,
×200) from eight rats, which were used to calculate the mean
(21,22).
Statistical analysis
Data are expressed as the mean ± standard error. The
total IOD of the two groups was compared using Kruskal-Wallis
analysis. P<0.05 was considered to indicate a statistically
significant difference. All analyses were performed in SPSS version
12.0 (SPSS Inc., Chicago, IL, USA).
Results
Histological examination
Hematoxylin and eosin staining did not reveal any
morphological differences in rat testes between the two groups
(data not shown).
Expression levels of ACE protein
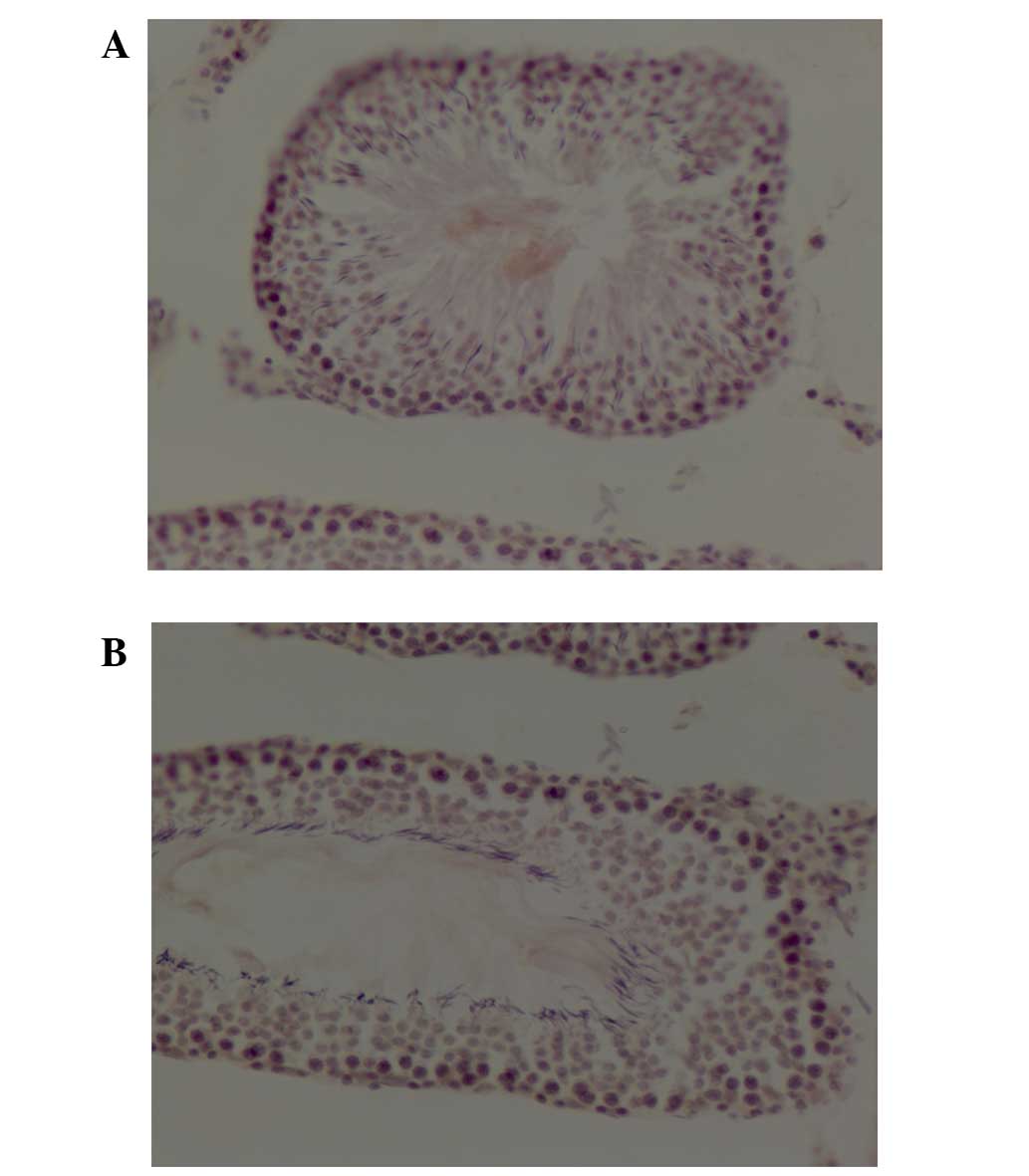
ACE staining was detected primarily in the tubule
lumen, as fine brown granular staining. Sections were independently
verified by two observers in order to confirm the results.
The photomicrographs in Fig. 1 reveal ACE staining in control
(Fig. 1A) and atropine-injured
(Fig. 1B) testes. Total IOD of ACE
in testes from rats that had undergone atropine intoxication was
significantly reduced compared with control rats (0.0049±0.00057
vs. 0.0063±0.00039; P=0.0001; Table
I).
 | Table I.IOD of ACE and ABCG2 proteins in rat
testes. |
Table I.
IOD of ACE and ABCG2 proteins in rat
testes.
| Group | ACE | ABCG2 |
|---|
| Control | 0.0063±0.00039 | 0.0059±0.00071 |
|
Atropine-treated |
|
|
| P-value | 0.0049±0.00057 | 0.0072±0.00063 |
|
| 0.0001 | 0.0017 |
Expression levels of ABCG2
protein
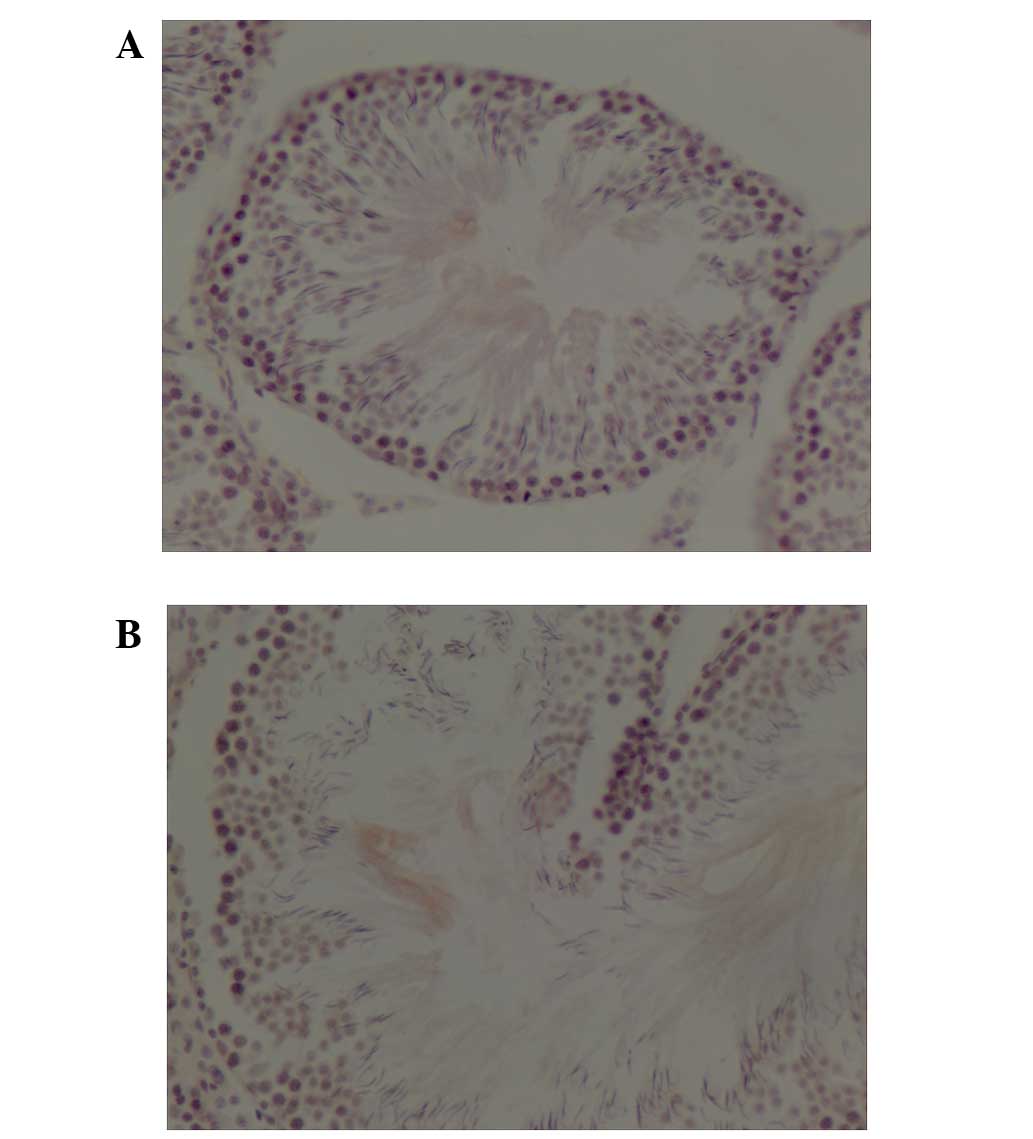
ABCG2 staining was detected primarily in the tubule
lumen, as fine brown granular staining.
ABCG2 staining was performed on the testes of
control (Fig. 2A) and
atropine-treated (Fig. 2B) rats.
Total IOD of ABCG2 in testes from rats subjected to atropine
intoxication was significantly increased compared with control rats
(0.0072±0.00063 vs. 0.0059±0.00071; P=0.0017; Table I).
Expression levels of ACE DNA
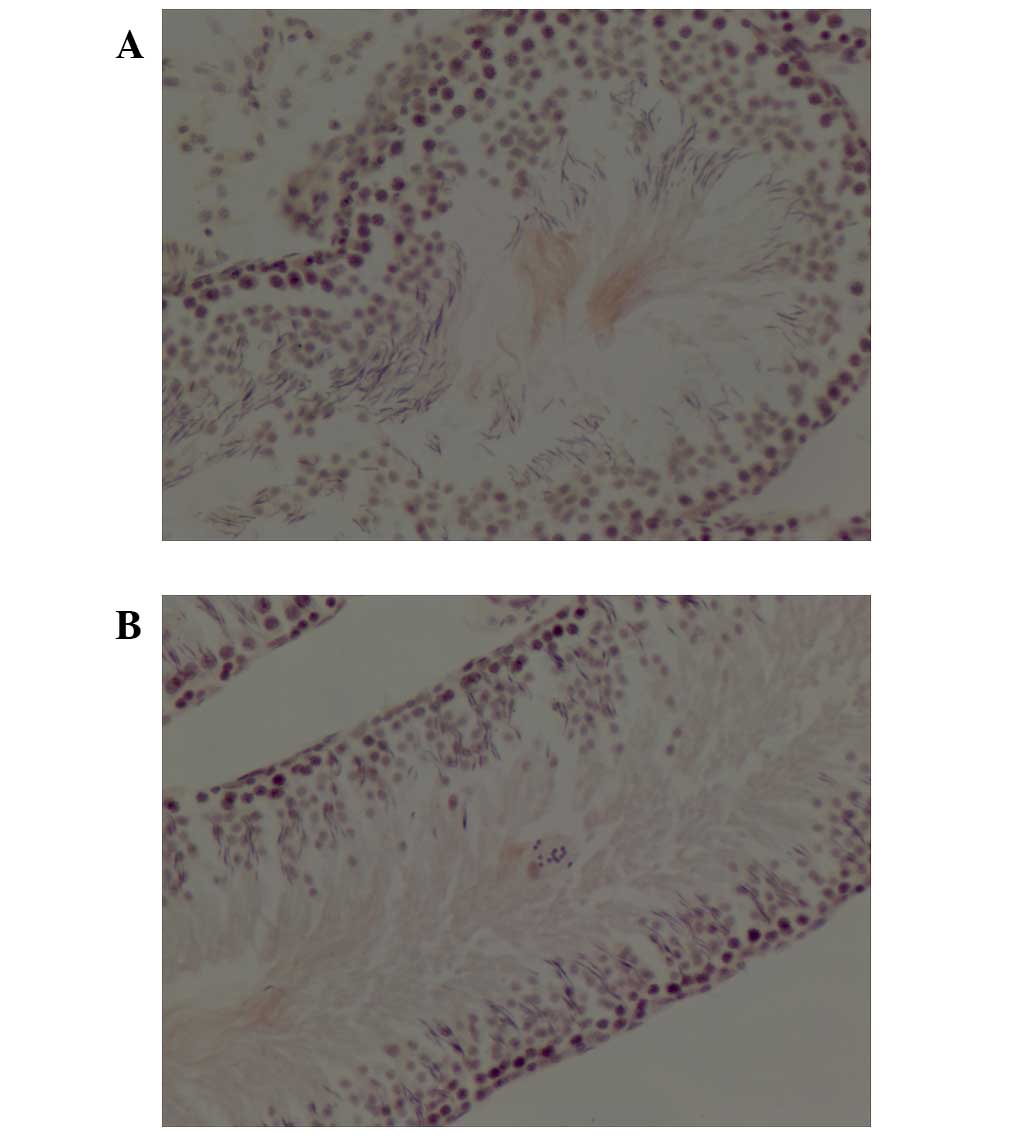
ISH of ACE DNA was detected primarily in the tubule
lumen of testicular tissue from control (Fig. 3A) and atropine-exposed (Fig. 3B) rats. Total IOD of ACE in testes
from rats subjected to atropine exposure was significantly reduced
compared with control rats (0.0047±0.00046 vs. 0.0062±0.00035:
P<0.001; Table II).
 | Table II.IOD of ACE and ABCG2 genes in rat
testes. |
Table II.
IOD of ACE and ABCG2 genes in rat
testes.
| Group | ACE | ABCG2 |
|---|
| Control | 0.0062±0.00035 | 0.0059±0.00016 |
|
Atropine-treated | 0.0047±0.00046 | 0.0070±0.00027 |
| P-value | <0.001 | <0.001 |
Expression levels of ABCG2 DNA
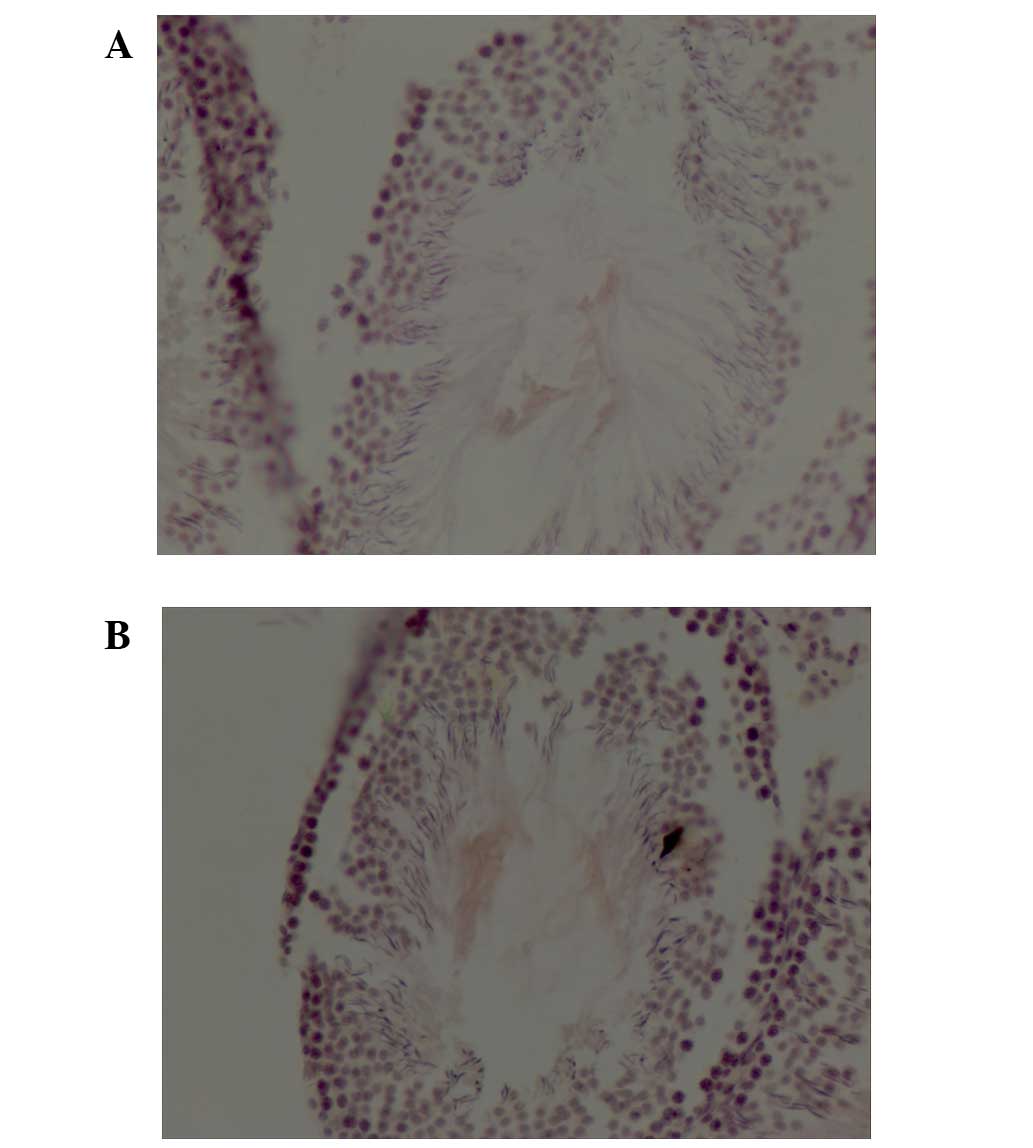
ISH of ABCG2 DNA was detected primarily in the
tubule lumen of testicular tissue from control (Fig. 4A) and atropine-exposed (Fig. 4B) rats. Total IOD of ABCG2 in
testes from rats subjected to atropine exposure was significantly
increased compared with control rats (0.0070±0.00027 vs.
0.0059±0.00016; P<0.001; Table
II).
Discussion
Although atropine is widely used, its undesirable
side effects may markedly decrease quality of life.
ACE is involved in the physiology of the
vasculature, blood pressure and inflammation (24). It has been demonstrated that the
insertion/deletion (I/D) ACE gene polymorphism is associated with
coronary restenosis (25), and may
also affect blood pressure (26)
and pregnancy-induced hypertension (27). ACE is one of the factors
controlling blood pressure (28).
The I/D polymorphism has been associated with nitric oxide
metabolite levels and systolic blood pressure in men (29), and high blood pressure at the end
of pregnancy in women (30).
Abnormal expression of ACE in rats resulted in inflammation,
pulmonary edema and histological changes in smoke
inhalation-induced lung injury (31). In humans, the I/D polymorphism of
the ACE gene has been associated with the development of high
altitude pulmonary edema (32).
The I/D ACE polymorphism has been demonstrated to be
independent of thrombosis formation (33); however, it may be associated with
osteoporosis (34), panic disorder
(35) and vitiligo (36).
In the present study, the expression levels of ACE
in the testes of atropine-exposed rats were significantly reduced
when compared with control rats. This suggests the ACE may be
associated with testicular injury (37). For example, atropine may inhibit
the muscarinic acetylcholine receptor (mACh) -receptor leading to
abnormal gland function (38).
These alterations may influence ACE expression and the subsequent
conversion of angiotensin (39,40).
ABCG2 actively transports numerous endogenous and
exogenous substrates across membranes (41). ABCG2 is involved in drug-resistance
in cancer (42), as overexpression
results in the ejection of drugs from cancer cells (43). In addition, ABCG2 overexpression
promotes proliferation and suppresses apoptosis (44,45).
Furthermore, ABCG2 may affect the oral availability, tissue
distribution and excretion of its substrates (46).
ABCG2 has been demonstrated to be overexpressed in
various solid tumors, acute myelogenous leukemia and chronic
myeloid leukemia (47), and is a
potential biomarker of multidrug resistance in non-small cell lung
cancer (48). In addition, ABCG2
is involved in amyloid β transport and was revealed to be
significantly upregulated in Alzheimer's disease (49). ABCG2 staining may be a potential
novel independent prognostic factor in colorectal cancer (50) and may be involved in hepatocellular
carcinoma drug resistance (51) It
has been demonstrated that ABCG2 is critical in cardiovascular and
cancer pathophysiology (52).
Furthermore, ABGG2 is overexpressed in acute myeloid leukemia
patients with an increased risk of relapse (53).
Targeted inhibition of ABCG2 has been demonstrated
to improve the efficacy of cancer therapeutics (54). Statins may downregulate ABCG2
expression and function by reducing low-density lipoprotein
cholesterol levels (55). However,
ABCG2 deficiency may increase oxidative stress, alter the
inflammatory response in the brain and exacerbate cognitive
deficits (56).
In the present study, the expression levels of ABCG2
in the testes of atropine-exposed rats were significantly increased
compared with control rats. This suggests that ABCG2 may be
associated with testicular injury, and influence the homeostasis of
testes tissues and cells, such as the blood-testis barrier
(57).
In conclusion, the results of the present study
demonstrate that ACE expression levels were significantly reduced,
while ABCG2 expression levels were significantly elevated, in
response to atropine exposure. These alterations may be reflected
in abnormal testicular function, including sperm production and
motility, due to disruption of the normal homeostasis of testes
tissues and cells. The proteins and genes investigated in the
present study may be useful to elucidate the mechanisms underlying
atropine-induced toxicity and provide directions for future
studies, such as the development of therapies that activate the
mACh receptor, as well as protect sperm production and motility
during atropine treatment.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81260466)
and Dali University (grant no. KYBS201104).
References
|
1
|
Byadagi KS, Nandibewoor ST and Chimatadar
SA: Catalytic activity of ruthenium (III) on the oxidation of an
anticholinergic drug-atropine sulfate monohydrate by copper (III)
periodate complex in aqueous alkaline medium - decarboxylation and
free radical mechanism. Acta Chim Slov. 60:617–627. 2013.PubMed/NCBI
|
|
2
|
Konickx LA, Bingham K and Eddleston M: Is
oxygen required before atropine administration in organophosphorus
or carbamate pesticide poisoning? - A cohort study. Clin Toxicol
(Phila). 52:531–537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Papoutsis I, Nikolaou P, Spiliopoulou C,
Pistos C, Stefanidou M and Athanaselis S: A simple and sensitive
GC/MS method for the determination of atropine during therapy of
anticholinesterase poisoning in serum samples. Drug Test Anal.
4:229–234. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stephenson M, Wong A, Rotella JA, Crump N,
Kerr F and Greene SL: Deliberate fingolimod overdose presenting
with delayed hypotension and bradycardia responsive to atropine. J
Med Toxicol. 10:215–218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li SM, Wu SS, Kang MT, Liu Y, Jia SM, Li
SY, Zhan SY, Liu LR, Li H, Chen W, et al: Atropine slows myopia
progression more in Asian than white children by meta-analysis.
Optom Vis Sci. 91:342–350. 2014.PubMed/NCBI
|
|
6
|
Baciarello M, Cornini A, Zasa M, Pedrona
P, Scrofani G, Venuti FS and Fanelli G: Intrathecal atropine to
prevent postoperative nausea and vomiting after Cesarean section: A
randomized, controlled trial. Minerva Anestesiol. 77:781–788.
2011.PubMed/NCBI
|
|
7
|
Jandrić Z, Rathor MN, Chhem-Kieth S,
Adu-Gyamfi J, Mayr L, Resch C, Bado S, Švarc-Gajić J and Cannavan
A: Uptake of (14)C-atropine and/or its transformation products from
soil by wheat (Triticum aestivum var Kronjet) and their
translocation to shoots. J Environ Sci Health B. 48:1034–1042.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilson ME, Lee GK, Chandra A and Kane GC:
Central anticholinergic syndrome following dobutamine-atropine
stress echocardiography. Echocardiography. 28:E205–E206. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tian CL, Wen Q and Fan TJ: Cytotoxicity of
atropine to human corneal epithelial cells by inducing cell cycle
arrest and mitochondrion-dependent apoptosis. Exp Toxicol Pathol.
67:517–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang QY, Li XF and Liu SP: E-cadherin and
5-HT alterations in the heart of rats having undergone
atropine-induced toxicity. Mol Med Rep. 5:700–704. 2012.PubMed/NCBI
|
|
11
|
Gaspari RJ and Paydarfar D: Pulmonary
effects of intravenous atropine induce ventilation perfusion
mismatch. Can J Physiol Pharmacol. 92:399–404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fry JR and Burr SA: A double-blind
atropine trial for active learning of autonomic function. Adv
Physiol Educ. 35:438–444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stellpflug SJ, Cole JB, Isaacson BA,
Lintner CP and Bilden EF: Massive atropine eye drop ingestion
treated with high-dose physostigmine to avoid intubation. West J
Emerg Med. 13:77–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cooper J, Eisenberg N, Schulman E and Wang
FM: Maximum atropine dose without clinical signs or symptoms. Optom
Vis Sci. 90:1467–1472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sato T, Ban Y, Uchida M, Gondo E, Yamamoto
M, Sekiguchi Y, Sakaue A, Kemi M and Nakatsuka T: Atropine-induced
inhibition of sperm and semen transport impairs fertility in male
rats. J Toxicol Sci. 30:207–212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hahnova-Cygalova L, Ceckova M and Staud F:
Fetoprotective activity of breast cancer resistance protein (BCRP,
ABCG2): Expression and function throughout pregnancy. Drug Metab
Rev. 43:53–68. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khalid A, Khudhair N, He H, Peng Z,
Yaguang T and Guixue Z: Effects of dietary selenium supplementation
on seminiferous tubules and SelW, GPx4, LHCGR and ACE expression in
chicken testis. Biol Trace Elem Res. 173:202–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Helin HO, Lundin ME, Laakso M, Lundin J,
Helin HJ and Isola J: Virtual microscopy in prostate
histopathology: Simultaneous viewing of biopsies stained
sequentially with hematoxylin and eosin and
alpha-methylacyl-coenzyme A racemase/p63 immunohistochemistry. J
Urol. 175:495–499. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Rossi A, Rocha LB and Rossi MA:
Application of fluorescence microscopy on hematoxylin and
eosin-stained sections of healthy and diseased teeth and supporting
structures. J Oral Pathol Med. 36:377–381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smith PS, Parkinson IH and Leong AS:
Principles of ploidy analysis by static cytometry. Clin Mol Pathol.
49:M104–M111. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong J, Yin H, Liu W, Wang P, Jiang Y and
Chen J: Congenital iodine deficiency and hypothyroidism impair LTP
and decrease C-fos and C-jun expression in rat hippocampus.
Neurotoxicology. 26:417–426. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Kuijk AW, Gerlag DM, Vos K, Wolbink G,
de Groot M, de Rie MA, Zwinderman AH, Dijkmans BA and Tak PP: A
prospective, randomised, placebo-controlled study to identify
biomarkers associated with active treatment in psoriatic arthritis:
Effects of adalimumab treatment on synovial tissue. Ann Rheum Dis.
68:1303–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seo HW, Han K, Oh Y, Kang I, Park C, Joo
HE, Kim SH, Lee BH and Chae C: Evaluation of commercial polyclonal-
and monoclonal-antibody-based immunohistochemical tests for 2
genotypes of Porcine circovirus type 2 and comparison with in-situ
hybridization assays. Can J Vet Res. 78:233–236. 2014.PubMed/NCBI
|
|
24
|
Rashed L, Hay R Abdel, Mahmoud R, Hasan N,
Zahra A and Fayez S: Association of Angiotensin-Converting Enzyme
(ACE) gene polymorphism with inflammation and cellular cytotoxicity
in vitiligo patients. PLoS One. 10:e01329152015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miao HW and Gong H: Association of ACE
insertion or deletion polymorphisms with the risk of coronary
restenosis after percutaneous coronary intervention: A
meta-analysis. J Renin Angiotensin Aldosterone Syst. 16:844–850.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goessler KF, Cornelissen VA, de Oliveira
EM, de Mota FG and Polito MD: ACE polymorphisms and the acute
response of blood pressure to a walk in medicated hypertensive
patients. J Renin Angiotensin Aldosterone Syst. 16:720–729. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miao HW and Gong H: Correlation of ACE
gene deletion/insertion polymorphism and risk of pregnancy-induced
hypertension: A meta-analysis based on 10,236 subjects. J Renin
Angiotensin Aldosterone Syst. 16:982–994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Betancur-Ancona D, Dávila-Ortiz G,
Chel-Guerrero LA and Torruco-Uco JG: ACE-I inhibitory activity from
phaseolus lunatus and phaseolus vulgaris peptide fractions obtained
by ultrafiltration. J Med Food. 18:1247–1254. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Avila-Vanzzini N, Posadas-Romero C,
Gonzalez-Salazar Mdel C, Maass-Iturbide C, Melendez-Ramirez G,
Perez-Mendez O, Del Valle-Mondragon L, Masso-Rojas F, Lopez E
Varela, Herrera-Bello H, et al: The ACE I/D polymorphism is
associated with nitric oxide metabolite and blood pressure levels
in healthy Mexican men. Arch Cardiol Mex. 85:105–110.
2015.PubMed/NCBI
|
|
30
|
Reshetnikov EA, Akulova LY, Dobrodomova
IS, Dvornyk VY, Polonikov AV and Churnosov MI: The
insertion-deletion polymorphism of the ACE gene is associated with
increased blood pressure in women at the end of pregnancy. J Renin
Angiotensin Aldosterone Syst. 16:623–632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yilin Z, Yandong N and Faguang J: Role of
angiotensin-converting enzyme (ACE) and ACE2 in a rat model of
smoke inhalation induced acute respiratory distress syndrome.
Burns. 41:1468–1477. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhagi S, Srivastava S, Tomar A, Bala SS
and Sarkar S: Positive association of D allele of ACE gene with
high altitude pulmonary edema in indian population. Wilderness
Environ Med. 26:124–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gorukmez O, Sag ŞO, Gorukmez Ö, Ture M,
Topak A, Sahinturk S, Ozkaya G, Gulten T, Ali R and Yakut T:
Association of the ACE I/D gene polymorphisms with
JAK2V617F-positive polycythemia vera and essential thrombocythemia.
Genet Test Mol Biomarkers. 19:303–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cakmak B, Inanir A, Karakus N, Ates O and
Yigit S: Association between the ACE gene I/D polymorphism and
osteoporosis in a Turkish population. Z Rheumatol. 74:346–350.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gulec-Yilmaz S, Gulec H, Dalan AB, Cetın
B, Tımırcı-Kahraman O, Ogut DB, Atasoy H, Dırımen GA, Gultekın GI
and Isbır T: The relationship between ACE polymorphism and panic
disorder. In Vivo. 28:885–889. 2014.PubMed/NCBI
|
|
36
|
Badran DI, Nada H and Hassan R:
Association of Angiotensin-Converting Enzyme ACE gene polymorphism
with ACE activity and susceptibility to Vitiligo in Egyptian
population. Genet Test Mol Biomarkers. 19:258–263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fujihara Y, Tokuhiro K, Muro Y, Kondoh G,
Araki Y, Ikawa M and Okabe M: Expression of TEX101, regulated by
ACE, is essential for the production of fertile mouse spermatozoa.
Proc Natl Acad Sci USA. 110:8111–8116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi CL, Täljedal IB and Mattsson MO:
Effect of carbachol on regulation of the mACh receptor mRNA
expression ADN insulin secretion in mouse pancreatic islets. Acta
Physiol Scand. 167:A181999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Balyasnikova IV, Metzger R, Franke FE,
Conrad N, Towbin H, Schwartz DE, Sturrock ED and Danilov SM:
Epitope mapping of mAbs to denatured human testicular ACE (CD143).
Tissue Antigens. 72:354–368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rushworth CA, Guy JL and Turner AJ:
Residues affecting the chloride regulation and substrate
selectivity of the angiotensin-converting enzymes (ACE and ACE2)
identified by site-directed mutagenesis. FEBS J. 275:6033–6042.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schnepf R and Zolk O: Effect of the
ATP-binding cassette transporter ABCG2 on pharmacokinetics:
Experimental findings and clinical implications. Expert Opin Drug
Metab Toxicol. 9:287–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Erdei Z, Sarkadi B, Brózik A, Szebényi K,
Várady G, Makó V, Péntek A, Orbán TI and Apáti Á: Dynamic ABCG2
expression in human embryonic stem cells provides the basis for
stress response. Eur Biophys J. 42:169–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang B, Ma YF and Liu Y: Elevated
expression of Nrf-2 and ABCG2 involved in multi-drug resistance of
lung cancer SP cells. Drug Res (Stuttg). 65:526–531.
2015.PubMed/NCBI
|
|
44
|
Xie J, Jin B, Li DW, Shen B, Cong N, Zhang
TZ and Dong P: ABCG2 regulated by MAPK pathways is associated with
cancer progression in laryngeal squamous cell carcinoma. Am J
Cancer Res. 4:698–709. 2014.PubMed/NCBI
|
|
45
|
Kalalinia F, Elahian F, Mosaffa F and
Behravan J: Celecoxib up regulates the expression of drug efflux
transporter ABCG2 in breast cancer cell lines. Iran J Pharm Res.
13:1393–1401. 2014.PubMed/NCBI
|
|
46
|
Lecerf-Schmidt F, Peres B, Valdameri G,
Gauthier C, Winter E, Payen L, Di Pietro A and Boumendjel A: ABCG2:
Recent discovery of potent and highly selective inhibitors. Future
Med Chem. 5:1037–1045. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Juvale K and Wiese M: Design of inhibitors
of BCRP/ABCG2. Future Med Chem. 7:1521–1527. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang DS, Patel A, Shukla S, Zhang YK, Wang
YJ, Kathawala RJ, Robey RW, Zhang L, Yang DH, Talele TT, et al:
Icotinib antagonizes ABCG2-mediated multidrug resistance, but not
the pemetrexed resistance mediated by thymidylate synthase and
ABCG2. Oncotarget. 5:4529–4542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fehér Á, Juhász A, László A, Pákáski M,
Kálmán J and Janka Z: Association between the ABCG2 C421A
polymorphism and Alzheimer's disease. Neurosci Lett. 550:51–54.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang X, Xia B, Liang Y, Peng L, Wang Z,
Zhuo J, Wang W and Jiang B: Membranous ABCG2 expression in
colorectal cancer independently correlates with shortened patient
survival. Cancer Biomark. 13:81–88. 2013.PubMed/NCBI
|
|
51
|
Hou H, Sun H, Lu P, Ge C, Zhang L, Li H,
Zhao F, Tian H, Zhang L, Chen T, et al: Tunicamycin potentiates
cisplatin anticancer efficacy through the DPAGT1/Akt/ABCG2 pathway
in mouse Xenograft models of human hepatocellular carcinoma. Mol
Cancer Ther. 12:2874–2884. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Deppe S, Ripperger A, Weiss J, Ergun S and
Benndorf RA: Impact of genetic variability in the ABCG2 gene on
ABCG2 expression, function, and interaction with AT1 receptor
antagonist telmisartan. Biochem Biophys Res Commun. 443:1211–1217.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Damiani D, Tiribelli M, Geromin A,
Michelutti A, Cavallin M, Sperotto A and Fanin R: ABCG2
overexpression in patients with acute myeloid leukemia: Impact on
stem cell transplantation outcome. Am J Hematol. 90:784–789. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lin YH, Chang HM, Chang FP, Shen CR, Liu
CL, Mao WY, Lin CC, Lee HS and Shen CN: Protoporphyrin IX
accumulation disrupts mitochondrial dynamics and function in
ABCG2-deficient hepatocytes. FEBS Lett. 587:3202–3209. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
To KK, Hu M and Tomlinson B: Expression
and activity of ABCG2, but not ABCB1 or OATP1B1, are associated
with cholesterol levels: Evidence from in vitro and in vivo
experiments. Pharmacogenomics. 15:1091–1104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zeng Y, Callaghan D, Xiong H, Yang Z,
Huang P and Zhang W: Abcg2 deficiency augments oxidative stress and
cognitive deficits in Tg-SwDI transgenic mice. J Neurochem.
122:456–469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Natarajan K, Xie Y, Baer MR and Ross DD:
Role of breast cancer resistance protein (BCRP/ABCG2) in cancer
drug resistance. Biochem Pharmacol. 83:1084–1103. 2012. View Article : Google Scholar : PubMed/NCBI
|