Introduction
Atrial fibrillation (AF) is a common cardiac
arrhythmia, characterized as an irregular and rapid heart rate. AF
has been identified to be associated with ischemic stroke,
hypertension, and heart failure (1–3). The
incidence of AF increases with increasing age (4), and with the increasing population of
elderly patients, AF is predicted to cause increased morbidity and
mortality. However, the etiology of AF is complex and unclear, and
inherited and environmental factors have been reported to be
involved (5,6).
The progression of AF is commonly accompanied with
alterations in gene expression, thus resulting in abnormal protein
expression expression (7,8). A previous study identified that long
non-coding RNAs (lncRNAs), endogenous RNAs >200 nucleotides in
length that do not code for functional proteins, regulate the gene
expression of numerous proteins (9). Several studies have demonstrated that
lncRNAs are associated with diseases including cancer (10), endocrine diseases (11), liver diseases (12) and heart diseases (13,14).
lncRNAs are important in the regulation of cardiogenesis (14) and associated with numerous cardiac
diseases such as myocardial infarction (15), heart failure (16) and left ventricular hypertrophy
(17). However, the association
between lncRNAs and AF has not been explored yet.
Previous studies have demonstrated that AF is
associated with a higher demand of energy in cardiomyocytes
(18,19). Additional studies have demonstrated
that AF is associated with impaired energy synthesis or consumption
(20–22). Therefore, alterations in the energy
metabolism may contribute to the pathogenesis of AF (22). lncRNAs have been observed to serve
a role in the energy metabolism in brown adipose tissues (23). It remains unclear whether lncRNAs
are involved in energy metabolism in cardiomyocytes.
It has been reported that compared with the left
atrial appendage (LAA), the pulmonary vein and the surrounding left
atrial area (LA-PV) exhibited with 391 differentially expressed
genes that included genes associated with arrhythmia cell death and
inflammation, suggesting that region-specific gene expression may
contribute to AF pathogenesis (8).
In the present study, microarray analysis was conducted to
investigate the differential lncRNA expression profiling in atrial
samples from the LA-PV and from the LAA in patients with AF. The
purpose of the present study was to identify region-specific
expression of lncRNAs in patients with AF and to define the
functional role of lncRNA in H9C2 cells.
Materials and methods
Patients
The Medical Ethics Committee of the First Affiliated
Hospital of Harbin Medical University (Harbin, China) approved the
experiments of the present study, and all patients gave their
informed consent prior to the study. The current study included
paired LA-PV and LAA samples from 16 patients with persistent AF
undergoing cardiac surgery. The LA-PV samples were used as the
experimental group and the LAA samples were used as the control
group. All patients had a history of AF >6 months prior to
surgery. AF was diagnosed by evaluation of medical records and
12-lead electrocardiogram observations.
Microarray analysis
Total RNAs were isolated from atrial samples in the
experimental and control groups using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to
manufacturer's protocol. The mRNAs were purified from total RNA
subsequent to removal of rRNA (mRNA-Only Eukaryotic mRNA Isolation
kit; Epicenter; Illumina, Inc., San Diego, CA, USA). Each sample
was then and transcribed into cRNA along the entire length of the
transcripts without 3′ bias using a SuperScript Double-Strand
Synthesis kit (Invitrogen; Thermo Fisher Scientific, Inc.). The
cDNAs were labeled with Cy3 using Quick-Ampl labeling kit (Agilent
Technologies, Inc., Santa Clara, CA, USA). Labeled miRNAs were
hybrided to the human microarray chip (Human LncRNA Microarray
V2.0). Hybridization signals were detected using an Agilent
scanner. Images were quantified using the Agilent Feature Extract
software, version 11.0 (Agilent Technologies, Inc.). Differential
expression of lncRNA between the experimental and control groups
was identified by volcano plot. The lncRNAs with ≥2 fold changes
between the experimental and control groups were selected.
Analysis of the association of lncRNAs
with target mRNAs
Pearson correlation analysis was used to determine
the association of the lncRNA AK055347 with direct regulated
expression of target mRNAs. mRNAs with high Pearson's correlation
coefficients (>0.75) were selected as the targets of lncRNA
AK055347.
Cell culture
H9C2 cells, a clonal cell line of cardiomyocytes
derived from embryonic rat heart tissues, were obtained from the
American Type Culture Collection (Manassas, VA, USA). Cells were
cultured in Dulbecco's modified Eagle's medium (Invitrogen; Thermo
Fisher Scientific, Inc.) containing 10% fetal bovine serum
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA), 100 U/ml
penicillin and 100 mg/ml streptomycin. The cells were maintained in
a humidified atmosphere with 5% CO2 at 37°C. Cells were subcultured
at 1:3 ratio every 3 days.
Small interfering RNA (siRNA)
The rat cDNA sequence was analyzed for potential
siRNA target sequences. Three oligonucleotides were analyzed for
the inhibition of the expression of AK055347. The siRNAs tested
were as follows: siRNA #1, 5′-gaggaucuac uguuaacaga-3′ (sense) and
5′-cuccuagaugacaauuguucu-3′ (antisense); siRNA #2,
5′-cauaccaccaagccuucuu-3′ (sense) and 5′-gua ugg ugg uuc gga aga
a-3′ (antisense); siRNA #3, 5′-cguguccucucugcugucucc-3′ and 5′-gca
cag gag acg aca gag g-3′. H9C2 cells were transfected with siRNAs
using Lipofectamine 2000.
Cell viability
Cell viability was analyzed using the Cell Counting
Kit 8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.,
Shanghai, China). Cells were seeded into a 96-well plate at a
density of 104 cells/well. Cells were transfected with
siRNAs and cultured in 5% CO2 at 37°C for 48 h. CCK-8 solution (10
µl) was added to each well and cultured for an additional 2 h.
Absorbance was measured at 490 nm using a MultiSkan 3 microplate
reader (Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from H9C2 cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. RNA was reverse transcribed into cDNA
using the reverse transcriptase of Moloney murine leukemia virus
(Promega Corporation, Madison, WI, USA). RT-qPCR was performed in a
final volume of 20 µl containing 2 µl cDNA, 1 µl of each primer,
and 10 µl SYBR Green (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Primers used for amplification of AK055347 were
5′-AACTCCTAACACATCTCT-3′ (sense) and 5′-CTAAGGTAGTCAGTCTCA-3′
(antisense). U6 was used as a housekeeping gene. The reaction
conditions were as follows: 95°C for 10 min; 95°C for 15 sec, 55°C
for 1 min with 40 cycles. Melting curve analyses were performed to
verify the amplification specificity. The gene expression ∆Cq
values of AK055347 from each sample were calculated by normalizing
with internal control U6. The relative expression of AK055347 was
calculated using 2−∆∆Cq method (24).
Western blotting
H9C2 cells were homogenized on ice in lysis buffer.
Lysates were centrifuged at 13,000 × g at 4°C for 20 min.
The supernatants were collected and protein concentrations were
determined using a Bicinchoninic Acid Protein Quantitation kit
(Abcam, Cambridge, MA, USA). Proteins were resolved using 10–12%
SDS-PAGE, and transferred onto polyvinylidene fluoride membranes by
electroblotting. Membranes were incubated with primary antibodies
against Cyp450 (ab196836; rabbit anti-rat; monoclonal; 1:100;
Abcam), adenosine triphosphate (ATP) synthase (ab54880; mouse
anti-rat; monoclonal; 1:100; Abcam) and MSS51 (ab63801; mouse
anti-rat; monoclonal; 1:100; Abcam). The antibodies were incubated
at 4°C overnight. GAPDH (cat. no. BM1623; Wuhan Boster Biological
Technology, Ltd., Wuhan, China) was used as a loading control.
Membranes were then incubated with horseradish
peroxidase-conjugated goat anti-mouse (cat. no. ab6789) or
anti-rabbit (cat. no. ab6721) secondary antibodies (1:10,000;
Abcam) at room temperature for 40 min. Bands were visualized by
exposure to X-ray film (Kodak, Rochester, NY, USA). Images were
acquired by scanning the films, and band gray values were analyzed
using ImageJ software (National Institutes of Health, Bethesda, MD,
USA; http://rsb.info.nih.gov/ij/index.html).
Immunofluorescence staining
H9C2 cells were grown on glass coverslips in sterile
6-well plates until confluence was reached. Cells were then rinsed
with phosphate-buffered saline (PBS) three times, and fixed in 4%
paraformaldehyde for 15 min at room temperature. The cells were
then permeabilized with 0.5% Triton X-100 for 20 min. Subsequent to
three washes with PBS, cells were incubated with primary antibodies
against MSS51 (ab165144; mouse anti-rat; polyclonal; 1:100; Abcam)
at 4°C overnight. PBS without primary antibodies was used as a
negative control. After the primary antibody was removed by washing
in PBS, immunoreactivity was detected by incubation in fluorescein
isothiocyanate-coupled secondary antibodies (goat anti-mouse IgG;
1:100; cat. no. ab6785; Abcam) at room temperature for 1 h. Cells
were counterstained with DAPI. Following washing of the coverslips
with PBS, the cells were examined and photographed with a
fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Statistical analysis
Analyses were performed using SPSS software, version
13.0 (SPSS, Inc., Chicago, IL, USA). All values are presented as
the mean ± standard deviation. One-way analysis of variance
followed by Bonferroni's test was used to compare the differences.
P<0.05 was considered to indicate a statistically significant
difference.
Results
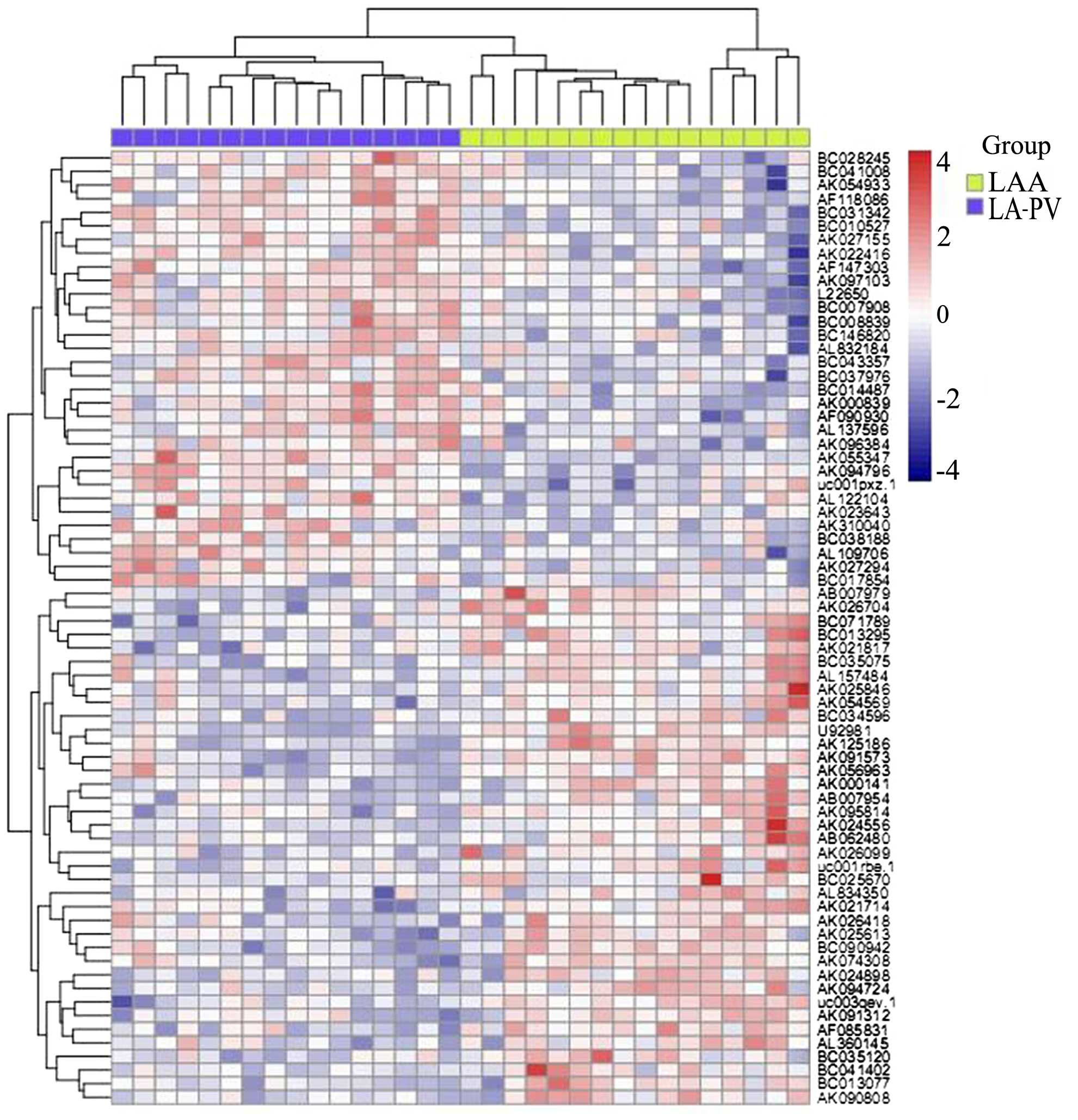
lncRNAs are abnormally expressed in
patients with AF
In order to investigate the role of lncRNAs in AF,
microarray-based profiling analysis was conducted using LA-PV and
LAA tissue samples in patients with AF. By comparing the expression
profiles between LA-PV and LAA tissue samples, 94 lncRNAs were
identifed that were either significantly upregulated or
downregulated (>2 fold change) in LA-PV samples compared with
LAA samples (Fig. 1). Table I presents the top 10 lncRNAs
including AK055347, AK310040, AK026494, BC010527, AK027294,
AB007979, AK091573, UC003qev.1, AK125186 and U9981. AK055347 was
selected for further analysis.
 | Table I.The expression levels of the top ten
lncRNAs with the most significant changes between LA-PV and LAA
tissues. |
Table I.
The expression levels of the top ten
lncRNAs with the most significant changes between LA-PV and LAA
tissues.
| lncRNAs | LAA | LA-PV | P-values |
|---|
| AK055347 | 5.178 | 6.565 | <0.00001 |
| AK310040 | 3.736 | 4.823 | 0.0006 |
| AK026494 | 6.273 | 7.159 | 0.004 |
| BC010527 | 5.309 | 6.156 | <0.0001 |
| AK027294 | 5.708 | 6.515 | 0.00187 |
| AB007979 | 4.898 | 3.710 | <0.0001 |
| AK091573 | 6.428 | 5.196 | <0.0001 |
| UC003qev.1 | 9.524 | 8.210 | <0.0001 |
| AK125186 | 6.391 | 4.969 | <0.0001 |
| U9981 | 8.788 | 7.069 | <0.0001 |
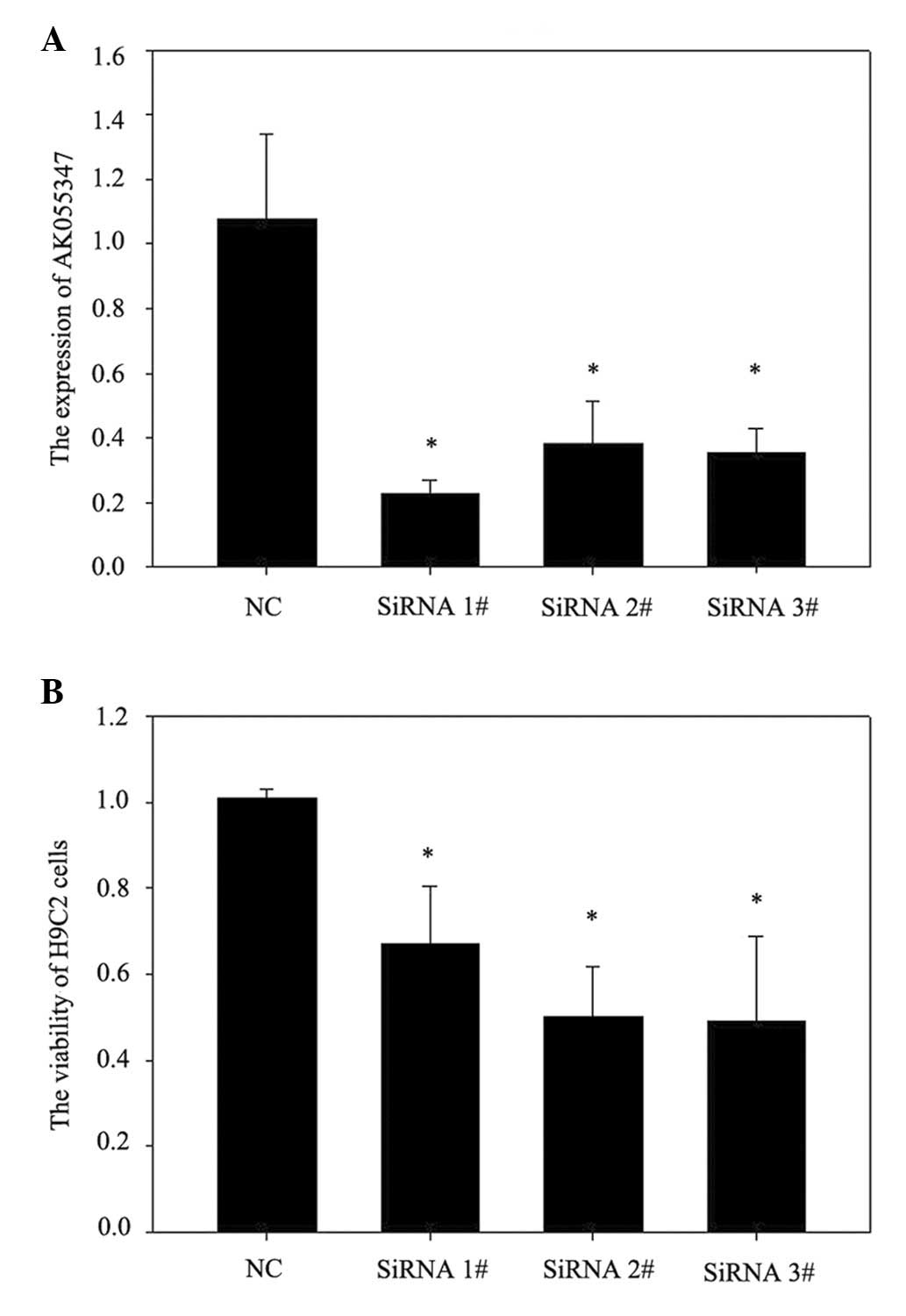
Knockdown of lncRNA AK055347 inhibited
cell viability in H9C2 cells
The role of lncRNA AK055347 in cell viability in
H9C2 cells was investigated using siRNA to knockdown lncRNA
AK055347. RT-qPCR results demonstrated that siRNA#1, #2 and #3
significantly downregulated AK055347 expression in H9C2 cells
(Fig. 2A). Knockdown of AK055347
significantly reduced cell viability of H9C2 cells (Fig. 2B).
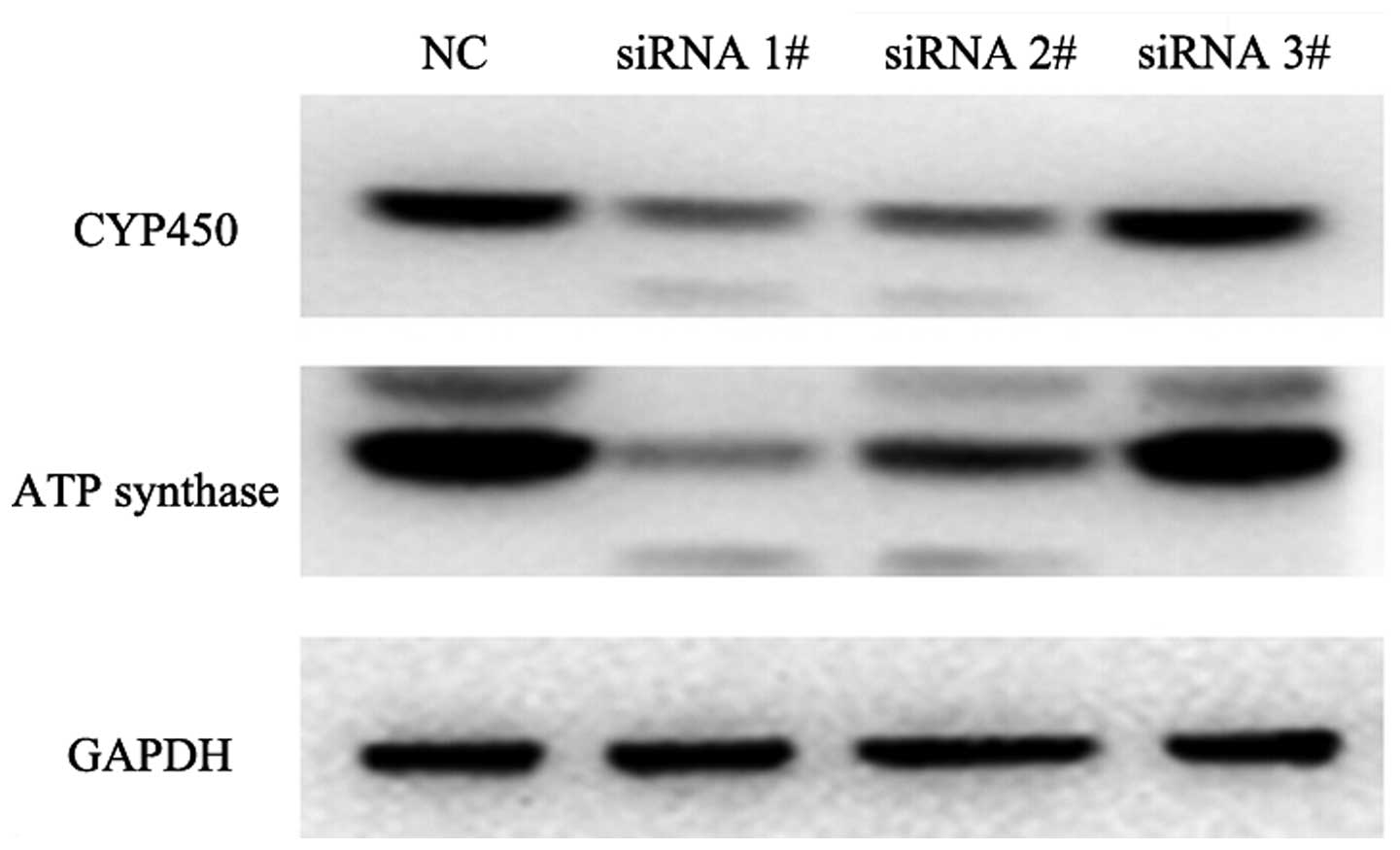
Knockdown of lncRNA AK055347 inhibited
the expression of Cyp450 and ATP synthase in H9C2 cells
The protein expression of Cyp450 and ATP synthase
was measured in H9C2 cells treated with siRNAs against AK055347.
Western blotting indicated that knockdown of AK055347 inhibited the
expression of Cyp450 and ATP synthases in H9C2 cells (Fig. 3).
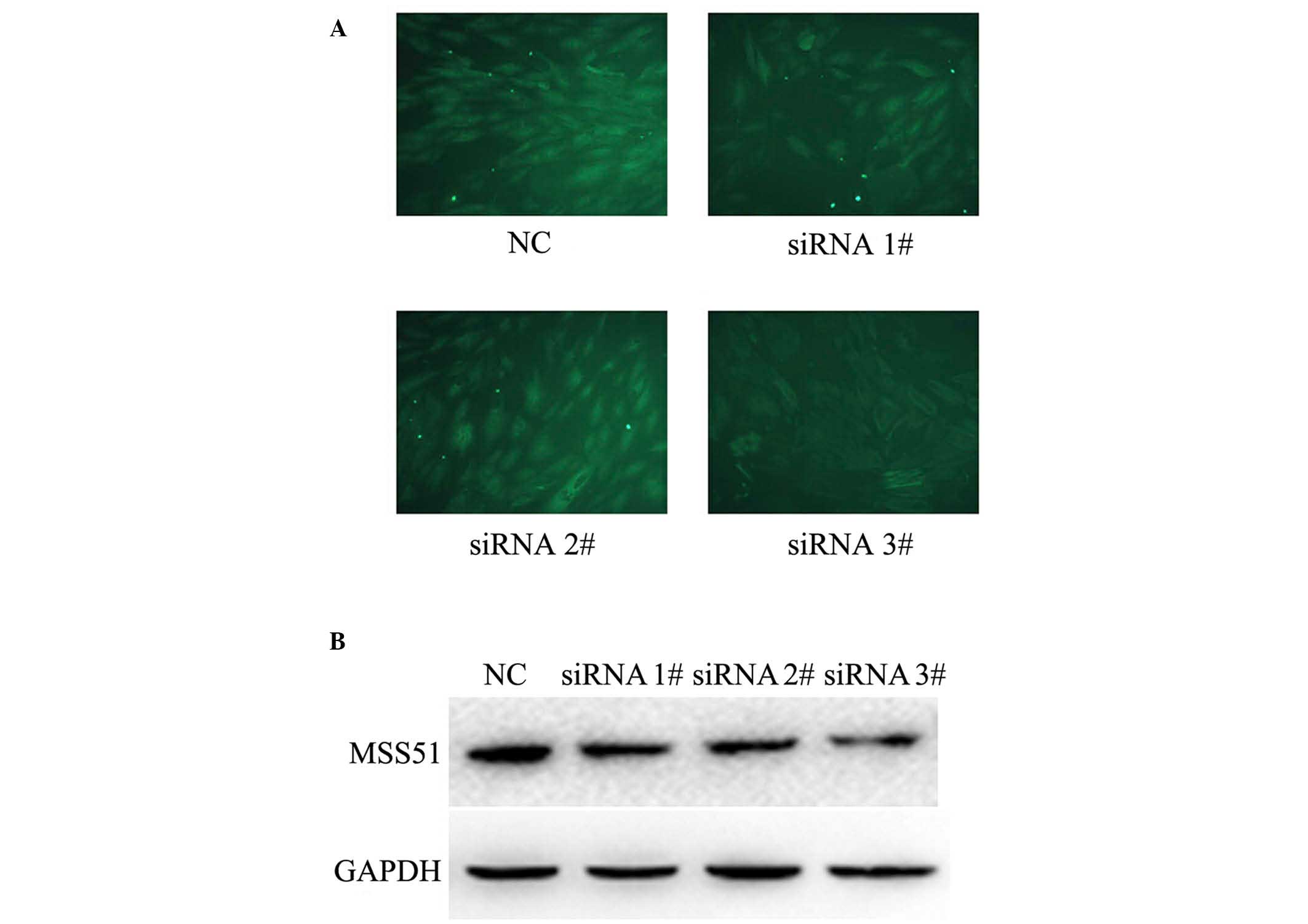
Knockdown of lncRNA AK055347 inhibited
the metabolism-associated protein MSS51 in H9C2 cells
Microarray analysis indicated that MSS51 protein was
associated with the expression of the lncRNA AK055347 (Table II). It was further investigated
whether lncRNA AK055347 regulated the expression of the
metabolism-associated protein MSS51 in H9C2 cells treated with
siRNAs against lncRNA AK055347, using immunofluorescence and
western blotting. Immunofluorescence staining indicated that
knockdown of AK055347 reduced the expression of MSS51 in H9C2 cells
(Fig. 4A). Consistent with
immunofluorescence results, western blotting results indicated that
knockdown of AK055347 inhibited the expression of MSS51 in H9C2
cells (Fig. 4B).
 | Table II.The proteins that are targets of
AK055347 predicted by Pearson correlation analysis. |
Table II.
The proteins that are targets of
AK055347 predicted by Pearson correlation analysis.
| Proteins | AK055347 |
|---|
| FAM78B | 0.991284 |
| MSS51 | 0.916019 |
| PPM1E | 0.886859 |
| CCDC19 | 0.865633 |
| AKR1B10 | 0.850339 |
| OSBPL6 | 0.823617 |
| GALNTL5 | 0.80761 |
| CENPN | 0.778999 |
| NUP62CL | 0.775631 |
| BOD1L2 | 0.766192 |
Discussion
Increasing evidence has demonstrated that lncRNAs
serve an important role in the control of the gene regulatory
network via transcriptional and post-transcriptional regulation and
epigenetic targeting (25,26). The tissue-specific gene expression
programs are finely controlled during heart development (27). Previously, lncRNAs have been
demonstrated to be important for cardiac lineage commitment and
heart development (28,29). The important role of lncRNAs in the
heart is further supported by several studies indicating that
lncRNAs are associated with numerous cardiac diseases including
myocardial infarction (15), heart
failure (16) and left ventricular
hypertrophy (17). However, it
remains unclear whether lncRNAs are involved in AF. In the present
study, microarray analysis was used to investigate the lncRNA
expression profiles between two left atrial regions, LA-PV and LAA
in patients with AF. A total of 94 lncRNAs were identified to be
differentially expressed between the LA-PV and LAA in patients with
AP. AK055347 was one of lncRNAs with the most significant
alterations, thus the function of A055347 in H9C2 cardiomyocytes
was assessed using siRNA to knock down AK055347. Knockdown of
AK055347 inhibited cell viability of H9C2 cells, accompanied by
downregulation of Cyp450 and ATP synthases. Furthermore, microarray
analysis identified that MSS51 was a target of AK055347. The
microarray result was confirmed by immunofluorescence and western
blot analysis results indicating knockdown of AK055347 inhibited
the expression of MSS51 in H9C2 cells. The results of the current
study suggest that the lncRNA AK055347 may contribute to the
pathogenesis of AF.
It has been reported that LA-PV is an important
region for AF (30). Yeh et
al (8) reported that 391 genes
were differentially expression between the LA-PV and LAA in
patients with persistent AF, including genes associated with
arrhythmia, cell death, inflammation and hypertrophy. Similarly, it
was identified that lncRNAs also exhibited this region-specific
expression between LA-PV and LAA. A total of 94 lncRNAs that were
differentially expressed between the two regions were identified.
In addition, it was observed that knockdown of AK055347 inhibited
cell viability in H9C2 cells, suggesting that AK055347 may be
associated with cell survival in cardiomyocytes.
Previous studies have demonstrated that AF is
associated with energy synthesis or consumption (20–22).
Mitochondria produce energy via the process of oxidative
phosphorylation, and mitochondrial dysfunction has been identified
to be associated with AF (31,32).
It has been reported that mitochondrial ATP synthase is upregulated
in an animal model of AF (19). In
the present study, it was demonstrated that lncRNA AK055347 was
upregulated in the LA-PV in patients with AF, and knockdown of
AK055347 significantly downregulated the expression of ATP synthase
in H9C2 cells, suggesting that AK055347 may regulate mitochondrial
energy production during AF. This hypothesis was further supported
by the observations that knockdown of AK055347 reduced the
expression of Cyp450, the terminal oxidase enzymes in electron
transfer chain.
MSS51 is a specific mitochondrial cytochrome
c oxidase (COX) regulator that is important for COX1
assembly (33,34). It has been reported that MSS51
promotes COX1 translation via interaction with the 5′-UTR of COX1
and inhibits translation via interaction with newly synthesized
COX1 (35,36). This dual effect of MSS51 is
important for correct assembly of COX in the respiratory complex
(37). In the present study,
microarray analysis demonstrated that MSS51 was the target of
lncRNA AK055347. Furthermore, the expression of MSS51 was
significantly downregulated in H9C2 cells subsequent to knockdown
of AK055347. The current study suggests that AK055347 may regulate
COX1 assembly via targeting its regulator MSS51.
In summary, a total of 94 lncRNAs were identified
that were differentially expressed between the LA-PV and LAA in
patients with AP. In addition, it was demonstrated that AK055347
was important for cell survival, due to the fact that knockdown of
AK055347 significantly inhibited viability of H9C2 cells.
Furthermore, knockdown of A055347 inhibited the expression of
mitochondrial Cyp450, ATP synthase, and MSS51, suggesting that
AK055347 may inhibit mitochondrial energy production. The present
study suggests that lncRNAs may contribute to AF pathogenesis, and
the lncRNA AK055347 may regulate mitochondrial energy production
via regulation of Cyp450, ATP synthase and MSS51.
Acknowledgements
The current study was supported by grants from the
Heilongjiang Province Natural Science Foundation of China (grant
no. H201443) and the First Affiliated Hospital of Harbin Medical
University.
References
|
1
|
Gialdini G, Nearing K, Bhave PD,
Bonuccelli U, Iadecola C, Healey JS and Kamel H: Perioperative
atrial fibrillation and the long-term risk of ischemic stroke.
JAMA. 312:616–622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Healey JS and Connolly SJ: Atrial
fibrillation: Hypertension as a causative agent, risk factor for
complications, and potential therapeutic target. Am J Cardiol.
91:9G–14G. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oluleye OW, Rector TS, Win S, McMurray JJ,
Zile MR, Komajda M, McKelvie RS, Massie B, Carson PE and Anand IS:
History of atrial fibrillation as a risk factor in patients with
heart failure and preserved ejection fraction. Circ Heart Fail.
7:960–966. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kannel WB, Wolf PA, Benjamin EJ and Levy
D: Prevalence, incidence, prognosis, and predisposing conditions
for atrial fibrillation: Population-based estimates. Am J Cardiol.
82:2N–9N. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nattel S: New ideas about atrial
fibrillation 50 years on. Nature. 415:219–226. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zöller B, Ohlsson H, Sundquist J and
Sundquist K: High familial risk of atrial fibrillation/atrial
flutter in multiplex families: A nationwide family study in Sweden.
J Am Heart Assoc. 2:e0033842013.
|
|
7
|
Ou F, Rao N, Jiang X, Qian M, Feng W, Yin
L and Chen X: Analysis on differential gene expression data for
prediction of new biological features in permanent atrial
fibrillation. PLoS One. 8:e761662013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yeh YH, Kuo CT, Lee YS, Lin YM, Nattel S,
Tsai FC and Chen WJ: Region-specific gene expression profiles in
the left atria of patients with valvular atrial fibrillation. Heart
Rhythm. 10:383–391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goodrich JA and Kugel JF: Non-coding-RNA
regulators of RNA polymerase II transcription. Nat Rev Mol Cell
Biol. 7:612–616. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu FJ, Zheng JJ, Dong PH and Fan XM: Long
non-coding RNAs and hepatocellular carcinoma. Mol Clin Oncol.
3:13–17. 2015.PubMed/NCBI
|
|
11
|
Sun M and Kraus WL: Minireview: Long
noncoding RNAs: New ‘links’ between gene expression and cellular
outcomes in endocrinology. Mol Endocrinol. 27:1390–1402. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Quagliata L and Terracciano LM: Liver
diseases and long non-coding RNAs: New insight and perspective.
Front Med (Lausanne). 1:352014.PubMed/NCBI
|
|
13
|
Li J, Xuan Z and Liu C: Long non-coding
RNAs and complex human diseases. Int J Mol Sci. 14:18790–18808.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scheuermann JC and Boyer LA: Getting to
the heart of the matter: Long non-coding RNAs in cardiac
development and disease. EMBO J. 32:1805–1816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ishii N, Ozaki K, Sato H, Mizuno H, Saito
S, Takahashi A, Miyamoto Y, Ikegawa S, Kamatani N, Hori M, et al:
Identification of a novel non-coding RNA, MIAT, that confers risk
of myocardial infarction. J Hum Genet. 51:1087–1099. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Salvo TG, Guo Y, Su YR, Clark T,
Brittain E, Absi T, Maltais S and Hemnes A: Right ventricular long
noncoding RNA expression in human heart failure. Pulm Circ.
5:135–161. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang L, Hamad EA, Vausort M, Funakoshi H,
Feldman AM, Wagner DR and Devaux Y: Identification of candidate
long noncoding RNAs associated with left ventricular hypertrophy.
Clin Transl Sci. 8:100–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ausma J, Coumans WA, Duimel H, Van der
Vusse GJ, Allessie MA and Borgers M: Atrial high energy phosphate
content and mitochondrial enzyme activity during chronic atrial
fibrillation. Cardiovasc Res. 47:788–796. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barbey O, Pierre S, Duran MJ, Sennoune S,
Lévy S and Maixent JM: Specific up-regulation of mitochondrial
F0F1-ATPase activity after short episodes of atrial fibrillation in
sheep. J Cardiovasc Electrophysiol. 11:432–438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuboi M, Hisatome I, Morisaki T, Tanaka
M, Tomikura Y, Takeda S, Shimoyama M, Ohtahara A, Ogino K, Igawa O,
et al: Mitochondrial DNA deletion associated with the reduction of
adenine nucleotides in human atrium and atrial fibrillation. Eur J
Clin Invest. 31:489–496. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cha YM, Dzeja PP, Shen WK, Jahangir A,
Hart CY, Terzic A and Redfield MM: Failing atrial myocardium:
Energetic deficits accompany structural remodeling and electrical
instability. Am J Physiol Heart Circ Physiol. 284:H1313–H1320.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seppet E, Eimre M, Peet N, Paju K, Orlova
E, Ress M, Kõvask S, Piirsoo A, Saks VA, Gellerich FN, et al:
Compartmentation of energy metabolism in atrial myocardium of
patients undergoing cardiac surgery. Mol Cell Biochem. 270:49–61.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Cui X, Shen Y, Pang L, Zhang A,
Fu Z, Chen J, Guo X, Gan W and Ji C: Distinct expression profiles
of LncRNAs between brown adipose tissue and skeletal muscle.
Biochem Biophys Res Commun. 443:1028–1034. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rinn JL and Chang HY: Genome regulation by
long noncoding RNAs. Annu Rev Biochem. 81:145–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ounzain S, Crippa S and Pedrazzini T:
Small and long non-coding RNAs in cardiac homeostasis and
regeneration. Biochim Biophys Acta. 1833:923–933. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bruneau BG: Signaling and transcriptional
networks in heart development and regeneration. Cold Spring Harb
Perspect Biol. 5:a0082922013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grote P, Wittler L, Hendrix D, Koch F,
Währisch S, Beisaw A, Macura K, Bläss G, Kellis M, Werber M and
Herrmann BG: The tissue-specific lncRNA Fendrr is an essential
regulator of heart and body wall development in the mouse. Dev
Cell. 24:206–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Klattenhoff CA, Scheuermann JC, Surface
LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey
L, Haas S, et al: Braveheart, a long noncoding RNA required for
cardiovascular lineage commitment. Cell. 152:570–583. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kabra R and Singh JP: Catheter ablation
targeting complex fractionated atrial electrograms for the control
of atrial fibrillation. Curr Opin Cardiol. 27:49–54. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Montaigne D, Marechal X, Lefebvre P,
Modine T, Fayad G, Dehondt H, Hurt C, Coisne A, Koussa M,
Remy-Jouet I, et al: Mitochondrial dysfunction as an arrhythmogenic
substrate: A translational proof-of-concept study in patients with
metabolic syndrome in whom post-operative atrial fibrillation
develops. J Am Coll Cardiol. 62:1466–1473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Slagsvold KH, Johnsen AB, Rognmo O, Høydal
MA, Wisløff U and Wahba A: Mitochondrial respiration and microRNA
expression in right and left atrium of patients with atrial
fibrillation. Physiol Genomics. 46:505–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fontanesi F, Clemente P and Barrientos A:
Cox25 teams up with Mss51, Ssc1, and Cox14 to regulate
mitochondrial cytochrome c oxidase subunit 1 expression and
assembly in Saccharomyces cerevisiae. J Biol Chem. 286:555–566.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pierrel F, Bestwick ML, Cobine PA,
Khalimonchuk O, Cricco JA and Winge DR: Coa1 links the Mss51
post-translational function to Cox1 cofactor insertion in
cytochrome c oxidase assembly. EMBO J. 26:4335–4346. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Perez-Martinez X, Broadley SA and Fox TD:
Mss51p promotes mitochondrial Cox1p synthesis and interacts with
newly synthesized Cox1p. EMBO J. 22:5951–5961. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barrientos A, Zambrano A and Tzagoloff A:
Mss51p and Cox14p jointly regulate mitochondrial Cox1p expression
in Saccharomyces cerevisiae. EMBO J. 23:3472–3482. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Perez-Martinez X, Butler CA,
Shingu-Vazquez M and Fox TD: Dual functions of Mss51 couple
synthesis of Cox1 to assembly of cytochrome c oxidase in
Saccharomyces cerevisiae mitochondria. Mol Biol Cell. 20:4371–4380.
2009. View Article : Google Scholar : PubMed/NCBI
|