Introduction
Macrodactyly is an uncommon congenital condition
characterized by an increase in the size of all the elements or
structures of the digits or toes, including phalanges, tendons,
vessels, subcutaneous fat and finger nails. The malformation often
occurs unilaterally or asymmetrically and affects more than one
digit or toe. The clinical conditions associated with this
deformity are carpal tunnel syndrome, syndactylism,
neurofibromatosis type 1 cafe au lait spots, lipoma and nevi
(1–3).
The malformations in the fingers or toes are
considered to be associated with other syndromes of macrodactyly,
including vascular malformations, multiple enchondromatosis,
maffuci syndrome, tuberous sclerosis and neurofibromatosis type 1
(4,5). The exact pathogenesis underlying this
condition and, in particular, the role of nerve growth stimulators
in macrodactyly, remains to be fully elucidated. The present study
aimed to determine nerve overgrowth stimuli using gene expression
profiling was performed in malformed enlarged nerve tissues from
the digits or toes of patients with macrodactyly. A total of six
overexpressed (>10-fold) genes, including creatine kinase,
mitochondrial 2 (CKMT2), vasoactive intestinal peptide
(VIP), FXYD domain-containing ion transport regulator 3
(FXYD3), Glutamate ionotropic receptor NMDA 3A
(GRIN3A), GSTT1 and microtubule-associated protein
tau (MAPT) were identified for their potential contribution
to abnormal nerve overgrowth. In addition, MAPT and
GRIN3A were identified as key regulators of nerve outgrowth.
These nerve growth stimulators may contribute to nerve regeneration
and reconstruction following nerve injury.
Materials and methods
Patients
The present study was reviewed and approved by the
Ethics Committee of the First Hospital of Jilin University,
(Changchun, China). Three male patients between the ages of 17 and
25 were recruited from the First Hospital of Jilin University
between May 2011 and September 2013. All enrolled patients
underwent surgical management for isolated nonsyndromic
macrodactyly. Normal nerve tissue samples, which served as healthy
controls, were obtained from five patients undergoing elective
surgery for unrelated reasons, including road traffic accidents or
fires. Written informed consent was obtained from guardians on the
behalf of all participants.
Tissue treatment and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
A sample (~10 mg) of enlarged nerve tissue from a
digit or toe was obtained from each of the patients with
macrodactyly, which were sectioned into smaller sections (~3
mm3), snap frozen in liquid nitrogen and stored at −80°C
until further use. The frozen tissues were homogenized in cold
normal saline (0.9% NaCl), using a ULTRA-TURRAX Tube Drive
Workstation (IKA Werke GmbH & Co. KG, Staufen, Germany). Next,
RNA was extracted from the nerve tissue samples using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA USA)
according to the manufacturer's protocol. A total of 500 ng RNA was
used for reverse transcription was performed using the GoScript
Reverse Transcription system (Promega Corporation, Madison, WI,
USA). Next, the products were amplified using Power SYBR Green
Master Mix (containing SYBR Green I Dye, AmpliTaq Gold®
DNA Polymerase, dNTPs, passive reference and optimized buffer) on
an ABI 7300 (Applied Biosystems; Thermo Fisher Scientific, Inc.).
RT-qPCR was performed with an initial 10 min at 95°C followed by 40
cycles of 95°C for 15 sec, and 60°C for 1 min. All experiments were
repeated three times. Relative gene expression was calculated with
the 2−ΔΔCq method (6)
following normalization to the expression of GAPDH. The primers
used for PCR are listed in Table
I.
 | Table I.Primer sequences used in reverse
transcription-polymerase chain reaction analysis. |
Table I.
Primer sequences used in reverse
transcription-polymerase chain reaction analysis.
| Gene | Accession no. | Forward primer | Reverse primer |
|---|
| CKMT2 | NM_001825.2 |
5′-GCCGCAATGCTTCTCTG-3′ |
5′-GGCCATCCCAGCACAT-3′ |
| VIP | NM_003381.3 |
5′-TCCTTGTGCTCCTGACTC-3′ | 5′-CTGCTCCTCTTTCCATTC
−3′ |
| FXYD3 | NM_005971.3 |
5′-GTGACCCTGGGCCTGCT-3′ |
5′-CAGCTTTGGGCTGAGCCT-3′ |
| GRIN3A | NM_133445.2 |
5′-GGAGGTAGATGATGAAGGC-3′ |
5′-AAACAAGAGGGCATAACAG-3′ |
| GSTT1 | NM_000853.2 |
5′-ATCTTTGCCAAGAAGAACG-3′ |
5′-TGTGAGGACCAGTAAGGAAG-3′ |
| MAPT | NM_016835.4 |
5′-GCCAAAGGGCAGGATG-3′ |
5′-TTCGGGAAGTGACAGAAGAG-3′ |
| GAPDH | NM_002046 |
5′-GGAGTCAACGGATTTGGTC-3′ |
5′-CCCCAGCCTTCTCCAT-3′ |
Cell culture
The SH-SY5Y cell line was obtained from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China), and
cultured in Dulbecco's modified Eagle's medium supplemented with
10% fetal bovine serum (all from Gibco; Thermo Fisher Scientific,
Inc.) and 1% penicillin/streptomycin (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) at 37°C with 5% CO2. The
cells were sub-cultured when they reached 85–90% confluence and
then seeded at a density of 5×103 cells/cm2
into 12-well plates (Nest Scientific USA, Rahway, NJ, USA) for 24 h
at 37°C. The cells were then induced to differentiate into neuronal
cells by treating the cells with 10 µM retinoic acid for 72 h at
37°C (7). Subsequently, the
differentiated cells were treated with protein kinase A (PKA)
inhibitor H89 or extracellular signal-regulated kinase (ERK)1/2
inhibitor PD98059 (Beyotime Institute of Biotechnology, Haimen,
China) for up to 4 h at 37°C.
Western blot analysis
The cells were collected and treated with lysis
buffer (Beyotime Institute of Biotechnology) supplemented with 1%
protease inhibitor mixture (Sigma-Aldrich; Merck Millipore).
Subsequently, cell lysates were centrifuged at 10,000 × g
for 15 min at 4°C. Protein quantification was performed using BCA
Protein Quantification kit (Thermo Fisher Scientific, Inc.) and 30
µg protein per lane were separated on 10% SDS PAGE gel and then
transferred onto PVDF membranes (Invitrogen; Thermo Fisher
Scientific, Inc.). The membranes were incubated with 5% skimmed dry
milk for 30 min and washed three times with TBST (10 mM Tris, 150
mM NaCl and 0.1% Tween-20). Next, the membranes were incubated with
rabbit anti-human GRIN3A polyclonal antibody (cat. no. sc-98986;
1:5,000) and rabbit anti-human MAPT polyclonal antibody (cat. no.
sc-32828; 1:1,000) at 4°C overnight. Then the goat anti-rabbit
IgG-HRP secondary antibody (cat. no. sc-2302; 1:1,000) was used to
incubate the membranes for 2 h at 37°C. All antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Densitometry scores were determined using Quantity One software,
version 4.6.9 (Bio-Rad Laboratories, Inc., CA, USA).
Microarray
The RNA from the nerve tissue samples was hybridized
and scanned using the Agilent Microarray Scanner (Agilent
Technologies, Inc., Santa Clara, CA, USA) at the National
Engineering Center for Biochip (Shanghai, China), according to the
manufacturer's protocol. The raw data were obtained using Feature
Extraction 10.7 software (Agilent Technologies, Inc.) with default
settings and normalized using the Quantile algorithm in Gene Spring
11.0 software (Agilent Technologies, Inc.).
Spring 11.0 software
Gene Ontology (GO) functional annotation clustering
analysis was used to process the microarray data. The GO project
database (geneontology.org/) was used following
the criteria of the Database for Annotation, Visualization and
Integrated Discovery (david.abcc.ncifcrf.gov/) for grouping genes according
with the relevant biological processes, molecular functions and
cellular component categories.
Statistical analysis
Statistical analyses were performed using GraphPad
software, version 5.0 (GraphPad, Inc., La Jolla, CA, USA). All data
are expressed as the mean ± standard deviation. Student's t-test
was performed to analyze the results of the gene expression
profiling assays. P<0.05 was considered to indicate a
statistically significant difference.
Results
Gene expression profile analysis in
macrodactyly
The present study first examined the gene expression
profile in nerve tissue samples from three patients with
macrodactyly. Of the 29,378 genes analyzed, 22 genes were
upregulated (≥5-fold) and 143 genes were downregulated (≥5-fold) in
the macrodactyly samples, compared with genes in the normal control
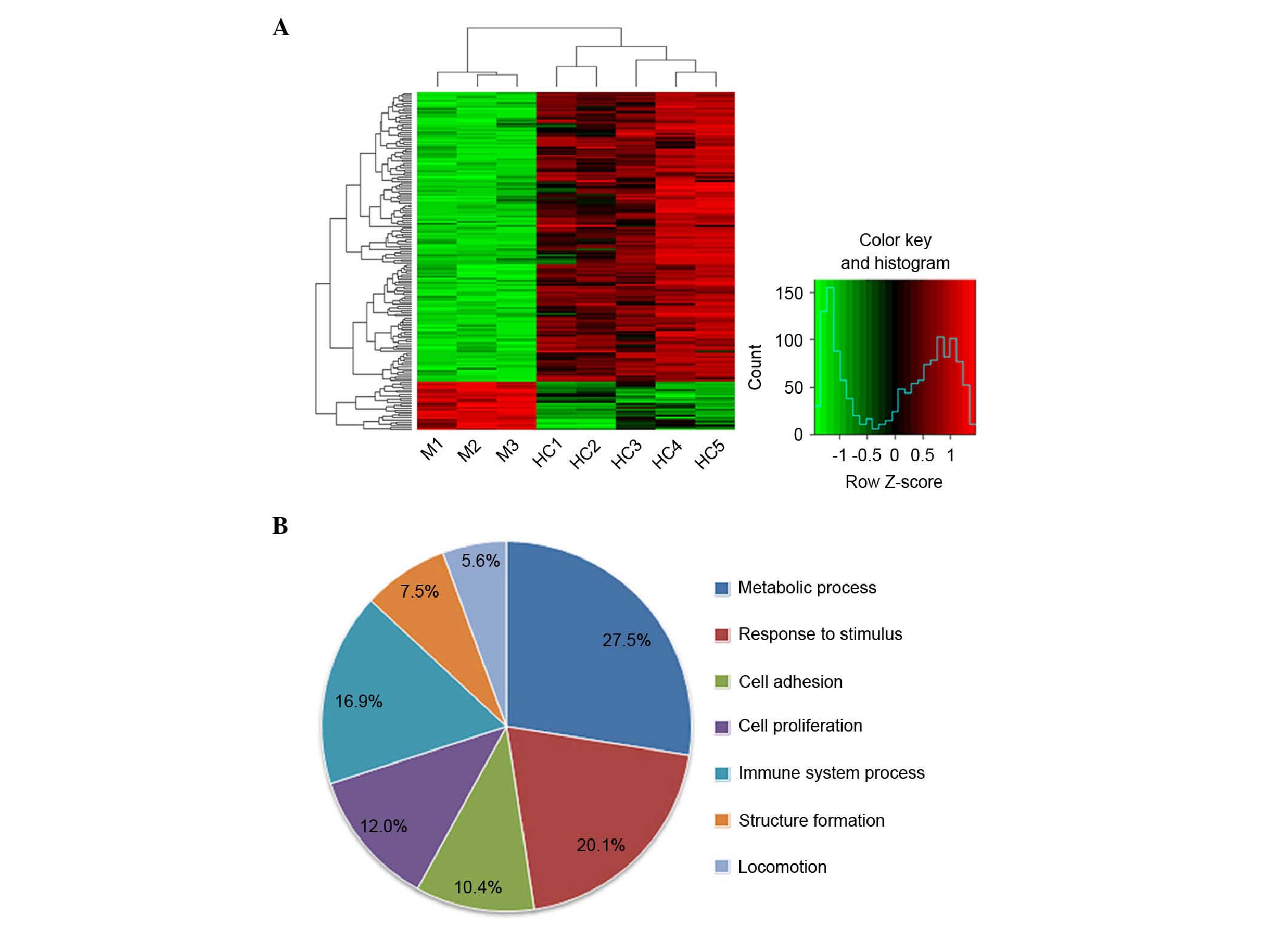
samples (Fig. 1A). In particular,
14 genes were downregulated (Table
II) and six genes were upregulated >10-fold in the
macrodactyly samples (Table
III). The genes upregulated in the samples from the patients
with macrodactyly were considered as possible targets, which may
promote nerve overgrowth.
 | Table II.Downregulated genes (>10-fold) in
the macrodactyly tissue samples. |
Table II.
Downregulated genes (>10-fold) in
the macrodactyly tissue samples.
| Gene symbol | Fold change | Description |
|---|
| ABL2 | 0.0901 | Similar to
ABL1; associated with cytoskeletal rearrangement |
| CCL20 | 0.0571 | Chemotactic factor;
associated with inflammation |
| CDC14B | 0.0523 | Serine/threonine
phosphatase; dephosphorylate p53 |
| LAMA1 | 0.0674 | α subunit of
laminin |
| IL1B | 0.0740 | Inflammatory
cytokine |
| LIF | 0.0613 | Inhibits embryonic
stem cell differentiation; promotes germ cell proliferation |
| MBP | 0.0945 | Myelin sheath
component |
| MFSD2 | 0.0872 | Mediates
syncytin-2-dependent cell-cell fusion |
| NR4A1 | 0.0764 | Nuclear
transcriptional activator |
| OSM | 0.0558 | Inhibits tumor
proliferation; regulates cytokines |
| PLAUR | 0.0825 | Degrades the
extracellular matrix |
| SPATA13 | 0.0836 | Activates
Rho-associated pathway; spermatogenesis-associated |
| SOD2 | 0.0947 | Superoxide
dismutase; |
| SOX11 | 0.0828 | Antigen in B
lymphomas; tumor suppressor |
 | Table III.Upregulated genes (>10-fold) in
the macrodactyly tissue samples. |
Table III.
Upregulated genes (>10-fold) in
the macrodactyly tissue samples.
| Gene symbol | Fold change | Description |
|---|
| CKMT2 | 10.4 | Creatine
kinase |
| VIP | 12.6 | Neuropeptide,
belongs to a glucagon/secretin superfamily |
| FXYD3 | 11.2 | Na, K-ATPase ion
channel regulator |
| GRIN3A | 10.8 | Ionotropic
glutamate receptor |
| GSTT1 | 13.4 | Catalyzes the
conjugation of reduced glutathione to electrophilic moeities of
xenobiotics or endogenous compounds |
| MAPT | 12.7 |
Microtubule-associated protein tau |
The differentially expressed genes were grouped
according to their biological process using Gene Ontology
annotation (Fig. 1B). In the
patients with macrodactyly, the majority of the differentially
expressed genes were involved in metabolic process (27.5%),
response to stimulus (20.1%) and immune system process (16.9%).
Confirmation of genes, which may
contribute to nerve overgrowth
The upregulated genes identified in the present
study (≥10-fold) may promote nerve overgrowth, therefore, these
target genes were further confirmed at the transcriptional level.
The results showed that, of the six candidate genes, VIP, FXYD3,
GRIN3A and MAPT were upregulated, which was consistent
with the microarray data (Fig.
2A). To further confirm the upregulation of the above genes,
retinoic acid was used to induce the neuronal differentiation of
SH-SY5Y cells (Fig. 2B), and the
mRNA levels of VIP, FXYD3, GRIN3A and MAPT in the
SH-SY5Y cells were measured using RT-qPCR analysis. As presented in
Fig. 2C and D, the transcriptional
expressions of GRIN3A and MAPT were increased along
with the induction. Subsequently, the translational levels of these
two genes (Fig. 2E and F) were
determined and the results also confirmed the result that
GRIN3A and MAPT were upregulated along with the
process of nerve axon growth during differentiation of the SH-SY5Y
cells.
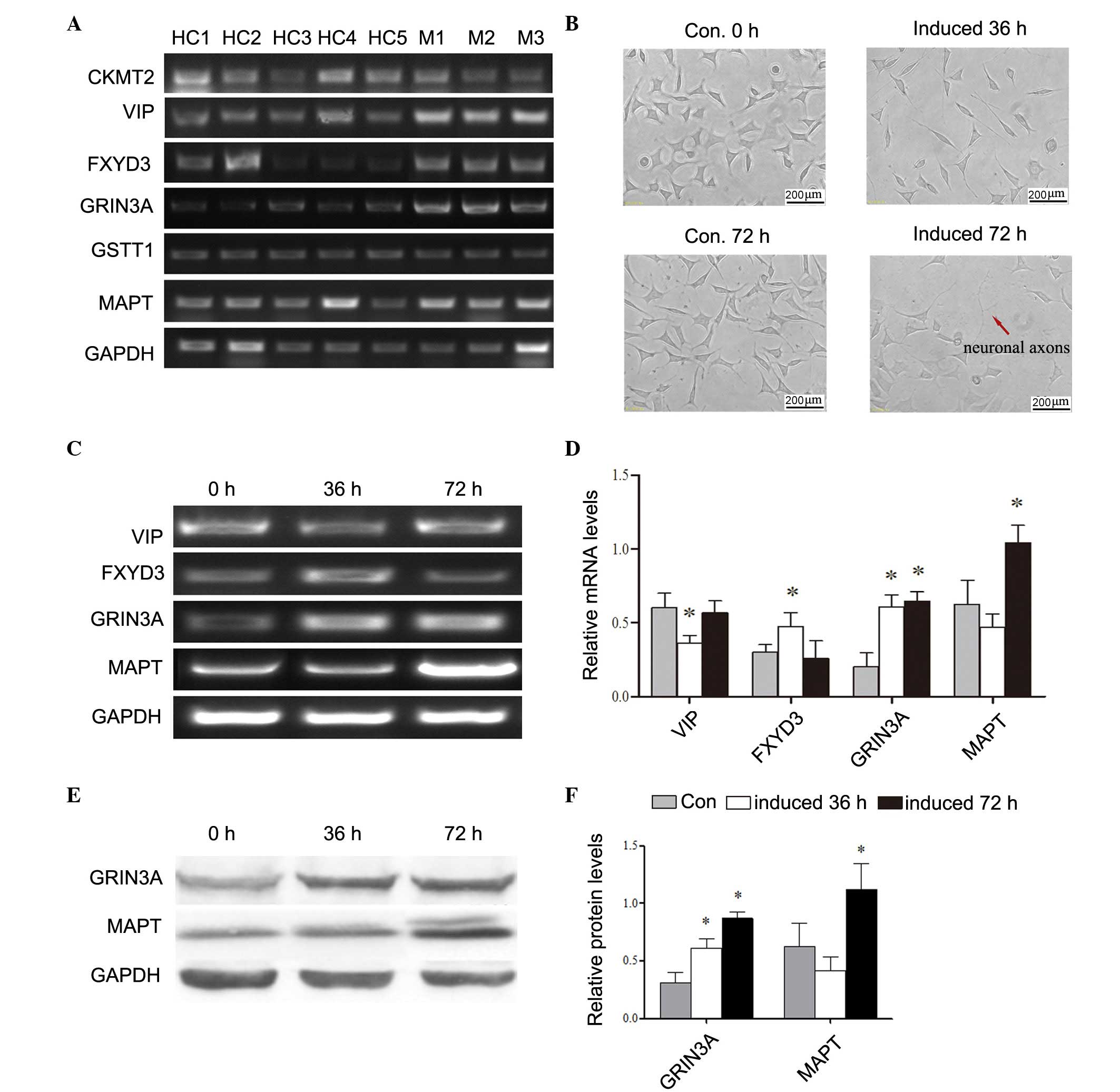 | Figure 2.Analysis of mRNA levels of candidate
genes associated with nerve outgrowth. mRNA levels were measured to
confirm the expression profile of the six candidate genes
(CKMT2, VIP, FXYD3, GRIN3A, GSTT1 and MAPT). (A) mRNA
levels of CKMT2, VIP, FXYD3, GRIN3A, GSTT1 and MAPT
were determined with GAPDH as the reference. (B)
Differentiation of SH-SY5Y cells induced by retinoic acid for 72 h.
Scale bar=200 µm. (C) Confirmation of selected genes following cell
differentiation. (D) Histogram showing results of the analysis of
relative mRNA levels of target genes. (E) Western blot analysis of
translational levels following differentiation. (F) Quantification
of western blotting. *P<0.05, vs. Con. CKMT2, creatine
kinase, mitochondrial 2; VIP, vasoactive intestinal peptide;
FXYD3, FXYD domain-containing ion transport regulator 3;
GRIN3A, glutamate ionotropic receptor NMDA 3A; GSTT1,
glutathione S-transferase θ1; MAPT, microtubule-associated
protein tau; Con, control. |
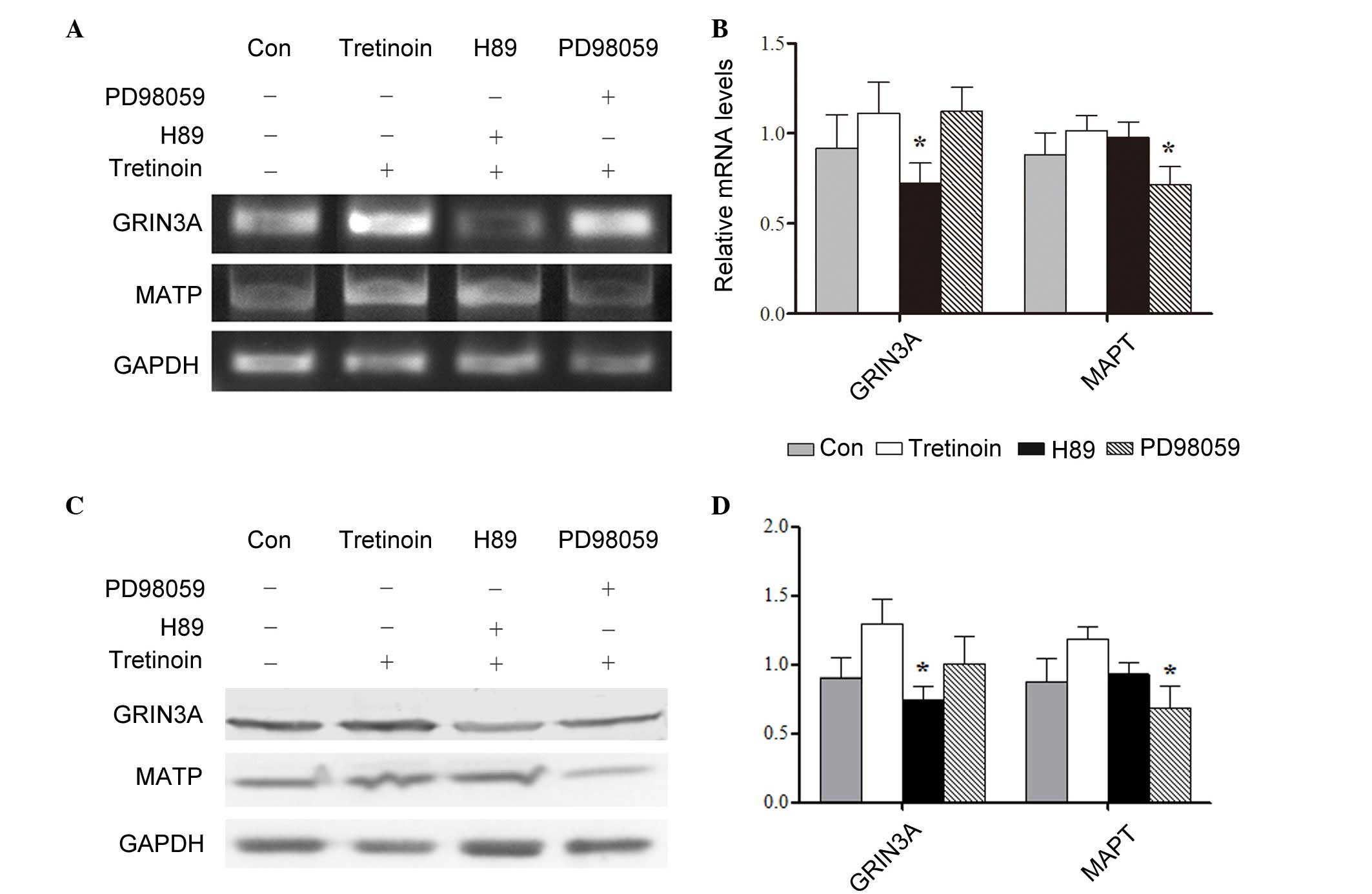
Signaling pathways responsible for the
upregulation of target genes
To investigate the signaling pathways and events,
which may stimulate nerve cell proliferation, the SH-SY5Y cells
were induced by retinoic acid for 72 h. Retinoic acid has been
shown to induce cell differentiation by sustained phosphorylation
of ERK1/2 or cAMP response element binding protein (8–10).
Therefore, PD98059 and H89 were added to the cells to inhibit the
ERK1/2 and cAMP/PKA pathways, respectively. The results showed that
at transcriptional and translational level, the expression of
GRIN3A was regulated by the cAMP/PKA pathway, whereas the
expression of MAPT was affected by the ERK1/2 pathway
(Fig. 3A-D).
Discussion
Macrodactyly is an uncommon congenital nervous
system disease, and is characterized by the proliferation of nerve
fibers, vessels, subcutaneous fat and other tissues (1–3). The
pathogenesis of macrodactyly remains to be fully elucidated,
therefore, the identification of genes expressed at high levels in
macrodactyly may reveal factors, which promote nerve overgrowth and
contribute to the pathogenesis of this condition.
In the present study, the gene expression profiles
were compared between patients with macrodactyly and healthy
controls. It was found that a significant number of genes (143;
87%) were downregulated (≥5-fold), whereas only 22 (13%) genes were
upregulated (≥5-fold). Among the downregulated genes,
apoptosis-associated factors, including B cell lymphoma 2, cell
division cycle 14 B and pentraxin 3, and microtubule formation
associated promoters, including MAP1, MAPT and sorting nexin
22, were downregulated, as expected. Of note, pro-inflammatory
cytokines or associated receptors, including interleukin
(IL)1β, IL1 receptor (R), IL7R and IL8, and
the regulators of cell-cell or cell-extracellular matrix
interactions, including chemokine (C-C motif) ligand 20, C-X-C
chemokine receptor type 4, integrin αX, integrin β8, major
facilitator superfamily domain-containing 2, plasminogen activator,
urokinase and selectin L, were significantly downregulated. It is
known that inflammatory cytokines and cell adhesion molecules are
frequently overexpressed in human cancer, and are involved in
oncogenesis and metastasis by improving extracellular matrix
degradation (11–15). The results of the present study
indicated that cell invasion and metastasis may be restrained in
macrodactyly, and this may explain why macrodactyly seldom develops
to malignancy.
In addition, the present study found that
angiogenesis-associated positive regulators, including vascular
endothelial growth factor (VEGF)A/B/C, Notch,
δ-like 4 and macrophage migration inhibitory factor, were not
expressed at high levels, and a number even showed decreased
expression, including fibroblast growth factor receptor 1,
microRNA 2 (mir21) and mir221. It is well known that
vessel overgrowth is common in tumors, particularly in malignancy
(16,17). Therefore, the present study
hypothesized that the abnormal vascular proliferation observed in
macrodactyly is not due to hyperplastic nerve tissue directly.
The genes, which identified as being upregulated in
the patients with macrodactyly were considered to be involved in
promoting nerve overgrowth. The present study found 14
downregulated (>10-fold) genes and six upregulated (>10-fold)
genes (CKMT2, VIP, FXYD3, GRIN3A, GSTT1 and MAPT) in
the macrodactyly samples. Subsequently, the upregulated levels of
VIP, FXYD3, GRIN3A and MAPT were confirmed using
RT-qPCR analysis. GRIN3A and MAPT have been shown to
be involved in stimulating nerve growth. MAPT, is involved
in improving nerve proliferation, and is expressed at high levels
in multiple neurodegenerative disorders, including progressive
supranuclear palsy, Parkinson's disease and Alzheimer's disease
(18,19). As an ionotropic glutamate receptor,
GRIN3A is involved in regulating nerve signal transduction
(20,21).
Nerve growth is a result of the combined effects of
multiple genes and signaling networks, thus selecting positive
regulators from a nerve outgrowth model may be a promising strategy
for identifying key factors and signaling pathways. To investigate
the signaling pathways responsible for promoting nerve growth,
retinoic acid was used to induce the differentiation of SH-SY5Y
cells (7). By inhibiting the
ERK1/2 pathway and cAMP-PKA pathway, which are involved in neurite
proliferation, it was found that GRIN3A showed a significant
correlation with the cAMP-PKA pathway, whereas MAPT was
affected by the ERK1/2 pathway.
Increased expression levels of MAPT and
GRIN3A may lead to the abnormal pathologic condition of
nerve tissue in macrodactyly and, to a certain extent, may have
potential applications in nerve regeneration. It is known that axon
continuity is always interrupted during nerve damage, and the
distal axonal tract finally undergoes degeneration. Thus,
identifying techniques to reconstruct the structure of nerve
tissue, and restore its function following nerve injury and repair
has been one of the key areas of investigation in tissue
engineering. Gene therapy is one potential approach in the
treatment of traumatic nerve injury. Various genes are being
considered as candidates for promoting nerve regeneration,
including VEGF (22,23). Based on the findings from the
present study, MAPT and GRIN3A warrant further
examination as two potential factors, which may contribute to nerve
regeneration and reconstruction following nerve injury.
Acknowledgements
The authors would like to thank Medjaden Bioscience
Limited (Hong Kong, China) for assistance with proofreading.
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 30972610 and
81273240), the Health Department Research Projects in Jilin
Province (grant no. 2009Z054) and the Norman Bethune Program of
Jilin University (grant no. 2012206).
References
|
1
|
Ho CA, Herring JA and Ezaki M: Long-term
follow-up of progressive macrodystrophia lipomatosa. A report of
two cases. J Bone Joint Surg Am. 89:1097–1102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lau FH, Xia F, Kaplan A, Cerrato F, Greene
AK, Taghinia A, Cowan CA and Labow BI: Expression analysis of
macrodactyly identifies pleiotrophin upregulation. PLoS One.
7:e404232012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Biesecker LG, Aase JM, Clericuzio C,
Gurrieri F, Temple IK and Toriello H: Elements of morphology:
Standard terminology for the hands and feet. Am J Med Genet A 149A.
93–127. 2009. View Article : Google Scholar
|
|
4
|
Rios JJ, Paria N, Burns DK, Israel BA,
Cornelia R, Wise CA and Ezaki M: Somatic gain-of-function mutations
in PIK3CA in patients with macrodactyly. Hum Mol Genet. 22:444–451.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rohilla S, Jain N, Sharma R and
Dhaulakhandi DB: Macrodystrophia lipomatosa involving multiple
nerves. J Orthop Traumatol. 13:41–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheung YT, Lau WK, Yu MS, Lai CS, Yeung
SC, So KF and Chang RC: Effects of all-trans-retinoic acid on human
SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research.
Neurotoxicology. 30:127–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang Y, Boskovic G and Niles RM: Retinoic
acid-induced AP-1 transcriptional activity regulates B16 mouse
melanoma growth inhibition and differentiation. J Cell Physiol.
194:162–170. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hung SP, Hsu JR, Lo CP, Huang HJ, Wang JP
and Chen ST: Genistein-induced neuronal differentiation is
associated with activation of extracellular signal-regulated
kinases and upregulation of p21 and N-cadherin. J Cell Biochem.
96:1061–1070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tegenge MA, Roloff F and Bicker G: Rapid
differentiation of human embryonal carcinoma stem cells (NT2) into
neurons for neurite outgrowth analysis. Cell Mol Neurobiol.
31:635–643. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bachireddy P, Rakhra K and Felsher DW:
Immunology in the clinic review series; focus on cancer: Multiple
roles for the immune system in oncogene addiction. Clin Exp
Immunol. 167:188–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mantovani A, Romero P, Palucka AK and
Marincola FM: Tumour immunity: Effector response to tumour and role
of the microenvironment. Lancet. 371:771–783. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moh MC and Shen S: The roles of cell
adhesion molecules in tumor suppression and cell migration: A new
paradox. Cell Adh Migr. 3:334–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nair KS, Naidoo R and Chetty R: Expression
of cell adhesion molecules in oesophageal carcinoma and its
prognostic value. J Clin Pathol. 58:343–351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hedlund EM, Hosaka K, Zhong Z, Cao R and
Cao Y: Malignant cell-derived PlGF promotes normalization and
remodeling of the tumor vasculature. Proc Natl Acad Sci USA.
13:17505–17510. 2009. View Article : Google Scholar
|
|
17
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Elbaz A, Ross OA, Ioannidis JP,
Soto-Ortolaza AI, Moisan F, Aasly J, Annesi G, Bozi M, Brighina L,
Chartier-Harlin MC, et al: Independent and joint effects of the
MAPT and SNCA genes in Parkinson's disease. Ann Neurol. 69:778–792.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Caffrey TM and Wade-Martins R: Functional
MAPT haplotypes: Bridging the gap between genotype and
neuropathology. Neurobiol Dis. 27:1–10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu XJ and Salter MW: Glutamate receptor
phosphorylation and trafficking in pain plasticity in spinal cord
dorsal horn. Eur J Neurosci. 32:278–289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oh MC, Kim JM, Safaee M, Kaur G, Sun MZ,
Kaur R, Celli A, Mauro TM and Parsa AT: Overexpression of
calcium-permeable glutamate receptors in glioblastoma derived brain
tumor initiating cells. PLoS One. 7:e478462012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wong WK, Cheung AW, Yu SW, Sha O and Cho
EY: Hepatocyte growth factor promotes long-term survival and axonal
regeneration of retinal ganglion cells after optic nerve injury:
Comparison with CNTF and BDNF. CNS Neurosci Ther. 20:916–929. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pelletier J, Roudier E, Abraham P, Fromy
B, Saumet JL, Birot O and Sigaudo-Roussel D: VEGF-A promotes both
pro-angiogenic and neurotrophic capacities for nerve recovery after
compressive neuropathy in rats. Mol Neurobiol. 51:240–251. 2015.
View Article : Google Scholar : PubMed/NCBI
|