Introduction
Systemic lupus erythematosus (SLE) is a complex
autoimmune disease, which is characterized by a loss of
self-tolerance and the production of autoantibodies against a host
of nuclear self-antigens (1). SLE
predominantly affects women of childbearing age, and ≤20% of cases
begin during teenage years. Lupus nephritis (LN) is kidney
inflammation caused by SLE, and is the leading cause of morbidity
and mortality in patients with SLE (2). One of the primary characteristics of
LN is excessive proliferation of mesangial cells; disordered
mesangial cell proliferation usually results in nephritic syndrome,
which eventually leads to renal failure (3,4). LN
is a severe disorder and the currently available therapeutic
methods are ineffective; therefore, it is essential to identify
novel useful targets for the treatment of LN.
MicroRNAs (miRNAs/miR) are small, non-coding RNAs
that endogenously control gene expression by partial complementary
binding to the 3′ untranslated region (3′-UTR) of target mRNAs
(5–8). Previous studies have reported that
miRNAs are involved in diverse physiological and pathophysiological
functions, including cell differentiation and development, cell
cycle and apoptosis, immune tolerance and carcinogenesis (9,10).
In addition, studies have demonstrated that several miRNAs exhibit
aberrant expression in LN. Dai et al identified 36
upregulated and 30 downregulated miRNAs in LN renal tissues
compared with in healthy kidney tissues (11). Denby et al reported that
miR-21 and miR-214 were induced by transforming growth factor-β
stimulation in an anti-Thy1.1 rat model (12). Furthermore, Te et al
revealed that five miRNAs (hsa-miR-371-5p, hsa-miR-423-5p,
hsa-miR-638, hsa-miR-1224-3p and hsa-miR-663) were abnormally
expressed in LN across various racial groups, as determined by
miRNA microarray analysis (13).
These results suggest that miRNA may function as a valuable novel
tool for understanding, diagnosing and treating LN.
Hsa-miR-371-5p is a novel miRNA that is present on
chromosome 19q13.42 in the human genome, the function of which has
not yet been elucidated. Hsa-miR-371-5p has previously been
reported to be dysregulated in LN (13). However, at present, the biological
role and molecular mechanisms of hsa-miR-371-5p in LN remain
unclear.
The present study aimed to identify the biological
function and molecular mechanisms of hsa-miR-371-5p in LN.
Hsa-miR-371-5p was shown to be downregulated in LN tissues.
Furthermore, exogenous restoration of hsa-miR-371-5p expression was
able to inhibit human mesangial cell proliferation and promote
apoptosis by directly targeting hypoxia-inducible factor 1α
(HIF-1α). The results of the present study indicate that
hsa-miR-495 may be considered a potential therapeutic target for
the future treatment of LN.
Materials and methods
Cell culture
The human mesangial cell line was obtained from the
Cell Resource Center, Shanghai Institutes for Biological Sciences
(Shanghai, China). The cells were cultured in Dulbecco's modified
Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Beyotime Institute of Biotechnology, Haimen, China) at 37°C in an
atmosphere containing 5% CO2. Penicillin (100 U/ml) and
streptomycin (100 µg/ml) were added to the culture medium to
prevent bacterial contamination.
Renal samples
A total of 35 SLE renal biopsy samples were
collected from the Fourth Hospital of Harbin Medical University
(Harbin, China) between January 2010 and January 2014. All SLE
patients (6 male and 29 female; mean age, 26.6±3.4) exhibited the
necessary criteria for a diagnosis of SLE. The classification of LN
was based on the International Society of Nephrology/Renal
Pathology Society criteria published in 2003 (14). Renal carcinoma-adjacent normal
kidney tissues were collected from 35 patients as the control group
(18 male and 17 female; mean age, 47.6±4.7). Written informed
consent was obtained from all of the participants or their
relatives. The present study was approved by the Ethics Committee
of the Fourth Hospital of Harbin Medical University, and was
conducted in compliance with the Helsinki Declaration.
Total RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from homogenized SLE renal
biopsy tissues, normal kidney tissues and mesangial cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Subsequently, RNA
was reverse transcribed using a miScript RT kit (Qiagen, Inc.,
Valencia, CA, USA) or a PrimeScript RT reagent kit (Takara Bio,
Inc., Otsu, Japan) according to the manufacturer's protocols. qPCR
was conducted using a TaqMan® miRNA assay kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). qPCR was performed on
an Applied Biosystems Prism® 7900HT sequence detection
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with a
total volume of 10 µl PCR mix, which consisted of: 1.0 µl template
cDNA, 4 µl SYBR Green mixture and 1.0 µl primers; the PCR mix was
supplemented with water to adjust the final volume to 20 µl.
The hsa-miR-371-5p, U6, HIF-1α and GAPDH primers
were purchased from Applied Biosystems (Thermo Fisher Scientific,
Inc.). The sequences were as follows: Hsa-miR-371-5p, forward
5′-ACTCAAAAGATGGCGGCAC-3′, the reverse primer was supplied by the
miScript RT kit; U6, forward 5′-CTCGCTTCGGCAGCACA-3′, reverse
5′-ACGCTTCACGAATTTGCGT-3′; HIF-1α, forward
5′-CTCAGAATGAAGTGTACCCTAA-3′, reverse 5′-CAAATCAGCACCAAGCAG-3′; and
GAPDH, forward 5′-GACCTGACCTGCCGTCTA-3′, reverse
5′-AGGAGTGGGTGTCGCTGT-3′. All reactions were incubated in a 96-well
plate at 95°C for 20 min, followed by 45 cycles at 95°C for 10 sec
and 60°C for 20 sec. The U6 gene was used as an internal control to
normalize differences in hsa-miR-371-5p expression in each sample.
The relative amount of hsa-miR-371-5p expression to U6 expression
was determined using the 2−ΔΔCq (15).
Western blot analysis
Total protein was extracted from the cultured cells
by using radioimmunoprecipitation assay buffer (Invitrogen; Thermo
Fisher Scientific, Inc.). The concentration of each protein was
detected by a Enhanced bicinchoninic acid Protein Assay kit
(Beyotime Institute of Biotechnology). Equal amounts of protein (40
µg) isolated from mesangial cells were separated by 10% SDS-PAGE
and were blotted onto a polyvinylidene difluoride membrane (Merck
Millipore, Darmstadt, Germany). After blocking with 5% non-fat milk
for 2 h at 37°C, the membrane was incubated with mouse monoclonal
anti-HIF-1α (cat. no. ab113642; 1:1,000; Abcam, Cambridge, UK) and
GAPDH (cat. no. ab8245; 1:10,000; Abcam) antibodies overnight at
4°C. Subsequently, the membrane was washed three times with TBS
containing 0.1% Tween for 15 min and was incubated with the
corresponding horseradish peroxidase-conjugated secondary antibody
(cat. no. ab6789; 1:2,000; Abcam) for 1 h at room temperature. The
results were visualized by enhanced chemiluminescence (Merck
Millipore).
Cell transfection assay
The synthesized hsa-miR-371-5p mimics and control
mimics (Shanghai GenePharma Co., Ltd., Shanghai, China) were
transfected into mesangial cells using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.), in order to
promote hsa-miR-371-5p expression, according to the manufacturer's
protocols. A total of 24 h post-transfection, the cells were
harvested and total RNA and protein were extracted for qPCR and
western blot analyses. The sequences were as follows:
Hsa-miR-371-5p mimics, 5′-ACUCAAAAGAUGGCGGCACUU-3′; and control
mimics, 5′-AGUACUUUUGUGUAGUACA-3′.
Analysis of mesangial cell
proliferation
Mesangial cell proliferation was detected using an
MTT assay (Sigma-Aldrich; Merck Millipore), according to the
manufacturer's protocol. Briefly, ~4,000 mesangial cells were
cultured in 96-well plates and treated with 10 pmol hsa-miR-371-5p
mimics or control mimics. At 0, 24, 48 or 72 h post-transfection,
the cells were treated with 30 µl MTT (5 µg/ml) for 4 h at 37°C,
then with 150 µl dimethyl sulfoxide for 15 min at 37°C. The results
were determined using a microplate reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) at a dual wavelength of 450/630 nm.
Analysis of mesangial cell
apoptosis
Mesangial cells (~8×105) were plated into
6-well plates and were transfected with hsa-miR-371-5p mimics or
control mimics within 24 h. All mesangial cells, including floating
and dead cells, were collected 48 h post-transfection, were washed
twice with cold PBS and were treated with 100 µl 1X binding buffer
(Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently, 5 µl
Annexin V-fluorescein isothiocyanate (Invitrogen; Thermo Fisher
Scientific, Inc.) and 4 µl propidium iodide were added to the
reactions. The results were analyzed using flow cytometry (Beckman
Coulter, Inc., Brea, CA, USA) at 488 nm excitation within 30 min of
staining, according to the manufacturer's protocols.
Plasmid construction and luciferase
activity analysis
PicTar (http://pictar.mdc-berlin.de/) and TargetScan
(http://www.targetscan.org/) databases
were used to predict the complementary region of hsa-miR-371-5p to
HIF-1α. Luciferase reporters were constructed using molecular
cloning technology. The target sequence was inserted into the
psiCHECK-2 luciferase reporter plasmid (Promega Corporation,
Madison, WI, USA) to obtain the psiCHECK-2-HIF-1α wild type (WT)
recombinant reporter vector, which contained the hsa-miR-371-5p
binding sequence. The psiCHECK-2-HIF-1α mutant (MUT) recombinant
reporter vector was chemically synthesized by Shanghai GenePharma
Co., Ltd.
Luciferase assays were then used to assess whether
HIF-1α was a direct target of hsa-miR-371-5p. Briefly, mesangial
cells were cultured in 24-well plates until they reached 70–80%
confluence, when they were transiently co-transfected with 0.2 µg
WT or MUT reporter plasmid, 10 nmol hsa-miR-371-5p mimics or
control mimics using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). A total of 48 h post-transfection,
all samples were harvested and analyzed using the Dual-Luciferase
Reporter Assay system (Promega Corporation), according to the
manufacturer's protocol. Renilla luciferase activity was
normalized to Firefly luciferase activity for each experiment. All
transfection assays were carried out in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
statistical software package (SPSS, Inc., Chicago, IL, USA). The
expression levels of hsa-miR-371-5p in LN renal tissues and normal
kidney tissues, luciferase assay, cell expression and flow
cytometry results were analyzed using Student's t-test. The
differences between hsa-miR-371-5p mimics- or control
mimics-transfected mesangial cells in the MTT assay were analyzed
using one-way analysis of variance with Dunnett's multiple
comparison test (>2 groups) or t test (2 groups). P<0.05 was
considered to indicate a statistically significant difference.
Results
Hsa-miR-371-5p expression is
downregulated in LN renal tissues
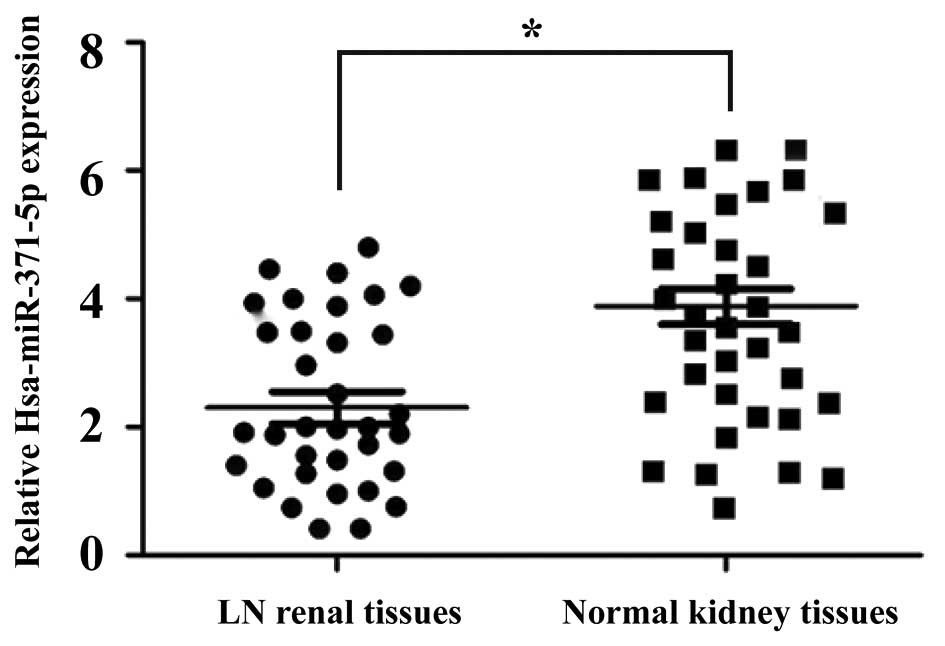
The expression levels of hsa-miR-371-5p were
detected in 35 LN renal tissues and 35 non-cancerous normal kidney
tissues using RT-qPCR. As shown in Fig. 1, the expression levels of
hsa-miR-371-5p were significantly decreased in the LN renal tissues
compared with in the non-cancerous normal kidney tissues.
Restoration of hsa-miR-371-5p
suppresses human mesangial cell proliferation
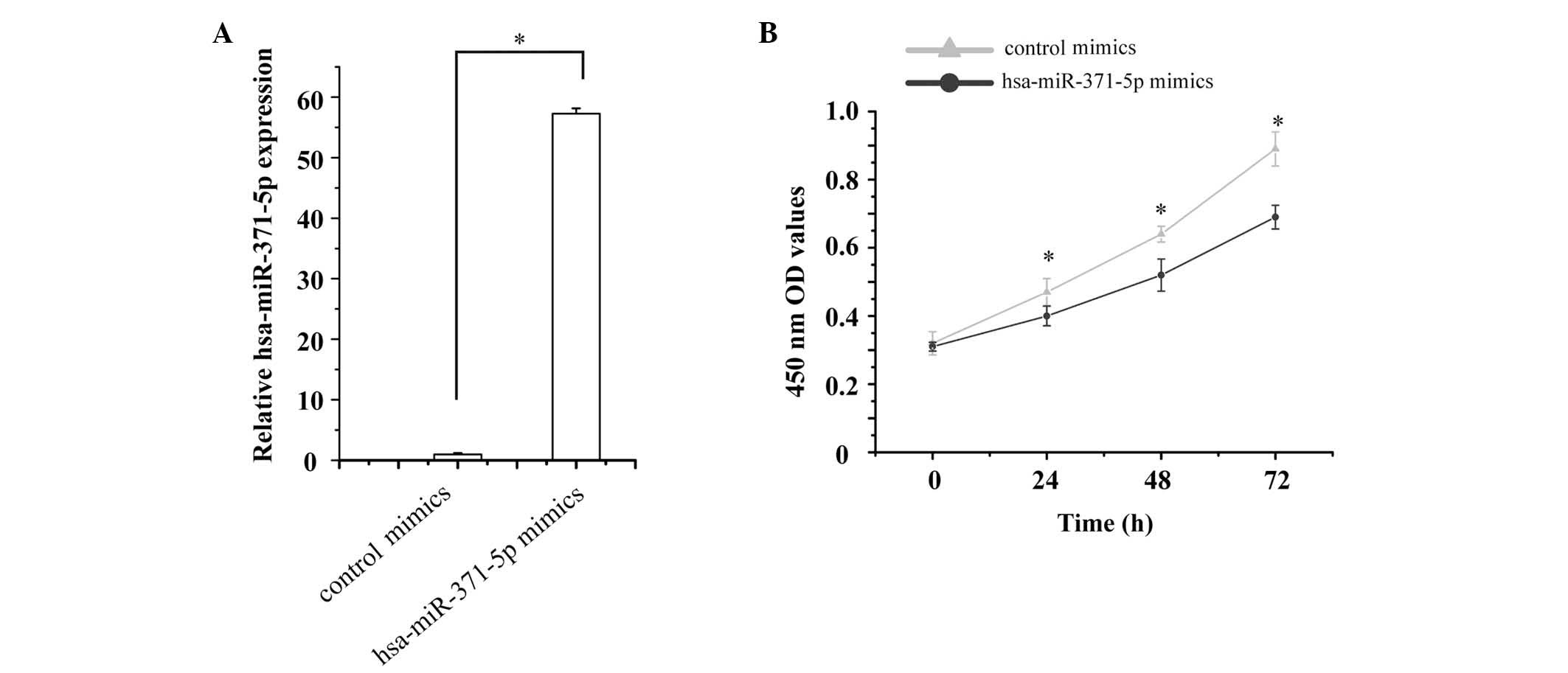
Since hsa-miR-371-5p expression was markedly
decreased in LN renal tissues, the present study hypothesized that
low levels of hsa-miR-371-5p may be associated with human mesangial
cell growth. To test this hypothesis, hsa-miR-371-5p mimics and
control mimics were transfected into human mesangial cells. The
relative expression levels of hsa-miR-371-5p were then detected by
RT-qPCR. The expression levels of hsa-miR-371-5p were 57.26-fold
higher in human mesangial cells transfected with hsa-miR-371-5p
mimics, as compared with in cells transfected with control mimics
(Fig. 2A).
Mesangial cell proliferation was detected using an
MTT assay. The effects of hsa-miR-371-5p overexpression were
determined on human mesangial cell proliferation. The results
revealed that transfection with hsa-miR-371-5p mimics significantly
inhibited human mesangial cell proliferation compared with in the
control group (Fig. 2B).
Restoration of hsa-miR-371-5p induces
human mesangial cell apoptosis
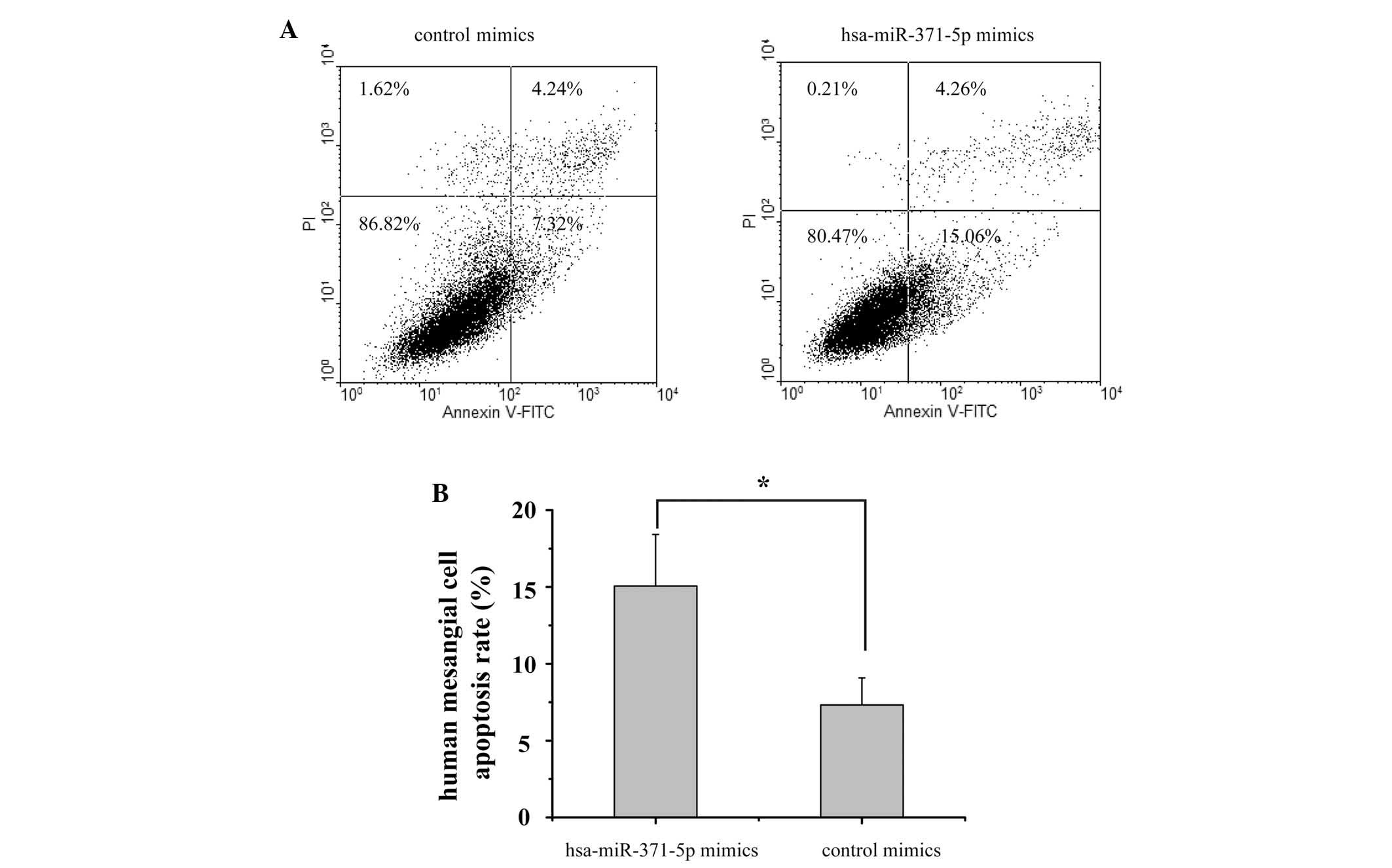
A flow cytometric assay was performed to examine the
apoptotic effects of hsa-miR-371-5p on human mesangial cells. As
shown in Fig. 3, 48 h
post-transfection the apoptotic rate of the hsa-miR-371-5p mimics
group was 15.06%, as compared with 7.32% in the control mimics
group. These results suggest that overexpression of hsa-miR-371-5p
may promote human mesangial cell apoptosis.
HIF-1α is a direct target of
hsa-miR-371-5p
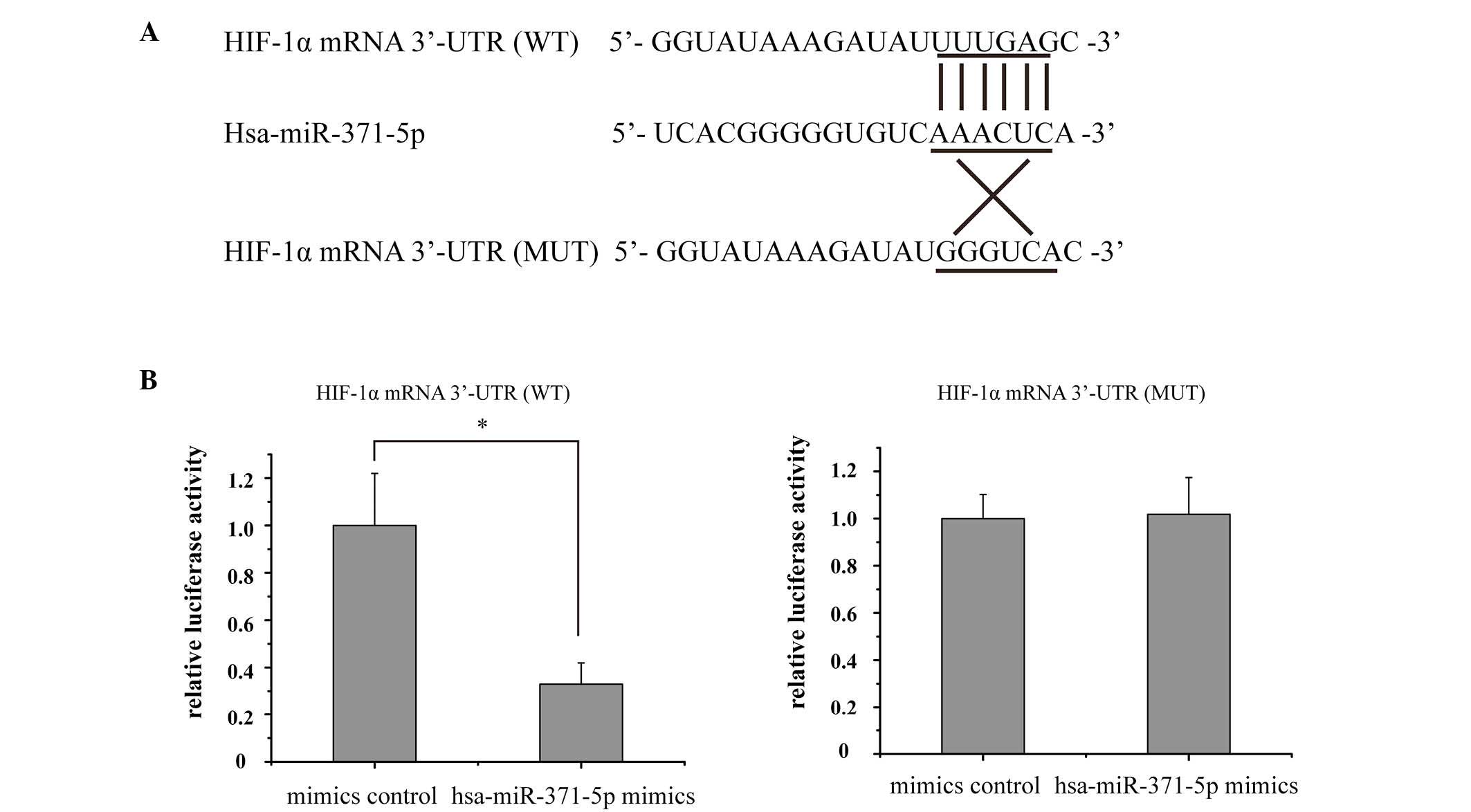
Bioinformatics analyses demonstrated that the HIF-1α
mRNA 3′-UTR contained a potential complimentary binding site to
hsa-miR-371-5p (Fig. 4A). In order
to verify this prediction, a luciferase assay was conducted in
human mesangial cells. Relative luciferase activity was markedly
decreased in the hsa-miR-371-5p-transfected human mesangial cells,
whereas there was no significant difference in luciferase activity
between the hsa-miR-371-5p mimics and control mimics group
following co-transfection with the MUT recombinant plasmid
(Fig. 4B). These results indicate
that HIF-1α is a direct target gene of hsa-miR-371-5p in human
mesangial cells.
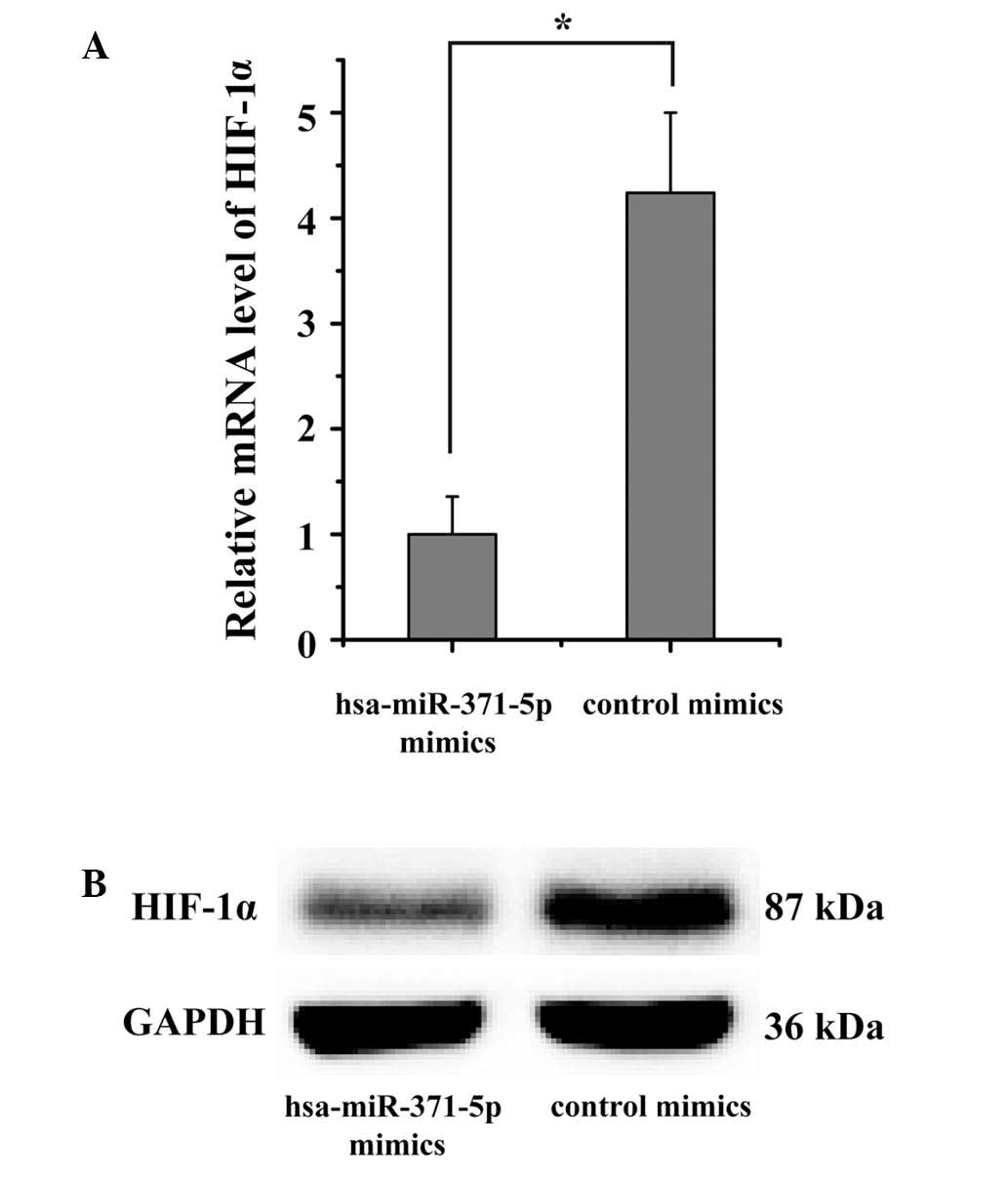
HIF-1α expression is downregulated by
hsa-miR-371-5p in human mesangial cells
The effects of hsa-miR-371-5p on the endogenous
expression of HIF-1α were subsequently confirmed in human mesangial
cells. The present study aimed to verify whether the mRNA and
protein expression levels of HIF-1α were regulated by
hsa-miR-371-5p. Hsa-miR-371-5p mimics were transfected into human
mesangial cells using Lipofectamine® 2000. RT-qPCR and
western blotting results indicated that HIF-1α expression levels
were markedly downregulated in human mesangial cells
post-transfection with hsa-miR-371-5p mimics (Fig. 5). These results further confirm
that the HIF-1α is a target gene of hsa-miR-371-5p, and
hsa-miR-371-5p can directly modulate HIF-1α expression in human
mesangial cells.
Discussion
miRNAs act as key regulators of gene expression. It
has previously been reported that a single miRNA or several miRNAs
serve key roles in regulating cell development and immunoregulation
(16). Previous studies have
demonstrated that dysregulated miRNA expression in specific cells
and tissues may contribute to LN immunopathogenesis (17–19).
At present, the possibility of altering miRNA expression for the
treatment of LN remains promising. Studies that alter pathogenic
miRNAs have hinted that miRNA-based treatment may be considered a
potential therapeutic tool for LN treatment (20,21).
The present study demonstrated that hsa-miR-371-5p
expression was downregulated in LN renal tissues compared with in
non-cancerous normal kidney tissues. Several miRNAs have been
demonstrated to inhibit cell growth and induce cell apoptosis
(22–24). The results of the present study
suggested that hsa-miR-371-5p suppressed cell proliferation and
promoted the apoptosis of human mesangial cells. These results
indicated that hsa-miR-371-5p may be pivotal in the development and
progression of LN.
HIF-1α has been reported to be a vital regulator for
the progression of kidney diseases (25). In acute renal injury, HIF-1α has an
important role in adaptation to acute hypoxia via the upregulation
of cell-protective HIF-1α target factors and promotion of anaerobic
adenosine triphosphate generation (26). In chronic renal injury, chronic
hypoxia leads to stabilized HIF-1α expression and enhances
interstitial fibrosis and tissue damage (27,28).
Furthermore, Deng et al demonstrated that HIF-1α was able to
promote mesangial cell growth in LN (29). The results of the present study
suggested that HIF-1α is a crucial downstream target of
hsa-miR-371-5p in LN. Hsa-miR-371-5p was able to directly bind to
the 3′-UTR of HIF-1α, as confirmed by luciferase assay. In
addition, restoration of hsa-miR-371-5p was able to markedly
downregulate HIF-1α expression in human mesangial cells. Taken
together, these data strongly indicated that hsa-miR-371-5p may
alleviate the progression of LN by directly targeting HIF-1α.
In conclusion, the present study suggested that
hsa-miR-371-5p was downregulated in LN tissues. Exogenous
overexpression of hsa-miR-371-5p inhibited human mesangial cell
proliferation and promoted apoptosis through directly targeting
HIF-1α. These results indicated that hsa-miR-371-5p may be a key
molecule in the development and progression of LN; therefore,
hsa-miR-371-5p may be considered a novel and valuable target in the
treatment of LN.
Acknowledgements
The present study was supported by the Youth Science
Foundation of Heilongjiang Province of China (grant no.
QC2010094).
References
|
1
|
Pan Y and Sawalha AH: Epigenetic
regulation and the pathogenesis of systemic lupus erythematosus.
Transl Res. 153:4–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yung S and Chan TM: Autoantibodies and
resident renal cells in the pathogenesis of lupus nephritis:
Getting to know the unknown. Clin Dev Immunol. 2012:1393652012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lewis EJ and Schwartz MM: Pathology of
lupus nephritis. Lupus. 14:31–38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aran AA and Putterman C: Treatment of
lupus nephritis: Facing the era of immunotherapy. Panminerva Med.
50:235–245. 2008.PubMed/NCBI
|
|
5
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davis-Dusenbery BN and Hata A: Mechanisms
of control of microRNA biogenesis. J Biochem. 148:381–392.
2010.PubMed/NCBI
|
|
8
|
Kim VN, Han J and Siomi MC: Biogenesis of
small RNAs in animals. Nat Rev Mol Cell Biol. 10:126–139. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mendell JT: MicroRNAs: Critical regulators
of development, cellular physiology and malignancy. Cell Cycle.
4:1179–1184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dai Y, Sui W, Lan H, Yan Q, Huang H and
Huang Y: Comprehensive analysis of microRNA expression patterns in
renal biopsies of lupus nephritis patients. Rheumatol Int.
29:749–754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Denby L, Ramdas V, McBride MW, Wang J,
Robinson H, McClure J, Crawford W, Lu R, Hillyard DZ, Khanin R, et
al: miR-21 and miR-214 are consistently modulated during renal
injury in rodent models. Am J Pathol. 179:661–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Te JL, Dozmorov IM, Guthridge JM, Nguyen
KL, Cavett JW, Kelly JA, Bruner GR, Harley JB and Ojwang JO:
Identification of unique microRNA signature associated with lupus
nephritis. PLoS One. 5:e103442010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Yu F, Song D, Wang SX and Zhao MH:
Podocyte involvement in lupus nephritis based on the 2003 ISN/RPS
system: A large cohort study from a single centre. Rheumatology
(Oxford). 53:1235–1244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chafin CB and Reilly CM: MicroRNAs
implicated in the immunopathogenesis of lupus nephritis. Clin Dev
Immunol. 2013:4302392013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou H, Hasni SA, Perez P, Tandon M, Jang
SI, Zheng C, Kopp JB, Austin H III, Balow JE, Alevizos I and Illei
GG: miR-150 promotes renal fibrosis in lupus nephritis by
downregulating SOCS1. J Am Soc Nephrol. 24:1073–1087. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akkina S and Becker BN: MicroRNAs in
kidney function and disease. Transl Res. 157:236–240. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen YQ, Wang XX, Yao XM, Zhang DL, Yang
XF, Tian SF and Wang NS: Abated microRNA-195 expression protected
mesangial cells from apoptosis in early diabetic renal injury in
mice. J Nephrol. 25:566–576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Trionfini P, Benigni A and Remuzzi G:
MicroRNAs in kidney physiology and disease. Nat Rev Nephrol.
11:23–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Costa-Reis P, Russo PA, Zhang Z, Colonna
L, Maurer K, Gallucci S, Schulz SW, Kiani AN, Petri M and Sullivan
KE: The role of microRNAs and human epidermal growth factor
receptor 2 in proliferative lupus nephritis. Arthritis Rheumatol.
67:2415–2426. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu W, Liu H, Li T and Xu G:
MicroRNA-490-43p regulates cell proliferation and apoptosis by
targeting HMGA2 in osteosarcoma. FEBS Lett. 589:3148–3153. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo H, Guo W, Wang F, You Y, Wang J, Chen
X, Wang J, Wang Y, Du Y, Chen X, et al: miR-1291 targets mucin 1
inhibiting cell proliferation and invasion to promote cell
apoptosis in esophageal squamous cell carcinoma. Oncol Rep.
34:2665–2673. 2015.PubMed/NCBI
|
|
24
|
Zhang S, Zhang C, Liu W, Zheng W, Zhang Y,
Wang S, Huang D, Liu X and Bai Z: MicroRNA-24 upregulation inhibits
proliferation, metastasis and induces apoptosis in bladder cancer
cells by targeting CARMA3. Int J Oncol. 47:1351–1360.
2015.PubMed/NCBI
|
|
25
|
Mao S and Huang S: The signaling pathway
of hypoxia inducible factor and its role in renal diseases. J
Recept Signal Transduct Res. 33:344–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fähling M, Mathia S, Paliege A, Koesters
R, Mrowka R, Peters H, Persson PB, Neumayer HH, Bachmann S and
Rosenberger C: Tubular von hippel-lindau knockout protects against
rhabdomyolysis-induced AKI. J Am Soc Nephrol. 24:1806–1819. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haase VH: Hypoxia-inducible factor
signaling in the development of kidney fibrosis. Fibrogenesis
Tissue Repair. 5:(Suppl 1). S162012.PubMed/NCBI
|
|
28
|
Kimura K, Iwano M, Higgins DF, Yamaguchi
Y, Nakatani K, Harada K, Kubo A, Akai Y, Rankin EB, Neilson EG, et
al: Stable expression of HIF-1alpha in tubular epithelial cells
promotes interstitial fibrosis. Am J Physiol Renal Physiol.
295:F1023–F1029. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng W, Ren Y, Feng X, Yao G, Chen W, Sun
Y, Wang H, Gao X and Sun L: Hypoxia inducible factor-1 alpha
promotes mesangial cell proliferation in lupus nephritis. Am J
Nephrol. 40:507–515. 2014. View Article : Google Scholar : PubMed/NCBI
|