Introduction
Obesity rates are increasing worldwide, and its
incidence has risen extensively in the last three decades (1). Obesity is an established risk factor
for metabolic diseases, including insulin resistance, type 2
diabetes mellitus, hypertension, nonalcoholic fatty liver disease
and various cancers (1).
Obesity is associated with a chronic low-grade
inflammatory state (2). In obese
individuals, adipocytes synthesize and secrete large quantities of
cytokines, including interleukin (IL)-6 and tumor necrosis factor α
(TNF-α), adipokines, including the hormones leptin and resistin,
and chemokines, which cause the migration of inflammatory cells
into adipose tissue. The abnormal release of hormones, cytokines
and chemokines by adipose tissue affects insulin sensitivity in an
endocrine manner in the liver and skeletal muscle and in an
auto-/paracrine fashion in adipose tissue. Previous studies have
demonstrated that insulin resistance and obesity are closely
associated with adipose tissue inflammation (3,4),
indicating that suppressing adipocyte inflammation may have a
beneficial effect on insulin sensitivity in obese individuals
(5,6). In contrast, a review by Ye and
McGuinness (4) suggested that
elevation of proinflammatory cytokines increases energy expenditure
and decreases the risk of obesity, indicating that proinflammatory
cytokines may have beneficial effects.
ILs, including IL-6 and IL-1β, affect adipocyte
function, contributing to insulin resistance due to obesity. IL-6
is abundantly expressed by adipose tissue and a negative
correlation has been demonstrated in humans between plasma IL-6
levels and insulin sensitivity (7). In addition, IL-6 has been revealed to
be important for mesenchymal stem cell (MSC) inflammatory function
(8,9). MSCs have a immunoregulatory capacity,
which may be induced by certain combinations of inflammatory
cytokines, including interferon-γ (IFNγ) and TNFα, or IFNγ and IL-1
(10).
miR-148a was identified as a DNA
methylation-associated silencing tumor suppressor involved in human
cancer metastasis (11).
Adipocytes are continuously stimulated by proinflammatory
cytokines, including TNF-α and IL-6, which contribute to the
inflammatory response and result in adipocyte dysfunction. Studies
by our laboratory and others have demonstrated that miR-148a is an
important regulator that contributes to adipogenesis via targeting
Wnt1 (12) and DNA
methyltransferase 1 (13). The
majority of previous studies have focused on the regulation of
miR-148a expression by inflammation in non-adipocytes (14). Therefore, little is known regarding
the underlying mechanisms that regulate miR-148a expression during
the obesity-induced inflammatory response.
The present study examined the expression of
miR-148a in differentiated human adipose tissue-derived MSCs
(hMSCs-Ad) and their response to proinflammatory cytokines.
miR-148a transcription was decreased in inflammatory and insulin
resistance microenvironments. The core promoter region of miR-148a
contains cyclic adenosine monophosphate-response element binding
protein (CREB) binding sites and various nuclear receptor response
elements, including the CCAAT enhancer binding protein and E2F
transcription factor. In addition, the CREB binding site is in the
core promoter region of miR-148a (12). A luciferase assay revealed that
CREB binding to the miR-148a promoter region was decreased
following TNF-α or IL-6 treatment, indicating that differentiated
hMSCs-Ad responded to inflammatory cytokines by decreasing miR-148a
expression, which may have resulted from an effect on the promoter
activity. The results of the present study indicated that the
inhibitionn of miR-148a during the proinflammatory response is
regulated by a transcriptional event.
Materials and methods
Cell culture and adipocyte
differentiation
hMSCs-Ad cells were obtained from ScienCell Research
Laboratories (Carlsbad, CA, USA) and maintained in MSC medium
(MSCM; ScienCell Research Laboratories) supplemented with 5% fetal
bovine serum (FBS, ScienCell Research Laboratories), 1% MSC growth
supplement (ScienCell Research Laboratories) and 1%
penicillin/streptomycin solution, at 37°C and 5% CO2. To induce
differentiation, the hMSCs-Ad were cultured in serum-free MSCM
supplemented with 50 nM insulin, 100 nM dexamethasone, 0.5 mM
3-isobutyl-1-methylxanthine and 100 µM rosiglitazone (all from
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) (day 0) and the
medium was replaced every 2 days for 4 days. Subsequently, cells
were cultured in serum-free MSCM supplemented with 50 nM insulin;
medium was replaced every 2 days until lipid accumulation occurred
(day 10).
Treatment with
cytokines/adipokines
Experiments were performed using differentiated
adipocytes, 15 days following the induction of differentiation. At
this time point >80% of cells exhibited the morphological and
biochemical properties of adipocytes. Following an overnight
incubation in serum-free MSCM, cells were treated with 5, 10 or 20
ng/ml TNF-α (Merck Millipore) (15), 10, 30 or 90 ng/ml IL-6 (Merck
Millipore) (16), 30 ng/ml leptin
or 60 ng/ml resistin (Merck Millipore) for 4, 8 or 24 h. Cells were
harvested at these time points for subsequent experiments.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was prepared from hMSCs-Ad at various time
points following adipocyte differentiation induction using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham MA, USA) according to the manufacturer's protocol,
followed by DNase treatment (Takara Bio, Inc., Otsu, Japan). The
quality and quantity of RNA was assessed using a NanoDrop 2.0
(Thermo Fisher Scientific, Inc.). cDNA was synthesized from 200 ng
RNA using the TaqMan MicroRNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). TaqMan Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and miRNA probe
(Applied Biosystems; Thermo Fisher Scientific, Inc.) were used to
amplify the cDNA by qPCR. qPCR was performed using an Applied
Biosystems 7500 Sequence Detection system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Cycling conditions were as follows: An initial
denaturation step at 95°C for 10 min was followed by 40 cycles of
denaturation at 95°C for 15 sec and annealing at 60°C for 1 min.
miR-148a expression was normalized to snoU6. The primer
identification numbers are 000470 (miR-148a) and 001973 (snoU6;
Applied Biosystems; Thermo Fisher Scientific, Inc.). Each sample
was measured in triplicate, and the mRNA expression levels were
calculated using the 2−ΔΔcq method (17).
Promoter reporter assays
The miR-148a promoter and control pGL3-basic
promoter (Promega Corporation, Madison, WI, USA) were established
in our previous study (12). Human
embryonic kidney 293T (HEK293T; American Type Culture Collection,
Manassas, VA, USA) cells were cultured in Dulbecco's modified
Eagle's medium supplemented with 10% FBS and 4 mM L-glutamine.
HEK293T cells were cultured to 60–70% confluence in 6-well plates
exposed to cytokines/adipokines. Promoter activity was assessed
using the Dual-Glo® Luciferase assay system (Promega
Corporation). Cells were transfected with 250 ng/well
promoter-Firefly luciferase reporter construct and 25 ng/well
Renilla luciferase vector (pRL-TK) using 0.6 µl
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) in
20 µl Opti-minimal essential medium® I Reduced Serum
(Thermo Fisher Scientific, Inc.). A total of 24 h later, cells were
lysed in 50 µl 1X Passive Lysis buffer (Promega Corporation) and
stored at −20°C until analysis. Assays were performed in
quadruplicate and repeated three times.
Statistical analysis
SPSS software version 17.0 (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. Representatives of
replicate experiments are presented in the figures and the data are
expressed as the mean ± standard error. Differences between groups
were analyzed using Student's two-tailed t-test when two groups
were compared, or one-way analysis of variance followed by the
least significant difference post hoc test when multiple groups
were compared. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-148a is regulated by the
adipokines leptin and resistin in differentiated hMSCs-Ad
Fig. 1 presents a
schematic representation of the chromosomal location of miR-148a.
Our previous study revealed that miR-148a was highly expressed in
mature adipocytes using a microRNA chip (12). To investigate the effects of
adipokines on the expression of miR-148a, the present study
simulated the adipokine microenvironment by exposing hMSCs-Ad to
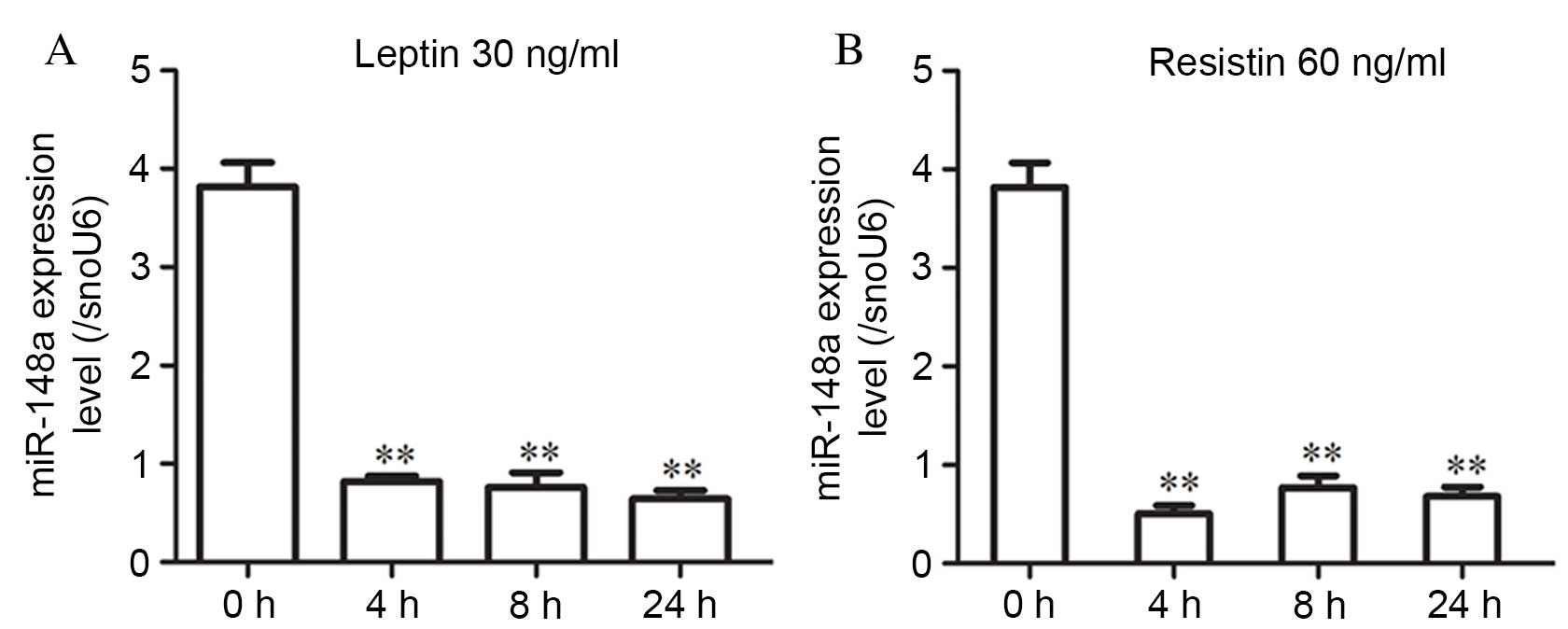
leptin or resistin. Differentiated hMSCs-Ad were treated with 30
ng/ml leptin or 60 ng/ml resistin; miR-148a expression was examined
at 4, 8 and 24 h and normalized to snoU6 expression. miR-148a
expression levels were significantly decreased at all time points
following treatment with leptin (P<0.001 at all time points;
Fig. 2A) or resistin (P=0.002, 4 h
vs. 0 h; P=0.003, 8 h vs. 0 h; P=0.001, 24 h vs. 0 h; Fig. 2B).
miR-148a is regulated by IL-6 in
hMSCs-Ad
The effect of the inflammatory cytokine IL-6 on
miR-148a expression levels in hMSCs-Ad was assessed. Differentiated
hMSCs-Ad were treated with IL-6, as in a previous study (16), and the miR-148a expression level
was examined at 4, 8 and 24 h and normalized to snoU6 expression.
miR-148a expression levels were significantly decreased following
treatment with low dose (10 ng/ml; P=0.004, 4 h vs. 0 h;
P<0.001, 8 h vs. 0 h; P<0.001, 24 h vs. 0 h; Fig. 3A), moderate dose (30 ng/ml;
P=0.008, 4 h vs. 0 h; P<0.001, 8 h vs. 0 h; P<0.001, 24 h vs.
0 h; Fig. 3B) and high dose (90
ng/ml; P<0.001 at all time points; Fig. 3C) IL-6, at all time points.
miR-148a expression levels were significantly reduced at 24 h of 10
ng/ml IL-6 stimulation, by >80% compared with the control
(Fig. 3A). However, IL-6 did not
decrease miR-148a expression levels in a dose-dependent manner.
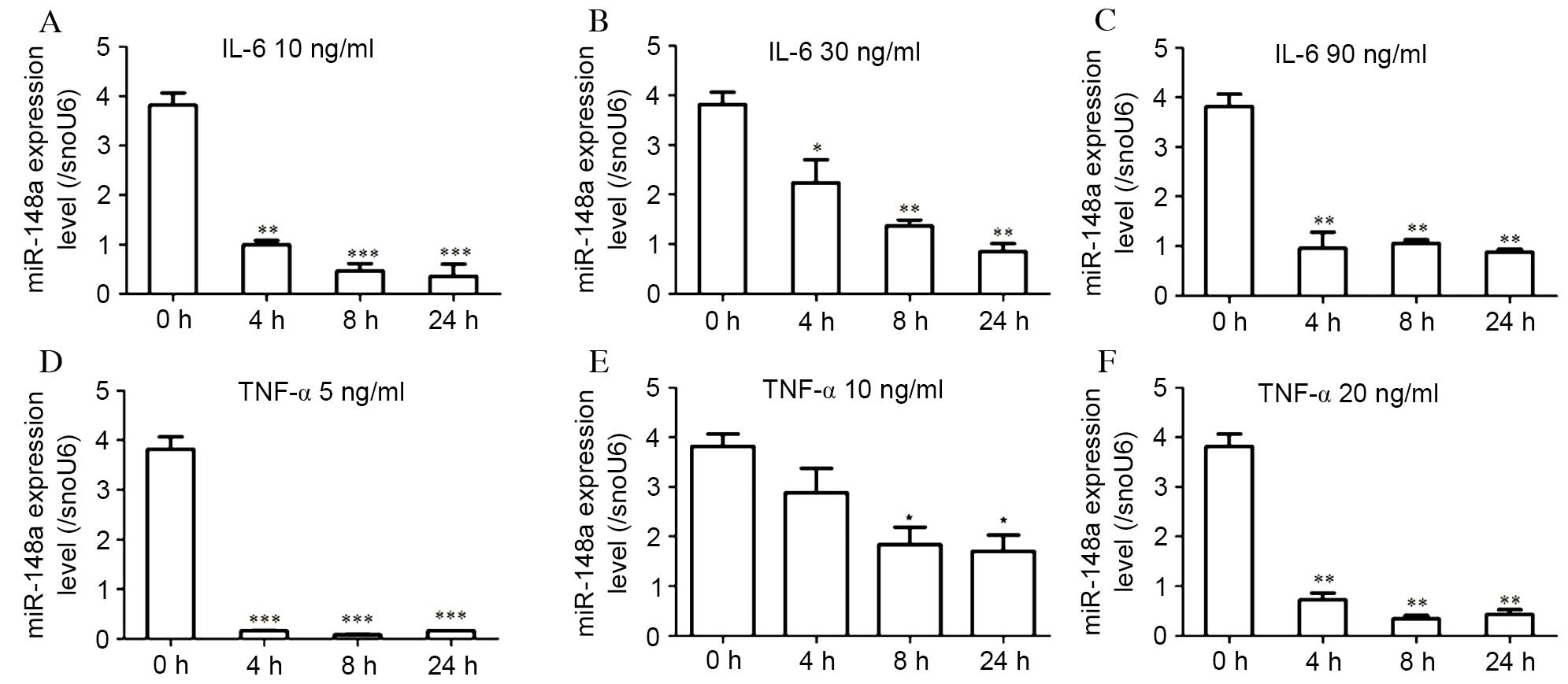 | Figure 3.miR-148a expression levels in hMSCs-Ad
are regulated by IL-6 and TNF-α. Cells were treated with (A) 10,
(B) 30 or (C) 90 ng/ml IL-6, or (D) 5, (E) 10 or (F) 20 ng/ml
TNF-α. Following 4, 8 or 24 h of incubation, miR-148a expression
levels were analyzed by reverse transcription-quantitative
polymerase chain reaction and normalized to snoU6 levels. miR-148a
expression levels were significantly decreased by IL-6 or TNF-α;
however, this effect was not dose-dependent. Data are expressed as
the mean ± standard error (n=3) and are representative of three
independent experiments. *P<0.05, **P<0.01 and ***P<0.001
vs. control (untreated cells, 0 h). miR, microRNA; hMSCs-Ad, human
adipose tissue-derived mesenchymal stem cells; IL, interleukin;
TNF, tumor necrosis factor. |
Effect of TNF-α on miR-148a expression
levels in hMSCs-Ad
In addition, the effect of treatment with low dose
(5 ng/ml; P<0.001 at all time points; Fig. 3D), moderate dose (10 ng/ml;
P=0.017, 4 h vs. 0 h; P=0.005, 8 h vs. 0 h; P=0.003, 24 h vs. 0 h;
Fig. 3E) and high dose (20 ng/ml;
P<0.001 at all time points; Fig.
3F) TNF-α was examined. miR-148a expression levels in
differentiated hMSCs-Ad treated with 10 ng/ml TNF-α, as in a
previous study (15), were
significantly downregulated compared with the control at 8
(P=0.005; Fig. 3E) and 24 h
(P=0.003; Fig. 3E). However,
miR-148a expression levels were not decreased in a dose-dependent
manner. Exposure of hMSCs-Ad to TNF-α and IL-6 resulted in
decreased miR-148a expression levels, suggesting that the
obesity-associated inflammatory microenvironment inhibited miR-148a
expression.
Decreased promoter activity in the
primary promoter of miR-148a as a result of inflammatory cytokine
or adipokine treatment
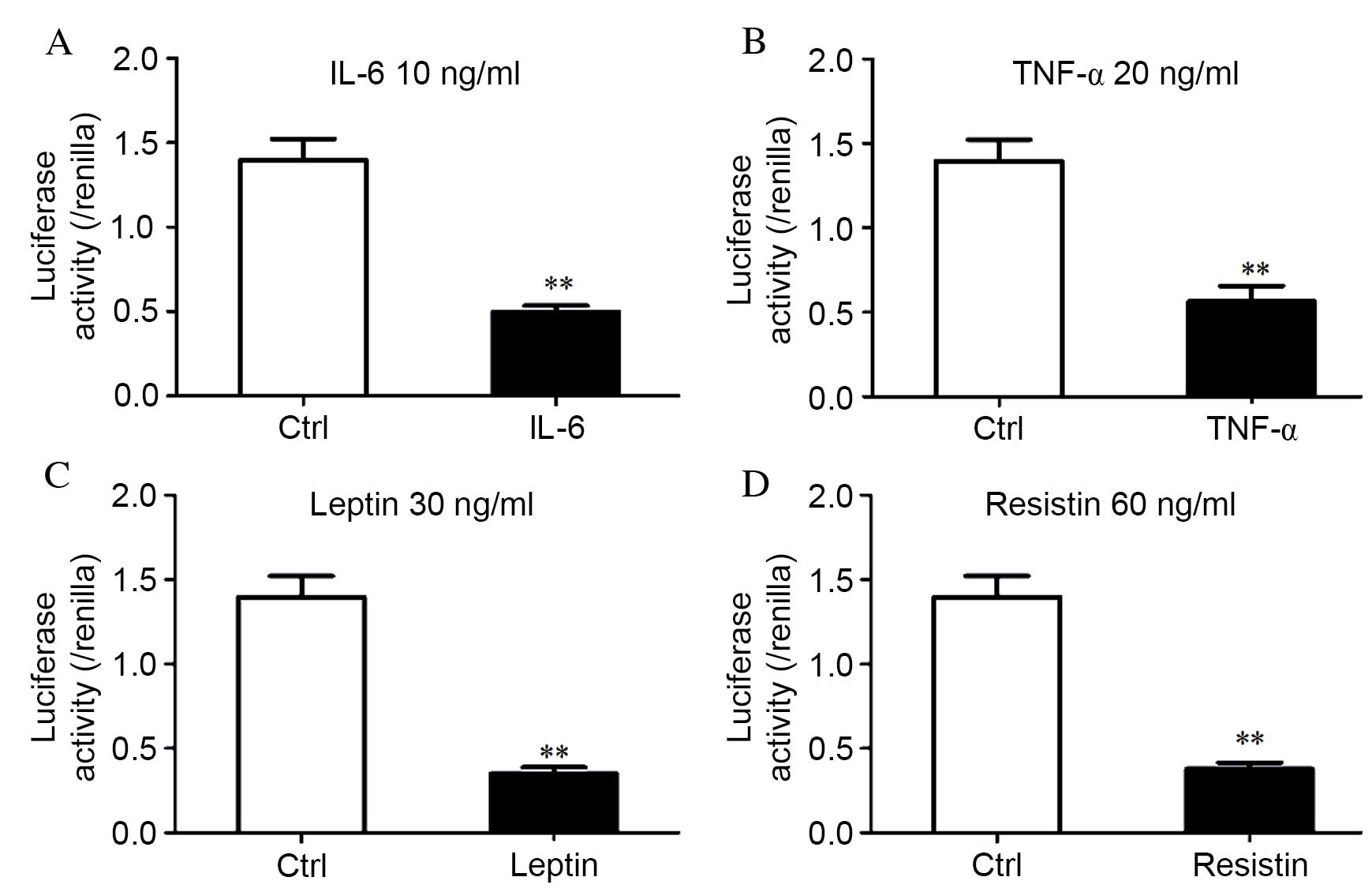
Our previous study determined that the primary
promoter of miR-148a is located at the promoter region-2947 to-2687
nt of pre-miR-148a (12). To
clarify the underlying mechanism by which adipokines and cytokines
regulate miR-148a expression, HEK293T cells were used to analyze
the effect of IL-6 (P<0.001; Fig.
4A), TNF-α (P=0.002; Fig. 4B),
leptin (P<0.001; Fig. 4C) and
resistin (P<0.001; Fig. 4D) on
the primary promoter activity of the gene encoding miR-148a.
miR-148a promoter activity was significantly reduced by ~60% (TNF-α
and resistin) or ~70% (IL-6 and leptin) compared with untreated
cells (P<0.001), which was normalized to pGL3-basic. A
luciferase assay revealed that the inflammatory cytokines decreased
miR-148a promoter activity, and this effect was consistent with
inflammatory cytokine levels that inhibited miR-148a at the
transcriptional level (Fig. 3).
Therefore, miR-148a expression levels were decreased in
differentiated hMSCs-Ad in response to treatment with inflammatory
cytokines, which may be the result of a decrease in the miR-148a
promoter activity.
Discussion
Although obesity has been associated with
inflammatory cytokine accumulation and altered insulin sensitivity
of adipocytes, these concepts remain controversial. miR-148a was
recently characterized as a novel obesity-associated microRNA with
a role in adipogenesis and energy metabolism (12,13).
Studies by our laboratory and others revealed miR-148a as an
important regulator involved in adipogenesis via targeting Wnt1
(12,18), and demonstrated that miR-148a was
highly expressed in obese individuals (12). However, the metabolic regulation of
miR-148a in adipocytes has not been comprehensively investigated.
The present study investigated the expression levels of miR-148a in
hMSCs-Ad in response to inflammatory cytokines and adipokines.
Certain studies have demonstrated that the
adipokines leptin and resistin are involved in obesity-associated
insulin resistance as well as in adipocyte differentiation
(19,20). Leptin, an adipocyte-derived hormone
and cytokine, is upregulated in patients with obesity-associated
type 2 diabetes mellitus, although leptin resistance has been
reported (21). However, the
association between miR-148a and adipokines remains to be fully
elucidated. The present study investigated the regulation of
miR-148a expression in adipocytes modulated by adipokines. Leptin
and resistin markedly downregulated miR-148a expression levels. Our
previous study revealed that miR-148a was overexpressed in obese
individuals (12). Therefore,
leptin and resistin may suppress miR-148a expression through
negative feedback; however, this association requires further
investigation.
IL-6 has been described as a resolution factor,
which affects the immune system by balancing inflammatory and
anti-inflammatory responses (22).
In addition, IL-6 is an important cytokine with extensive
biological activities in processes including immune regulation,
inflammation, hematopoiesis and oncogenesis. TNF-α has numerous
adverse effects on adipocyte functions, including increased basal
lipolysis and reduced insulin sensitivity, as reviewed in (23). IL-6 and TNF-α additionally modulate
miR-148a expression levels. The present study revealed that
miR-148a expression levels were decreased in differentiated
hMSCs-Ad treated with IL-6. However, this effect was not
dose-dependent. This may be due to the fact that miR-148a is highly
expressed in differentiated hMSC-Ad. Alternatively, there may be
other compensatory mechanisms involved. These findings were
consistent with a previous study that demonstrated that miR-148a
expression levels were decreased in IL-6-overexpressing malignant
cholangiocytes in vitro, and in tumor cell xenografts
(14). In the present study, TNF-α
significantly downregulated miR-148a expression compared with the
control, suggesting that obesity-associated inflammation inhibits
miR-148a expression.
Our previous study determined that the primary
miR-148a promoter is located at the promoter region −2947 to −2687
nt of pre-miR-148a (12).
Therefore, the present study investigated whether the primary
promoter was involved in the downregulation of miR-148a by
inflammatory cytokines and adipokines. miR-148a promoter activity
was significantly reduced following treatment with IL-6, TNF-α,
leptin and resistin compared with the control. These results
suggested that miR-148a expression levels were decreased in
differentiated hMSCs-Ad in response to inflammatory cytokines and
adipokines, potentially due to an effect on its promoter
activity.
A previous study revealed that adipocyte
inflammation is essential for healthy adipose tissue expansion and
remodeling (24); this does not
contradict results from a model in which chronic inflammation is an
important contributor toward metabolic syndrome (25). In conclusion, the results of the
present study revealed the miR-148a expression levels and promoter
activity are regulated by inflammatory cytokines and adipokines.
These findings suggested a novel role for miR-148a in adipocyte
inflammation, indicating that miR-148a may be involved in obesity
complications via its own underlying transcriptional mechanism.
Acknowledgements
The present study was supported by grants from the
National Key Basic Research Program of China (grant no.
2013CB530604), the Key Project of the National Natural Science
Foundation of China (grant no. 81330067), the National Natural
Science Foundation of China (grant nos. 81270928, 81370964 and
81500674), the National Natural Science Foundation of Jiangsu
Province (grant no. BK20150082), the Program for Innovative
Research Teams of Jiangsu Province (grant no. LJ201108), the 333
High Level Talents Training Project of Jiangsu Province, the
Nanjing Technological Development Program (grant no. 201104013) and
the Science and Technology Development Fund of Nanjing Medical
University (grant no. 2014NJMUZD041).
Glossary
Abbreviations
Abbreviations:
|
CREB
|
cyclic adenosine
monophosphate-response element binding protein
|
|
MSCs
|
mesenchymal stem cells
|
|
hMSCs-Ad
|
human adipose tissue-derived
mesenchymal stem cells
|
|
IL
|
interleukin
|
|
TNF
|
tumor necrosis factor
|
References
|
1
|
Ogden CL, Carroll MD, Lawman HG, Fryar CD,
Kruszon-Moran D, Kit BK and Flegal KM: Trends in obesity prevalence
among children and adolescents in the United States, 1988–1994
Through 2013–2014. JAMA. 21:2292–2299. 2016. View Article : Google Scholar
|
|
2
|
Glass CK and Olefsky JM: Inflammation and
lipid signaling in the etiology of insulin resistance. Cell Metab.
15:635–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patsouris D, Li PP, Thapar D, Chapman J,
Olefsky JM and Neels JG: Ablation of CD11c-positive cells
normalizes insulin sensitivity in obese insulin resistant animals.
Cell Metab. 8:301–309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ye J and McGuinness OP: Inflammation
during obesity is not all bad: Evidence from animal and human
studies. Am J Physiol Endocrinol Metab. 304:E466–E477. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Osborn O and Olefsky JM: The cellular and
signaling networks linking the immune system and metabolism in
disease. Nat Med. 18:363–374. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Romeo GR, Lee J and Shoelson SE: Metabolic
syndrome, insulin resistance, and roles of inflammation-mechanisms
and therapeutic targets. Arterioscler Thromb Vasc Biol.
32:1771–1776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kern PA, Ranganathan S, Li C, Wood L and
Ranganathan G: Adipose tissue tumor necrosis factor and
interleukin-6 expression in human obesity and insulin resistance.
Am J Physiol Endocrinol Metab. 280:E745–E751. 2001.PubMed/NCBI
|
|
8
|
Xu G, Zhang Y, Zhang L, Ren G and Shi Y:
The role of IL-6 in inhibition of lymphocyte apoptosis by
mesenchymal stem cells. Biochem Biophys Res Commun. 361:745–750.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Djouad F, Charbonnier LM, Bouffi C,
Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C and
Noël D: Mesenchymal stem cells inhibit the differentiation of
dendritic cells through an interleukin-6-dependent mechanism. Stem
Cells. 25:2025–2032. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ren G, Zhang L, Zhao X, Xu G, Zhang Y,
Roberts AI, Zhao RC and Shi Y: Mesenchymal stem cell-mediated
immunosuppression occurs via concerted action of chemokines and
nitric oxide. Cell Stem Cell. 2:141–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lujambio A, Calin GA, Villanueva A, Ropero
S, Sánchez-Céspedes M, Blanco D, Montuenga LM, Rossi S, Nicoloso
MS, Faller WJ, et al: A microRNA DNA methylation signature for
human cancer metastasis. Proc Natl Acad Sci USA. 105:13556–13561.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi C, Zhang M, Tong M, Yang L, Pang L,
Chen L, Xu G, Chi X, Hong Q, Ni Y, et al: miR-148a is associated
with obesity and modulates adipocyte differentiation of mesenchymal
stem cells through Wnt signaling. Sci Rep. 5:99302015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Londoño Gentile T, Lu C, Lodato PM, Tse S,
Olejniczak SH, Witze ES, Thompson CB and Wellen KE: DNMT1 is
regulated by ATP-citrate lyase and maintains methylation patterns
during adipocyte differentiation. Mol Cell Biol. 33:3864–3878.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Braconi C, Huang N and Patel T:
MicroRNA-dependent regulation of DNA methyltransferase-1 and tumor
suppressor gene expression by interleukin-6 in human malignant
cholangiocytes. Hepatology. 51:881–890. 2010.PubMed/NCBI
|
|
15
|
Wellen KE, Fucho R, Gregor MF, Furuhashi
M, Morgan C, Lindstad T, Vaillancourt E, Gorgun CZ, Saatcioglu F
and Hotamisligil GS: Coordinated regulation of nutrient and
inflammatory responses by STAMP2 is essential for metabolic
homeostasis. Cell. 129:537–548. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kralisch S, Klein J, Lossner U, Bluher M,
Paschke R, Stumvoll M and Fasshauer M: Interleukin-6 is a negative
regulator of visfatin gene expression in 3T3-L1 adipocytes. Am J
Physiol Endocrinol Metab. 289:E586–E590. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qin L, Chen Y, Niu Y, Chen W, Wang Q, Xiao
S, Li A, Xie Y, Li J, Zhao X, et al: A deep investigation into the
adipogenesis mechanism: Profile of microRNAs regulating
adipogenesis by modulating the canonical Wnt/beta-catenin signaling
pathway. BMC Genomics. 11:3202010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Steppan CM, Bailey ST, Bhat S, Brown EJ,
Banerjee RR, Wright CM, Patel HR, Ahima RS and Lazar MA: The
hormone resistin links obesity to diabetes. Nature. 409:307–312.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim KH, Lee K, Moon YS and Sul HS: A
cysteine-rich adipose tissue-specific secretory factor inhibits
adipocyte differentiation. J Biol Chem. 276:11252–11256. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maffei M, Halaas J, Ravussin E, Pratley
RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R and Ranganathan S:
Leptin levels in human and rodent: Measurement of plasma leptin and
ob RNA in obese and weight-reduced subjects. Nat Med. 1:1155–1161.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jones SA: Directing transition from innate
to acquired immunity: Defining a role for IL-6. J Immunol.
175:3463–3468. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sethi JK and Hotamisligil GS: The role of
TNF alpha in adipocyte metabolism. Semin Cell Dev Biol. 10:19–29.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wernstedt Asterholm I, Tao C, Morley TS,
Wang QA, Delgado-Lopez F, Wang ZV and Scherer PE: Adipocyte
inflammation is essential for healthy adipose tissue expansion and
remodeling. Cell Metab. 20:103–118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lumeng CN and Saltiel AR: Inflammatory
links between obesity and metabolic disease. J Clin Invest.
121:2111–2117. 2011. View
Article : Google Scholar : PubMed/NCBI
|