Introduction
S100 genes include at least 19 members of a
calcium-binding protein family, which are located as a cluster on
chromosome 1q21 (1–3). S100 gene expression predominantly
takes place in the bone marrow-derived cells, such as granulocytes,
macrophages and monocytes, endothelial cells, and is involved in
the cell cycle, differentiation, inflammation, mobility and
mobility (4–7). S100 proteins serve important
intracellular and extracellular functions (8). The proteins typically consist of
homodimers, each monomer of S100 contains two helix-loop-helix
structural domains, so-called EF-hand, calcium-binding domains,
which are connected by a central hinge region. The high affinity
binding to calcium of the C-terminal classical EF-hand domain
induces a conformational change in the S100 proteins to expose a
hydrophobic binding site for targeting a large number of proteins
(9). S100 proteins are localized
in the cytoplasm and/or nucleus, and may be involved in the
regulation of cellular processes, including cell cycle progression
and differentiation in a wide range of cells (10). The chromosomal rearrangements and
altered expression of the S100 gene have been implicated in tumor
metastasis. Over 50 target proteins have been identified to
interact with various S100 proteins, including transcription
factors, metabolic enzymes, kinases, annexins and contractile
proteins (11). In general, the
majority of these interactions are dependent on calcium signaling,
however a subset of interactions is independent of calcium
activation (12). S100 proteins
are typically expressed in a tissue specific manner and their up or
downregulation has been associated with numerous diseases,
including several types of cancer (13). For example, elevated serum levels
of S100 calcium binding protein B have been detected in patients
with melanoma, and to be associated with metastasis and a poor
prognosis (14,15).
S100 calcium binding protein A4 (S100A4) is one of
the multiple alternative splice variants of the S100 gene, creating
a 109 amino acid protein (12 kDa), particularly involved in cell
mobility and motility. In vivo studies demonstrated that
S100A4 was involved in the development of metastasis (16–19)
and overexpression of S100A4 has been detected in various types of
cancer, including breast, pancreatic, colorectal, ovarian, and
prostate and lung cancer (20).
S100A4 has also been widely detectable in blood samples from
patients with inflammation, neoplasia or cancer. S100A4 protein
expression has been suggested as a criterion for clinical diagnosis
despite the potential for false positive results (21,22).
However, the high sensitivity of S100A4 to inflammation and
metastasis has led to a potential target in cancer therapy,
frequently by inhibiting the dimerization (23–25).
However, the various mechanisms of S100A4 in and outside the cells
are not fully understood. For instance, various types of cancer
cells may adjust to respond to external factors such as
polysaccharides. By examining the S100A4 protein structure, it has
been demonstrated that carcinogenesis is particularly associated
with the mobility and motility of S100A4 by aggregation with a
non-muscle myosin IIA (NMIIA) tail fragment complex protein. The
S100A4-NMIIA complex is the functional conformation for inducing
migration (26–29). However, despite the inferences of
an important role in cancer development, the mode of action of
S100A4 proteins remains unclear.
A large number of foodborne or naturally derived
compounds, particularly non-starch polysaccharides, have exhibited
antioxidation activity in vitro (30–32)
and inhibition of cancer cell migration and invasion (33–35).
Our previous studies demonstrated that the coix polysaccharide CP1
fraction extracted from adlay seeds inhibits A549 cell
proliferation and induces cell apoptosis via a mechanism primarily
involving activation of the intrinsic mitochondrial pathway
(36). The current study analyzed
the inhibition of cancer cell migration and invasion by CP1, the
association with the inhibition of S100A4 gene expression and the
potential mechanism of the interference of the location of S100A4
targeted by a polysaccharide CP1 analog through in silico
analysis.
Materials and methods
Preparation of coix polysaccharide,
CP1, and cell culture
The CP1 polysaccharide was extracted from adlay
seeds (C. lachryma-jobi L.) by decoction and alcohol
precipitation as described in a previous study (36). The human A549 non-small cell lung
cancer cell line was obtained from the Cell Bank of Type Culture
Collection of Chinese Academy of Sciences (Shanghai, China) and
cultured in RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with L-glutamine (1 mM), 10%
(v/v) heat-inactivated fetal bovine serum (FBS), penicillin (100
U/ml), and streptomycin (100 µg/ml) at 37°C in 5% (v/v)
CO2 incubator. In general, all experiments were
conducted when cells reached 80–90% confluence. The cells were at
<20 passages, remaining normal and with healthy cell morphology,
and without mycoplasma contamination throughout the
experiments.
Cell viability and proliferation
The effect of CP1 on the viability of A549 cells was
assessed by MTT assay (36).
Briefly, exponentially growing cells in 96-well plates were treated
with different concentrations (10–300 µg/ml) of CP1 in complete
RPMI-1640 medium. Control cells were cultured in medium not
containing CP1. MTT (20 µl, 5 mg/ml) was added following incubation
of the cells for 24 and 48 h, and subsequently the cells were
incubated for 4 h. The medium was then aspirated and 150 µl
dimethyl sulfoxide (DMSO) was added into each well. The absorbance
was measured at 570 nm using a 96-well microplate reader. All
experiments were performed three times. The cell viability was
calculated as follows: Ratio of cell viability (%) = (A-B / C-B) ×
100; where A is the average optical density of CP1-treated cells, B
is the average optical density of the control wells (culture medium
without cells), and C is the average optical density of the
negative control (culture medium containing DMSO and no CP1).
Cell scratch wound healing assay in
vitro
The cell scratch wound healing assay was performed
based on the Yarrow method (37).
The cells were seeded in 24-well plates for 24 h and cell density
reached ~70–80% confluence as a monolayer. Gently and slowly, a
scratch was made in the monolayer across the center of the well
using a 1 µl pipette tip. While scratching across the surface of
the well, the long-axial of the tip was always perpendicular to the
bottom of the well. The resulting gap distance is therefore equal
to the outer diameter of the end of the tip, and then the cells
were washed with PBS buffer three times to remove cell debris.
Scratch healing rate (%) = (0 h scratch width-12 or 24 h scratch
width) / 0 h scratch width × 100 was photographically recorded and
cell confluence area was measured to calculate the cell
migration.
Cell invasion assay
A Transwell migration chamber assay was performed to
observe cell invasion, particularly the motility capability of
tumor cell transmigration across Matrigel in vitro. For the
assay, 1×105 cells in FBS-free RPMI-1640 medium were
plated in the top chamber of the Transwell insert with a
Matrigel-coated polycarbonate membrane. RPMI-1640 medium with 10%
FBS was added to the lower chamber as a chemoattractant. After
incubation for 24 h (migration assay) or 36 h (invasion assay),
cells on the lower surface of the membrane were fixed with 10%
formalin and stained with 0.2% crystal violet. Cells that did not
migrate through the pores were mechanically removed using a cotton
swab (38). The images of migrated
cells were acquired using an inverted light microscope at ×200
magnification. The number of invaded cells was counted from five or
six randomly selected fields in a blind manner.
S100A4 gene expression
Total RNA was extracted from ~2×106 cells
for each test using an RNeasy Mini kit (Qiagen, Inc., Valencia, CA,
USA) according to the manufacturer's protocol. The integrity of the
total RNA was determined electrophoresis on 1% agarose gel. Reverse
transcription (RT) was performed using 1 µl Ribolock™ RNase
Inhibitor, 1 µl Oligo (dT) 18 primer, 2 µl 10 mM dNTP mix, 4 µl 5X
RevertAid reaction buffer, 2 µl template RNA (100 ng/µl), 1 µl
RevertAid™ reverse transcriptase (Thermo Fisher Scientific, Inc.)
and nuclease-free water was added to a final volume of 20 µl.
Reagents were mixed, and incubated at 42°C for 1 h, then at 70°C
for 5 min to terminate the reaction. The cDNA was stored at −20°C.
The polymerase chain reaction (PCR) product of S100A4 was detected
using the primers listed in Table
I, designed with Primer 3 software version 0.3.0 (frodo.wi.mit.edu). Primers were synthesized by
Invitrogen (Thermo Fisher Scientific, Inc.). PCR of S100A4 was
performed using 5 µl 10X Taq reaction buffer, 2 µl template cDNA,
1.5 µl primers each (forward and reverse), 1 µl dNTP mix (10 mM), 1
µl Taq DNA polymerase (Promega Corporation, Madison, WI, USA) and
nuclease-free water to a final volume of 50 µl. The reaction was
performed at 94°C for 30 sec, then 35 cycles of 94°C for 30 sec,
58°C for 30 sec and 72°C for 45 sec, with final extension at 72°C
for 10 min, and then maintained at 4°C. The PCR fragments were
visualized on a 1.2% agarose gel stained with ethidium bromide,
semi-quantitatively analyzed using an ImageQuant LAS 4000 (GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA), and sequenced by a
commercial sequencing service company (Beijing Genomics Institute,
Beijing, China) to identify it as S100A4.
 | Table I.Polymerase chain reaction primers and
the products lengths. |
Table I.
Polymerase chain reaction primers and
the products lengths.
| Gene | Primers
sequences | Product length |
|---|
| β-actin | Sense:
5′-AAATCTGGCACCACACCTT-3′ | 646 bp |
|
| Antisense:
5′-AGCACTGTGTTGGCGTAGAG-3′ |
|
| S100A4 | Sense:
5′-TCAGAACTAAAGGAGCTGCTGACC-3′ | 198 bp |
|
| Antisense:
5′-TTTCTTCCTGGGCTGCTTATCTGG-3′ |
|
Western blot detection of S100A4
protein expression
A549 cells were incubated to the logarithmic growth
phase and 1×106 cells were synchronized for another 10
h, then CP1 was added to a final concentration of 200 and 300
µg/ml, and incubated for 48 h. Cells were collected, washed twice
with ice-cold PBS and lysed with lysis buffer [50 mM Tris, pH 7.4,
150 mM NaCl, 1 mM EDTA, 0.2 mM PMSF, 1.0% Triton X-100, protease
inhibitor cocktail (Sigma-Aldrich; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA)]. Lysates were incubated for 10 min on ice,
sonicated and centrifuged for 15 min at 12,000 × g.
Subsequently, protein concentrations were determined using the
Bradford assay and the samples were boiled for 10 min. Equal
amounts of protein (20 µg/lane) were separated by SDS-PAGE,
transferred to nitrocellulose membranes and immunoblotted with a
1:1,000 dilution of a rabbit primary antibody against human S100A4
(catalog no. ab41532; Abcam, Cambridge, MA, USA) and 1:4,000
dilution of a rabbit primary antibody against human β-actin
(catalog no. ab8227; Abcam) at 4°C overnight. The secondary
antibody was a fluorescein-conjugated goat anti-rabbit IgG antibody
(H+L; catalog no. 111-035-144; Jackson ImmunoResearch Laboratories,
Inc., West Grove, PA, USA), diluted 1:5,000 in blocking solution
for 1 h at room temperature. Immunoreactivity of PVDF membranes was
visualized by scanning on LI-COR infrared laser imaging system
(LI-COR, Inc., Lincoln, NE, USA). The values of the band density
were normalized to β-actin using Multi-Gauge software version 2.0
(FujiFilm Corporation, Tokyo, Japan), therefore, the background was
subtracted and only the non-saturated signals were quantified,
resulting in a ratio which indicated the relative expression levels
of the target protein for statistical analysis (n=3).
Molecular docking of S100A4 and
polysaccharides
The protein structure of S100A4 used in the docking
studies was obtained from the Protein Data Bank (http://www.rcsb.org/pdb/home/home.do
cod3CGA) (21,22). All hydrogen atoms were added and
the calcium ion, in each subunit, and an active site of a sphere
was set around the following seven residues: Phe72, Tyr75, Phe78,
Leu79, Met12, Val13 and Phe16, the coordinates of the sphere were
7.985, 6.865, −4.913. Iota-carrageenan (1CAR) was used as surrogate
polysaccharide (37) for the
simulation, it was energy minimized using ‘Powell algorithms’
method with a convergence gradient value of 0.05 kcal/(mol Å), 100
max interations were saved as mol2 format using the SYBYL-X 2.0
package (Tripos, Inc., St. Louis, MO, USA). Molecular docking was
performed using GOLD 3.0.1 software (www.ccdc.cam.ac.uk/solutions/csd-discovery/components/gold/)
that applied genetic algorithm. The number of generic algorithm
runs was set to 10. Other parameters were the default.
Motility mechanism by analyzing the
S100A4-NMIIA complex with 1CAR
The protein structure used in the docking studies
was obtained from the Protein Data Bank (code 3ZWH). All hydrogen
atoms were added, then the internal NMIIA peptide was removed. The
polysaccharide in 1CAR was energy minimized using ‘Powell
algorithms’ method with a convergence gradient value of 0.05
kcal/(mol Å), 100 max iterations, saved as mol2 format using
SYBYL-X 2.0 package. Molecular docking was performed using GOLD
3.0.1 software that applied genetic algorithm, and the binding site
was defined to encompass all atoms within a 10 Å sphere, whose
origin (point 16.579, 1.084, 23.581) was located at the center of
the residues at N-terminal of the NMIIA peptide (residues from
Tyr1893 to Ala1907). The number of generic algorithm runs was set
to 20. Other parameters were the default.
Statistical analysis
SPSS 19.0 software (IBM SPSS, Armonk, NY, USA) was
used for statistic analysis. All experimental results were
expressed as the mean ± standard deviation, and were analyzed by
one-way analysis of variance with Dunnett's multiple comparison
tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
CP1 inhibits A549 cell
proliferation
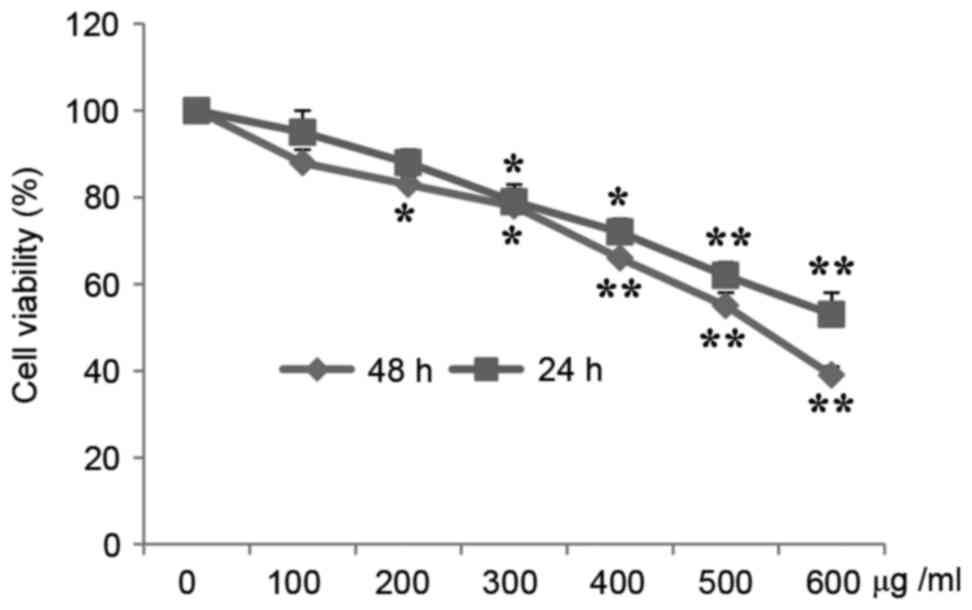
MTT assays demonstrated that A549 cell proliferation
was significantly inhibited by CP in a time- and
concentration-dependent manner. Fig.
1 presents the viability of cells treated with CP at various
concentrations for 24 and 48 h. The cell viability was reduced
84.11 and 76.33% of the control following treatment with 200 and
300 µg/ml CP, respectively, for 24 h, and was 83.88 and 71.23%
following treatment with 200 and 300 µg/ml CP, respectively, for 48
h.
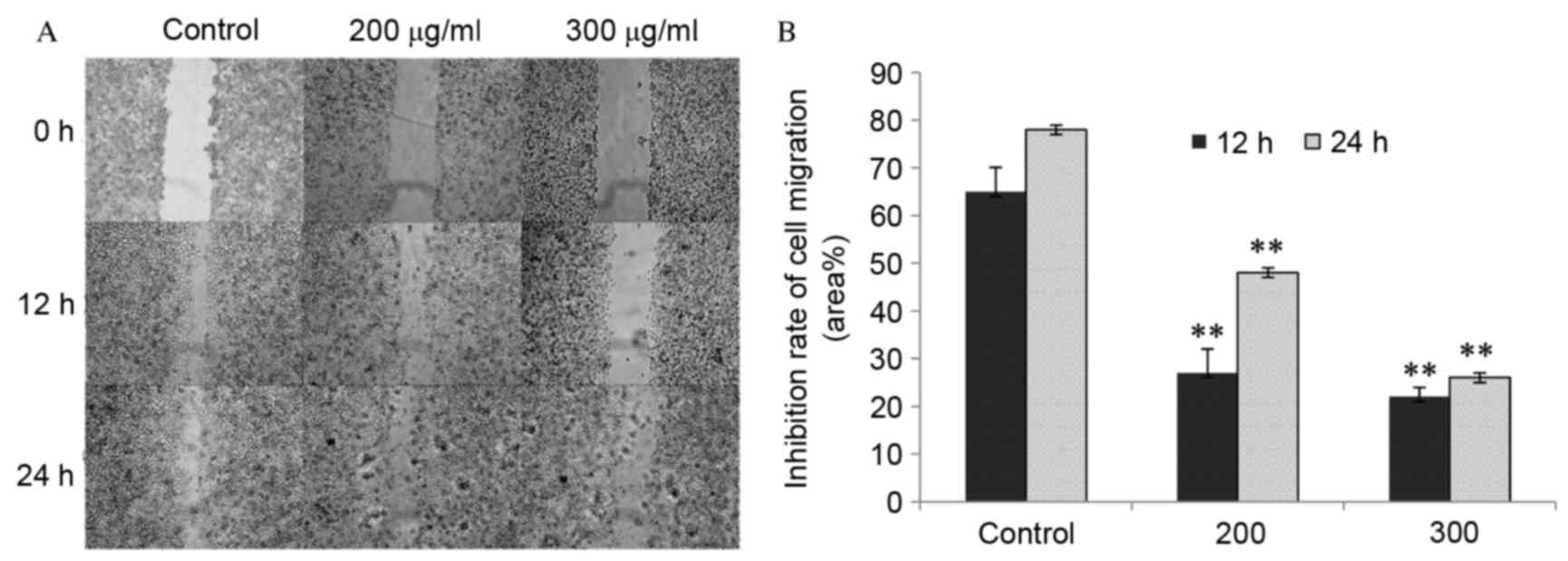
CP1 inhibits A549 cell migration
The effects of CP1 on cell migration were determined
using a cell scratch wound healing assay. The cells gradually grow
back to confluence over scratch wound and were almost healed in the
control group after 24 h. However, in the CP treatment groups wound
healing was significantly reduced compared with the control
(P<0.01), as shown my measuring the cell free area.
Additionally, 300 µg/ml CP exerted a more potent effect on cell
migration compared with 200 µg/ml CP following scratches. This
indicated that CP1 inhibited cell mobility in a dose-dependent
manner (Fig. 2).
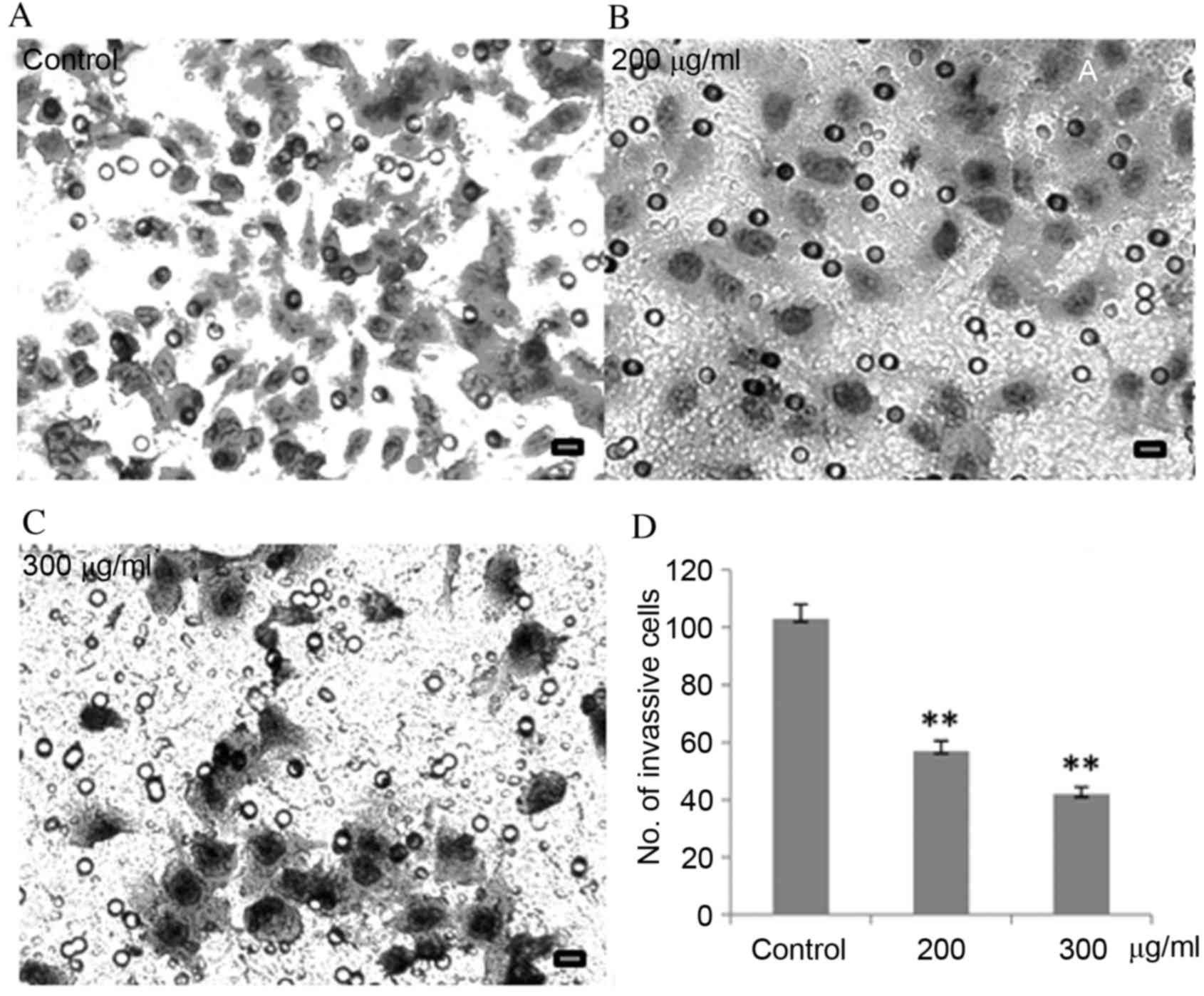
CP1 inhibits invasion of A549
cells
Aggressive cancer cells can penetrate surrounding
tissues or different organs by epithelial invasion, which directly
and indirectly reflects clinical progression. The results of
Fig. 3 demonstrated that, compared
with untreated cells, as the CP1 concentration increases, the
number of cells penetrating through the Matrigel was significantly
reduced in a dose-dependent manner (P<0.01).
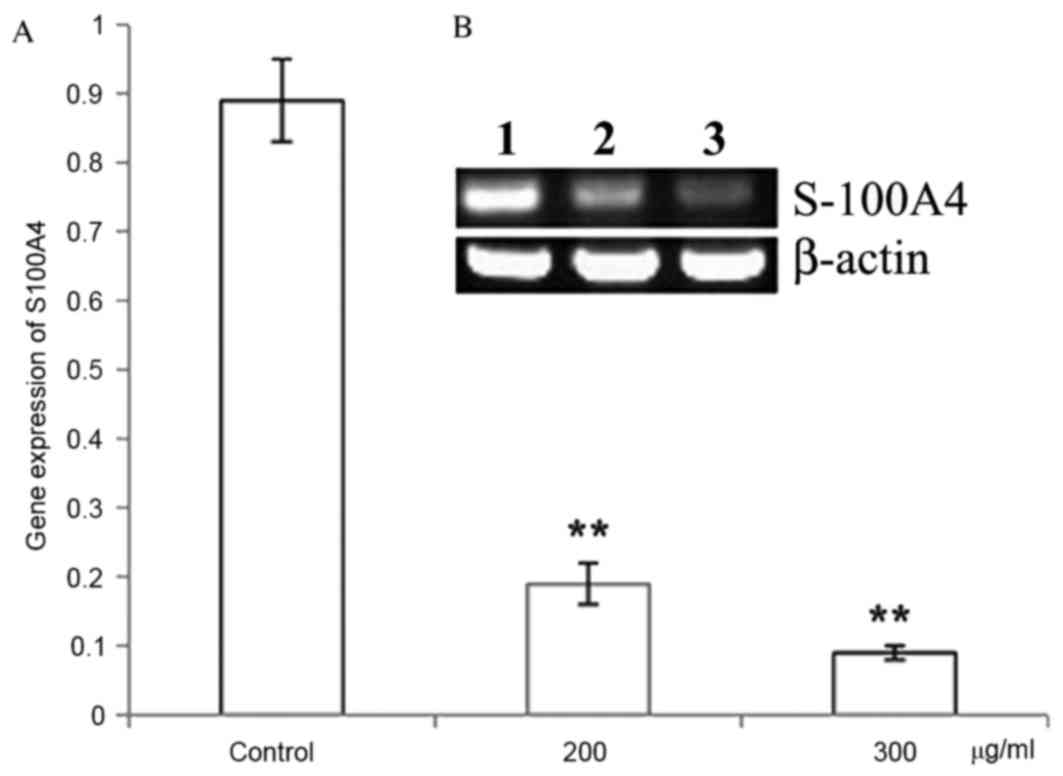
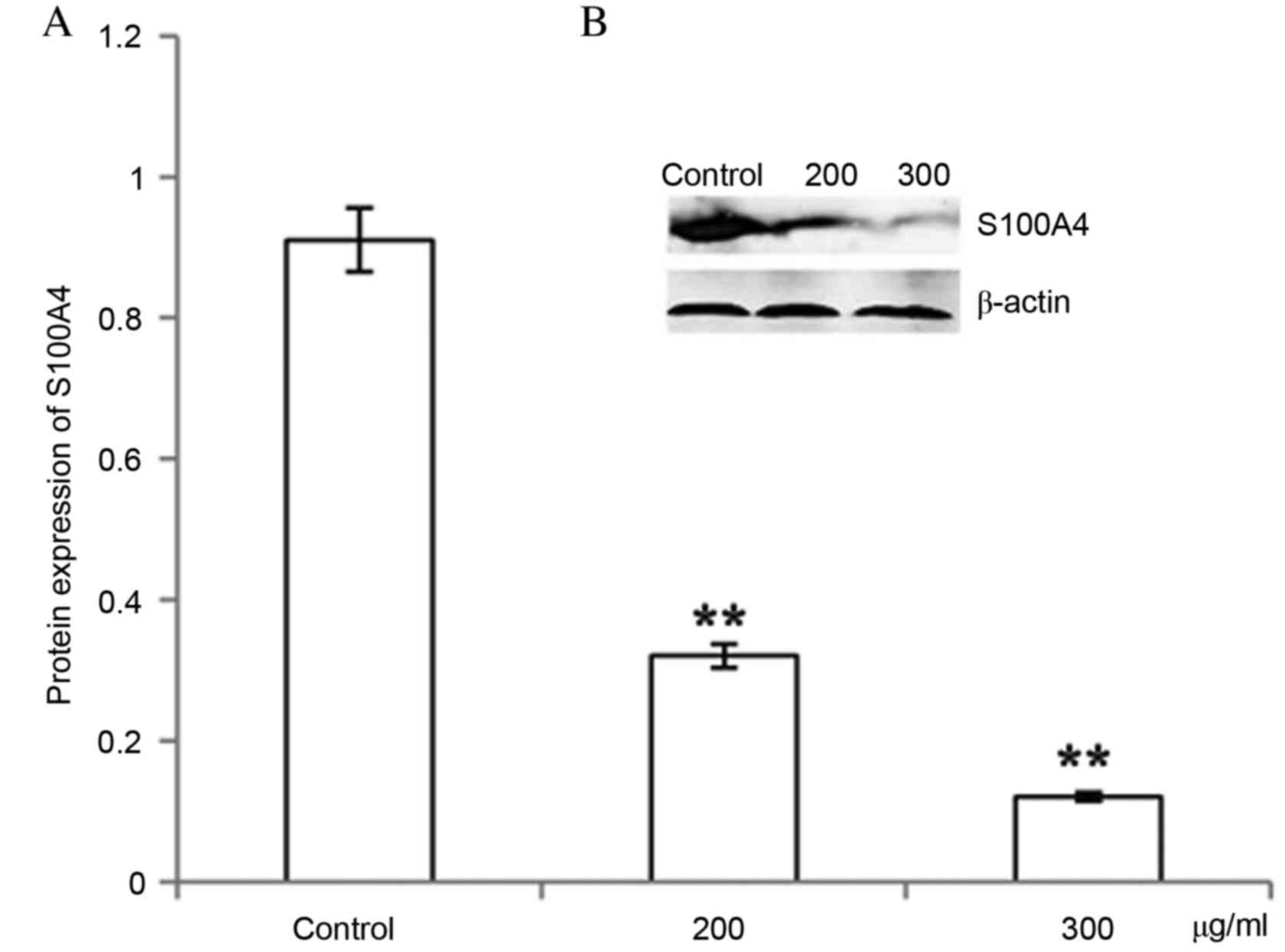
CP1 inhibits S100A4 gene and protein
expression
Different concentrations of CP1 were incubated with
A549 cells for 24 h. The relative S100A4 mRNA level was
significantly decreased compared with the control in a
dose-dependent manner (P<0.01), which demonstrated that CP1
inhibited the expression of the S100A4 gene (Fig. 4). Western blot analysis was
performed to detect the expression of S100A4 protein, which
demonstrated that treatment with CP1 polysaccharides of 24 h
inhibited the S100A4 protein expression in a dose-dependent manner
(P<0.01; Fig. 5).
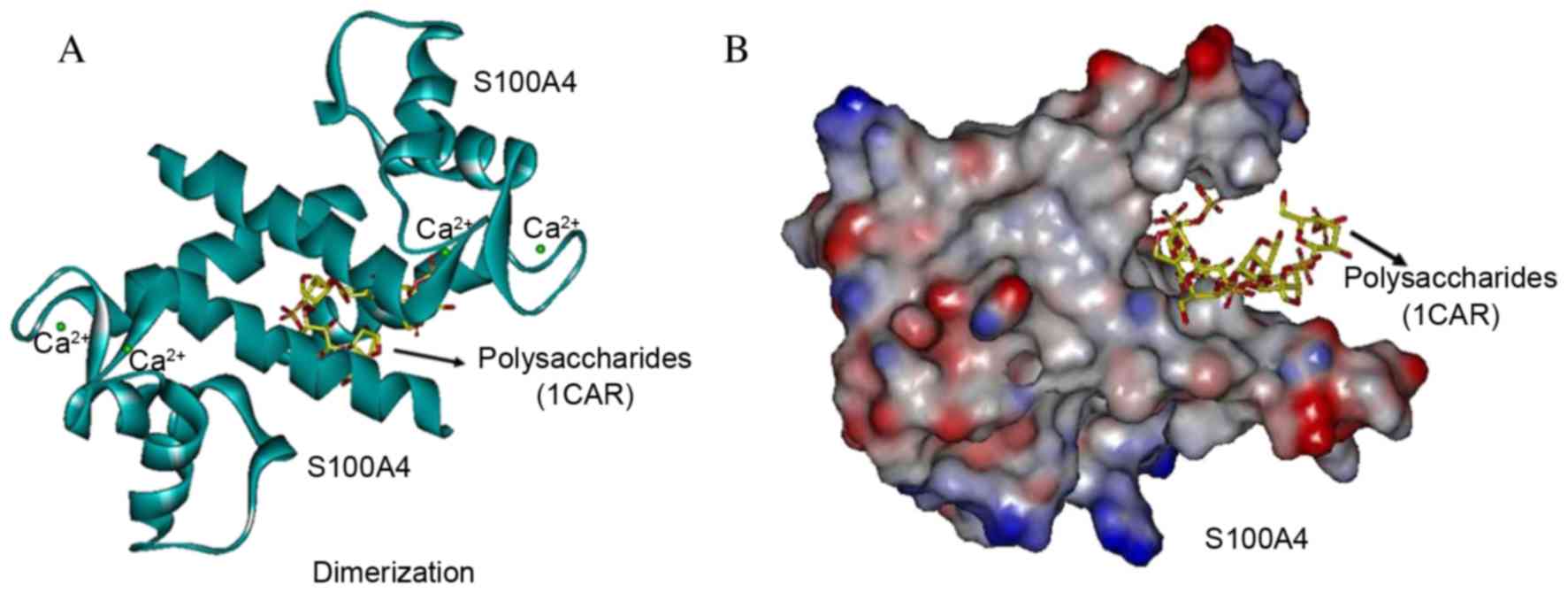
Interaction with polysaccharides in
the dimerization site of S100A4
Each S100A4 subunit contains two calcium-binding
motifan N-terminal pseudo-EF hand and a C-terminal canonical
EF-hand. The polysaccharide analog did not target the dimerization
site as it was demonstrated that 1CAR interacted between the two
helices from each subunit of S100 homodimers, stabilized by
noncovalent that form an X-type four-helix bundle dimerization
motif. With full flexibility to be as close as possible to a
natural conformation of S100A4 (Protein Bank Database, 3CGA), 1CAR
GOLD docking occurred in the dimerization site at the turning point
of the amino acids Phe72, Tyr75, Phe78 and Leu79 of the H4 helix
with Met12, Val13 and Phe16 of the H1 helix of the 2nd S100A4 dimer
protein (Fig. 6). The 1CAR docking
pocket was more clearly demonstrated with the molecular surface
(Fig. 6B) compared with the helix
(Fig. 6A). The three strongest
bonds between S100A4 and 1CAR were scored at 20.26, 17.12 and 9.92,
respectively, which indicate a low affinity, and weak interaction
with the S100A4 dimer, however affinity may be markedly effected by
in the situation of the electric charge, for instance when cell
senescence, over oxidation or carcinogenesis occur (37).
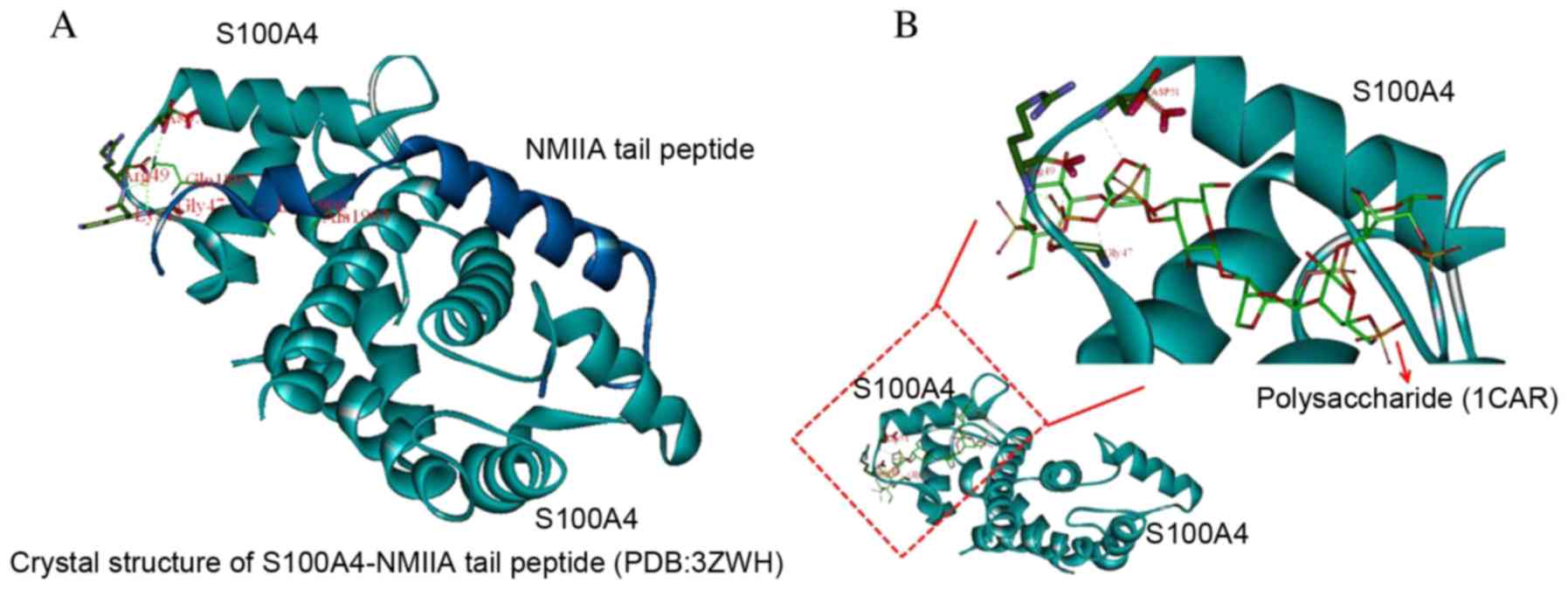
Interaction of the S100A4-NMII complex
with polysaccharides
The original setting (point 16.579, 1.084, 23.581)
was located at the center of the residues at the N-terminal of the
NMIIA peptide (residues from Tyr1893 to Ala1907), the number of
generic algorithm runs was set to 20. Other parameters were the
default. 1CAR was targeted to S100A4 Asp-51 Arg-49 Gly-47 epitopes,
revealing the potential for an intermolecular hydrogen bond by
computational chemistry. The in vivo structure of S100A4 is
associated with NMIIA protein. The most rigid portion of the
structure is the N-terminal half of the S100A4 chains (residues
2–44) with no marked differences between subunit A and B. These
residues are partially involved in subunit-subunit interactions and
in forming the surface of the dimer on the diagonally opposite side
to the NMIIA peptide tail binding interface (Fig. 7).
Discussion
The effect of coix polysaccharides, CP1, on cell
proliferation was demonstrated to corroborate our previous results,
which indicated that CP1 predominantly reduced cell viability by
inducing apoptosis (36). Thus,
the present study aimed to investigate how CP1 would influence cell
migration and invasion, particularly the potential target genes
involved.
S100A4 is localized in the nucleus, cytoplasm and
extracellularly, and possesses a wide range of biological
functions, including regulation of angiogenesis, cell
senescence/survival, motility and invasion (39). S100A4 promotes metastasis in
several experimental animal models, and S100A4 protein expression
is associated with patient outcome in a number of tumor types
(40). Thus, inhibition of S100A4
would be an applicable approach for cancer chemotherapy,
particularly the compounds in food that may help to reduce, or
reverse, the progression of metastasis without high toxicity or
side effects.
The mechanism of S100A4 as metastatic factor is
unclear, even though its association with motility-associated
proteins has being previously demonstrated (41). The inhibition of A549 lung cancer
cell line proliferation in the presence of CP1 coix polysaccharide
was repeatedly observed by MTT assay, and S100A4 expression was
increased in the migratory and invasive cancer cells (42,43).
As CP1 inhibition of S100A4 was associated with inhibition of
cancer cell migration and invasion, it is important further examine
the precise molecular mechanism of S100A4 in mobility and motility.
The data of the current study demonstrated that CP1 inhibited the
migration of A549 cells in a scratch wound healing assay and
Matrigel assay. Furthermore, S100A4 expression was reduced by CP1
as demonstrated by RT-PCR and western blot analysis. However, it
remains unclear how the molecules interact in vivo. It is
known that S100A4 acts in a complex of calcium-bound dimers and is
secreted into the extracellular environment and the blood stream
(34). It is also known that the
effects of S100A4 on cell motility require interaction with NMIIA
(44). Molecular docking
simulation suggested that polysaccharide analog, 1CAR, interacted
with one of the pockets of the S100A4 dimer, and interestingly the
analog was able to dock to the N-terminal pocket of the
S100A4-NIIMA complex, the site of the interaction S100A4 and NMIIA,
even though the GOLD score was relatively high (26.04), the analog
access to the pocket of the S100A4 dimer with an relative low free
energy (20.26, 17.12 and 9.92).
The precise structure of CP1 is unknown, even though
the polysaccharides in the extract are rhamnose, arabinose, xylose,
galactose, galacturonic acid and glucuronic acid in molar ratios of
1.8:43.8:10.8:33.2:3.2:7.2 (45),
however the molecular unity is low for the polysaccharides. Due to
this reason a 1CAR, a main cell wall polysaccharide of red algae
was used as a CP1 surrogate for molecular docking analysis.
Carrageenans are linear polymers of ~25,000 galactose derivatives
with regular but imprecise structures, dependent on the source and
extraction conditions. The iota-carrageenase bound to 1CAR
fragments was resolved at 2.0A resolution (Protein Bank Database,
1KTW), the first direct determination of a 3D structure of a
polysaccharide (46). For
simulation and simplification, the 6-mer polysaccharide, 1CAR, is
widely used for the docking. The docking occurred at the interface
of dimerization with the following 3D structural sequence: Phe72,
Tyr75, Phe78, Leu79, Met12, Val13, Phe16; however the affinity with
1CAR was low.
S100A4 is frequently interacts with different
proteins, and one of the most investigated interaction partners of
S100A4 is NMIIA. Metastasis-associated cellular motility is
associated with S100A4-NMIIA interaction (47). The calcium-dependent interaction of
S100A4 with NMIIA prevents filament assembly and promotes filament
disassembly (8,10,28,29).
The increased cytoskeletal dynamics lead to the formation of side
protrusions and extensive forward protrusions in S100A4 expressing
cells. S100A4 binds to the C-terminal end of the coiled-coil tail
of NMIIA (31) overlapping the
assembly competence domain. The complex of S100A4-NMIIA was
demonstrated interact with polysaccharides in the current
study.
Taking together all the above biological and docking
simulation data, the inhibition of cancer cell migration and
invasion by CP1 may be caused by interaction with S100A4. Even
though the large molecules of polysaccharides interacted weakly,
they exhibited a tendency and ability to inhibit S100A4,
potentially due to de-dimerization or destabilization of the
S100A4-NMIIA complex. CP1 may therefore have potential as an
alternative cancer chemotherapeutic via targeting of S100A4.
However, further studies are required to understand the precise
mechanisms involved.
Acknowledgements
This work was financed by National Natural Science
Foundation of China (grant no. 31471609), the International Science
and Technology Cooperation Program of China (grant no.
2012DFA30600), the Special Program for the Science and Technology
Plan of Zhejiang Province (grant no. 2011C02003).
References
|
1
|
Maletzki C, Bodammer P, BreitrÜck A and
Kerkhoff C: S100 prteins as diagnostic and prognostic markers in
colorectal and hepatocellular carcinoma. Hepat Mon.
12:e72402012.PubMed/NCBI
|
|
2
|
Schäfer BW, Wicki R, Engelkamp D, Mattei
MG and Heizann CW: Isolation of a YAC clone covering a cluster of
nine S100 genes on human chromosome 1q21: Rationale for a new
nomenclature of the S100 calcium-binding protein family. Genomics.
25:638–643. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamamura T, Hitomi J, Nagasaki K, Suzuki
M, Takahashi E, Saito S, Tsukada T and Yamagchi K: Human CAAF1
gene-molecular cloning, gene structure, and chromosome mapping.
Bichem Biophys Res Commun. 221:356–360. 1996. View Article : Google Scholar
|
|
4
|
Roth J, Vogl T, Sorg C and Sunderkötter C:
Phagocyte-specific S100 proteins: A novel group of proinflammatory
molecules. Trends Immunol. 24:155–158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guignard F, Mauel J and Markert M:
Identification and characterization of a novel human
neutrophilprotein related to the S100 family. Biochem. 309:395–401.
1995. View Article : Google Scholar
|
|
6
|
Marti T, Erttmann KD and Gallin MY:
Host-parasite interaction in human onchocerciasis: Identification
and sequence analysis of a novel human calgranulin. Biochem Biophys
Res Commun. 221:454–458. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ryckman C, Robichaud GA, Roy J, Cantin R,
Tremblay MJ and Tessier PA: HIV-1 transcription and virus
production are both accentuated by the proinflammatory
myeloid-related proteins in human CD4+ T lymphocytes. J Immunol.
169:3307–3313. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Donato R: S100: A multigenic family of
calcium-modulated proteins of the EF-hand type with intracellular
and extracellular functional roles. Int J Biochem Cell Biol.
33:637–668. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Donato R, Cannon BR, Sorci G, Riuzzi F,
Hsu K, Weber DJ and Geczy CL: Functions of S100 proteins. Curr Mol
Med. 13:24–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marenholz I, Heizmann CW and Fritz G: S100
proteins in mouse and man: From evolution to function and pathology
(including an update of the nomenclature). Biochem Biophys Res
Comun. 322:1111–1122. 2004. View Article : Google Scholar
|
|
11
|
Orre LM, Panizza E, Kaminskyy VO, Vernet
E, Gräslund T, Zhivotovsky B and Lehtiö J: S100A4 interacts with
p53 in the nucleus and promotes p53 degradation. Oncogene.
32:5531–5540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ilg EC, Troxler H, Burgisser DM, Kuster T,
Markert M, Guignard F, Hunziker P, Birchler N and Heizmann CW:
Amino acid sequence determination of human S100A12 (P6, calgranulin
C, CGRP, CAAF1) by tandem mass spectrometry. Biochem Biophys Res
Commun. 225:146–150. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cole AM, Kim YH, Tahk S, Hong T, Weis P,
Waring AJ and Ganz T: Calcitermin, a novel antimicrobial peptide
isolated from human airway secretions. FEBS Lett. 504:5–10. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Filipek A, Jastrzebska B, Nowotny M and
Kuznicki J: CacyBP/SIP, a calcyclin and Siah-1-interacting protein,
binds EF-hand proteins of the S100 family. J Biol Chem.
277:28848–28852. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schäfer BW and Heizmann CW: The S100
family of EF-hand calcium-binding proteins: Functions and
pathology. Trends Biochem Sci. 21:134–140. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sherbet GV and Lakshmi MS: S100A4 (MTS1)
calcium binding protein in cancer growth, invasion and metastasis.
Anticancer Res. 18:2415–2421. 1998.PubMed/NCBI
|
|
17
|
Mazzuccheli L: Protein S100A4: Too long
overlooked by pathologists? Am J Pathol. 160:7–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gribenko AV, Hopper JE and Makhatadze GI:
Molecular characterization and tissue distribution of a novel
member of the S100 family of EF-hand proteins. Biochemistry.
40:15538–15548. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taylor S, Herrington S, Prime W, Rudland
PS and Barraclough R: S100A4 (p9Ka) protein in colon carcinoma and
liver metastasis: Sssociation with carcinoma cell and
T-lymphocytes. Br J Cancer. 86:409–416. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boye K and Maelandsmo GM: S100A4 and
metastasis: A small actor playing many roles. Am J Pathol.
176:528–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cerezo LA, Kuncová K, Mann H, Tomcík M,
Zámecník J, Lukanidin E, Neidhart M, Gay S, Grigorian M, Vencovsky
J and Senolt L: The metastasis promoting protein S100A4 is
increased in idiopathic inflammatory myopathies. Rheumatology
(Oxford). 50:1766–1772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klingelhöfer J, Møller HD, Sumer EU, Berg
CH, Poulsen M, Kiryushko D, Soroka V, Ambartsumian N, Grigorian M
and Lukanidin EM: Epidermal growth factor receptor ligands as new
extracellular targets for the metastasis-promotingS100A4 protein.
FEBS J. 276:5936–5948. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pathuri P, Vogeley L and Luecke H: Crystal
structure of metastasis-associated protein S100A4 in the active
calcium-bound form. J Mol Biol. 383:62–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kiss B, Duelli A, Radnai L, Kékesi KA,
Katona G and Nyitray L: Crystal structure of the S100A4-nonmuscle
myosin IIA tail fragment complex reveals an asymmetric target
binding mechanism. Proc Natl Acad Sci USA. 109:6048–6053. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li ZH and Bresnick AR: The S100A4
metastasis factor regulates cellular motility via a direct
interaction with myosin-IIA. Cancer Res. 66:5173–5180. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tarabykina S, Scott DJ, Herzyk P, Hill TJ,
Tame JR, Kriajevska M, Lafitte D, Derrick PJ, Dodson GG, Maitland
NJ, et al: The dimerization interface of the metastasis-associated
protein S100A4 (Mts1): In vivo and in vitro studies. J Biol Chem.
276:24212–24222. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abdali S, Laere BD, Poulsen M, Grigorian
M, Lukanidin E and Klingelhöfer J: Toward methodology for detection
of cancer-promoting S100A4 protein conformations in subnanomolar
concentrations using Raman and SERS. J Phys Chem. 114:7274–7279.
2010.
|
|
28
|
Fang Z, Forslund N, Takenaga K, Lukanidin
E and Kozlova EN: Sensory neurite outgrowth on white matter
astrocytes is influenced by intracellular and extracellular S100A4
protein. J Neurosci Res. 83:619–626. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bettum IJ, Vasiliauskaite K, Nygaard V,
Clancy T, Pettersen SJ, Tenstad E, Maelandsmo GM and Prasmickaite
L: Metastasis-associated protein S100A4 induces a network of
inflammatory cytokines that activate stromal cells to acquire
pro-tumorigenic properties. Cancer Lett. 344:28–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo C, Urgard E, Vooder T and Metspalu A:
The role of COX-2 and Nrf2/ARE in anti-inflammation and
antioxidative stress: Aging and anti-aging. Med Hypotheses.
77:174–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takahashi M, Konno C and Hikino H:
Isolation and hypoglycemic of coixan A, B and C, glycans of Coix
lachrymal-jobi var. Ma-yuen. seeds. Planta med. 64–65.
1986.PubMed/NCBI
|
|
32
|
Hsia SM, Chiang W, Ku YH and Wang PS:
Downregulation of progesterone biosynthesis in rat granulosa cells
by adlay(Coix lachrymal-jobi L. var. Ma-yuen Stapf.) bran extracts.
Int J Impot Res. 18:264–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baas P: Inductive and adjuvant treatment
strategies for localized nonsmall cell lung cancer in operable and
inoperable patients. Curr Opin Oncol. 14:180–184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yarrow JC, Perlman ZE, Westwood NJ and
Mitchison TJ: A high-throughput cell migration assay using scratch
wound healing, a comparison of image-based readout methods. BMC
Biotechnol. 4:212014.
|
|
35
|
Yao R, Davidson DD, Lopez-Beltran A,
MacLennan GT, Montironi R and Cheng L: The S100 proteins for
screening and prognostic grading of bladder cancer. Histol
Histopathol. 22:1025–1032. 2007.PubMed/NCBI
|
|
36
|
Lu X, Liu W, Wu J, Li M, Wang J, Wu J and
Luo C: A polysaccharide fraction of adlay seed (Coix lachryma-jobi
L.) induces apoptosis in human non-small cell lung cancer A549
cells. Biochem Biophys Res Commun. 430:846–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Arnott S, Scott WE, Rees DA and McNab CG:
Iota-carrageenan: Molecular structure and packing of polysaccharide
double helices in oriented fibres of divalent cation salts. J Mol
Biol. 90:253–267. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pathuri P, Vogeley L and Luecke H: Crystal
structure of metastasis-associated protein S100A4 in the active
calcium-bound form. J Mol Biol. 383:62–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Van Deursen JM: The role of senescent
cells in ageing. Nature. 509:439–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim YJ, Kim MA, Im SA, Kim TM, Kim DW,
Yang HK, Heo DS, Lee KU, Choe KJ, Kim NK, et al:
Metastasis-associated protein S100A4 and p53 predict relapse in
curatively resected stage III and IV (M0) gastric cancer. Cancer
Invest. 26:152–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Garrett SC, Varney KM, Weber DJ and
Bresnick AR: S100A4, a mediator of metastasis. J Biol Chem.
281:677–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang L, Gao S, Jiang W, Luo C, Xu M,
Bohlin L, Rosendahl M and Huang W: Antioxidative dietary compounds
modulate gene expression associated with apoptosis, DNA repair,
inhibition of cell proliferation and migration. Int J Mol Sci.
15:16226–16245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Michel G, Helbert W, Kahn R, Dideberg O
and Kloareg B: The structural bases of the processive degradation
of iota-carrageenan, a main cell wall polysaccharide of red algae.
J Mol Biol. 334:421–433. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Abrantes VE, da Rocha BA Matias, da
Nóbrega Batista R, Silva-Filho JC, Teixeira CS, Cavada BS, Gadelha
CA, Ferreira SH, Figueiredo JG, Santi-Gadelha T and Delatorre P:
Molecular modeling of lectin-like protein from Acacia farnesiana
reveals a possible anti-inflammatory mechanism in
carrageenan-induced inflammation. Biomed Res Int.
2013:2534832013.PubMed/NCBI
|
|
46
|
Bowers RR, Manevich Y, Townsend DM and Tew
KD: Sulfiredoxin redox-sensitive interaction with S100A4 and
non-muscle myosin IIA regulates cancer cell motility. Biochemistry.
51:7740–7754. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Elliott PR, Irvine AF, Jung HS, Tozawa K,
Pastok MW, Picone R, Badyal SK, Basran J, Rudland PS, Barraclough
R, et al: Asymmetric mode of Ca2+-S100A4 interaction
with nonmuscle myosin IIA generates nanomolar affinity required for
filament remodeling. Structure. 20:654–666. 2012. View Article : Google Scholar : PubMed/NCBI
|