Introduction
Atopic dermatitis (AD) involves a complex immune
response mediated by T helper (Th) 1 and Th2 T cell subsets
(1). Chronic skin lesions of AD
are dominated by Th1-type cytokine responses with high levels of
interferon (IFN)-γ. The Th2-type cytokines, interleukin (IL)-4, −6
and −13, serve important roles in the increase in the levels of
serum immunoglobulin E (IgE) and mast cells during AD development
(2). Therefore, Th1 and Th2
cytokines may contribute to AD lesions (3).
The topical application of steroids is typically
recommended for the treatment of AD (4). However, topical steroids have various
well-known adverse effects, including skin atrophy, a tendency for
bruising, tachyphylaxis and the rebound phenomenon (5). Therefore, herbal medicines have been
investigated as potential topical agents for the treatment of
AD.
Pseudostellaria heterophylla (Miquel) Pax has
been described as a type of ‘qi-enriching and tonic drug’ (6), which has a beneficial effect for the
immune system (7). In addition,
Pseudostellaria heterophylla (PH) has mild toning effects on
the body, leading to its widespread use in herbal medicines and
healthcare products (8,9). The primary components of PH include
polysaccharides, cycle peptides, amino acids, volatile compounds,
saponins, amino acids and minerals. In particular, macromolecule
polysaccharides are considered to be the primary active component
of PH (10). Previous studies have
suggested that PH has immunomodulatory, anti-inflammatory and
anti-oxidant properties (7,11).
These diverse biological activities may be beneficial for the
treatment of AD.
The present study therefore aimed to evaluate the
immunomodulatory activity of PH water extracts in a
2,4-dinitrochlorobenzene (DNCB)-induced mouse model of AD.
Materials and methods
Preparation of sample
PH was supplied by Jung-do Herb Inc. (Guri, Korea)
and was authenticated by Professor Woong Mo Yang (Kyung Hee
University, Seoul, Korea). The crude drug (100 g) was extracted
with 1,000 ml distilled water for 24 h at room temperature.
Following filtration, the extracted solution was concentrated in a
rotary evaporator, freeze-dried for three days, and stored in
aliquots at −70°C. The yield from the dried roots was 23.09%. The
authenticated voucher specimens (no. PH131201) were stored in the
College of Pharmacy of Kyung Hee University. The PH extracts was
dissolved in distilled water immediately prior to use.
Animal model
A total of 25 female BALB/c mice (age, 6 weeks;
weight, 18–22 g) were obtained from Raon Bio Inc. (Yongin, Korea)
and housed in an air-conditioned room at a temperature of 23±1°C
and relative humidity of 60±5%, under a 12-h light/dark cycle.
During the experimental period, all mice received standard mouse
chow and water ad libitum. All experiments were conducted
according to the guidelines of the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The
protocol was approved by Committee on Care and Use of Laboratory
Animals of the Kyung Hee University (approval no. KHUASP
(SE)-14-030; Seoul, Korea). Following an acclimation period of 1
week, mice were randomly divided into five groups (n=5): NOR, in
which mice were challenged with vehicle treatment as a baseline
control; DNCB, in which mice were sensitized with 100 µl/day 1 or
0.5% DNCB (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) as
negative control; DEX, in which mice were topically treated with
100 µl/day 10 µM dexamethasone (DEX; Sigma-Aldrich; Merck
Millipore) following DNCB sensitization as positive control; PH1,
in which mice were topically treated with 100 µl/day 1 mg/ml PH
following DNCB sensitization; and PH100, in which mice were
topically treated with 100 µl/day 100 mg/ml PH following DNCB
sensitization.
To induce dermatitis, 100 µl 1% DNCB solution [in
acetone: olive oil at a ratio of 4:1 (v/v)] was applied onto the
shaved dorsal skin of all mice excluding the NOR group once a day
for 3 days (day 1–3). Following the first challenge, mice were left
untreated for 4 days (day 4–7). In the second challenge, PH (1 or
100 mg/ml) or DEX was topically applied to mice prior to the
application of 0.5% DNCB for 11 consecutive days (day 8 to day 18).
An equal volume of vehicle was applied to the NOR group at the same
time points. All animals were sacrificed 24 h after the last
treatment (day 19) under anesthesia with intraperitoneal injection
of a tiletamine/zolazepam mixture (30 mg/kg, Zoletil 50; Virbac
Lab, Carros, France). Serum was collected by cardiac puncture and
the dorsal skin of mice was biopsied for histopathology and
measurement of tissue cytokines levels.
Histopathology
To evaluate skin thickening and mast cell
infiltration, the dorsal skin samples (1×0.4 cm2) were
prepared at sacrifice (day 19). Samples were fixed in 10% buffered
formalin (Sigma-Aldrich; Merck Millipore), embedded in paraffin and
cut into 4-µm thick sections. Following dewaxing and dehydration of
paraffin sections, hematoxylin and eosin staining was performed to
assess skin thickening and toluidine blue staining to detect mast
cells as previously described (12). Histopathological alterations were
examined using the Leica Application Suite (LAS; Leica
Microsystems, Inc., Buffalo Grove, IL, USA) and viewed at
magnification, ×100. Skin thickness was measured in >5 fields,
at intervals of 200 µm, in each sample (n=5). The number of mast
cells was measured in the entire area of each section (19.05×25.4
cm) for each sample (n=5).
IgE concentrations in plasma, as
detected by ELISA
Blood was allowed to clot for 30 min at room
temperature. The serum was prepared by centrifugation (10,000 × g
for 10 min at room temperature) and stored at −70°C until use. The
concentrations of IgE in serum were determined using a Mouse IgE
ELISA kit (cat. no. 555248; BD Biosciences, Franklin Lakes, NJ,
USA) according to the manufacturer's protocol.
Cluster of differentiation
(CD)4+ cell expression, as detected by
immunohistochemistry (IHC)
IHC was performed according to the manufacturer's
protocol. To retrieve antigens in the skin tissues, the
deparaffinized sections were boiled in 10 mM sodium citrate buffer
for 30 min. Endogenous peroxidase was subsequently blocked by
incubation in 3% H2O2 for 30 min and slides
were blocked with 5% normal goat serum (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) in phosphate-buffered saline (PBS)
containing 5% fetal bovine serum (Corning, Inc., Corning, NY, USA),
2% bovine serum albumin (BSA; Vector Laboratories, Burlingame, CA,
USA) and 0.1% Triton X-100 for 1 h. Following washing in PBS three
times, the sections were incubated overnight with an antibody
against biotin anti-mouse CD4 (cat. no. 100403; BioLegend, Inc.,
San Diego, CA, USA), diluted 1:100 in normal goat serum, in a
humidified chamber. After washing with PBS, the biotinylated goat
anti-mouse IgG (cat. no. BA-9200; diluted 1:200 in TBST; Vector
Laboratories) was applied for 2 h at room temperature. The slides
were subsequently incubated with an Avidin/Biotinylated Enzyme
Complex kit (Vector Laboratories) for 1 h, followed by the
substrate chromogen (3,3-diaminobenzidine; Dako, Glostrup,
Denmark), and counterstained by hematoxylin staining. CD4 staining
was visualized using LAS (magnification, ×200).
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
To detect mRNA expression levels of various
inflammatory cytokines, RT-PCR was performed. Total RNA was
extracted from excised dorsal skin using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol, and quantified by
determining the optical density at a wavelength of 260 nm. Isolated
total RNA (1 µg) was used as a template for cDNA synthesis. RT was
performed using a cDNA Synthesis kit (Invitrogen) at 45°C for 1 h.
The reaction was terminated by incubation at 95°C for 5 min, and
cDNA was stored at −20°C. PCR was performed using the synthesized
cDNA as a template and Maxime PCR premix kit (Invitrogen; Thermo
Fisher Scientific, Inc.) and specific primers for mouse IFN-γ,
IL-4, IL-6, IL-8, tumor necrosis factor (TNF)-α, IL-1β and GAPDH.
Cycling conditions were as follows: Initial denaturation cycle for
5 min at 94°C, 30 cycles of amplification for 2 min at 72°C, 1 min
at 94°C, 1 min at 60°C, and a final extension phase consisting of 1
cycle of 10 min at 72°C. The primers were designed using Primer
Express software version 3.0 (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and the sequences of primers are presented in
Table I. Amplicons were visualized
on 2% agarose gel stained with ethidium bromide using a GDS-200D
Gel Image System version PSRem164 (UVP, Inc., CA, USA). All mRNA
levels were normalized to the housekeeping gene GAPDH by a
computerized densitometry system (ImageJ version 1.38e; National
Institutes of Health, Bethesda, MD, USA).
 | Table I.Primer sequences used for reverse
transcription polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription polymerase chain reaction.
| Target gene | Direction | Sequence (5′-3′) | Amplicon size
(bp) |
|---|
| Interferon-γ | Forward |
AGCGGCTGACTGAACTCAGATTGTAG | 330 |
|
| Reverse |
GTCACAGTTTTCAGCTGTATAGGG |
| Interleukin-4 | Forward |
ATGGGTCTCAACCCCCAGC | 300 |
|
| Reverse |
GCTCTTTACGCTTTCCAGGAAGTC |
| Interleukin-6 | Forward | ATCAACTCCTTCTCCACA
AGCGC | 628 |
|
| Reverse |
GAAGAGCCCTCAGGCTGGACTG |
| Interleukin-8 | Forward |
TGTGGGAGGCTGTGTTTGTA | 200 |
|
| Reverse |
ACGAGACCAGGAGAAACAGG |
| Tumor necrosis
factor-α | Forward |
GGTGCAATGCAGAGCCTTCC | 173 |
|
| Reverse |
CAGTGATGTAGCGACAGCCTGG |
| Interleukin-1β | Forward |
CTCTAGACCATGCTACAGAC | 291 |
|
| Reverse |
TGGAATCCAGGGGAAACACTG |
| GAPDH | Forward |
GGCATGGACTGTGGTCATGA | 376 |
|
| Reverse |
TTCACCACCATGGAGAAGGC |
|
Analysis of inflammatory protein
expression by western blot analysis
To determine the mechanisms underlying the
development of AD-like skin lesions, the protein expression levels
of the pro-inflammatory mediators, such as nuclear factor (NF)-κB,
phosphorylated (p)-inhibitor of (I)κBα and p-mitogen-activated
protein kinases (MAPKs) were examined in skin homogenates. To
detect p-IκBα the dorsal skin samples were homogenized by the
addition of the cytoplasmic buffer [10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) at pH
7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM ethylene glycol-bis
(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA), 1 mM
dithiothreitol (DTT), 0.15% Nonidet P-40, 50 mM β-glycerophosphate,
10 mM NaF and 5 mM Na3VO4] containing
protease inhibitors. The cytoplasmic protein extracts were
collected following sample centrifugation at 10,000 × g for 30 min
at 4°C. Cytoplasmic proteins were collected from the supernatant,
and nuclear lysis buffer (20 mM HEPES at pH 7.9, 400 mM NaCl, 1 mM
EDTA, 1 mM EGTA, 1 mM DTT, 0.50% Nonidet P-40, 50 mM
β-glycerophosphate, 10 mM NaF and 5 mM
Na3VO4) was added to the pellet, homogenized
on ice for 20 min, and centrifuged at 16,000 × g for 30 min at 4°C.
The resultant homogenate was carefully removed without disturbing
the pellet for assay of activated NF-κB. The protein expression
levels of the MAPKs extracellular signal-regulated kinase (ERK)
1/2, c-Jun N-terminal kinase (JNK) and p38 were confirmed in whole
extracts using radioimmunoprecipitation assay buffer containing a
protease inhibitor cocktail. The cytoplasmic, nuclear and whole
protein extracts were subsequently used for western blotting.
Proteins were quantified by the bicinchoninic acid method according
to the manufacturer's protocol (Pierce; Thermo Fisher Scientific,
Inc.). The proteins (30 µg) were resolved in 10% SDS-PAGE gels and
electroblotted onto polyvinylidene difluoride membranes. The
membranes were blocked with 5% BSA for 1 h at room temperature and
incubated with antibodies against β-actin (cat. no. sc-47778; Santa
Cruz Biotechnology, Inc.), lamin-B (cat. no. sc-374015; Santa Cruz
Biotechnology, Inc.), NF-κB (cat. no. sc-372; Santa Cruz
Biotechnology, Inc.), p-IκBα (cat. no. sc-8404; Santa Cruz
Biotechnology, Inc.), ERK1/2 (cat. no. 4695; Cell Signaling
Technology, Inc., Danvers, MA, USA), p-ERK1/2 (cat. no. 4370; Cell
Signaling, Inc.), JNK (cat. no. 9252; Cell Signaling Technology,
Inc.), p-JNK (cat. no. 9251; Cell Signaling Technology, Inc.), p38
(cat. no. 9212; Cell Signaling Technology, Inc.) and p-p38 (cat.
no. 4631; Cell Signaling Technology, Inc.), diluted 1:1,000 in TBS
containing Tween-20 (TBST) for overnight at 4°C. Membranes were
subsequently incubated with a horseradish peroxidase-conjugated
secondary anti-mouse antibody (cat. no. sc-2005; Santa Cruz
Biotechnology, Inc.) and anti-rabbit antibody (cat. no. sc-2004;
Santa Cruz Biotechnology, Inc.) diluted 1:2,000 in TBST for 2 h at
room temperature. The immunoreactive proteins were detected with an
Enhanced Chemiluminescence reagent (Pierce; Thermo Fisher
Scientific, Inc.). Band intensities were quantified by a
computerized densitometry software ImageJ version 1.38e.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Comparisons between the values for different variables were
analyzed by one-way analysis of variance followed by Dunnett's
t-test. P<0.05 was considered to indicate a statistically
significant difference using a GraphPad PRISM 5 software version 5
(GraphPad Software, Inc., La Jolla, CA, USA).
Results
PH treatment reduces dorsal skin
thickness
To evaluate AD symptoms, histological analysis was
performed on the dorsal skin of each group (Fig. 1A). Repeated DNCB-treatment caused a
thickening of the epidermis and dermis (P<0.001). Topically
applied 1 and 100 mg/ml PH suppressed epidermal hyperplasia and
dermal thickening, as did 10 µM DEX. Quantitative analysis revealed
that the PH1, PH100 and DEX groups (P<0.001) demonstrated a
27.4, 44.6 and 47.2% reduction in epidermal thickness (Fig. 1B). In addition, 12.3, 27.0 and
27.1% reduction of dermal thickness was observed in PH1
(P<0.01), PH100 (P<0.01) and DEX (P<0.001; Fig. 1C).
 | Figure 1.Pseudostellaria heterophylla
treatment reduces dorsal skin thickness. (A) Histological
assessment of the dorsal skin of each group following hematoxylin
and eosin staining. Magnification, ×100; scale bar=200 µm. (B)
Epidermal and (C) dermal thicknesses were quantified in 35 randomly
selected fields per group. Data are presented as the mean ±
standard deviation (n=5). ###P<0.001 vs. NOR;
***P<0.001 and **P<0.01 vs. DNCB. NOR, vehicle-treated mice;
DNCB, 2,4-dinitrochlorobenzene-treated mice; DEX,
2,4-dinitrochlorobenzene- and dexamethasone-treated mice; PH1,
2,4-dinitrochlorobenzene- and 1 mg/ml Pseudostellaria
heterophylla-treated mice; PH100, 2,4-dinitrochlorobenzene- and
100 mg/ml P. heterophylla-treated mice. |
PH inhibits the infiltration of mast
cells into the dermis
DNCB-treated skin lesions (P<0.001) revealed
increased infiltration of mast cells compared with the NOR group
(Fig. 2A and B). Topical
application of PH1, PH100 or DEX markedly decreased this
infiltration (P<0.001).
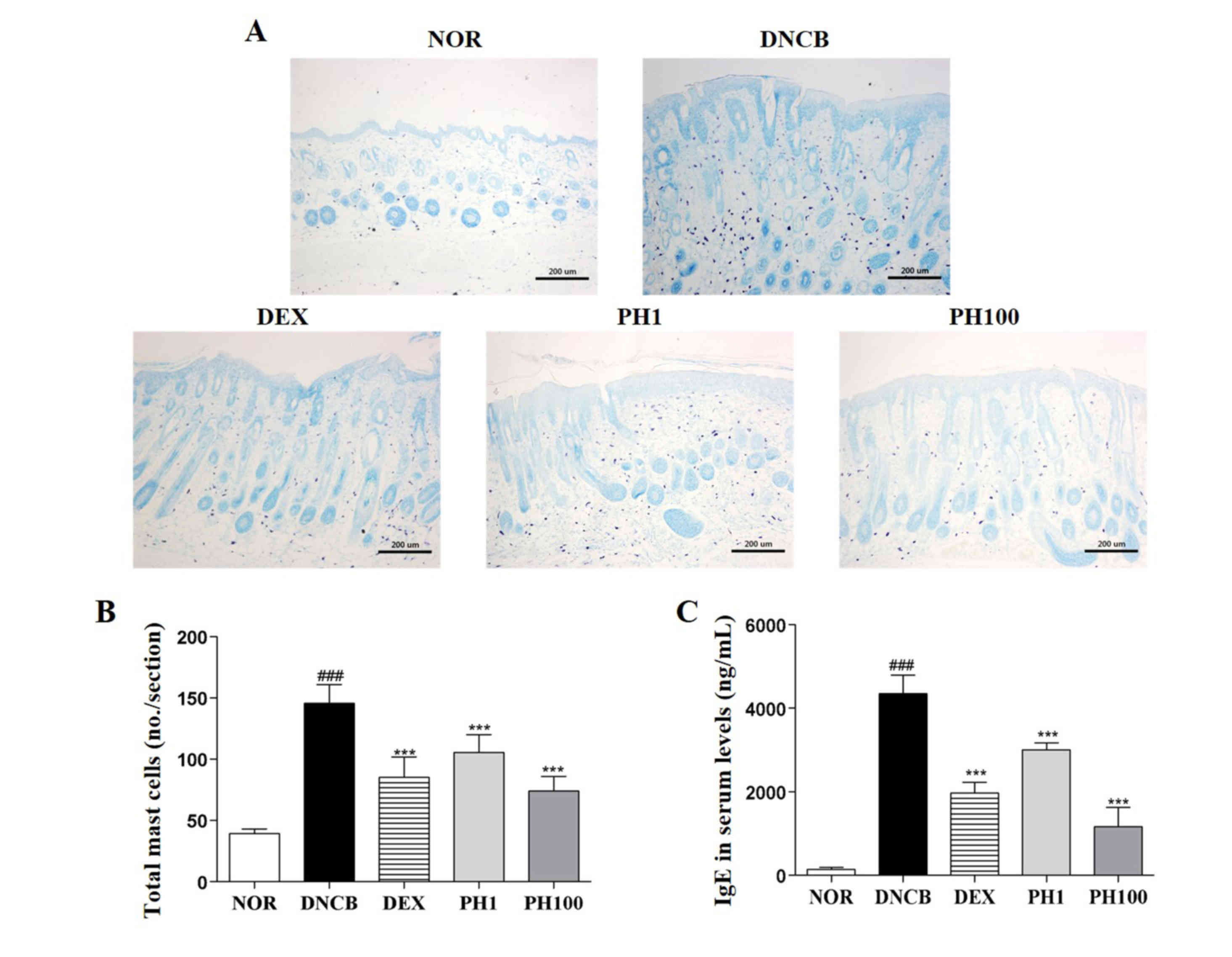 | Figure 2.Pseudostellaria heterophylla
decreases the number of mast cells in dorsal skin and serum IgE
levels. (A) Mast cells were detected by toluidine blue staining.
Magnification, ×100; scale bar=200 µm. (B) Infiltration of mast
cells was quantified by counting the mast cells in the slides
(n=5). (C) Serum levels of IgE were measured by ELISA. Data are
presented as the mean ± standard deviation (n=5).
###P<0.001 vs. NOR; ***P<0.001 and **P<0.01 vs.
DNCB. NOR, vehicle-treated mice; DNCB,
2,4-dinitrochlorobenzene-treated mice; DEX,
2,4-dinitrochlorobenzene- and dexamethasone-treated mice; PH1,
2,4-dinitrochlorobenzene- and 1 mg/ml Pseudostellaria
heterophylla-treated mice; PH100, 2,4-dinitrochlorobenzene- and
100 mg/ml P. heterophylla-treated mice; IgE,
immunoglobulin E. |
PH reduces serum IgE levels
The concentration of IgE in the plasma of the NOR
group was 143.4±51.49 ng/ml. This increased to 4348.0±446.05 ng/ml
in the DNCB group (P<0.001; Fig.
2C). The serum IgE levels in the PH1, PH100 and DEX groups was
reduced ~1.4-, ~3.7- and ~2.2-fold compared with the DNCB group
(P<0.001).
PH suppresses the infiltration of
CD4+ cells
To examine the accumulation of CD4+ cells
in allergic inflamed skin, the expression of CD4 in dorsal skin
sections was detected by IHC (Fig.
3). Increased immunostaining was observed in the DNCB group
compared with the NOR group. The detection of CD4 expression was
markedly reduced in mice treated with PH1, PH100 or DEX compared
with the DNCB group.
 | Figure 3.Pseudostellaria heterophylla
suppresses the infiltration of CD4+ T cells into dorsal
skin. Representative images revealing CD4 staining, as detected by
immunohistochemistry. Magnification, ×100; scale bar=200 µm. NOR,
vehicle-treated mice; DNCB, 2,4-dinitrochlorobenzene-treated mice;
DEX, 2,4-dinitrochlorobenzene- and dexamethasone-treated mice; PH1,
2,4-dinitrochlorobenzene- and 1 mg/ml Pseudostellaria
heterophylla-treated mice; PH100, 2,4-dinitrochlorobenzene- and
100 mg/ml P. heterophylla-treated mice; CD, cluster
of differentiation. |
PH inhibits the mRNA expression levels
of Th1 and Th2 cytokines in dorsal skin lesions
To elucidate the effect of PH treatment on cytokine
expression, alterations in the mRNA expression levels of various
cytokines were analyzed, including Th1-specific (IFN-γ),
Th2-specific (IL-4) and pro-inflammatory (IL-6, IL-8, TNF-α and
IL-1β) cytokines (Fig. 4). The
DNCB group (P<0.001) expressed increased expression levels of
Th1 and pro-inflammatory cytokine mRNA in skin tissues compared
with the NOR group. PH (1 and 100 mg/ml) and DEX suppressed the Th1
and pro-inflammatory cytokine production stimulated by DNCB
application. The inhibitory effects of the PH1, PH100 and DEX
groups were as follows: 32.5, 51.0 and 47.7% for IFN-γ (PH1 and
PH100; P<0.001); 26.8, 36.2 and 27.5% for TNF-α (PH1, P<0.01;
PH100, P<0.001); and 14.7, 29.8 and 44.2% for IL-1β (PH100,
P<0.001), respectively. Similarly, the mRNA expression levels of
IL-4, IL-6 and IL-8 markedly increased following DNCB application
compared with the NOR group (P<0.001). PH treatment suppressed
IL-4 and IL-6 mRNA expressions at all concentrations (PH1,
P<0.05; PH100, P<0.001). In PH-treated AD legion, elevated
IL-8 mRNA levels were decreased (PH1, P<0.01; PH100,
P<0.001). In addition, DEX treatment significantly reduced the
expression levels of these cytokines (P<0.001).
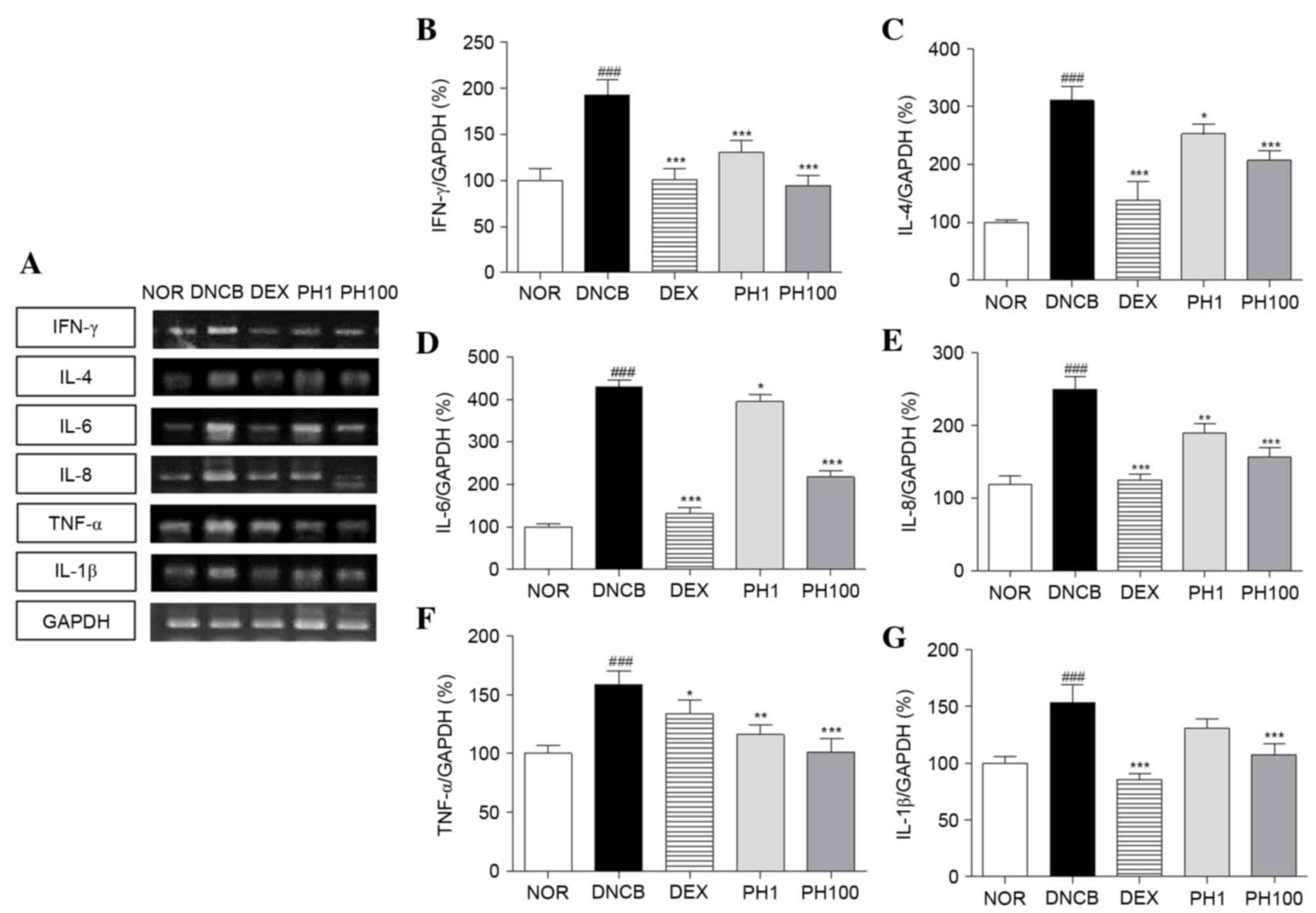 | Figure 4.Pseudostellaria heterophylla
inhibits the mRNA expression levels of Th1, Th2 and
pro-inflammatory cytokines in dorsal skin. (A) The expression of
mRNA was quantified using reverse transcription-polymerase chain
reaction. (B) IFN-γ, (C) IL-4, (D) IL-6, (E) IL-8, (F) TNF-α and
(G) IL-1β mRNA expression levels were normalized to GAPDH. Data are
presented as the mean ± standard deviation (n=5).
###P<0.001 vs. NOR; ***P<0.001, **P<0.01 and
*P<0.05 vs. DNCB. NOR, vehicle-treated mice; DNCB,
2,4-dinitrochlorobenzene-treated mice; DEX,
2,4-dinitrochlorobenzene- and dexamethasone-treated mice; PH1,
2,4-dinitrochlorobenzene- and 1 mg/ml Pseudostellaria
heterophylla-treated mice; PH100, 2,4-dinitrochlorobenzene- and
100 mg/ml P. heterophylla-treated mice; Th, T helper;
IFN, interferon; IL, interleukin; TNF, tumor necrosis factor. |
PH decreases the protein expression
levels of NF-κB, p-IκBα and MAPKs in dorsal skin lesions
The expression of inflammatory cytokines is
regulated by the transcription factor p65 NF-κB. PH treatment (1
and 100 mg/ml) inhibited the nuclear translocation of p65 NF-κB
about 34.4 and 56.0%, respectively (P<0.001). DEX group showed
50.4% reduction of NF-κB protein expression (P<0.001). Also,
p-IκBα expressions in PH1 (P<0.05), PH100 (P<0.001) and DEX
(P<0.001) groups were significantly decreased (Fig. 5A). Similarly, the protein
expression levels of p-ERK1/2 (P<0.001, Fig. 5B), p-p38 (P<0.01, Fig. 5C) and p-JNK (P<0.001, Fig. 5D) were dose-dependently
downregulated following PH treatment.
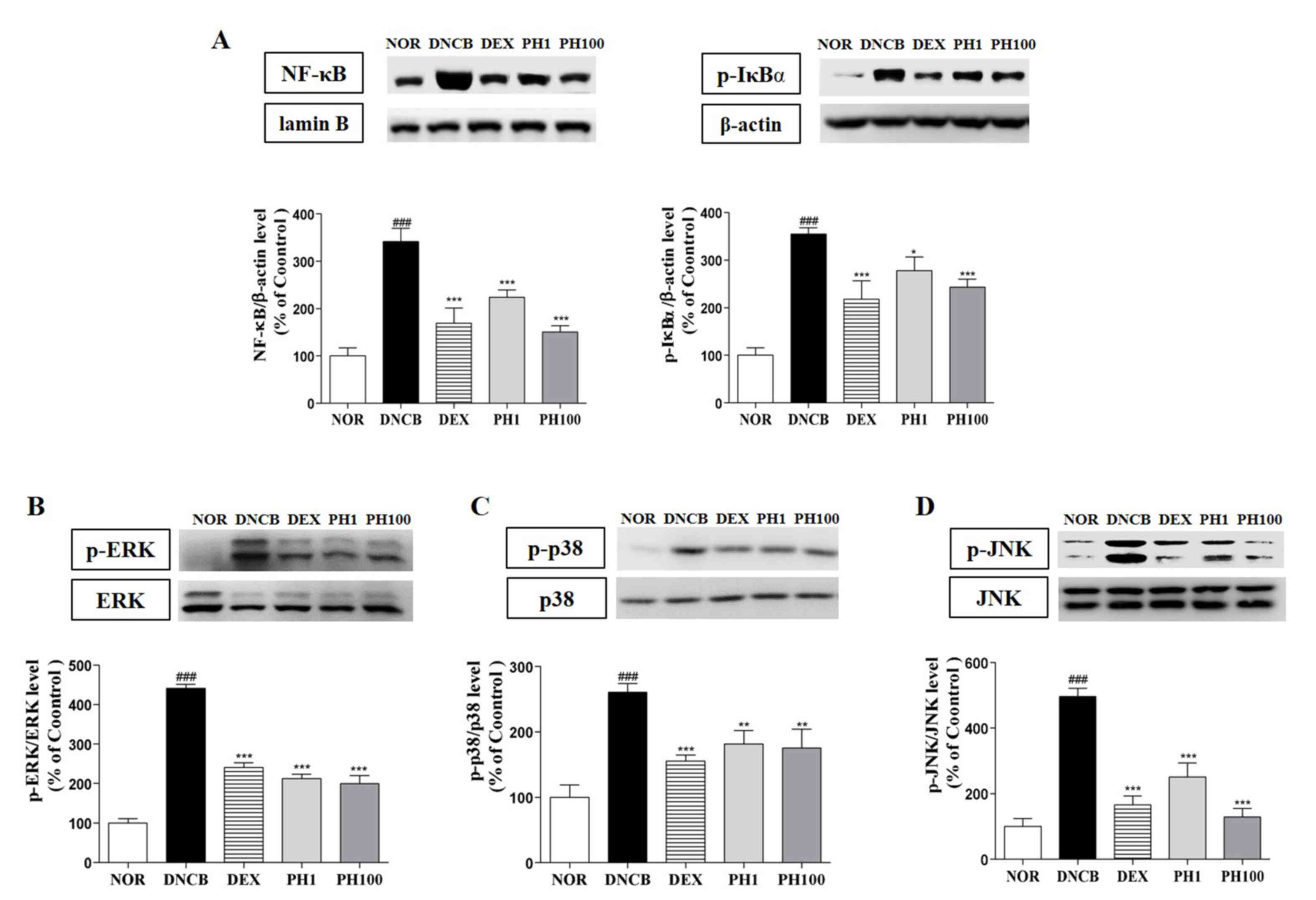 | Figure 5.Pseudostellaria heterophylla
decreases the protein expression levels of NF-κB, p-IκBα and MAPKs
in dorsal skin lesions. (A) NF-κB protein expression levels in
nuclear extracts and p-IκBα protein expression levels in cytosolic
extracts. The protein expression levels of the MAPKs (B) p-ERK1/2,
(C) p-p38 and (D) p-JNK were analyzed in whole extracts. Data are
presented as the mean ± standard deviation (n=5).
###P<0.001 vs. NOR; ***P<0.001, **P<0.01 and
*P<0.05 vs. DNCB. NOR, vehicle-treated mice; DNCB,
2,4-dinitrochlorobenzene-treated mice; DEX,
2,4-dinitrochlorobenzene- and dexamethasone-treated mice; PH1,
2,4-dinitrochlorobenzene- and 1 mg/ml Pseudostellaria
heterophylla-treated mice; PH100, 2,4-dinitrochlorobenzene- and
100 mg/ml P. heterophylla-treated mice; NF, nuclear
factor; I, inhibitor; p, phosphorylated; MAPK, mitogen-activated
protein kinase; ERK, extracellular signal-regulated kinase; JNK,
c-Jun N-terminal kinase. |
Discussion
The present study demonstrated an immunomodulatory
effect of PH against AD progression and elucidated the underlying
mechanism of action. The morphology of the DNCB-induced AD model
revealed increased epidermal thickening and dermal infiltration of
CD4+ T cells and mast cells, consistent with AD skin
lesions. Repeated treatment with PH effectively improved the
symptoms of AD by reducing hyperkeratosis and hyperplasia in the
epidermis and dermis.
The high levels of IgE measured in AD are associated
with the activation of underlying inflammatory cascades (13). In addition, mast cells exhibit
immunomodulatory activity via cross-talk with various biological
mediators involved in IgE-mediated AD (14,15).
Therefore, the present study investigated whether topical treatment
of PH affected the serum level of IgE and the infiltration of mast
cells. The DNCB group expressed the greatest levels of IgE in serum
and numbers of mast cells in skin. Topical application of 1 and 100
mg/ml PH suppressed serum IgE levels by 30.9 and 73.2%,
respectively. The reduction of IgE concentrations is consistent
with the suppression of mast cell infiltration into the dermis
following PH treatment.
T cells are crucial for immune responses, and the
dysregulation of the CD4+ T cell subset is an important
aspect of AD pathophysiology (16). A previous study determined that
CD4+ T cells increased in numbers in the AD-like skin
lesions in DNCB-induced mice model (17). Accordingly, the elevation of CD4
expression in the dermis may suggest that Th1 and Th2 cells are
involved (4). The present study
revealed that PH suppressed increases in CD4+ cell
numbers, were assumed to be CD4+ T cells in the skin.
Activated CD4+ T cells have two distinct subfamilies,
Th1 cells and Th2 cells, classified by the cytokines they secrete.
The topical treatment of PH significantly suppressed the expression
levels of Th1-(IFN-γ) and Th2-(IL-4 and −6) specific cytokines. In
addition, IFN-γ may contribute to the proliferation and
differentiation of infiltrating macrophages, which may aggravate
AD-like skin lesions (18). The
reduction of IFN-γ expression levels may be associated with the
immunosuppressive effects of PH, via inhibition of macrophage
recruitment to the site of inflammation. Th2 cells produce IL-4 and
−6, and are associated with cell and membrane destruction in acute
atopic dermatitis (19,20). PH treatment decreased the increased
expression levels of IL-4 and −6 induced by DNCB application.
The modulating effect of PH on Th1 and Th2 immune
responses appears to have been demonstrated by the effects on serum
IgE levels. In addition, overexpression of IL-8, TNF-α and IL-1β
may be associated with a high number of infiltrated macrophages and
basophils in the dermis (21).
Topical treatment with PH significantly reduced IL-8, TNF-α and
IL-1β expression levels and these findings were more pronounced in
the PH100 group.
NF-κB is critical for inflammatory cytokine
transcriptional regulation and is important for immune responses.
The activation of the NF-κB signaling pathway results in the
phosphorylation and therefore degradation of IκB (22). In the DNCB-induced mouse model of
the present study, PH inhibited the phosphorylation of IκBα and the
nuclear translocation of NF-κB. In addition, MAPKs represent a
critical signaling cascade in immune responses, interacting with
NF-κB to regulate inflammation (23,24).
In the present study, DNCB-induced phosphorylation of all three
MAPKs was reduced by PH treatment. These results support the
hypothesis that the inhibitory effects of PH on pro-inflammatory
cytokine expression are due to suppression of the MAPK and NF-κB
signaling pathways.
In conclusion, the results of the present study
demonstrated a reduction in the pathological features of AD,
including dorsal skin thickness, mast cell infiltration and release
of IgE following treatment with PH, supporting the clinical
potential of PH as an alternative therapeutic strategy for the
management of AD. The inhibition of Th1 and Th2 inflammatory
cytokines suggests that PH may exert anti-AD activity by regulating
a series of immune pathological events, including the expression of
NF-κB, p-IκBα and MAPKs. PH may therefore affect Th1/Th2 immune
dysregulation, leading to the suppression of the features of
AD.
Acknowledgements
The present study was supported by the National
Research Foundation of Korea funded by the Korean government (grant
no. NRF-2015R1A4A1042399).
References
|
1
|
Levy ML: Atopic dermatitis: Understanding
the disease and its management. Curr Med Res Opin. 23:3091–3103.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minnicozzi M, Sawyer RT and Fenton MJ:
Innate immunity in allergic disease. Immunol Rev. 242:106–127.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mosmann TR and Coffman RL: Th1 and th2
cells: Different patterns of lymphokine secretion lead to different
functional properties. Annu Rev Immunol. 7:145–173. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leung DY, Boguniewicz M, Howell MD, Nomura
I and Hamid QA: New insights into atopic dermatitis. J Clin Invest.
113:651–657. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakamura K: Pathogenesis and treatment of
allergic skin disease-atopic dermatitis. Arerugi. 64:703–706.
2015.(In Japanese). PubMed/NCBI
|
|
6
|
Liu J, Pei M, Zheng C, Li Y, Wang Y, Lu A
and Yang L: A systems-pharmacology analysis of herbal medicines
used in health improvement treatment: Predicting potential new
drugs and targets. Evid Based Complement Alternat Med.
2013:9387642013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ng TB, Liu F and Wang HX: The antioxidant
effects of aqueous and organic extracts of panax quinquefolium,
panax notoginseng, codonopsis pilosula, pseudostellaria
heterophylla and glehnia littoralis. J Ethnopharmacol. 93:285–288.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pang W, Lin S, Dai Q, Zhang H and Hu J:
Antitussive activity of pseudostellaria heterophylla (miq.) pax
extracts and improvement in lung function via adjustment of
multi-cytokine levels. Molecules. 16:3360–3370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li X, Chen Y, Lai Y, Yang Q, Hu H and Wang
Y: Sustainable utilization of traditional chinese medicine
resources: Systematic evaluation on different production modes.
Evid Based Complement Alternat Med. 2015:2189012015.PubMed/NCBI
|
|
10
|
Li Y and Yang XW: Studies on chemical
constituents of root tuber of cultivated pseudostellaria
heterophylla (Zheshen No1). Zhongguo Zhong Yao Za Zhi.
33:2353–2355. 2008.(In Chinese). PubMed/NCBI
|
|
11
|
Wong CK, Leung KN, Fung MC, Fung KP and
Choy YM: The induction of cytokine gene expression in murine
peritoneal macrophages by pseudostellaria heterophylla.
Immunopharmacol Immunotoxicol. 16:347–357. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi YY, Kim MH, Hong J, Kim K and Yang
WM: Effect of Dangguibohyul-Tang, a mixed extract of astragalus
membranaceus and angelica sinensis, on allergic and inflammatory
skin reaction compared with single extracts of astragalus
membranaceus or angelica sinensis. Evid Based Complement Alternat
Med. 2016:59363542016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shin HS, See HJ, Jung SY, Choi DW, Kwon
DA, Bae MJ, Sung KS and Shon DH: Turmeric (curcuma longa)
attenuates food allergy symptoms by regulating type 1/type 2 helper
t cells (th1/th2) balance in a mouse model of food allergy. J
Ethnopharmacol. 175:21–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang H, Lee CH, Kim JR, Kwon JY, Seo SG,
Han JG, Kim BG, Kim JE and Lee KW: Chlorella vulgaris attenuates
dermatophagoides farinae-induced atopic dermatitis-like symptoms in
nc/nga mice. Int J Mol Sci. 16:21021–21034. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park S, Kim da S, Kang S and Shin BK:
Synergistic topical application of salt-processed phellodendron
amurense and sanguisorba officinalis linne alleviates atopic
dermatitis symptoms by reducing levels of immunoglobulin e and
pro-inflammatory cytokines in nc/nga mice. Mol Med Rep.
12:7657–7664. 2015.PubMed/NCBI
|
|
16
|
Vocanson M, Hennino A, Chavagnac C,
Saint-Mezard P, Dubois B, Kaiserlian D and Nicolas JF: Contribution
of cd4(+)and cd8(+) T-cells in contact hypersensitivity and
allergic contact dermatitis. Expert Rev Clin Immunol. 1:75–86.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang EY, Chen AY and Zhu BT: Mechanism of
dinitrochlorobenzene-induced dermatitis in mice: Role of specific
antibodies in pathogenesis. PLoS One. 4:e77032009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee HS, Kim SK, Han JB, Choi HM, Park JH,
Kim EC, Choi MS, An HJ, Um JY, Kim HM and Min BI: Inhibitory
effects of Rumex japonicus Houtt. On the development of atopic
dermatitis-like skin lesions in Nc/Nga mice. Br J Dermatol.
155:33–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen L, Martinez O, Overbergh L, Mathieu
C, Prabhakar BS and Chan LS: Early up-regulation of Th2 cytokines
and late surge of Th1 cytokines in an atopic dermatitis model. Clin
Exp Immunol. 138:375–387. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee HJ, Lee HP, Ha SJ, Byun DG and Kim JW:
Spontaneous expression of mrna for iL-10, GM-CSF, TGF-beta,
TGF-alpha, and IL-6 in peripheral blood mononuclear cells from
atopic dermatitis. Ann Allergy Asthma Immunol. 84:553–558. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szegedi K, Lutter R, Res PC, Bos JD,
Luiten RM, Kezic S and Middelkamp-Hup MA: Cytokine profiles in
interstitial fluid from chronic atopic dermatitis skin. J Eur Acad
Dermatol Venereol. 29:2136–2144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li W, Zhao Y, Xu X, Ma W, Gao P, Wang Y,
Liang K and Li R: Rebamipide suppresses TNF-α mediated inflammation
in vitro and attenuates the severity of dermatitis in mice. FEBS J.
282:2317–2326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee J, Choi YY, Kim MH, Han JM, Lee JE,
Kim EH, Hong J, Kim J and Yang WM: Topical application of angelica
sinensis improves pruritus and skin inflammation in mice with
atopic dermatitis-like symptoms. J Med Food. 19:98–105. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mishra S, Tripathi A, Chaudhari BP,
Dwivedi PD, Pandey HP and Das M: Deoxynivalenol induced mouse skin
cell proliferation and inflammation via MAPK pathway. Toxicol Appl
Pharmacol. 279:186–197. 2014. View Article : Google Scholar : PubMed/NCBI
|



















