Introduction
Pseudomonas aeruginosa is one of the most
prevalent opportunistic gram-negative bacteria that lead to
pneumonia in immunocompromised patients through the production of
numerous virulence factors, including enzymes and exotoxin, such as
lipopolysaccharides (LPS) (1–5).
P. aeruginosa accounts for 10–20% of all hospital-acquired
infection (6), 18.1% of nosocomial
pneumonia (7), 9.3% of
ventilator-associated pneumonia (8) and 0.9–1.9% of community-acquired
pneumonia requiring hospitalization (9–11).
LPS are the most important pathogenic factors produced by P.
aeruginosa and serve a major role in the interplay between the
host and the pathogen. Type II alveolar epithelial cells (AECs) are
major target cells of LPS locating in the alveolar corners,
exhibiting various important functions including initiation of
immune responses, fluid balance, mucus and surfactant production,
and progenitor action to maintain the normal function of the
alveoli (12–14).
Sirtuin1 (SIRT1), an NAD+-dependent class
III protein deacetylase, belongs to the silent information
regulator (Sir) family (15) and
is suggested to serve diverse roles in gene silencing, stress
resistance, apoptosis, senescence, aging and inflammation (16–19).
Previous studies have demonstrated that SIRT1 can regulate cellular
oxidative stress and its toxicity (20–23).
Modulation of the acetylation of the p65 subunit of nuclear factor
kB (NF-κB) in cells or tissues is the major means via which SIRT1
regulates cellular oxidative status (24).
At present, to the best of our knowledge, no reports
have focused on the potential roles of SIRT1 in the regulation of
oxidative stress induced by P. aeruginosa LPS in human AECs.
In the current study, the effects of SIRT1 on the regulation of
reactive oxidative stress, which is indicated by reactive oxygen
species (ROS) generation, was examined in LPS-stimulated A549
cells.
Materials and methods
Cell culture and drug treatment
A549 cells were maintained as previously described
(25). Briefly cells were
maintained in Dulbecco's modified Eagle's medium (DMEM) F-12
culture medium (GE Healthcare Life Sciences, Logan, UT, USA)
containing 10% heat-inactivated fetal calf serum, 100 U/ml
penicillin, and streptomycin respectively, in 25-cm2
culture flasks at 37°C in a humidified atmosphere with 5%
CO2. LPS (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) was dissolved in sterilized phosphate-buffered saline
(PBS). Cells were pretreated with nicotinamide (NAM) and
resveratrol [Res; dissolved in dimethyl sulfoxide (DMSO); all from
Sigma-Aldrich; Merck Millipore) for 1 h prior to LPS stimulation.
The concentration of DMSO in the medium never exceeded 0.1% to
avoid the toxicity of this solvent towards the A549 cells. Cells
were divided into four groups, including a DMSO group (control), a
Res group, a LPS treatment plus DMSO group (DMSO+LPS), and a
LPS-treated Res group (Res+LPS).
Fluorescence staining
A549 cells were cultured on six-well chamber slides
and were washed with PBS three times for 5 min per wash, then
subsequently incubated with ROS Fluorescent Probe-DHE (Vigorous
Biotechnology Beijing Co., Ltd., Beijing, China) in serum-free DMEM
F-12 medium for 30 min at 37°C in darkness and fixed in 4%
paraformaldehyde for 30 min at room temperature. The slides were
washed again and mounted. The slides were finally examined using a
fluorescence microscope.
Quantification of intracellular
ROS
Intracellular ROS levels were quantified using ROS
Fluorescent Probe-Dihydroethidium (DHE) to determine the oxidative
stress towards the A549 cells in response to LPS stimulation.
Following drug treatment, A549 cells were treated with
dichlorofluorescin diacetate, a ROS-sensitive dye, and incubated
for 30 min at 37°C in a humidified and dark atmosphere. A549 cells
were harvested and suspended in PBS (0.14 M NaCl, 2.6 mM KCl, 8 mM
Na2HPO4, and 1.5 mM
KH2PO4). Relative fluorescence intensities in
the A549 cells were analyzed with flow cytometry (BD Biosciences,
Franklin Lakes, NJ, USA).
Protein extraction and western
blotting analysis
Following treatments, the cells were washed with
ice-cold PBS three times. Proteins were extracted from the A549
cells in radioimmunoprecipitation assay buffer [1% Triton X-100,
150 mmol/l NaCl, 5 mmol/l EDTA and 10 mmol/l Tris-HCl (pH 7.0)]
containing a protease inhibitor cocktail. Following sonication,
cell lysates were subjected to centrifugation at 12,000 × g at 4°C
for 15 min and the supernatants were collected as the total
protein. Total protein (10–50 µg/lane) was electrophoresed and
separated on a 10% SDS-polyacrylamide gel and transferred to a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA), which was then soaked in 8% non-fat milk in Tris-buffered
saline with 1% Tween-20 (TBST; pH 7.6) at room temperature for 2 h
to block non-specific binding sites. The membranes were then
incubated at 4°C overnight with a rabbit SIRT1 polyclonal antibody
(EMD Millipore; cat. no. 07-131) at a dilution of 1:3,000, a rabbit
NF-κB polyclonal antibody (Santa Cruz Biotechnology, Inc., Dallas,
TX, USA; cat. no. sc-7178) at a dilution of 1:1,000 or a rabbit
acetyl-NF-κB polyclonal antibody (Cell Signaling Technology, Inc.,
Danvers, MA, USA; cat. no. 3045) at a dilution of 1:1,000 on a
rotating platform at 4°C. Subsequently, the membrane was rinsed in
TBST (pH 7.6) 3 times and incubated with horseradish
peroxidase-conjugated IgG antibodies diluted in TBST (1:5,000;
Abmart, Inc., Shanghai, China; cat. no. M21003) for 2 h on a
rotating platform at room temperature. Bands were visualized using
a horseradish peroxidase developer, and background-subtracted
signals were quantified on a laser densitometer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). A β-actin antibody (Santa
Cruz Biotechnology, Inc.) was used to normalize the signal obtained
for the total protein extracts. The protein bands were quantified
using a PhosphorImager and ImageQuant (GE Healthcare Life Sciences)
software analysis.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Multiple comparisons were evaluated by one-way analysis
of variance followed by Tukey's multiple-comparison test. P<0.05
was considered to indicate a statistically significant
difference.
Results
SIRT1 expression was reduced and ROS
generation was elevated by LPS in A549 cells
In order to investigate the associations between ROS
and SIRT1, the effects of LPS on SIRT1 protein expression and the
generation of intracellular ROS following LPS treatment were
assessed. A549 cells were treated with 10 µg/ml LPS for 12, 24 or
48 h or with 0.1, 1, or 10 µg/ml LPS for 48 h. Western blotting
indicated that when the A549 cells were treated with 10 µg/ml LPS
for 48 h, SIRT1 protein expression was reduced by 50% (Fig. 1A). LPS significantly reduced the
level of SIRT1 expression in a dose-dependent manner. At doses of 1
and 10 µg/ml, the SIRT1 protein expression was downregulated to 28
and 51% compared with the control group, respectively (Fig. 1B). In addition, the generation of
intracellular ROS was investigated. The results demonstrated that
LPS elevated the generation of intracellular ROS in a
time-dependent manner. At the 48 h time point, the generation of
ROS was increased by approximately three times.
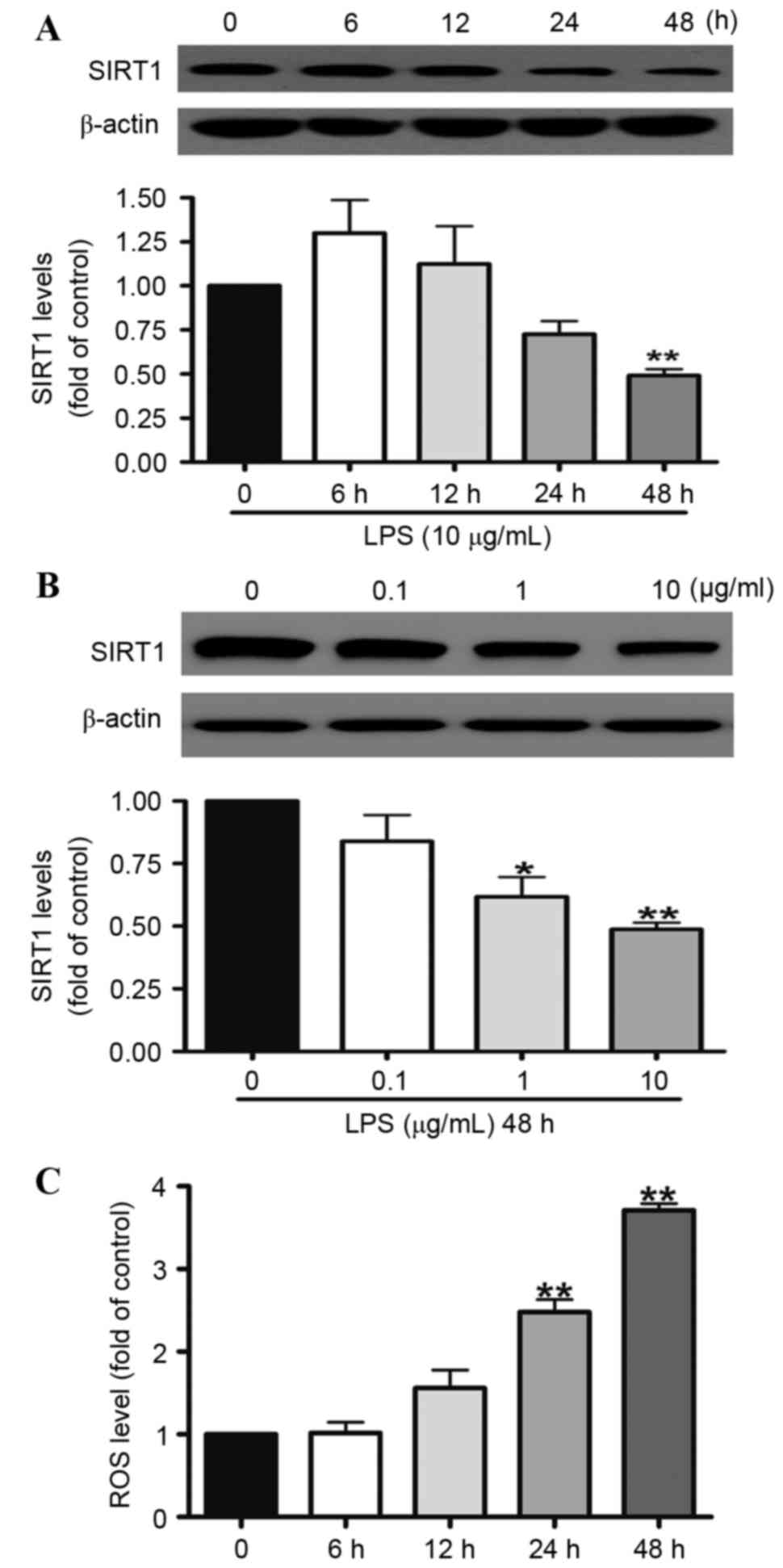 | Figure 1.SIRT1 protein expression was reduced
by LPS in the A549 cells. A549 cells were exposed to 10 µg/ml LPS
for (A) 6, 12, 24 or 48 h or (B) 0.1, 1 or 10 µg/ml LPS for 48 h.
Western blotting illustrated that SIRT1 protein expression in the
A549 cells was reduced significantly at 48 h (A), and was reduced
by LPS in the A549 cells in a dose-dependent manner (B). (C) A549
cells were treated with 10 µg/ml LPS for 6, 12, 24 and 48 h. Levels
of intracellular ROS in the A549 cells were quantified using flow
cytometry. ROS generation was increased by LPS in the A549 cells in
a time-dependent manner. Data are presented as the mean ± standard
error, n=3 independent experiments. *P<0.05, **P<0.01 vs.
control. SIRT1, sirtuin 1; LPS, lipopolysaccharides. |
SIR1 regulates LPS-induced A549 cell
ROS generation associated with the activation of NF-κB pathway
In order to determine the level of oxidative stress
induced by LPS, cells were treated with 10 µg/ml LPS for 48 h.
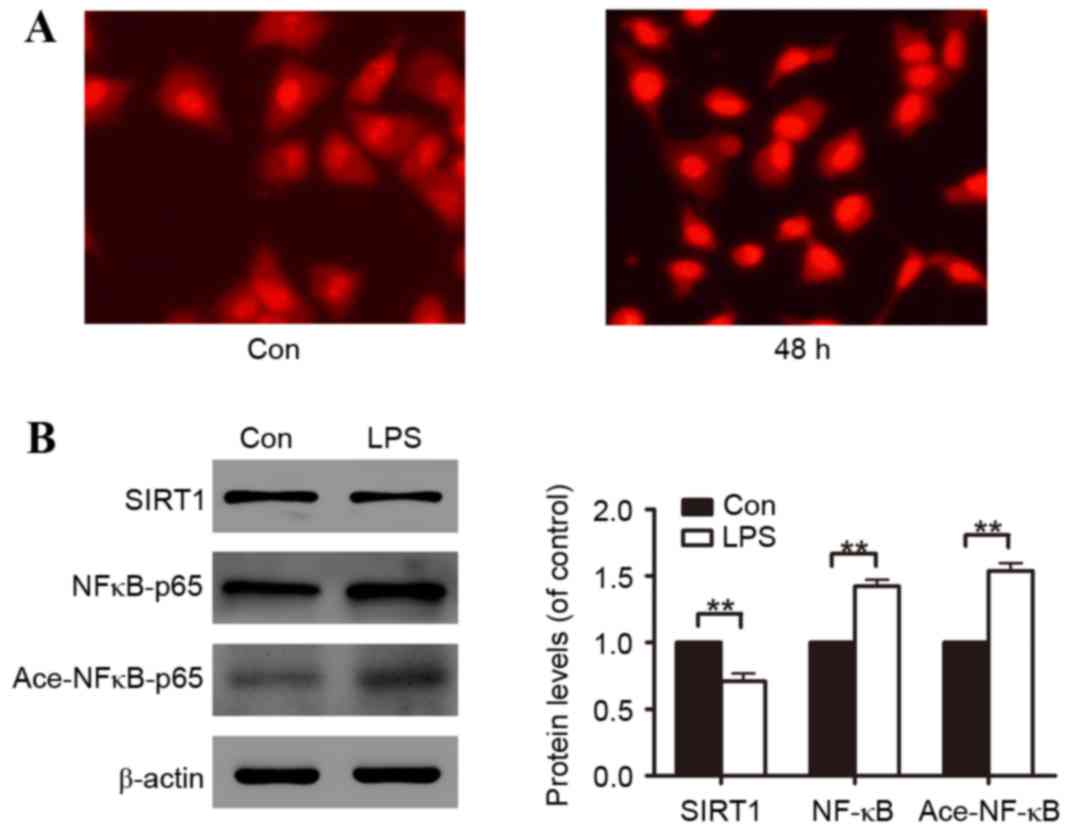
Fluorescence staining illustrated that A549 cells incubated with
LPS had increased ROS generation (Fig.
2A). Subsequently, the molecular mechanisms involved in A549
cell oxidative stress induced by LPS were investigated. Through
48-h LPS exposure, it was identified that LPS reduced SIRT1 protein
expression and promoted NF-κB protein expression and NF-κB protein
acetylation (Fig. 2B).
A549 cell oxidative stress is
attenuated by Res through the SIRT1-mediated deacetylation of
NF-κB
Res, a natural polyphenol compound widely found in
grapes, pine trees, peanuts and other plants and fruits, has
comprehensive biochemical and physiological functions, including
anti-cancer, anti-inflammatory and anti-oxidant activities, that
regulate lipid actions (26–31).
Res is widely accepted to activate SIRT1, thus, is frequently
adopted as a SIRT1 enhancer. In order to determine the pivotal role
of SIRT1 in regulating A549 cell oxidative stress induced by LPS,
the cells were divided into four groups, including a DMSO group
(control), a Res group, a LPS treatment plus DMSO group (DMSO+LPS),
and a LPS-treated Res group (Res+LPS). The results suggested that
Res induces SIRT1 protein expression in A549 cells in a
dose-dependent manner. At doses of 5 and 20 µM, the increases in
SIRT1 protein expression reached 58% and 91%, respectively
(Fig. 3A). According to the
results above, 20 µM was selected as the dose used in the
subsequent experiments. It was demonstrated that ROS generation was
reduced by Res in the A549 cells (Fig.
3C). The effects of Res on the activation of the SIRT1 pathways
were partially reversed by LPS-stimulated downregulation of NF-κB
deacetylation in the A549 cells (Fig.
3B).
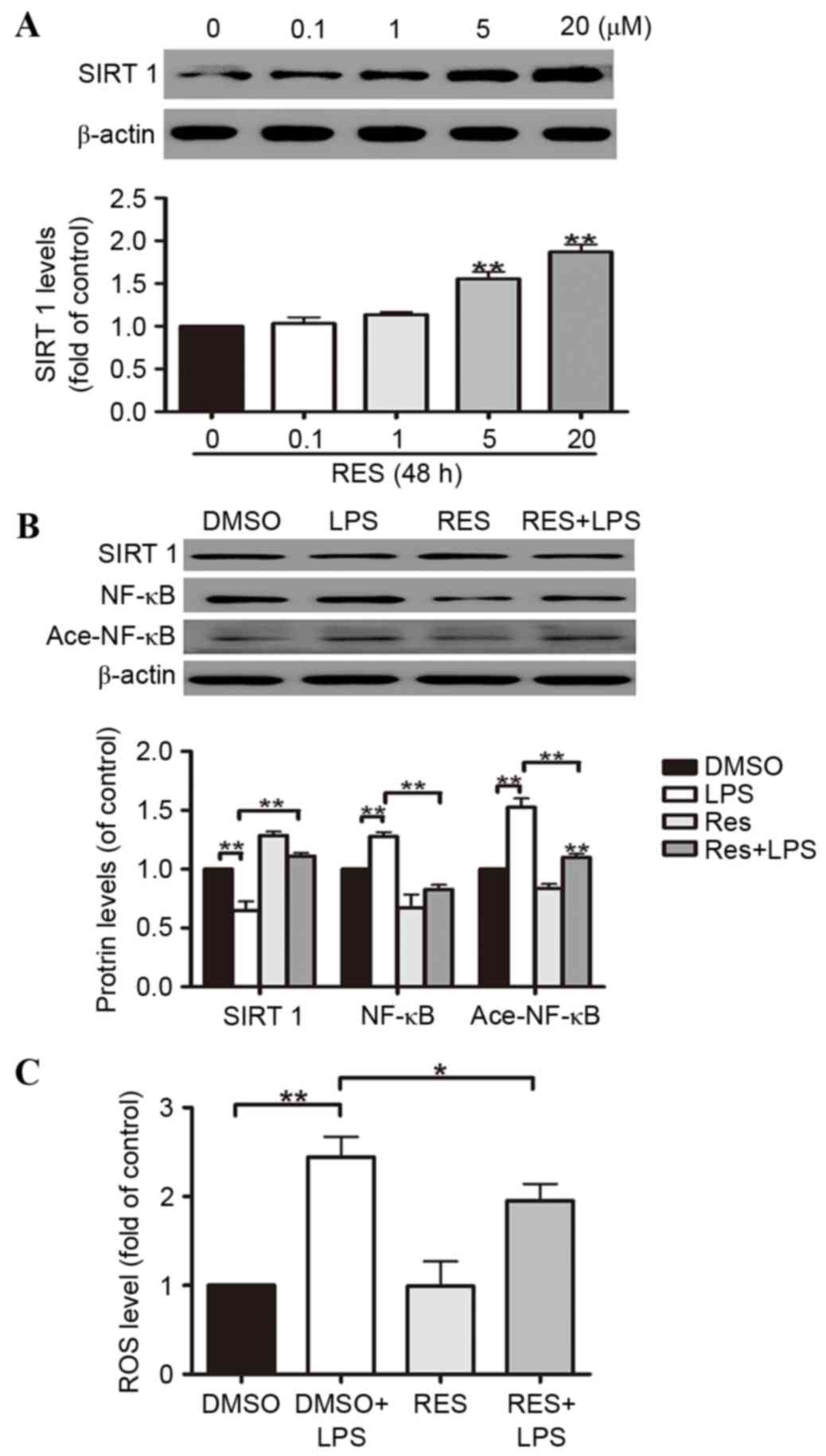 | Figure 3.A549 cell oxidative stress is
attenuated by Res through the SIRT1-mediated deacetylation of
NF-κB. A549 cells were exposed to 0.1, 1, 5 and 20 µM Res for 48 h.
(A) Western blotting illustrated that SIRT1 protein expression was
increased by Res in the A549 cells in a dose-dependent manner. (B)
The effects of Res on the activation of the SIRT1 pathways were
partially reversed by LPS via NF-κB deacetylation in the A549
cells. (C) Res reduces ROS generation in A549 cells. Data are
presented as the mean ± standard error, n=3 independent
experiments. *P<0.05, **P<0.01 vs. control. Res, resveratrol;
SIRT1, sirtuin 1; NF-κB, nuclear factor κB; LPS,
lipopolysaccharides; DMSO, dimethyl sulfoxide; Ace-NF-κB, acetyl-
NF-κB. |
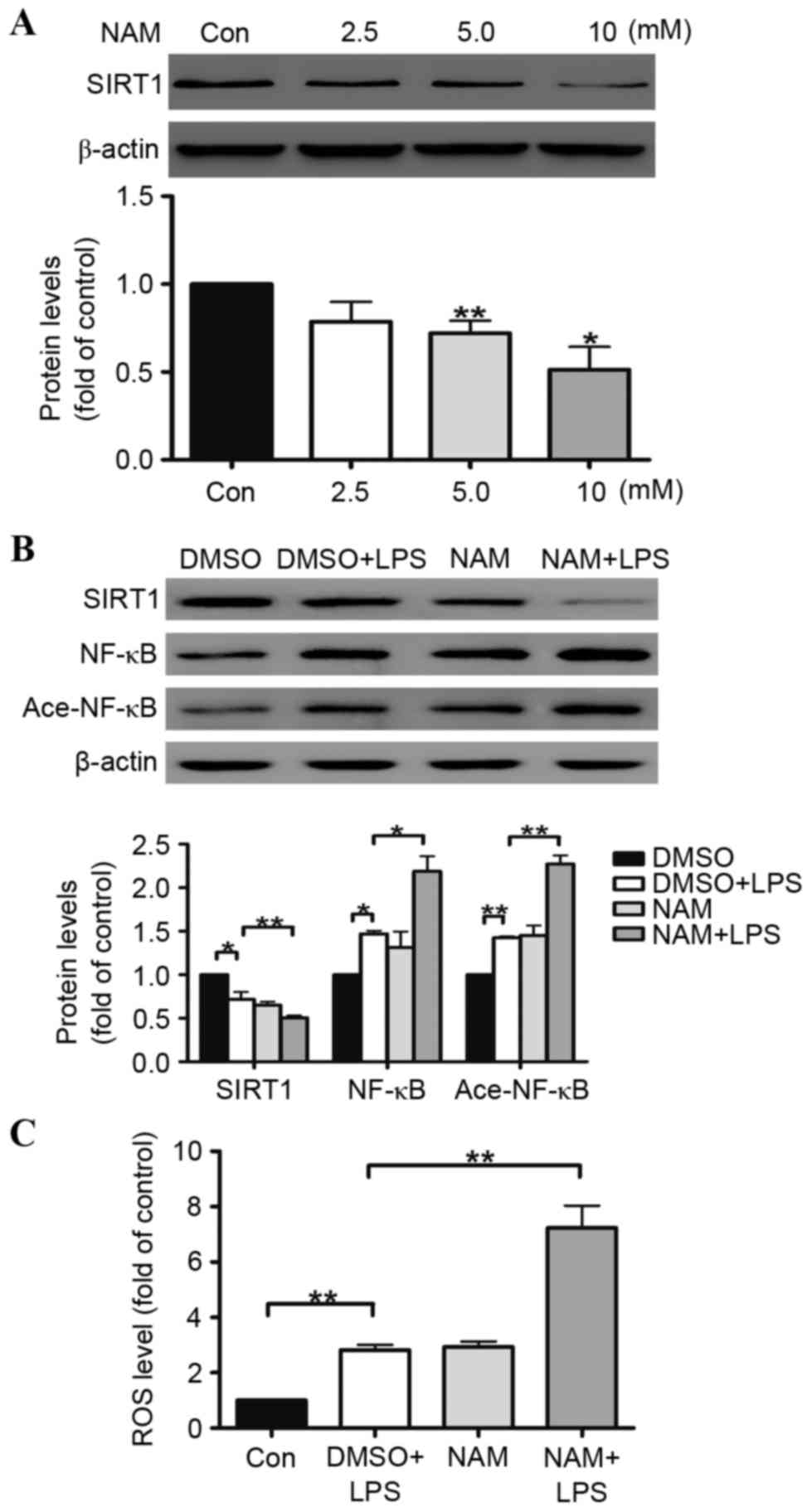
NAM aggravates LPS-induced A549 cell oxidative
stress by inhibiting the SIRT1 pathway. To further determine the
role of SIRT1 in regulating the human alveolar epithelial A549 cell
oxidative stress induced by LPS, NAM was selected as an inhibitor
of SIRT1. The results illustrated that SIRT1 protein expression in
the A549 cells was reduced by NAM in a dose-dependent manner. At
doses of 5 and 10 mmol/ml, the reductions in SIRT1 protein
expression were 27 and 51%, respectively (Fig. 4A). The ROS generation was examined
in the A549 cells, indicating that the ROS generation in the A549
cells was increased (Fig. 4C). The
effects of LPS on the inhibition of the SIRT1 pathway were
aggravated by NAM in the A549 cells (Fig. 4B).
Discussion
P. aeruginosa is a conditional Gram-negative
bacterium leading to pneumonia in immunosuppressed patients and is
colonized in the lower respiratory tract of patients with primary
pulmonary diseases. In addition, ventilated patients are
particularly susceptible to developing P. aeruginosa
pneumonia (32,33). The mortality of
ventilator-associated pneumonia due to P. aeruginosa has
been observed to be significantly higher than that of other
pathogens (34). LPS is one of
predominant virulence factors produced by P. aeruginosa. The
current study clarified the role of SIRT1 in regulating oxidative
stress induced by P. aeruginosa LPS in human alveolar
epithelial A549 cells.
Few studies have elucidated the association between
SIRT1 and P. aeruginosa pneumonia-induced lung injury. The
present study demonstrated SIRT1 expression had been significantly
reduced in A549 cells that had been treated with LPS, in a
dose-dependent manner. Following 48 h stimulation, the expression
of SIRT1 was significantly reduced. Previous studies have reported
that LPS increases the generation of reactive oxygen species in
lungs, leading to lung damage (35,36).
The results of the current study suggested that the generation of
ROS was elevated in the A549 cells in a time-dependent manner
following exposure to LPS, which is consistent with previous
studies (37–40). Accordingly, it was hypothesized
that the possible mechanisms of P. aeruginosa-induced lung
damage may be associated with reactive oxidative stress in the
alveolar type II epithelial cells induced by LPS.
SIRT1 is a NAM adenine dinucleotide (NAD+)-dependent
histone deacetylase involved in multiple cellular functions
(41,42). Previous studies have elucidated
that SIRT1 serves an important role in oxidative stress (43–48).
In addition, SIRT1 participates in adjusting the inflammatory
response through modulation of the acetylation status of the p65
subunit of NF-κB, which is activated by oxidative stress (24). However, it is not currently clear
whether SIRT1 is inhibited or activated under oxidative stress. In
the current study, SIRT1 expression was reduced during the process
of LPS-induced A549 cell oxidative stress, associated with
upregulation of NF-κB and acetylation of NF-κB. This indicates that
SIRT1 serves a crucial inhibitory role during the process of A549
cell oxidative stress induced by LPS through regulating the NF-κB
signaling pathway. Therefore, reductions in SIRT1 expression
induced by LPS may be associated with lung injury.
To further verify the pivotal role of SIRT1 in
LPS-induced oxidative stress, a SIRT1 activator and inhibitor were
used to result in functional gain and loss. Res has been previously
reported as a widely used activator of SIRT1 (49,50).
The current study demonstrates that A549 cell exposure to LPS
increases SIRT1 expression. In addition, levels of intracellular
ROS were reduced. The current study also hypothesized that the
protective effect of Res against LPS-induced A549 cell oxidative
stress may be associated with SIRT1 activation and modulation of
deacetylation of NF-κB; and this was verified by western blotting
analysis of SIRT1, NF-κB and acetylated NF-κB. To further verify
the above hypothesis, NAM was utilized as a SIRT1 inhibitor to
examine the role of SIRT1 regulation in LPS-induced A549 oxidative
stress. The results demonstrated that treatment of A549 cells with
NAM reduced SIRT1 protein expression in the A549 cells in a
dose-dependent manner. The A549 cell oxidative stress induced by
LPS was aggravated by NAM, and ROS generation was increased in the
A549 cells. The effects of LPS on SIRT1 pathway inhibition were
aggravated by NAM in the A549 cells. Taken together, these results
indicate the central and pivotal role of SIRT1 in LPS-induced
oxidative stress in A549 cells via modulation of the NF-κB pathway,
which maybe a potential translational target for treating P.
aeruginosa pneumonia.
In conclusion, the results of the current study
suggest that A549 cell oxidative stress is induced by LPS. SIRT1
serves a central role in the oxidative stress induced by LPS
through inducing NF-κB deacetylation. Res defends against
LPS-induced A549 cell oxidative stress via activation of SIRT1 and
NAM aggravates the A549 cell oxidative stress induced by LPS. SIRT1
activators are suggested as a promising therapeutic interventional
target for P. aeruginosa infections.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81270495).
References
|
1
|
Quartin AA, Scerpella EG, Puttagunta S and
Kett DH: A comparison of microbiology and demographics among
patients with healthcare-associated, hospital-acquired and
ventilator-associated pneumonia: A retrospective analysis of 1184
patients from a large, international study. BMC Infect Dis.
13:5612013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Restrepo MI and Anzueto A: The role of
gram-negative bacteria in healthcare-associated pneumonia. Semin
Respir Crit Care Med. 30:61–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zilberberg MD and Shorr AF: Epidemiology
of healthcare-associated pneumonia (HCAP). Semin Respir Crit Care
Med. 30:10–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Di Pasquale M, Ferrer M, Esperatti M,
Crisafulli E, Giunta V, Li Bassi G, Rinaudo M, Blasi F, Niederman M
and Torres A: Assessment of severity of ICU-acquired pneumonia and
association with etiology. Crit Care Med. 42:303–312. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sadikot RT, Blackwell TS, Christman JW and
Prince AS: Pathogen-host interactions in Pseudomonas aeruginosa
pneumonia. Am J Respir Crit Care Med. 171:1209–1223. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramos GP, Rocha JL and Tuon FF: Seasonal
humidity may influence Pseudomonas aeruginosa hospital-acquired
infection rates. Int J Infect Dis. 17:e757–e761. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gaynes R and Edwards JR: National
Nosocomial Infections Surveillance System: Overview of nosocomial
infections caused by gram-negative bacilli. Clin Infect Dis.
41:848–854. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rello J, Ollendorf DA, Oster G,
Vera-Llonch M, Bellm L, Redman R and Kollef MH: VAP Outcomes
Scientific Advisory Group: Epidemiology and outcomes of
ventilator-associated pneumonia in a large US database. Chest.
122:2115–2121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fang GD, Fine M, Orloff J, Arisumi D, Yu
VL, Kapoor W, Grayston JT, Wang SP, Kohler R and Muder RR: New and
emerging etiologies for community-acquired pneumonia with
implications for therapy. A prospective multicenter study of 359
cases. Medicine (Baltimore). 69:307–316. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blanquer J, Blanquer R, Borrás R, Nauffal
D, Morales P, Menéndez R, Subías I, Herrero L, Redón J and Pascual
J: Aetiology of community acquired pneumonia in Valencia, Spain: A
multicentre prospective study. Thorax. 46:508–511. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neill AM, Martin IR, Weir R, Anderson R,
Chereshsky A, Epton MJ, Jackson R, Schousboe M, Frampton C, Hutton
S, et al: Community acquired pneumonia: Aetiology and usefulness of
severity criteria on admission. Thorax. 51:1010–1016. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crapo JD, Barry BE, Gehr P, Bachofen M and
Weibel ER: Cell number and cell characteristics of the normal human
lung. Am Rev Respir Dis. 126:332–337. 1982.PubMed/NCBI
|
|
13
|
Herzog EL, Brody AR, Colby TV, Mason R and
Williams MC: Knowns and unknowns of the alveolus. Proc Am Thorac
Soc. 5:778–782. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Perlman CE and Bhattacharya J: Alveolar
expansion imaged by optical sectioning microscopy. J Appl Physiol
(1985). 103:1037–1044. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Imai S, Armstrong CM, Kaeberlein M and
Guarente L: Transcriptional silencing and longevity protein Sir2 is
an NAD-dependent histone deacetylase. Nature. 403:795–800. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chung S, Yao H, Caito S, Hwang JW,
Arunachalam G and Rahman I: Regulation of SIRT1 in cellular
functions: Role of polyphenols. Arch Biochem Biophys. 501:79–90.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rahman I, Kinnula VL, Gorbunova V and Yao
H: SIRT1 as a therapeutic target in inflammaging of the pulmonary
disease. Prev Med. 54 Suppl:S20–S28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao H and Rahman I: Perspectives on
translational and therapeutic aspects of SIRT1 in inflammaging and
senescence. Biochem Pharmacol. 84:1332–1339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao H, Chung S, Hwang JW, Rajendrasozhan
S, Sundar IK, Dean DA, McBurney MW, Guarente L, Gu W, Rönty M, et
al: SIRT1 protects against emphysema via FOXO3-mediated reduction
of premature senescence in mice. J Clin Invest. 122:2032–2045.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dey N, Chattopadhyay DJ and Chatterjee IB:
Molecular mechanisms of cigarette smoke-induced proliferation of
lung cells and prevention by vitamin C. J Oncol. 2011:5618622011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kume S, Haneda M, Kanasaki K, Sugimoto T,
Araki S, Isono M, Isshiki K, Uzu T, Kashiwagi A and Koya D: Silent
information regulator 2 (SIRT1) attenuates oxidative stress-induced
mesangial cell apoptosis via p53 deacetylation. Free Radic Biol
Med. 40:2175–2182. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chua KF, Mostoslavsky R, Lombard DB, Pang
WW, Saito S, Franco S, Kaushal D, Cheng HL, Fischer MR, Stokes N,
et al: Mammalian SIRT1 limits replicative life span in response to
chronic genotoxic stress. Cell Metab. 2:67–76. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Y, Xu W, McBurney MW and Longo VD:
SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and
protects neurons. Cell Metab. 8:38–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hah YS, Cheon YH, Lim HS, Cho HY, Park BH,
Ka SO, Lee YR, Jeong DW, Kim HO, Han MK and Lee SI: Myeloid
deletion of SIRT1 aggravates serum transfer arthritis in mice via
nuclear factor-κB activation. PLoS One. 9:e877332014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang T, Chen M and Sun T: Simvastatin
attenuates TGF-β1-induced epithelial-mesenchymal transition in
human alveolar epithelial cells. Cell Physiol Biochem. 31:863–874.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsieh TC, Wu ST, Bennett DJ, Doonan BB, Wu
E and Wu JM: Functional/activity network (FAN) analysis of
gene-phenotype connectivity liaised by grape polyphenol
resveratrol. Oncotarget. 7:38670–38680. 2016.PubMed/NCBI
|
|
27
|
Blanquer-Rosselló MD, Hernández-López R,
Roca P, Oliver J and Valle A: Resveratrol induces mitochondrial
respiration and apoptosis in SW620 colon cancer cells. Biochim
Biophys Acta. Oct 17–2016.(Epub ahead of print).
|
|
28
|
Caddeo C, Nacher A, Vassallo A, Armentano
MF, Pons R, Fernàndez-Busquets X, Carbone C, Valenti D, Fadda AM
and Manconi M: Effect of quercetin and resveratrol co-incorporated
in liposomes against inflammatory/oxidative response associated
with skin cancer. Int J Pharm. 513:153–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Riveiro-Naveira RR, Valcárcel-Ares MN,
Almonte-Becerril M, Vaamonde-García C, Loureiro J, Hermida-Carballo
L, López-Peláez E, Blanco FJ and López-Armada MJ: Resveratrol
lowers synovial hyperplasia, inflammatory markers and oxidative
damage in an acute antigen-induced arthritis model. Rheumatology
(Oxford). 55:1889–9000. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jeong H, Samdani KJ, Yoo DH, Lee DW, Kim
NH, Yoo IS and Lee JH: Resveratrol cross-linked chitosan loaded
with phospholipid for controlled release and antioxidant activity.
Int J Biol Macromol. 93:757–766. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang L, Yang F, Fang Z and Hu C:
Resveratrol Ameliorates Alcoholic Fatty Liver by Inducing
Autophagy. Am J Chin Med. 44:1207–1220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arabi Y, Al-Shirawi N, Memish Z and
Anzueto A: Ventilator-associated pneumonia in adults in developing
countries: A systematic review. Int J Infect Dis. 12:505–512. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee MS, Walker V, Chen LF, Sexton DJ and
Anderson DJ: The epidemiology of ventilator-associated pneumonia in
a network of community hospitals: A prospective multicenter study.
Infect Control Hosp Epidemiol. 34:657–662. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tumbarello M, De Pascale G, Trecarichi EM,
Spanu T, Antonicelli F, Maviglia R, Pennisi MA, Bello G and
Antonelli M: Clinical outcomes of Pseudomonas aeruginosa pneumonia
in intensive care unit patients. Intensive Care Med. 39:682–692.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chian CF, Chiang CH, Yuan-Jung C, Chuang
CH, Liu SL, Yi-Han J, Zhang H and Ryu JH: Apocynin attenuates
lipopolysaccharide-induced lung injury in an isolated and perfused
rat lung model. Shock. 38:196–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Boots AW, Gerloff K, Bartholomé R, van
Berlo D, Ledermann K, Haenen GR, Bast A, van Schooten FJ, Albrecht
C and Schins RP: Neutrophils augment LPS-mediated pro-inflammatory
signaling in human lung epithelial cells. Biochim Biophys Acta.
1823:1151–1162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cho RL, Yang CC, Lee IT, Lin CC, Chi PL,
Hsiao LD and Yang CM: Lipopolysaccharide induces ICAM-1 expression
via a c-Src/NADPH oxidase/ROS-dependent NF-κB pathway in human
pulmonary alveolar epithelial cells. Am J Physiol Lung Cell Mol
Physiol. 310:L639–L657. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kinoshita M, Sanuki T, Yamada Y, Sasaki A,
Yoshie T, Kuroda D, Kanzawa M and Itou T: A case of esophageal
mucosa-associated lymphoid tissue lymphoma diagnosed using
endoscopic ultrasound-guided fine-needle aspiration. Nihon
Shokakibyo Gakkai Zasshi. 113:63–70. 2016.PubMed/NCBI
|
|
39
|
Yang L, Li D, Zhuo Y, Zhang S, Wang X and
Gao H: Protective Role of Liriodendrin in Sepsis-Induced Acute Lung
Injury. Inflammation. 39:1805–1813. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu X, Pei C, Yan S, Liu G, Liu G, Chen W,
Cui Y and Liu Y: NADPH oxidase 1-dependent ROS is crucial for TLR4
signaling to promote tumor metastasis of non-small cell lung
cancer. Tumour Biol. 36:1493–1502. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yamamoto H, Schoonjans K and Auwerx J:
Sirtuin functions in health and disease. Mol Endocrinol.
21:1745–1755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kong XX, Wang R, Liu XJ, Zhu LL, Shao D,
Chang YS and Fang FD: Function of SIRT1 in physiology. Biochemistry
(Mosc). 74:703–708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hwang JW, Sundar IK, Yao H, Sellix MT and
Rahman I: Circadian clock function is disrupted by environmental
tobacco/cigarette smoke, leading to lung inflammation and injury
via a SIRT1-BMAL1 pathway. FASEB J. 28:176–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Guo H, Chen Y, Liao L and Wu W:
Resveratrol protects HUVECs from oxidized-LDL induced oxidative
damage by autophagy upregulation via the AMPK/SIRT1 pathway.
Cardiovasc Drugs Ther. 27:189–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hu YX, Cui H, Fan L, Pan XJ, Wu JH, Shi
SZ, Cui SY, Wei ZM and Liu L: Resveratrol attenuates left
ventricular remodeling in old rats with COPD induced by cigarette
smoke exposure and LPS instillation. Can J Physiol Pharmacol.
91:1044–1054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang Y, Duan W, Lin Y, Yi W, Liang Z, Yan
J, Wang N, Deng C, Zhang S, Li Y, et al: SIRT1 activation by
curcumin pretreatment attenuates mitochondrial oxidative damage
induced by myocardial ischemia reperfusion injury. Free Radic Biol
Med. 65:667–679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lim HD, Kim YS, Ko SH, Yoon IJ, Cho SG,
Chun YH, Choi BJ and Kim EC: Cytoprotective and anti-inflammatory
effects of melatonin in hydrogen peroxide-stimulated CHON-001 human
chondrocyte cell line and rabbit model of osteoarthritis via the
SIRT1 pathway. J Pineal Res. 53:225–237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Brandl A, Meyer M, Bechmann V, Nerlich M
and Angele P: Oxidative stress induces senescence in human
mesenchymal stem cells. Exp Cell Res. 317:1541–1547. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Howitz KT, Bitterman KJ, Cohen HY, Lamming
DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL,
et al: Small molecule activators of sirtuins extend Saccharomyces
cerevisiae lifespan. Nature. 425:191–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wood JG, Rogina B, Lavu S, Howitz K,
Helfand SL, Tatar M and Sinclair D: Sirtuin activators mimic
caloric restriction and delay ageing in metazoans. Nature.
430:686–689. 2004. View Article : Google Scholar : PubMed/NCBI
|