Introduction
IgA nephropathy (IgAN) is the most common primary
glomerular disease worldwide and contributes significantly to
end-stage renal disease (1). The
diagnostic hallmark of IgAN is mesangial deposition of IgA,
predominantly polymeric IgA of the IgA1 subclass (pIgA1) (2). IgA1 contains a hinge region in its
heavy chain, which serves as the site of attachment for five
O-linked glycan chains comprised of N-acetylgalactosamine with a 1,
3-linked galactose, which is sometimes sialylated (3,4).
Patients with IgAN exhibit elevated circulating levels of IgA1
linked to O-glycans consisting of galactose-deficient
N-acetylgalactosamine (GalNAc) with or without N-acetylneuraminic
acid (5). These aberrantly
galactosylated IgA1 molecules have an increased tendency to
self-aggregate and to form antigen-antibody complexes with IgG
antibodies directed against IgA1 hinge epitopes (6,7). In
addition, Ig-containing complexes are particularly prone to
mesangial trapping. Therefore, the formation of circulating
IgA-containing immune complexes may promote mesangial IgA
deposition, eventually resulting in glomerular injury (8). There is increasing evidence
supporting the involvement of circulating immune complexes
containing aberrantly glycosylated IgA1 in the pathogenesis of
IgAN. However, the mechanisms underlying the overproduction of
abnormal O-glycosylated IgA1 in IgAN remain to be fully
elucidated.
The production of abnormal galactosylated IgA1 in
patients with IgAN is caused by defective B lymphocytes (9). Core1 β1, 3-galactosyltransferase
(C1GalT1) is a key enzyme, which transfers galactose from
UDP-galactose to N-acetylgalactosamine residues. The stability of
this enzyme is dependent on its interaction with the chaperone,
Cosmc (10,11). The decreased expression and
activity of C1GalT1, the decreased expression of its dedicated
chaperone, Cosmc, and/or the increased expression and activity of a
specific sialyltransferase have been demonstrated in the B cells of
patients with IgAN (12–14). The production of aberrantly
glycosylated IgA1 is likely due to the altered expression of these
specific glycosyltransferases in B cells.
The production of IgA in B cells is regulated by T
cells (15). Previous
investigations have primarily focused on the imbalance of T helper
(Th)1/Th2. It has been demonstrated that Th2 cytokines, including
IL-4, 5 and 6, promote B cell class switching to IgA, and promote
the subsequent proliferation and differentiation of IgA-producing
cells (16). In addition, it has
been reported that the Th2 cytokines IL-4 and IL-5 alter the
N-glycosylation of murine IgA (17). As mice have only one subclass of
IgA, which generally lacks O-glycans, Yamada et al (18) used an IgA1-positive human B-cell
line (DAKIKI) to observe the effect of Th2 cytokines on the
production of IgA1. The results revealed that IL-4 increases the
galactose deficiency of secreted IgA1 by downregulating the
expression of C1GalT1 and Cosmc. Furthermore, Suzuki et al
(19) found that cytokines IL-6
and IL-4 accentuate IgA1 galactose deficiency via modulation of key
glycosyltransferases in IgA1-producing cells isolated from patients
with IgAN.
TGF-β1 is a key cytokine, which promotes IgA
production and is elevated in patients with IgAN. A previous study
examining the cytokine expression of peripheral blood monocuclear
cells (PBMCs) showed increased levels of TGF-β1 and enhanced Th2
cytokine production in single cells isolated from patients with
IgAN (20). In addition,
circulating γδ T cells are increased in patients with IgAN and
produce higher levels of TGF-β1, which promotes the production of
IgA by B cells in culture (21). A
previous study of a Chinese patient cohort also suggested that an
increase in TGF-β1 in the sera of patients with IgAN was correlated
with the levels of IgA, SIgA and galactose-deficient IgA1
(Gd-IgA1), and with pathologic classification (22). Yang et al (23) found that the number of type 3,
TGF-β-secreting helper T cells was significantly elevated during
the acute stage of Henoch-Schönlein purpura, which has a similar
pathogenesis to IgAN. Our preliminary investigation demonstrated a
significant increase in the percentages of Th2 and Th3 cells in
patients with IgAN, compared with healthy controls, and that these
percentages were positively correlated with serum levels of IgA
(24). Based on these data, it was
hypothesized that the Th3 cytokine, TGF-β1, has a similar role to
IL-4 in the pathogenesis of IgAN. The present study aimed to
determine the effect of TGF-β1 on the production and
under-glycosylation of IgA1, and to explain the mechanisms
underlying this effect in DAKIKI cells.
Materials and methods
Cell cultures and experimental
protocols
The surface IgA1-positive human B lymphoma cell
line, DAKIKI, was purchased from America Type Culture Collection
(Manassas, VA, USA) and cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
heat-inactivated fetal calf serum (FCS; Gibco; Thermo Fisher
Scientific, Inc.), 1 mM sodium pyruvate, 2 mM glutamine, 100 U/ml
penicillin and 100 mg/ml streptomycin in a humidified atmosphere at
37°C with 5% CO2. To assess the effect of cytokine
stimulation on the production of IgA1, and to analyze the
glycosylation and sialylation of IgA1, the cells were cultured in
different concentrations (0, 5, 10, 15, 30 ng/ml) of recombinant
human TGF-β1 (PeproTech, Inc., Rocky Hill, NJ, USA) at a density of
5×105/ml per well in 24-well culture plates at 37°C for 3 or 7
days. To assess the effect of cytokine stimulation on the mRNA
expression of C1GalT1 and Cosmc, the cells were cultured under the
same conditions for 12, 24 or 72 h. Previous studies have shown
that IL-4 increases the levels of Gd-IgA1 by suppressing the
expression of C1GalT1 and its chaperone Cosmc (18,19).
Thus, 10 ng/ml of recombinant human IL-4 (PeproTech, Inc.) was used
as a positive control in the present study (18). In each experiment, triplicate
culture wells were used for each stimulation condition.
Enzyme-linked immunosorbent assay
(ELISA) for IgA1
The level of IgA1 in the supernatant of each culture
well was determined in duplicate using ELISA, as previously
described (25). Briefly, 96-well
immunoplates were coated with 5 µg/ml goat anti-human IgA
polyclonal antibody (1:200; Southern Biotechnology Associates,
Birmingham, AL, USA) and incubated at 4°C overnight. Following
three washes with PBS containing 0.05% Tween-20 (TBST), the plates
were blocked in PBS containing 1% bovine serum albumin (BSA)
(Sigma-Aldrich Shanghai Trading Co., Ltd., Shanghai, China) for 1
h. Following washing with 0.05% PBST, 50 µl of supernatant samples
centrifuged at 100 × g for 5 min at 20°C or standard human IgA1
(Merck Millipore, Darmstadt, Germany) was added in duplicate and
incubated for 2 h at room temperature, followed by incubation with
horseradish peroxidase-conjugated mouse anti-human IgA1 antibody
(1:1,000 Southern Biotechnology Associates) for 1 h at 37°C. Color
was developed using tetramethylbenzidine solution (BD Biosciences,
Franklin Lakes, NJ, USA) and detected using a microplate reader
(Spectramax Plus384; Molecular Devices LLC, Sunnyvale, CA, USA) at
450 nm. Standard curves were constructed by serial dilution of an
IgA1 standard serum.
Enzyme-linked lectin binding assays
for IgA1 glycosylation and sialylation
O-glycosylation of the IgA1 samples was measured by
the binding of Vicia villosa lectin (VVL; Vector Laboratories, Ltd.
Peterborough, UK), which is specific for terminal GalNAc (26). The sialylation of IgA1 was measured
by the binding of peanut agglutinin lectin (PNA; Vector
Laboratories, Ltd.), which binds to the core1 disaccharide
Gal-GalNAc (27). The procedures
used to coat, block and wash the plates were the same as for the
measurement of IgA1 concentration. Biotinylated VVL and PNA (5
µg/ml) were added to the reaction wells, which were then incubated
for 2 h at 37°C. The plates were washed with 0.05% PBST five times,
and lectin binding was detected with avidin-horseradish peroxidase
conjugate (Vector Laboratories, Ltd.). The color was developed and
measured, as above.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis for C1GalT1 and Cosmc
mRNA
Total RNA was extracted from the cells using an
E.Z.N.A. Total RNA kit II (Omega Bio-Tek, Inc., Norcross, GA, USA).
cDNA was synthesized from the total RNA using EasyScript
First-Strand cDNA Synthesis SuperMix (TransGen Biotech Co., Ltd.,
Beijing, China). The resulting cDNAs (1 µg) were amplified by real
time qPCR analysis using a StepOne Plus™ Real-Time PCR system
(Thermo Fisher Scientific, Inc.). The 20 µl reaction mixture
contained: 10 µl SYBR Green PCR Master mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) 0.4 µl forward primer, 0.4 µl
reverse primer, 2 µl cDNA template, 0.4 µl passive reference, 6.8
µl ddH2O. The samples were incubated at 94°C for 30 sec,
followed by 40 cycles of denaturation for a 5 sec at 94°C, and
annealing and extension for 31 sec at 60°C. All samples were
examined in triplicate against the human GAPDH reference gene,
which was used to normalize the expression data. The primer pairs
for C1GalT1, Cosmc and human GAPDH are listed in Table I. The results were analyzed using
the 2−ΔΔCq method (28).
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer
sequence | Fragment size
(bp) |
|---|
| C1GalT1 | Forward
5′-AAGGTTGACACCCAGCCTAA-3′ | 226 |
|
| Reverse
5′-CTTTGACGTGTTTGGCCTTT-3′ |
|
| Cosmc | Forward
5′-AATGGTTCTGACAATGACTG-3′ | 272 |
|
| Reverse
5′-GCTGTATTGGATATGTAGTTACT-3′ |
|
| Human GAPDH | Forward
5′-CAGGGCTGCTTTTAACTCTGGT-3′ | 203 |
|
| Reverse
5′-GATTTTTGGAGGGATCTCGCT-3′ |
|
Statistical analysis
All data are presented as the mean ± standard
deviation. Comparisons among groups were evaluated by using one way
analysis of variance and least significant difference t-tests.
P<0.05 was considered to indicate a statistically significant
difference. SPSS software (version, 18.0; SPSS, Inc., Chicago, IL,
USA) was used for all calculations.
Results
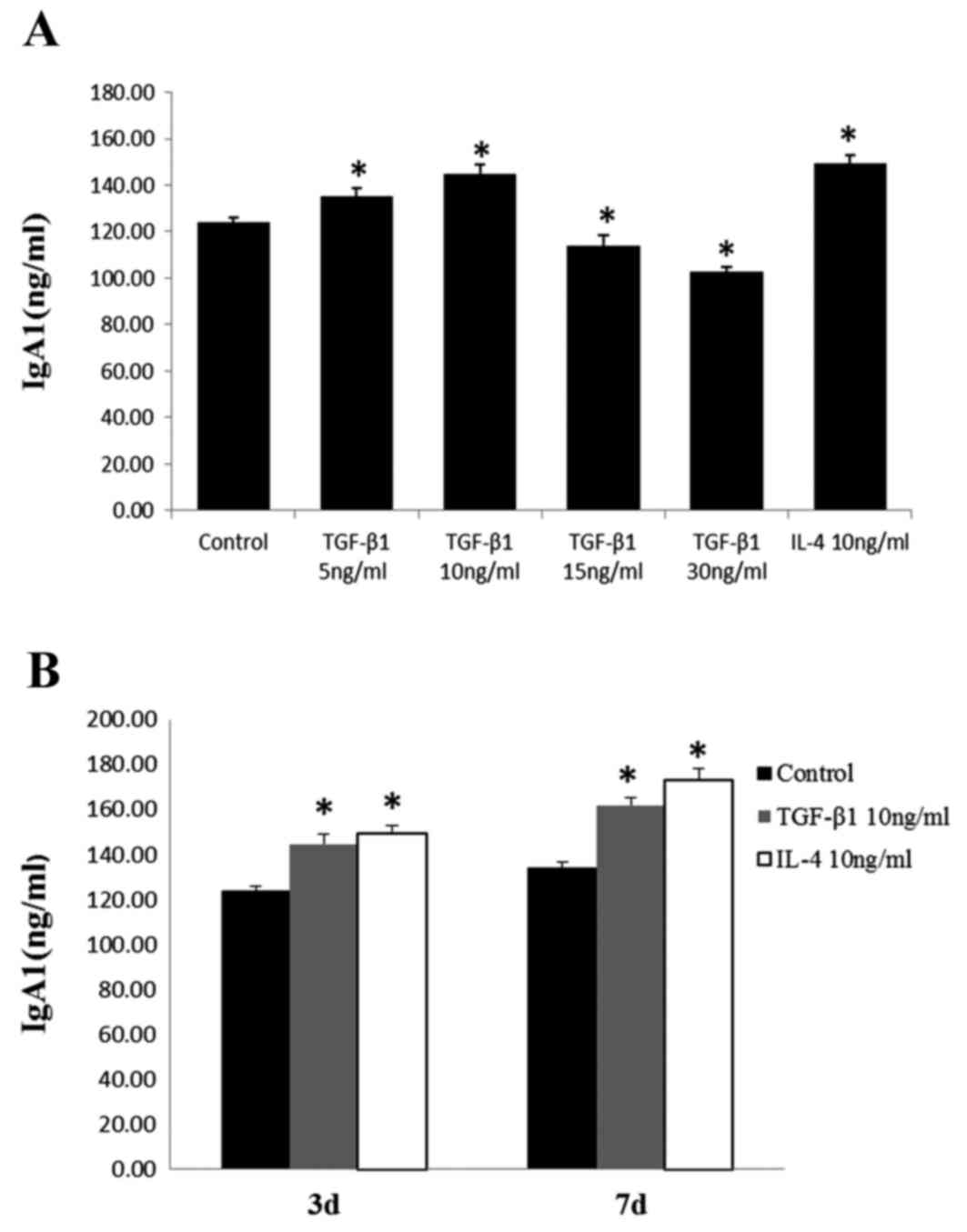
IgA1 production in DAKIKI cells
treated with TGF-1
The concentrations of IgA1 in the supernatants from
cells cultured with 5 or 10 ng/ml TGF-β1 were significantly higher,
compared with those in the control cells. Culture with 10 ng/ml
TGF-β1 had the most marked effect on the production of IgA1,
similar to the effect observed with IL-4. By contrast, IgA1
concentrations decreased following treatment with 15 or 30 ng/ml of
TGF-β1 (Fig. 1A). These results
indicated that low doses of TGF-β1 stimulated the production of
IgA1, whereas high doses suppressed the production of IgA1. It was
also observed that the concentration of IgA1 increased following
prolonged stimulation for 7 days (Fig.
1B).
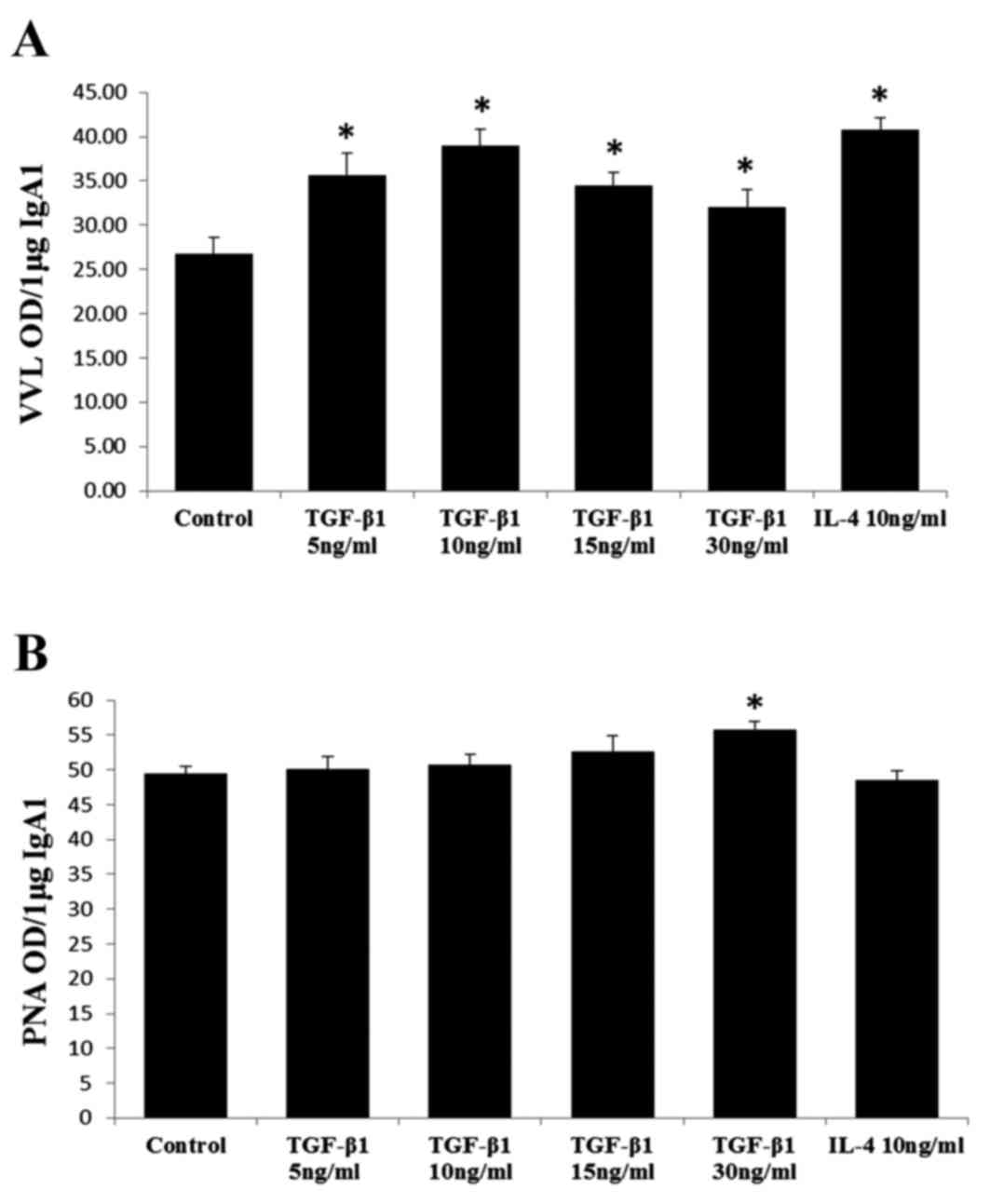
Effects of TGF-β1 stimulation on the
glycosylation and sialylation of IgA1
The VVL reactivity of IgA1 in each sample was
calculated as the optical density (OD) U/µg of IgA1, which
indicated the degree of glycosylation. The PNA reactivity of IgA1
was also calculated as OD U/µg of IgA1, which indicated the degree
of sialylation. Unlike the dose-dependent effect observed on the
secretion of IgA1, TGF-β1 had a more direct effect on the
glycosylation of IgA1, wherein each dose stimulated the
under-glycosylation of IgA1 (Fig.
2A). Regarding sialylation, the binding of PNA to IgA1 derived
from cells stimulated by 30 ng/ml of TGF-β1 was significantly
higher, compared with that for IgA1 from cells stimulated by other
doses of TGF-β1 or IL-4, or from unstimulated cells (Fig. 2B). No significant differences were
found between samples treated for 3 or 7 days.
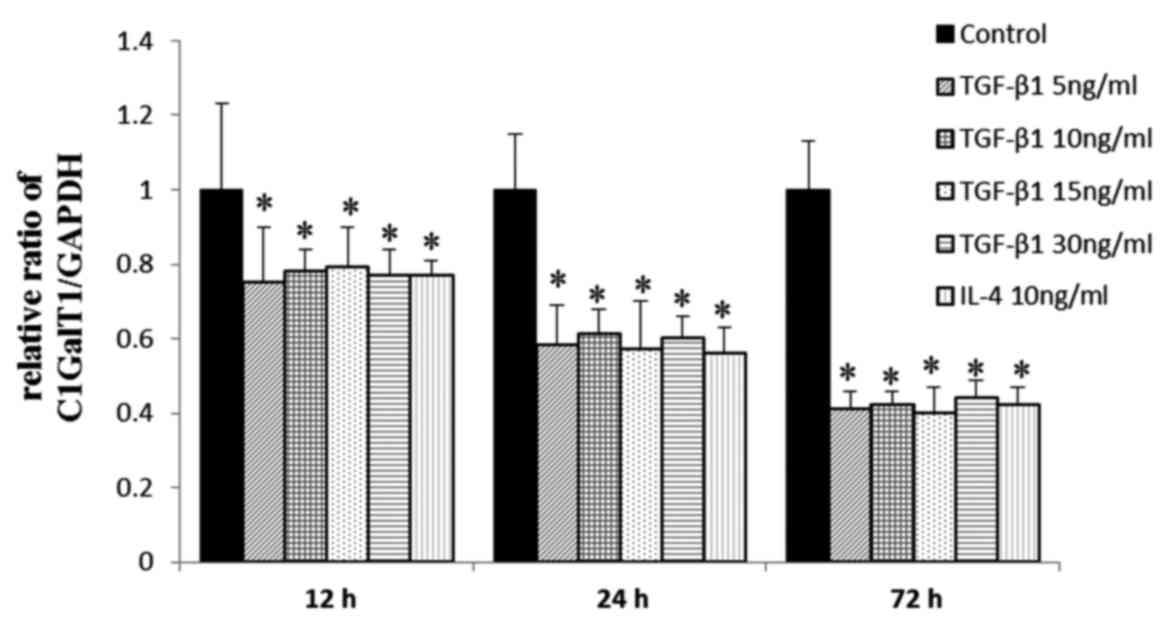
Effects of TGF-β1 on the gene
expression of C1GalT1
The mRNA expression levels of C1GalT1 in cells
incubated with TGF-β1 or IL-4 were significantly lower, compared
with those in the negative controls at 12–72 h, indicating that
TGF-β1 downregulated the mRNA expression of C1GalT1 at 12, 24 and
72 h in a time-dependent manner (Fig.
3). No significant difference was observed between the
stimulation doses.
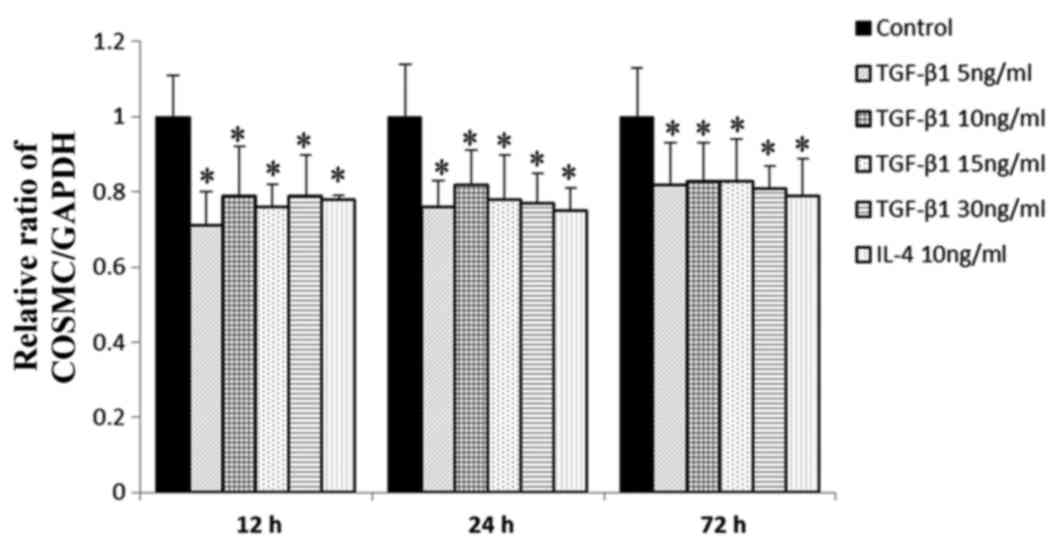
Effects of TGF-β1 on gene expression
of Cosmc
The mRNA expression levels of Cosmc, which is the
C1GalT1 chaperone, were also examined. Compared with the negative
control, the mRNA level of Cosmc was markedly reduced by
stimulation with TGF-β1 or IL-4 at each incubation time point
(Fig. 4). No significant
difference was found between incubation durations or incubation
concentrations.
Discussion
TGF-β1 is a multifunctional cytokine, which affects
a variety of biological processes, including extracellular matrix
accumulation, embryonic development, tumorigenesis, inflammation,
wound healing, differentiation and immunoregulation (29). Extensive clinical and experimental
evidence shows that the expression of TGF-β1 is increased in human
patients with IgAN, compared with normal control groups (30–33).
Although these studies demonstrated that TGF-β1 mediates the
progression of IgAN by inducing extracellular matrix accumulation,
the effect of TGF-β1 on IgA in the context of IgAN has not been
investigated.
TGF-β1 is a known physiological mediator of IgA
isotype switching (34,35). In the present study, it was found
that low concentrations of TGF-β1 (5 and 10 ng/ml) promoted the
production of IgA1 in the DAKIKI human IgA1-producing B cell line.
By contrast, high concentrations of TGF-β1 (15 and 30 ng/ml)
inhibited the production of IgA1. TGF-β1 inhibits the
proliferation, differentiation and activity of various immune
cells, including B cells (36).
The B cell surface presents receptors for TGF-β1, the stimulation
of which leads to the inhibition of B cell proliferation (37). The degree of inhibition of cell
proliferation by TGF-β1 depends on the cell type, cytokine
concentration and interactions with biologically active substances
(38). In the present study,
TGF-β1 had an effect on the production of IgA1 in IgA+ B
cells, which may be due, in part, to an increase in cell numbers
following proliferation. Thus, the inhibited production of IgA1 by
high doses of TGF-β1 may have been caused by the suppression of B
cell proliferation.
It has been suggested that aberrant glycosylation of
IgA1, for example galactose deficiency in the hinge-region of
O-linked glycans, is directly involved in the pathogenesis of IgAN
(39–41). Lectin binding assays have been
extensively used to examine the glycosylation patterns of
circulating glycoproteins with O-glycans. In the present study, the
degree of glycoslylation and sialylation were assessed by measuring
the binding activities of VVL and PNA. Although different
concentrations of TGF-β1 induced different levels of IgA1, they had
a consistent effect on the glycosylation of IgA1. The results
indicated that TGF-β1 promoted the under-glycosylation of IgA1,
which may not be accounted for by the overproduction of IgA1. The
effect of TGF-β1 on IgA1 sialylation was not confirmed in the
present study.
C1GalT1 and its chaperone, Cosmc, are key in protein
glycosylation (42). Abnormal
expression of C1GalT1 and Cosmc can result in aberrant protein
glycosylation. Functional abnormality of C1GALT1, which is
responsible for the O-glycosylation of IgA1, has been suggested as
a mechanism for the altered O-glycosylation observed in IgAN
(43). Cosmc is essential for the
folding and stability of C1GalT1 (11). In PBMCs, tonsil tissue and
tonsillar B cells of patients with IgAN, the expression levels of
C1GalT1 and Cosmc are significantly downregulated (44–46).
In addition, the expression level of C1GalT1 in the tonsillar B
cells of patients with IgAN is correlated with the estimated
glomerular filtration rate, proteinuria and the histological injury
score (46). Thus, aberrant IgA1
O-glycosylation in patients with IgAN may be a consequence of
reduced expression levels of C1GalT1 and Cosmc in B lymphocytes
(26). The results of the present
study demonstrated that the mRNA expression levels of C1GalT1 and
Cosmc in DAKIKI cells were reduced by TGF-β1 treatment, which may
have contributed to the under-glycosylation of IgA1.
In conclusion, the results of the present study
showed that certain concentrations of TGF-β1 stimulated the
production of IgA1 in DAKIKI cells. In addition, TGF-β1 promoted
the under-glycosylation of IgA1 through the downregulation of
C1GalT1 and Cosmc. Further investigation is required to clarify the
mechanisms by which TGF-β1 downregulates the mRNA levels of C1GalT1
and Cosmc, and whether the same results are observed in other B
cell lines. The findings of the present study indicated a novel
role for TGF-β1 in IgAN, which may form the basis of IgAN
therapies.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of Jiangxi Province for Youth project (grant no.
2010GQY0324) and the Natural Science Foundation of China (grant no.
81160091). The manuscript was edited for language at American
Journal Experts (Durham, NC, USA).
References
|
1
|
D'Amico G: Natural history of idiopathic
IgA nephropathy and factors predictive of disease outcome. Semin
Nephrol. 24:179–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barratt J, Feehally J and Smith AC:
Pathogenesis of IgA nephropathy. Semin Nephrol. 24:197–217. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tarelli E, Smith AC, Hendry BM,
Challacombe SJ and Pouria S: Human serum IgA1 is substituted with
up to six O-glycans as shown by matrix assisted laser desorption
ionisation time-of-flight mass spectrometry. Carbohydr Res.
339:2329–2335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Renfrow MB, Cooper HJ, Tomana M, Kulhavy
R, Hiki Y, Toma K, Emmett MR, Mestecky J, Marshall AG and Novak J:
Determination of aberrant O-glycosylation in the IgA1 hinge region
by electron capture dissociation Fourier transform-ion cyclotron
resonance mass spectrometry. J Biol Chem. 280:19136–19145. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moldoveanu Z, Wyatt RJ, Lee JY, Tomana M,
Julian BA, Mestecky J, Huang WQ, Anreddy SR, Hall S, Hastings MC,
et al: Patients with IgA nephropathy have increased serum
galactose-deficient IgA1 levels. Kidney Int. 71:1148–1154. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kokubo T, Hiki Y, Iwase H, Horii A, Tanaka
A, Nishikido J, Hotta K and Kobayashi Y: Evidence for involvement
of IgA1 hinge glycopeptide in the IgA1-IgA1 interaction in IgA
nephropathy. J Am Soc Nephrol. 8:915–919. 1997.PubMed/NCBI
|
|
7
|
Yan Y, Xu LX, Zhang JJ, Zhang Y and Zhao
MH: Self-aggregated deglycosylated IgA1 with or without IgG were
associated with the development of IgA nephropathy. Clin Exp
Immunol. 144:17–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barratt J, Smith AC and Feehally J: The
pathogenic role of IgA1 O-linked glycosylation in the pathogenesis
of IgA nephropathy. Nephrology. 12:275–284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Floege J: The pathogenesis of IgA
nephropathy: What is new and how does it change therapeutic
approaches? Am J Kidney Dis. 58:992–1004. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ju T, Brewer K, D'Souza A, Cummings RD and
Canfield WM: Cloning and expression of human core 1
beta1,3-galactosyltransferase. J Biol Chem. 277:178–186. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ju T and Cummings RD: A unique molecular
chaperone Cosmc required for activity of the mammalian core 1 beta
3-galactosyltransferase. Proc Natl Acad Sci USA. 99:16613–16618.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Allen AC, Topham PS, Harper SJ and
Feehally J: Leucocyte beta 1,3 galactosyltransferase activity in
IgA nephropathy. Nephrol Dial Transplant. 12:701–706. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Suzuki H, Moldoveanu Z, Hall S, Brown R,
Vu HL, Novak L, Julian BA, Tomana M, Wyatt RJ, Edberg JC, et al:
IgA1-secreting cell lines from patients with IgA nephropathy
produce aberrantly glycosylated IgA1. J Clin Invest. 118:629–639.
2008.PubMed/NCBI
|
|
14
|
Takahashi K, Raska M, Horynova M
Stuchlova, Hall SD, Poulsen K, Kilian M, Hiki Y, Yuzawa Y,
Moldoveanu Z, Julian BA, et al: Enzymatic sialylation of IgA1
O-Glycans: Implications for studies of IgA nephropathy. PLoS One.
9:e990262014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagasawa R, Maruyama N, Imasawa T and
Mitarai T: Role of T cells in murine IgA nephropathy. Nephrol Dial
Transplant. 14 Suppl 1:S12–S13. 1999. View Article : Google Scholar
|
|
16
|
Lycke N: T cell and cytokine regulation of
the IgA response. Chem Immunol. 71:209–234. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chintalacharuvu SR and Emancipator SN: The
glycosylation of IgA produced by murine B cells is altered by Th2
cytokines. J Immunol. 159:2327–2333. 1997.PubMed/NCBI
|
|
18
|
Yamada K, Kobayashi N, Ikeda T, Suzuki Y,
Tsuge T, Horikoshi S, Emancipator SN and Tomino Y: Down-regulation
of core 1 beta 1,3-galactosyltransferase and Cosmc by Th2 cytokine
alters O-glycosylation of IgA1. Nephrol Dial Transplant.
25:3890–3897. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suzuki H, Raska M, Yamada K, Moldoveanu Z,
Julian BA, Wyatt RJ, Tomino Y, Gharavi AG and Novak J: Cytokines
Alter IgA1 O-Glycosylation by Dysregulating C1GalT1 and
ST6GalNAc-II Enzymes. J Biol Chem. 289:5330–5339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu HH, Chu KH, Yang YH, Lee JH, Wang LC,
Lin YT and Chiang BL: Genetics and Immunopathogenesis of IgA
Nephropathy. Clinic Rev Allerg Immunol. 41:198–213. 2011.
View Article : Google Scholar
|
|
21
|
Toyabe S, Harada W and Uchiyama M:
Oligoclonally expanding gammadelta T lymphocytes induce IgA
switching in IgA nephropathy. Clin Exp Immunol. 124:110–117. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meng H, Zhang L, E XQ, Ye F, Li H, Han C,
Yamakawa M and Jin X: Application of Oxford classification, and
overexpression of transforming growth factor-β1 and immunoglobulins
in immunoglobulin A nephropathy: Correlation with World Health
Organization classification of immunoglobulin A nephropathy in a
Chinese patient cohort. Transl Res. 163:8–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang YH, Huang MT, Lin SC, Lin YT, Tsai MJ
and Chiang BL: Increased transfroming growth factor-beta
(TGF-beta)-secreting T cells and IgA anti-cardiolipin antibody
levels during acute stage of childhood Henoch-Schönlein purpura.
Clin Exp Immunol. 122:285–290. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao J, Cao CY, Zhou J and Wang WH:
Changes in levels of Th1, Th2 and Th3 cells in peripheral blood in
patients with IgA nephropathy and their significance. Prac Clin
Med. 13:28–31. 2012.(In Chinese).
|
|
25
|
Qin W, Zhou Q, Yang LC, Li Z, Su BH, Luo H
and Fan JM: Peripheral B lymphocyte beta1, 3-galactosyltransferase
and chaperone expression in immunoglobulin A nephropathy. J Intern
Med. 258:467–477. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie LS, Qin W, Fan JM, Huang J, Xie XS and
Li Z: The role of C1GALT1C1 in lipopolysaccharide-induced IgA1
aberrant O-glycosylation in IgA nephropathy. Clin Invest Med.
33:E5–E13. 2010.PubMed/NCBI
|
|
27
|
De Wolff JF, Dickinson SJ, Smith AC,
Molyneux K, Feehally J, Simon A and Barratt J: Abnormal IgD and
IgA1 O-glycosylation in hyperimmunoglobulinaemia D and periodic
fever syndrome. Clin Exp Med. 9:291–296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prud'homme GJ and Piccirillo CA: The
inhibitory effects of transforming growth factor-beta-1(TGF-beta1)
in autoimmune diseases. J Autoimmun. 14:23–42. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Del Prete D, Gambaro G, Lupo A, Anglani F,
Brezzi B, Magistroni R, Graziotto R, Furci L, Modena F, Bernich P,
et al: Precocious activation of genes of the renin-angiotensin
system and the fibrogenic cascade in IgA glomerulonephritis. Kidney
Int. 64:149–159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leung JC, Chan LY, Tsang AW, Liu EW, Lam
MF, Tang SC and Lai KN: Anti-macrophagemigration inhibitory factor
reduces transforming growth factor-beta 1 expression in
experimental IgA nephropathy. Nephrol Dial Transplant.
19:1976–1985. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhong Q, Leung JC, Chan LY, Tsang AW, Chen
X and Lai KN: The study of Chinese medicinal herbal formula Shen
San Fang in the treatment of experimental IgA nephropathy. Am J
Chin Med. 33:613–626. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chihara Y, Ono H, Ishimitsu T, Ono Y,
Ishikawa K, Rakugi H, Ogihara T and Matsuoka H: Roles of TGF-beta 1
and apoptosis in the progression of glomerulosclerosis in human IgA
nephropathy. Clin Nephrol. 65:385–392. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim PH and Kagnoff MF: Transforming growth
factor beta 1 increases IgA isotype switching at the clonal level.
J Immunol. 145:3773–3778. 1990.PubMed/NCBI
|
|
35
|
Cazac BB and Roes J: TGF-beta receptor
controls B cell responsiveness and induction of IgA in vivo.
Immunity. 13:443–451. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Boratyńska M: Urine excretion of
transforming growth factor-beta 1 in chronic allograft nephropathy.
Ann Transplant. 4:23–28. 1999.
|
|
37
|
Ibelgaufts H: Cytokines and Cells Online
Pathfinder Encyclopaedia (COPE). http://www.copewithcytokines.de/cope.cgi2007.
|
|
38
|
Kajdaniuk D, Marek B, Borgiel-Marek H and
Kos-Kudła B: Transforming growth factor β1 (TGF-β1) in physiology
and pathology. Endokrynol Pol. 64:384–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Barratt J and Feehally J: IgA nephropathy.
J Am Soc Nephrol. 16:2088–2097. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Coppo R and Amore A: Aberrant
glycosylation in IgA nephropathy (IgAN). Kidney Int. 65:1544–1547.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Julian BA and Novak J: IgA nephropathy: An
update. Curr Opin Nephrol Hypertens. 13:171–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lai KN: Pathogenesis of IgA nephropathy.
Nat Rev Nephrol. 8:275–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ju T, Brewer K, D'Souza A, Cummings RD and
Canfield WM: Cloning and expression of human core 1 beta1,
3-galactosyltransferase. J Biol Chem. 277:178–186. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Serino G, Sallustio F, Cox SN, Pesce F and
Schena FP: Abnormal miR-148b expression promotes aberrant
glycosylation of IgA1 in IgA nephropathy. J Am Soc Nephrol.
23:814–824. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
He L, Peng Y, Liu H, Yin W, Chen X, Peng
X, Shao J, Liu Y and Liu F: Activation of the interleukin-4/signal
transducer and activator of transcription 6 signaling pathway and
homeodomain-interacting protein kinase 2 production by tonsillar
mononuclear cells in IgA nephropathy. Am J Nephrol. 38:321–332.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Inoue T, Sugiyama H, Hiki Y, Takiue K,
Morinaga H, Kitagawa M, Maeshima Y, Fukushima K, Nishizaki K, Akagi
H, et al: Differential expression of glycogenes in tonsillar B
lymphocytes in association with proteinuria and renal dysfunction
in IgA nephropathy. Clin Immunol. 136:447–455. 2010. View Article : Google Scholar : PubMed/NCBI
|