Alterations of mRNAs have been implicated in a
variety of human diseases, including cancer, cardiovascular
diseases and neurodegenerative disorders, due to the changes in
protein expression levels (1–4). By
contrast, ncRNAs represent a diverse family of ribonucleic acid
transcripts that do not have protein-coding potential (5). The ENCODE Project established that
the majority of the human transcriptomes are ncRNAs, which are
involved in almost all physiological or pathological processes
(6–8). With the development of genome-wide
sequencing technology, more ncRNAs are being identified and
characterized (9).
ncRNAs include microRNA (miRNA), small nucleolar RNA
(snoRNA), small interfering RNA (siRNA), PIWI-interacting RNA
(piRNA), the heterogeneous group of long non-coding RNA (lncRNA),
transcribed ultraconserved region (T-UCR) and numerous other types
of ncRNAs (10–13). Previously, dysfunctional
miRNA-mediated regulation has been implicated in the pathogenesis
of various disorders including cancer and diabetes, oncomiRs
including miRNA-21 (miR-21) and miR-221/222 regulate the expression
of their targets such as estrogen receptor a to modulate the
progression of cancer (14–16).
On the other hand, autoimmune diseases such as scleroderma have
been associated with autoantibodies to protein components specific
to U3 small nucleolar ribonucleoproteins (snoRNPs) (17,18);
lncRNAs were closely associated with the differentiation of nerve,
muscle and skin (8); and types of
ncRNAs including T-UCRs, piRNAs and long intergenic noncoding RNAs
(lincRNAs) were associated with human diseases. Overall, the role
of ncRNAs in human diseases is gaining interest.
Ovarian diseases have a wide variety of clinical
pathological types, including ovarian tumors, polycystic ovary
syndrome (PCOS), premature ovarian failure (POF) and other
disorders. Previous studies have demonstrated that ncRNAs were
differentially expressed in ovarian diseases (19–22).
For example, miR-132, miR-320, miR-520c-3p, miR-24 and miR-222
regulated estradiol concentrations, while miR-24, miR-193b and
miR-483-5p regulated progesterone concentrations. In addition,
miR-132 and miR-320 were expressed at significantly lower levels in
the follicular fluid of patients with PCOS than in the healthy
controls (20). miR-23a was highly
expressed in the plasma of patients with POF and it targeted SMAD5
to regulate apoptosis in human granulosa cells (22). The present review aimed to
summarize recent studies that reported the changes of several
ncRNAs in ovarian diseases, which may contribute to the
pathogenesis of ovarian diseases.
In the early 1970s, the non-coding sequences were
identified and referred as ‘junk DNA’ (23). Over the past decade, however,
understanding of the non-coding genomes has increased (24,25).
The comprehensive understanding of non-coding sequences was
initiated in the 1990s, epitomized by the ENCODE consortium, which
claimed complete genome sequences of numerous species (6). Subsequent to this, the ENCODE
consortium identified that the majority of non-coding genomes may
affect cellular and large-scale phenotypes and thus should be
considered as having biochemical function. In addition, some
representative ncRNAs, such as miRNAs and piRNAs, have been
identified.
ncRNAs, a diverse family of transcripts that have no
potential of coding protein, include miRNA, snoRNA, siRNA, piRNA,
lncRNA, T-UCR and numerous other types of ncRNAs (Table I) (10–13).
miRNAs, the most widely studied class of ncRNAs, act
as endogenous 18–24 nucleotide long RNA molecules that recruit RNP
complexes to the complementary RNAs (26). miRNAs regulate particular mRNA
targets through binding to specific sequences. The outcome of miRNA
binding is to inhibit target protein expression. However, the
effect is not to silence mRNA expression, rather is a more nuanced
effect to decrease protein levels. This can be amplified by binding
multiple miRNAs to a single target, or by the targeting of multiple
proteins in the same pathway (26). Numerous processes, including
proliferation, differentiation, apoptosis and development, have
been reported to be regulated by miRNAs (27,28).
piRNAs are small RNA molecules 24–32 nucleotides
long, which are abundant in the germline across animal species
(29). piRNAs are transcribed from
intergenic transcripts that are enriched in transcribed
transposable elements and other repetitive elements (30–32).
Previously, two piRNA-associated mechanisms have been described in
Drosophila melanogaster: The cleavage of transposable element
transcripts by PIWI proteins (33)
and heterochromatin-mediated gene silencing (34).
snoRNAs, 60–300 base pairs (bp) long, were the first
identified components of snoRNPs (35). The complexes are responsible for
post-transcriptional modifications of ribosomal RNAs (rRNAs)
through sequence-specific 2′-O-methylation and pseudouridylation of
rRNA (35,36). snoRNAs are responsible for
targeting the assembled snoRNPs to facilitate rRNA folding and
stability (37).
As 20–30 nucleotide long RNA molecules, siRNAs have
emerged as critical regulators in the expression and function of
eukaryotic genomes (38). Previous
studies suggest widespread usage of endo-siRNAs as endogenous
regulators of gene expression (39,40).
siRNAs protect genome integrity in response to foreign or invasive
nucleic acids including viruses, transposons, and transgenes
(38). Almost all siRNAs, whether
endo-siRNAs or virus siRNAs, silence the same locus from which they
are derived (38).
lncRNAs, a heterogeneous group of non-coding
transcripts more than 200 nucleotides long, are typically
transcribed and frequently spliced and polyadenylated (41,42).
lncRNAs account for the majority of the ncRNAs in mammals (43). lncRNAs are predominantly localized
in the nucleus and act as signals, scaffolds for protein-protein
interactions, molecular decoys and guides to target elements in the
genomes or transcriptomes (44).
The earliest discovered lncRNAs include H19 (45) and Xist (12).
Numerous other classes of ncRNA have been reported,
for example endo-siRNA (22 nucleotide-long small RNAs that arise
from sense-antisense RNA hybrids, pseudogene transcripts,
transposable elements and mRNA exons or introns) (46), T-UCRs (DNA segments that are longer
than 200 bp and are completely conserved) (47) and telomeric repeat containing RNAs
(maintain the integrity of telomeric heterochromatin by regulating
telomerase activity) (48). The
biological functions for these ncRNAs remain poorly defined.
Ovarian growth is a series of complex and
coordinated processes, accompanied by significant morphological and
functional changes in different follicular cells. Among the small
ncRNAs associated with ovarian development, miRNAs are the most
widely studied and first elucidated ncRNAs.
Dicer1 is an important RNase III enzyme that
processes pre-miRNA into the shorter miRNA duplex (49). Mice with conditional knockout of
Dicer exhibited increased primordial follicle pool endowment,
increased degeneration of follicles and decreased ovulation rates
(50). Functional studies using
inhibition of miRNA biogenesis have demonstated the occurrence of
developmental arrest and female infertility in various species.
Otsuka et al (51) reported
that miRNAs were associated with the secretion of progesterone by
disturbing the function of ovarian corpus. Furthermore, Hossain
et al (52) suggested that
miRNAs were involved in the regulation of steroid hormone receptors
in ovarian follicle growth.
In multicellular organisms, lncRNAs participated in
cell differentiation and individual development as promoters or
inhibitors (56). In germ cell
development, lncRNAs regulate the expression of specific genes and
serve key roles in the complex epigenetic process.
The mouse oocyte pseudogenegene siRNA system has
been observed to preferentially target genes that are involved in
microtubule dynamics (40).
miRNAs serve important roles in tumorigenesis by
acting as oncogenes or tumor suppressor genes (57–59).
The members of the let-7 family of miRNAs were observed to be lost
in ovarian cancer (59). Calin
et al (60) reported that
miRNAs were frequently located in fragile regions of the
chromosomes associated with the development of ovarian
carcinomas.
Steroid receptor RNA activator (SRA) was initially
characterized as an lncRNA by Lanz et al in 1999 (61). SRAs were strongly upregulated in
ovarian tumors (62,63), suggesting their potential role in
ovary tumorigenesis. H19 acted as a type of lncRNA whose
methylation imprinting was reported to be associated with the
development of human benign ovarian teratomas (64). Yiya was identified as a 1.9 kb long
ncRNA gene located 69 kb upstream of the transcription factor
prospero-related homeobox 1 (65).
The potential role of snoRNAs in types of cancer,
including non small cell lung cancer and breast cancer, has been
reported previously (67–69), however the role of snoRNAs in the
development of ovarian tumors remains unclear.
Mice lacking the progesterone receptor exhibited
pleiotropic reproductive abnormalities (73). In the letrozole-induced rat PCOS
model, Zurvarra et al (74)
observed increased expression levels of androgen receptor and
decreased expression of the estrogen and progesterone receptors.
These data indicate that steroid receptors serve important roles in
the etiology of PCOS. Furthermore, several studies demonstrated
that lncRNA SRA was capable of promoting the activity of these
steroid receptors (75,76). Further studies are necessary to
confirm the association between lncRNA SRA and PCOS.
To understand the molecular mechanism of POF,
several studies analyzed miRNAs and their target mRNAs (22,77).
Profiling of differentially expressed miRNAs in POF provided novel
insight into the molecular events in POF development, with the
upregulation of miR-151 and miR-672 targeting expression of TNFSF10
and FNDC1, which had been demonstrated to positively regulate cell
apoptosis (77).
At present, it is not possible to elucidate whether
altered ncRNA expression profiles are associated with the
occurrence of ovarian diseases. However, miRNAs have been isolated
from the blood, saliva, urine, feces, follicular fluid and other
body fluids, and secreted miRNAs remain stable in body fluids, thus
may serve as biomarkers for associated diseases (78). Several serum miRNAs (miR-222,
miR-29a and let-7) have been suggested to act as novel non-invasive
biomarkers for ovarian diseases (59,77,79).
The technological development in the field,
particularly bead-based flow cytometry, single molecule detection
and massively parallel sequencing coupled with the mirage approach,
may aid in the development of automated and high-speed ncRNAs
profiling in the near future.
ncRNAs are highly abundant in living organisms and
serve important roles in numerous biological processes. Therefore,
there has been an increasing requirement to investigate the entire
ncRNAomes and their biological function in further detail. In
addition, aberrant ncRNA expression profiles are considered to be
associated with the pathogenesis of ovarian diseases (Table II). To better understand the role
of ncRNAs in ovarian diseases, future studies should focus on the
molecular mechanisms by which abnormal expression of ncRNAs
contributes to ovarian diseases, particularly the identification of
the ncRNA regulation network and the interaction between ncRNAs and
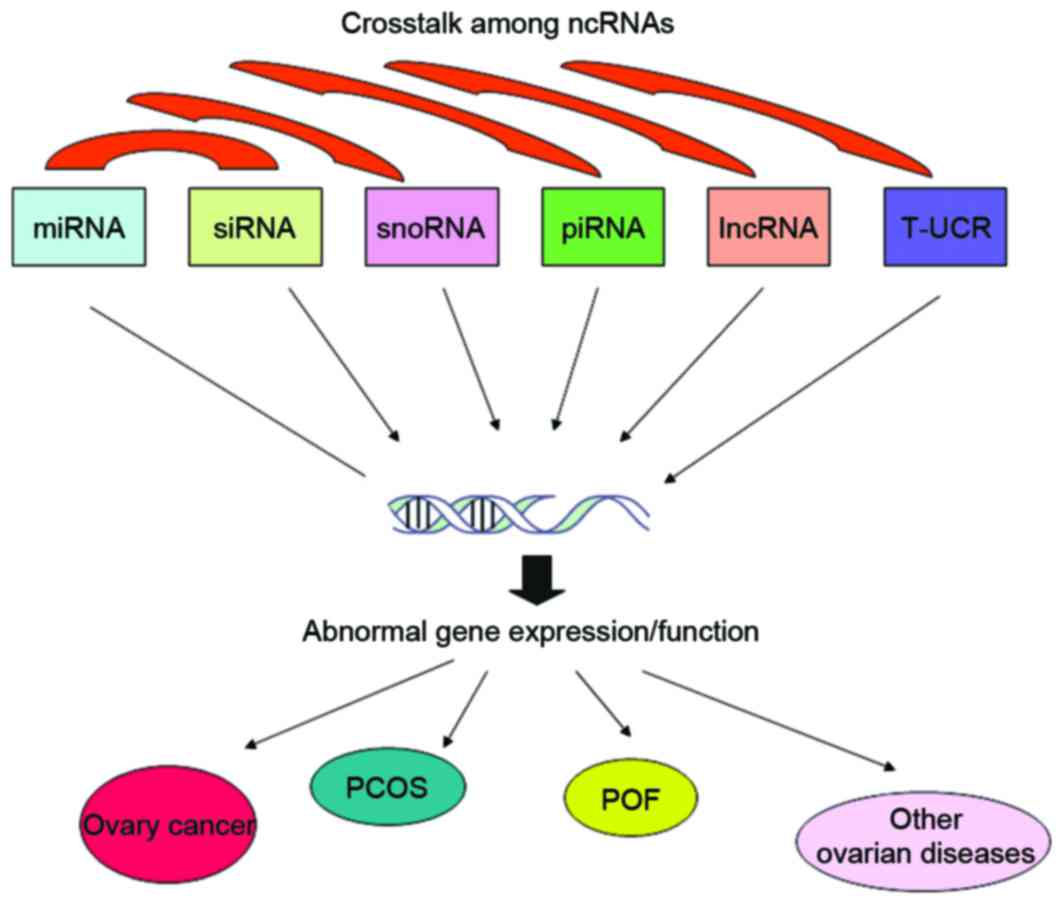
DNAs (Fig. 1). These
investigations will aid in the identification of ncRNAs as novel
diagnostic markers and therapeutic targets for ovarian
diseases.
The current study was supported by the
Pharmaceutical Industry Development Fund of Jiin Province (grant
no. 20150311035YY).
|
1
|
Lee TI and Young RA: Transcriptional
regulation and its misregulation in disease. Cell. 152:1237–1251.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lawrence MS, Stojanov P, Mermel CH,
Robinson JT, Garraway LA, Golub TR, Meyerson M, Gabriel SB, Lander
ES and Getz G: Discovery and saturation analysis of cancer genes
across 21 tumour types. Nature. 505:495–501. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
MacLellan WR, Wang Y and Lusis AJ:
Systems-based approaches to cardiovascular disease. Nat Rev
Cardiol. 9:172–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bai B, Hales CM, Chen PC, Gozal Y, Dammer
EB, Fritz JJ, Wang X, Xia Q, Duong DM, Street C, et al: U1 small
nuclear ribonucleoprotein complex and RNA splicing alterations in
Alzheimer's disease. Proc Natl Acad Sci USA. 110:16562–16567. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang F, Yi F, Zheng Z, Ling Z, Ding J, Guo
J, Mao W, Wang X, Ding X, Wang X, et al: Characterization of a
carcinogenesis-associated long non-coding RNA. RNA Biol. 9:110–116.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
ENCODE Project Consortium: An integrated
encyclopedia of DNA elements in the human genome. Nature.
489:57–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flynn RA and Chang HY: Long noncoding RNAs
in cell-fate programming and reprogramming. Cell Stem Cell.
14:752–761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kung JT, Colognori D and Lee JT: Long
noncoding RNAs: Past, present, and future. Genetics. 193:651–669.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bachellerie JP, Cavaillé J and Hüttenhofer
A: The expanding snoRNA world. Biochimie. 84:775–790. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Stijn T and Galloway S: A BamHI
polymorphism at the ovine inactive X-specific transcript locus
(XIST). Anim Genet. 26:279–280. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brockdorff N, Ashworth A, Kay GF, McCabe
VM, Norris DP, Cooper PJ, Swift S and Rastan S: The product of the
mouse Xist gene is a 15 kb inactive X-specific transcript
containing no conserved ORF and located in the nucleus. Cell.
71:515–526. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E and
Chang HY: Functional demarcation of active and silent chromatin
domains in human HOX loci by noncoding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dehwah MA, Xu A and Huang Q: MicroRNAs and
type 2 diabetes/obesity. J Genet Genomics. 39:11–18. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Medina PP, Nolde M and Slack FJ: OncomiR
addiction in an in vivo model of microRNA-21-induced pre-B-cell
lymphoma. Nature. 467:86–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao JJ, Lin J, Yang H, Kong W, He L, Ma
X, Coppola D and Cheng JQ: MicroRNA-221/222 negatively regulates
estrogen receptor alpha and is associated with tamoxifen resistance
in breast cancer. J Biol Chem. 283:31079–31086. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Herrera-Esparza R, Kruse L, von Essen M,
Campos L, Barbosa O, Bollain JJ, Badillo I and Avalos-Díaz E: U3
snoRNP associates with fibrillarin a component of the scleroderma
clumpy nucleolar domain. Arch Dermatol Res. 294:310–317.
2002.PubMed/NCBI
|
|
18
|
Yang JM, Hildebromdt B, Luderschmidt C and
Pollard KM: Human scleroderma sera contain autoantibodies to
protein components specific to the U3 small nucleolar RNP complex.
Arthritis Rheum. 48:210–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nicoloso MS, Spizzo R, Shimizu M, Rossi S
and Calin GA: MicroRNAs-the micro steering wheel of tumour
metastases. Nature Rev Cancer. 9:293–302. 2009. View Article : Google Scholar
|
|
20
|
Sang Q, Yao Z, Wang H, Feng R, Wang H,
Zhao X, Xing Q, Jin L, He L, Wu L and Wang L: Identification of
microRNAs in human follicular fluid: Characterization of microRNAs
that govern steroidogenesis in vitro and are associated with
polycystic ovary syndrome in vivo. J Clin Endocrinol Metab.
98:3068–3079. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan Z, Hu HY, Jiang X, Maierhofer V, Neb
E, He L, Hu Y, Hu H, Li N, Chen W and Khaitovich P: Widespread
expression of piRNA-like molecules in somatic tissues. Nucleic
Acids Res. 39:6596–6607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang X, Zhou Y, Peng S, Wu L, Lin HY, Wang
S and Wang H: Differentially expressed plasma microRNAs in
premature ovarian failure patients and the potential regulatory
function of mir-23a in granulosa cell apoptosis. Reproduction.
144:235–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohno S: So much ‘junk’ DNA in our genome.
Brookhaven Symp Biol. 23:366–370. 1972.PubMed/NCBI
|
|
24
|
Zhang R, Zhang L and Yu W: Genome-wide
expression of non-coding RNA and global chromatin modification.
Acta Biochim Biophys Sin (Shanghai). 44:40–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hertel J, De Jong D, Marz M, Rose D, Tafer
H, Tanzer A, Schierwater B and Stadler PF: Non-coding RNA
annotation of the genome of Trichoplax adhaerens. Nucleic Acids
Res. 37:1602–1615. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mohr AM and Mott JL: Overview of MicroRNA
biology. Semin Liver Dis. 35:3–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sirotkin AV, Lauková M, Ovcharenko D,
Brenaut P and Mlyncek M: Identification of microRNAs controlling
human ovarian cell proliferation and apoptosis. J Cell Physiol.
223:49–56. 2010.PubMed/NCBI
|
|
28
|
Jiang L, Huang J, Li L, Chen Y, Chen X,
Zhao X and Yang D: MicroRNA-93 promotes ovarian granulosa cells
proliferation through targeting CDKN1A in polycystic ovarian
syndrome. J Clin Endocrinol Metab. 100:E729–E738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thomson T and Lin H: The biogenesis and
function of PIWI proteins and piRNAs: Progress and prospect. Annu
Rev Cell Dev Biol. 25:355–376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aravin AA, Hannon GJ and Brennecke J: The
Piwi-piRNA pathway provides an adaptive defense in the transposon
arms race. Science. 318:761–764. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aravin AA, Sachidanandam R, Girard A,
Fejes-Toth K and Hannon GJ: Developmentally regulated piRNA
clusters implicate MILI in transposon control. Science.
316:744–747. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brennecke J, Aravin AA, Stark A, Dus M,
Kellis M, Sachidanandam R and Hannon GJ: Discrete small
RNA-generating loci as master regulators of transposon activity in
Drosophila. Cell. 128:1089–1103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gunawardane LS, Saito K, Nishida KM,
Miyoshi K, Kawamura Y, Nagami T, Siomi H and Siomi MC: A
slicer-mediated mechanism for repeat-associated siRNA 5′ end
formation in Drosophila. Science. 315:1587–1590. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pal-Bhadra M, Leibovitch BA, Gandhi SG,
Chikka MR, Bhadra U, Birchler JA and Elgin SC: Heterochromatic
silencing and HP1 localization in Drosophila are dependent on the
RNAi machinery. Science. 303:669–672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kiss-László Z, Henry Y, Bachellerie JP,
Caizergues-Ferrer M and Kiss T: Site-specific ribose methylation of
preribosomal RNA: A novel function for small nucleolar RNAs. Cell.
85:1077–1088. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ni J, Tien AL and Fournier MJ: Small
nucleolar RNAs direct site-specific synthesis of pseudouridine in
ribosomal RNA. Cell. 89:565–573. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
King TH, Liu B, McCully RR and Fournier
MJ: Ribosome structure and activity are altered in cells lacking
snoRNPs that form pseudouridines in the peptidyl transferase
center. Mol Cell. 11:425–435. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Diederichs S and Haber DA: Dual role for
argonautes in microRNA processing and posttranscriptional
regulation of microRNA expression. Cell. 131:1097–1108. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tam OH, Aravin AA, Stein P, Girard A,
Murchison EP, Cheloufi S, Hodges E, Anger M, Sachidanandam R,
Schultz RM and Hannon GJ: Pseudogene-derived small interfering RNAs
regulate gene expression in mouse oocytes. Nature. 453:534–538.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rinn JL and Chang HY: Genome regulation by
long noncoding RNAs. Annu Rev Biochem. 81:145–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guttman M, Amit I, Garber M, French C, Lin
MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al:
Chromatin signature reveals over a thousand highly conserved large
non-coding RNAs in mammals. Nature. 458:223–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Derrien T, Johnson R, Bussotti G, Tanzer
A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG,
et al: The GENCODE v7 catalog of human long noncoding RNAs:
Analysis of their gene structure, evolution, and expression. Genome
Res. 22:1775–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brannan CI, Dees EC, Ingram RS and
Tilghman SM: The product of the H19 gene may function as an RNA.
Mol Cell Biol. 10:28–36. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Okamura K and Lai EC: Endogenous small
interfering RNAs in animals. Nat Rev Mol Cell Biol. 9:673–678.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ørom UA, Derrien T, Guigo R and
Shiekhattar R: Long noncoding RNAs as enhancers of gene expression.
Cold Spring Harb Symp Quant Biol. 75:325–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Feuerhahn S, Iglesias N, Panza A, Porro A
and Lingner J: TERRA biogenesis, turnover and implications for
function. FEBS Lett. 584:3812–3818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kanellopoulou C, Muljo SA, Kung AL,
Ganesan S, Drapkin R, Jenuwein T, Livingston DM and Rajewsky K:
Dicer-deficient mouse embryonic stem cells are defective in
differentiation and centromeric silencing. Genes Dev. 19:489–501.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lei L, Jin S, Gonzalez G, Behringer RR and
Woodruff TK: The regulatory role of Dicer in folliculogenesis in
mice. Mol Cell Endocrinol. 315:63–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Otsuka M, Zheng M, Hayashi M, Lee JD,
Yoshino O, Lin S and Han J: Impaired microRNA processing causes
corpus luteum insufficiency and infertility in mice. J Clin Invest.
118:1944–1954. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hossain MM, Cao M, Wang Q, Kim JY,
Schellander K, Tesfaye D and Tsang BK: Altered expression of miRNAs
in a dihydrotestosterone-induced rat PCOS model. J Ovarian Res.
6:362013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Malone CD, Brennecke J, Dus M, Stark A,
McCombie WR, Sachidanandam R and Hannon GJ: Specialized piRNA
pathways act in germline and somatic tissues of the Drosophila
ovary. Cell. 137:522–535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li C, Vagin VV, Lee S, Xu J, Ma S, Xi H,
Seitz H, Horwich MD, Syrzycka M, Honda BM, et al: Collapse of
germline piRNAs in the absence of Argonaute3 reveals somatic piRNAs
in flies. Cell. 137:509–521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Saito K, Inagaki S, Mituyama T, Kawamura
Y, Ono Y, Sakota E, Kotani H, Asai K, Siomi H and Siomi MC: A
regulatory circuit for piwi by the large Maf gene traffic jam in
Drosophila. Nature. 461:1296–1299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sasaki H and Matsui Y: Epigenetic events
in mammalian germ-cell development: Reprogramming and beyond. Nat
Rev Genet. 9:129–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Esquela-Kerscher A and Slack FJ:
OncomiRs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hammond SM: MicroRNAs as tumor
suppressors. Nat Genet. 39:582–583. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lanz RB, McKenna NJ, Onate SA, Albrecht U,
Wong J, Tsai SY, Tsai MJ and O'Malley BW: A steroid receptor
coactivator, SRA, functions as an RNA and is present in an SRC-1
complex. Cell. 97:17–27. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cooper C, Guo J, Yan Y,
Chooniedass-Kothari S, Hube F, Hamedani MK, Murphy LC, Myal Y and
Leygue E: Increasing the relative expression of endogenous
non-coding Steroid Receptor RNA Activator (SRA) in human breast
cancer cells using modified oligonucleotides. Nucleic Acids Res.
37:4518–4531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lanz RB, Chua SS, Barron N, Söder BM,
DeMayo F and O'Malley BW: Steroid receptor RNA activator stimulates
proliferation as well as apoptosis in vivo. Mol Cell Biol.
23:7163–7176. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Miura K, Obama M, Yun K, Masuzaki H, Ikeda
Y, Yoshimura S, Akashi T, Niikawa N, Ishimaru T and Jinno Y:
Methylation imprinting of H19 and SNRPN genes in human benign
ovarian teratomas. Am J Hum Genet. 65:1359–1367. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yang F, Yi F, Zheng Z, Ling Z, Ding J, Guo
J, Mao W, Wang X, Wang X, Ding X, et al: Characterization of a
carcinogenesis-associated long non-coding RNA. RNA Biol. 9:110–116.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lee JH, Schütte D, Wulf G, Füzesi L,
Radzun HJ, Schweyer S, Engel W and Nayernia K: Stem-cell protein
Piwil2 is widely expressed in tumors and inhibits apoptosis through
activation of Stat3/Bcl-XL pathway. Hum Mol Genet. 15:201–211.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chang LS, Lin SY, Lieu AS and Wu TL:
Differential expression of human 5S snoRNA genes. Biochem Biophys
Res Commun. 299:196–200. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liao J, Yu L, Mei Y, Guarnera M, Shen J,
Li R, Liu Z and Jiang F: Small nucleolar RNA signatures as
biomarkers for non-small-cell lung cancer. Mol Cancer. 9:1982010.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dong XY, Guo P, Boyd J, Sun X, Li Q, Zhou
W and Dong JT: Implication of snoRNA U50 in human breast cancer. J
Genet Genomics. 36:447–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Roth LW, McCallie B, Alvero R, Schoolcraft
WB, Minjarez D and Katz-Jaffe MG: Altered microRNA and gene
expression in the follicular fluid of women with polycystic ovary
syndrome. J Assist Reprod Genet. 31:355–362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liu S, Zhang X, Shi C, Lin J, Chen G, Wu
B, Wu L, Shi H, Yuan Y, Zhou W, et al: Altered microRNAs expression
profiling in cumulus cells from patients with polycystic ovary
syndrome. J Transl Med. 13:2382015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lin L, Du T, Huang J, Huang LL and Yang
DZ: Identification of differentially expressed microRNAs in the
ovary of polycystic ovary syndrome with hyperandrogenism and
insulin resistance. Chin Med J (Enql). 128:169–174. 2015.
View Article : Google Scholar
|
|
73
|
Lydon JP, DeMayo FJ, Funk CR, Mani SK,
Hughes AR, Montgomery CA Jr, Shyamala G, Conneely OM and O'Malley
BW: Mice lacking progesterone receptor exhibit pleiotropic
reproductive abnormalities. Genes Dev. 9:2266–2278. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zurvarra FM, Salvetti NR, Mason JI,
Velazquez MM, Alfaro NS and Ortega HH: Disruption in the expression
and immunolocalisation of steroid receptors and steroidogenic
enzymes in letrozole-induced polycystic ovaries in rat. Reprod
Fertil Dev. 21:827–839. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhao X, Patton JR, Davis SL, Florence B,
Ames SJ and Spanjaard RA: Regulation of nuclear receptor activity
by a pseudouridine synthase through posttranscriptional
modification of steroid receptor RNA activator. Mol Cell.
15:549–558. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Takayama K, Tsutsumi S, Katayama S,
Okayama T, Horie-Inoue K, Ikeda K, Urano T, Kawazu C, Hasegawa A,
Ikeo K, et al: Integration of cap analysis of gene expression and
chromatin immunoprecipitation analysis on array reveals genome-wide
androgen receptor signaling in prostate cancer cells. Oncogene.
30:619–630. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kuang H, Han D, Xie J, Yan Y, Li J and Ge
P: Profiling of differentially expressed microRNAs in premature
ovarian failure in an animal model. Gynecol Endocrinol. 30:57–61.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: A novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Long W, Zhao C, Ji C, Ding H, Cui Y, Guo
X, Shen R and Liu J: Characterization of serum microRNAs profile of
PCOS and identification of novel non-invasive biomarkers. Cell
Physiol Biochem. 33:1304–1315. 2014. View Article : Google Scholar : PubMed/NCBI
|