Introduction
As loose connective tissues surrounding the
developing tooth germ, dental follicles not only control tooth
eruption, but also contain progenitor cells that give rise to the
cementum, periodontal ligaments and alveolar bones (1,2).
Progenitor cells in dental follicles expressing Notch-1 and nestin
proteins are believed to possess mesenchymal stem cell-like
properties (3). Dental follicle
cells (DFCs) can differentiate into osteoblasts and cementoblasts
through the regulation of specific signaling pathways (4,5).
Using suitable biocompatible scaffolds, DFCs form dentin and
cementum-like matrices, indicating that DFCs may potentially serve
as seed cells in tooth regeneration engineering (6–8).
Previous studies have identified several effectors
that have been implicated in the osteogenic differentiation of
DFCs, including bone morphogenetic protein (BMP), Wnt, Sonic
hedgehog and the Notch signaling pathway (4,9–11).
Previously, the canonical and non-canonical Wnt pathways have been
demonstrated to promote DFC differentiation into
osteoblasts/cementoblasts (9,12).
Results have indicated that the Wnt signaling pathway branches into
at least three distinct pathways: The canonical, non-canonical and
planar-cell polarity pathways (13,14).
The canonical Wnt pathway is the most extensively studied, and
refers to Wnt ligands causing β-catenin accumulation in the
cytoplasm, leading to eventual nuclear translocation, where it acts
as a transcriptional co-activator of transcription factors
belonging to the T-cell factor/lymphoid enhancer factor family
(15).
The canonical Wnt/β-catenin pathway serves a
critical function in the cell cycle and cell growth, which mediate
oral tissue development (16).
Previous research has suggested that Wnt/β-catenin signaling
inhibits dental pulp stem cell differentiation into odontoblasts
(17), and enhances the
mineralization capacity of ameloblasts (18). However, the biological role of the
canonical Wnt pathway in dental follicles has not been fully
elucidated. To investigate the effects of Wnt pathway activation on
DFCs, a polymerase chain reaction (PCR) array was implemented to
compare the expression levels of 84 Wnt-associated genes maintained
in routine or mineral-induction media for 4 weeks. The results
indicated that the Dishevelled 3 (DVL3), cyclin D2 (CCND) and
Dickkopf-related protein 3 (DKK3) genes were downregulated upon
mineral induction, thus providing novel areas to study the
osteogenic differentiation of DFCs.
The DVL protein family function as scaffold proteins
that bridge receptors and distinct downstream signaling components
(19). CCND proteins are members
of the cyclin protein family, which regulate cell cycle progression
(20). In the present study, there
was a particular focus on the role of DKK3, since DKK family
members are secreted glycoproteins known to antagonize Wnt
signaling (21,22). Specifically, DKK3 is downregulated
in various tumor cells, including hepatocellular carcinoma,
lymphoblastic leukemia, prostate cancer, renal cell carcinoma, and
melanoma cells (23–28). It has also previously been reported
that DKK-family members participate in tooth development (29) DKK1 has previously been reported to
be markedly expressed in the distal, incisor-bearing mesenchyme
area of mouse mandibular processes during the initial stages of
tooth formation. However, during molar morphogenesis, DKK1 was
detected in the dental mesenchyme, DKK2 was expressed in the dental
papilla, and DKK3 was specifically expressed in the primary and
secondary enamel knots. In addition, DKK3 was transiently expressed
postnatally in pre-ameloblasts, prior to the onset of enamel matrix
secretion (29). However, the
supportive roles of DKK family members in tooth development or
dental-related cells remain elusive. Considering the evidence
provided by the Wnt PCR array in the present study, the role of
DKK3 in rat DFCs during osteogenesis was investigated.
Materials and methods
Ethical approval
All procedures performed in the present study
involving animals were approved by the Ethics Committee of the
Guanghua College of Stomatology at Sun Yat-sen University
(Guangzhou, China).
DKK3 expression assay of DFCs
with/without mineral induction
DFCs were cultured from 6–7-day-old Sprague Dawley
rats, as described previously (2).
A total of 5 rats [2 female/3 male, ~10 to 15 g, bred at 24°C on a
12 h light/dark cycle with ad libitum access to food and
water, supplied by the Animal Research Center of Sun Yat-sen
University (Guangzhou, China)] were sacrificed by cervical
dislocation. The mandibles were dissected to separate the dental
follicles. Following this, the dental follicles were digested by
0.1% collagenase type I (cat. no. C0130; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) and 10 U/ml dispase (cat. no. D4818;
Sigma-Aldrich; Merck Millipore) for 30 min at 37°C to obtain DFCs.
The DFCs were identified by Alizarin red staining and Oil Red O
staining. For the Oil Red O Staining, DFCs were treated with
Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS, 0.5
mmol/l 3-isobutyl-1-methylxanthine, 200 µm/l indometacin, 10 µg/ml
insulin, and 1 µm/l dexamethasone (Sigma-Aldrich; Merck-Millipore).
The cells were stained with Oil Red O (Sigma-Aldrich;
Merck-Millipore) 2 weeks subsequent to this. For the DKK3 assay,
DFCs were cultured in 10% DMEM or mineral-induction medium (10%
DMEM, 10 µl β-glycerophosphate, 50 µM ascorbate-2 phosphate and 0.1
µM dexamethasone; Sigma-Aldrich; Merck-Millipore) for 4 weeks at
37°C. Subsequently, the rat Wnt signaling pathway profile (cat. no.
PARN-043Z; Qiagen, Inc., Valencia, CA, USA) was used to detect the
expression of 84 genes associated with Wnt-mediated signal
transduction on DFCs with/without mineral induction for 4 weeks.
Briefly, the experiment was assayed on 96-well plates by running
the reverse transcription-quantitative PCR (RT-qPCR) cycling
program, according to the manufacturer's protocol.
Based on the results of the RT-qPCR, DKK3 was
selected for further study. DFCs were cultured using DMEM or
mineral-induction medium for 1, 2 or 4 weeks at 37°C. DKK3 gene
expression in each group was measured by RT-qPCR and western blot
(WB) analysis.
RT-qPCR analysis
Cells were collected and total RNA was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's protocol. Reverse
transcription was performed using the 1st Strand cDNA Synthesis kit
with random hexamer primers (Invitrogen; Thermo Fisher Scientific,
Inc.) as follows: The RNA/primer mixture was incubated at 65°C for
5 min, then placed on ice for 1 min. 2X reaction mix was added to
the prepared RNA/primer mixture followed with incubation at room
temperature (~25°C) for 2 min. The mixture with 1 µl SuperScript II
RT was incubated at 42°C for 50 min and the reaction was terminated
at 70°C for 15 min. The obtained cDNA was collected by brief
centrifugation (4,989.5 × g, 30 sec, room temperature) and the
quantification of DKK3 expression was analyzed by RT-qPCR using the
TaqMan Gene Expression Assay kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with the following parameters: 95°C for 3 min for
initial denaturation, 40 cycles at 95°C for 3 sec, 57°C for 30 sec,
68°C for 1 min. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
was used as the internal control and the primers were as follows:
Forward 5′-GCAAGAGAGAGGCCCTCAG-3′ and reverse
5′-TGTGAGGGAGATGCTCAGTG-3′. The primer sequences of the DKK3 gene
were as follows: Forward 5′-TATACATGTGCAAGCCAGCC-3′ and reverse
5′-TCCTCAAATGCCATCTCCTG-3′. Threshold cycle values (Cq) were
determined and data were analyzed with the 2−∆∆Cq method
(30).
Western blotting analysis
DFCs from each group were obtained and protein was
extracted using a NE-PER Extraction kit (Pierce; Thermo Fisher
Scientific, Inc.). Protein concentrations were measured with a
bicinchoninic acid (BCA) protein assay (Beyotime Institute of
Biotechnology, Haimen, China). Proteins (20 µg) were separated by
10% sodium dodecylsulfate-polyacrylamide gel electrophoresis and
electrophoretically transferred onto a polyvinylidene fluoride
membrane (EMD Millipore, Billerica, MA, USA). The membrane was
incubated for 1 h at room temperature in TBST containing 5% skim
milk to block nonspecific protein binding and incubated at 4°C
overnight with the primary antibodies. The following primary
antibodies were used: Rabbit anti-DKK3 polyclonal antibody
(dilution, 1:1,000; cat. no. ABIN411466; Biorbyt, Ltd., Cambridge,
UK) and rabbit anti-GAPDH polyclonal antibody (dilution, 1:5,000;
cat. no. ab9485; Abcam, Cambridge, UK), which was used as internal
control. Following washing for 20 min, the membrane was incubated
with horseradish peroxidase-conjugated goat anti-rabbit IgG
(dilution, 1:2,000; cat. no. ab6721; Abcam) for 1 h at room
temperature. Blots were visualized using an enhanced
chemiluminescence system (Millipore ECL Western Blotting Detection
System; EMD Millipore), and band densities were obtained and
normalized to GAPDH and the background using ImageJ software
version 2 (National Institutes of Health, Bethesda, MD, USA).
Construction of DFCs expressing a
lentiviral vector containing short hairpin RNA (shRNA) against
DKK3
A lentiviral vector expressing shRNA against DKK3
mRNA was constructed. The DKK3-targeting shRNA sequence (forward,
5′-TGCCACAGTCTGGTATACATCTTCCTGTCAATGTATACCAGACTGTGGCTTTTTTC-3′ and
reverse,
5′-TCGAGAAAAAAGCCACAGTCTGGTATACATTGACAGGAAGATGTATACCAGACTGTGGCA-3′)
was designed and cloned into the PLL3.79 lentiviral vector
(Addgene, Inc., Cambridge, MA, USA), which encodes a green
fluorescence protein (GFP) tag, and was validated by sequence
analysis. The packaging 293T cell line (cat. no. CMH010; Shanghai
Gaining Biotechnology Co., Ltd., Shanghai China) was transfected
with the lentiviral vector using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The viral supernatant was harvested and
the lentiviral particle titer was determined. The DFCs were seeded
into 75 cm2 culture flasks at a density of 1×106 cells/ml.
Following overnight culture, the cells were infected for 12 h at
37°C with the lentiviral vector in the presence of polybrene (final
concentration, 8 mg/ml). Subsequently, the cells were washed and
cultured in fresh medium for 3 days. Infected DFCs were selected by
fluorescence activated cell sorting (FACS). Cells were trypsinized
with 0.05% Trypsin-EDTA (cat. no. 25300-062; Gibco; Thermo Fisher
Scientific, Inc.) for 40 sec at 37°C, harvested, and resuspended in
PBS. FACS analyses were performed with the BD Influx Cell Sorter
(BD Biosciences, Franklin Lakes, NJ, USA) and samples were analyzed
by collecting 10,000 events using FlowJo software version 1.0 (BD
Biosciences). Cells transfected with control vectors served as
negative controls. The GFP+ transduced cells were also isolated by
FACS with the BD Influx Cell Sorter (BD Biosciences).
Alkaline phosphatase (ALP) activity
assays and Alizarin Red staining of DKK3-shRNA DFCs
DKK3-shRNA DFCs and control vector DFCs were placed
in 24-well plates at a density of 3×104 cells/well. After 24 h, the
cells were grown in mineral-induction medium for 3, 7, 14 or 21
days at 37°C. Each experimental group of cells was washed with
phosphate-buffered saline at the appointed time and lysed by the
addition of 200 µl/well 1% Triton X-100. Cellular ALP activities
and total protein concentrations were determined with an ALP kit
(Nanjing Jiancheng Chemical Industrial Co., Ltd., Nanjing, China)
and the Bicinchoninic Acid Protein Assay kit (Boshide, Wuhan,
China), respectively.
During the Alizarin Red staining experiments, the
DKK3-shRNA DFCs and control vector DFCs were seeded in 6-well
plates at a density of 1×105 cells/well. Following 24 h,
the cells were grown in mineral-induction medium for 3 weeks at
37°C. The DFCs were subsequently fixed in formaldehyde for 15 min
and stained with 0.5% Alizarin Red. Mineral deposits were
visualized under a light microscope (Zeiss GmbH, Jena,
Germany).
Mineral capability assays of
DKK3-shRNA DFCs
RT-qPCR and WB analysis were employed to compare the
respective expression levels of osteogenic markers in DKK3-shRNA
DFCs and control DFCs in vitro. Following mineral induction
for 1 or 3 weeks in shRNA DFCs and control vector DFCs, the mRNA
expression levels of ALP (forward 5′-GGAAGCTAGATGCGGACAAG-3′ and
reverse 5′-TCCCTGACATCGAAGTACCC-3′) and collagen-type-I (Col-I;
forward, 5′-AGAGCATGACCGATGGATTCC-3′ and reverse,
5′-TTGCCAGTCTGCTGGTCCATG-3′) were measured by RT-qPCR.
In addition, the protein expression levels of DKK3
(dilution, 1:1,000; cat. no. ABIN411466; Biorbyt, Ltd.), β-catenin
(dilution, 1:500; cat. no. ab16051; Abcam), runt-related
transcription factor 2 (RUNX2; dilution, 1:1,000; cat. no. ab23981;
Abcam), and osteocalcin (OCN; dilution, 1:1,000; cat. no. ab93876;
Abcam) were investigated using WB analysis.
To detect the osteogenic differentiation of
DKK3-shRNA DFCs or vector DFCs in vivo, cells at a density
of 4×106 cells were mixed with 40 mg
hydroxyapatite/b-tricalcium phosphate powder (HA/TCP; Engineering
Research Centre in Biomaterials, Sichuan University, Chengdu,
China) Each mixture (1 ml) was centrifuged (148.8 × g, 3
min, room temperature) and transplanted into the subcutaneous
tissue of 4 severe combined immunodeficiency (SCID) mice (6 weeks
old; ~30 g; 2 female/2 male, supplied by Animal Research Center of
Sun Yat-sen University, Guangzhou, China). The mice were maintained
in a clean animal room under a temperature of ~24°C, humidity of 40
to 70%, on a 12 h light/dark cycle. At 5 weeks post-implantation,
the mice were sacrificed by cervical dislocation and the
transplants were separated, then fixed with 4% paraformaldehyde for
24 h, and decalcified in 5% methanoic acid for 5 days. The sections
were embedded in paraffin and cut longitudinally at 5-µm intervals.
Initially, hematoxylin and eosin (H&E) staining was performed
to observe the general view of transplants. The paraffin sections
were deparaffinized, rehydrated and incubated with hematoxylin to
stain the nuclei. After washing with tap water, the sections were
stained with eosin. Finally, the samples were dehydrated, cleared
and mounted. To further explore the collagen fiber of the
transplants, Masson staining was applied with a similar protocol to
the H&E staining. A total of three sections from each group
were selected randomly, and three independent visual fields were
captured to observe the newly formed collagen and mineralized
matrices. The images captured by light microscope (Zeiss GmbH) were
analyzed by Image-Pro Plus software (version, 6.0; Media
Cybernetics, Rockville, MD, USA) to calculate the integrated
optical density (IOD) and area of new collagen and mineralized
matrices. The mineralized capability was obtained using the
following formula: IOD/area × 100.
Statistical analysis
Results were presented as the mean ± standard
deviation of at least 3 independent experiments. Data analysis was
performed using SPSS software (version 20.0; IBM SPSS, Armonk, NY,
USA). A Student's t-test was used to compare two means. One-way
analysis of variance was applied to compare two or more means,
followed by Student-Newman-Keuls test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Decreased DKK3 expression in DFCs upon
mineral induction
DFCs were successfully cultured and induced to form
mineral deposits and adipocytes (Fig.
1A-D). The Wnt RT-qPCR array detected the expression levels of
84 Wnt-associated genes in DFCs maintained in routine or
mineral-induction media for 4 weeks. The results indicated that
DVL3, CCND2 and DKK3 genes were downregulated upon mineral
induction (Table I;
P<0.05).
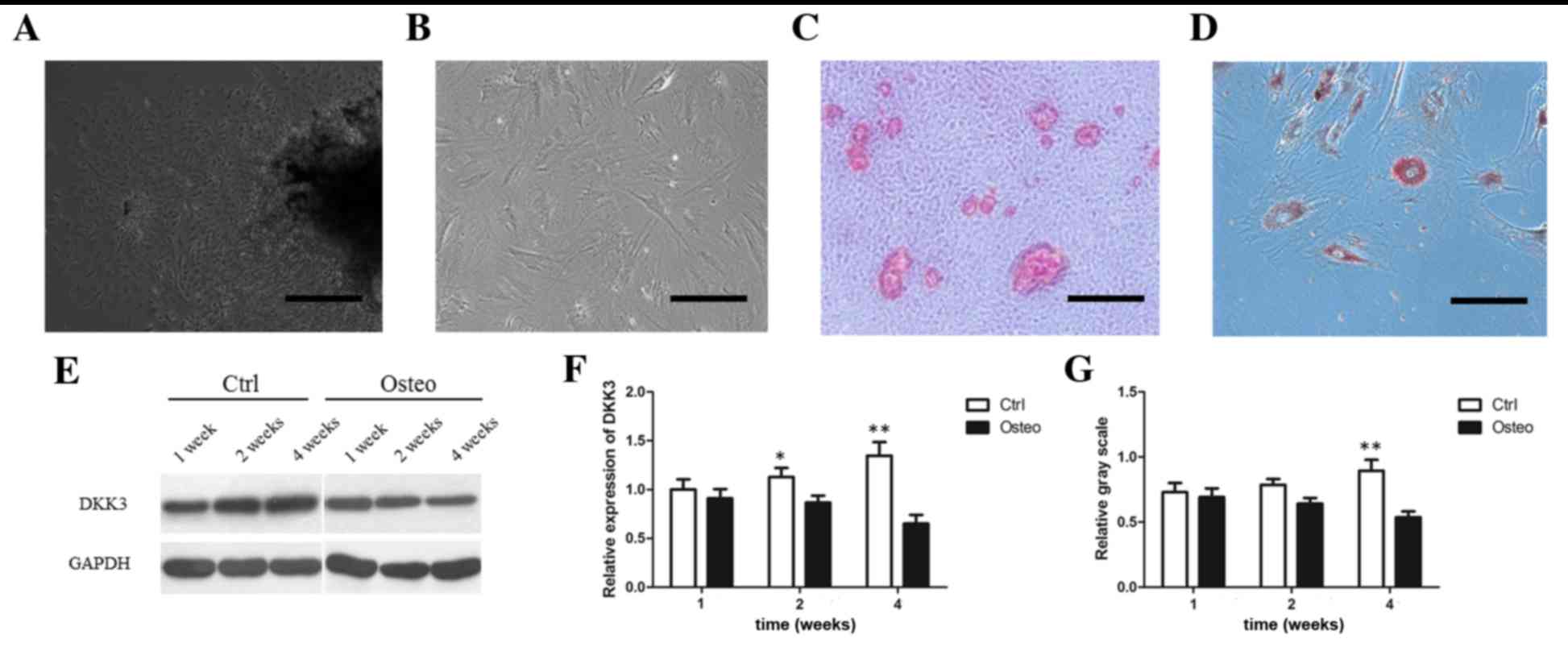 | Figure 1.DKK3 expression in DFCs during
osteogenic differentiation. (A) Primary culture of DFCs after 3
days. Scale bar, 200 µm. (B) Most DFCs at passage 3 presented
fibroblast-like features. Scale bar, 100 µm. (C) Mineral deposits
on DFCs grown in osteogenic medium for 3 weeks, as detected by
Alizarin Red staining. Scale bar, 100 µm. (D) Adipocytes stained
with Oil Red O following adipocyte induction for 4 weeks. Scale
bar, 100 µm. (E) WB analysis demonstrating DKK3 protein
downregulation following osteogenic differentiation. (F) DKK3 mRNA
downregulation following osteogenic differentiation. (G) Relative
gray scale of DKK3 expression determined following WB analysis.
*P<0.05, **P<0.01 vs. Osteo. DKK3, Dickkopf-related protein
3; DFCs, dental follicle cells; WB, western blot; Control, DFCs
grown in 10% Dulbecco's modified Eagle's medium; Osteo, DFCs grown
in mineral induction medium. |
 | Table I.Wnt polymerase chain reaction array
results of dental follicle cells cultured in mineral induction
medium or 10% Dulbecco's modified Eagle's medium for 4 weeks. |
Table I.
Wnt polymerase chain reaction array
results of dental follicle cells cultured in mineral induction
medium or 10% Dulbecco's modified Eagle's medium for 4 weeks.
|
| Average ∆∆Cq |
2−∆∆Cq | t-test | Fold up or
downregulation |
|---|
|
|
|
|
|
|
|---|
| Gene | Mineral 4
weeks | 10% DMEM 4
weeks | Mineral 4
weeks | 10% DMEM 4
weeks | P-value | Mineral 4 weeks/10%
DMEM 4 weeks |
|---|
| Apc | 5.88 | 5.68 | 1.7E-02 | 2.0E-02 | 0.716282 | −1.15 |
| Apc2 | 11.35 | 10.39 | 3.8E-04 | 7.5E-04 | 0.715525 | −1.95 |
| Axin1 | 6.93 | 6.93 | 8.2E-03 | 8.2E-03 | 0.854303 | 1.00 |
| Axin2 | 8.82 | 9.12 | 2.2E-03 | 1.8E-03 | 0.570925 | 1.23 |
| Bcl9 | 6.56 | 5.89 | 1.1E-02 | 1.7E-02 | 0.273815 | −1.59 |
| Btrc | 6.53 | 6.17 | 1.1E-02 | 1.4E-02 | 0.198065 | −1.28 |
| Ctnnb1 | 2.80 | 2.48 | 1.4E-01 | 1.8E-01 | 0.794771 | −1.25 |
| Ccnd1 | 2.20 | 2.38 | 2.2E-01 | 1.9E-01 | 0.520418 | 1.13 |
| Ccnd2 | 4.18a | 3.07a |
5.5E-02a |
1.2E-01a | 0.031100 | −2.15a |
| Ccnd3 | 4.22 | 3.92 | 5.3E-02 | 6.6E-02 | 0.719887 | −1.24 |
| Csnk1a1 | 3.29 | 2.99 | 1.0E-01 | 1.3E-01 | 0.476922 | −1.23 |
| Csnk1d | 4.32 | 4.50 | 5.0E-02 | 4.4E-02 | 0.475343 | 1.13 |
| Csnk2a1 | 4.52 | 4.48 | 4.3E-02 | 4.5E-02 | 0.940791 | −1.03 |
| Csnk2b | 3.71 | 3.69 | 7.6E-02 | 7.7E-02 | 0.940995 | −1.02 |
| Daam1 | 6.20 | 6.04 | 1.4E-02 | 1.5E-02 | 0.632151 | −1.12 |
| Dixdc1 | 8.45 | 7.99 | 2.9E-03 | 3.9E-03 | 0.971372 | −1.38 |
| Dkk1 | 11.79 | 12.24 | 2.8E-04 | 2.1E-04 | 0.414675 | 1.37 |
| Dkk3 | 3.15a | 2.21a |
1.1E-01a |
2.2E-01a |
0.049257a | −1.91a |
| Dkk4 | 7.09 | 6.59 | 7.3E-03 | 1.0E-02 | 0.299526 | −1.42 |
| Dvl1 | 6.15 | 6.02 | 1.4E-02 | 1.5E-02 | 0.955741 | −1.10 |
| Dvl2 | 4.48 | 4.26 | 4.5E-02 | 5.2E-02 | 0.426044 | −1.17 |
| Dvl3 | 7.08a | 6.09a |
7.4E-03a |
1.5E-02a |
0.011969a | −1.99a |
| Ep300 | 6.05 | 5.82 | 1.5E-02 | 1.8E-02 | 0.976422 | −1.17 |
| Fbxw11 | 4.56 | 4.29 | 4.2E-02 | 5.1E-02 | 0.585973 | −1.21 |
| Fbxw2 | 3.24 | 3.16 | 1.1E-01 | 1.1E-01 | 0.902830 | −1.06 |
| Fgf4 | 13.88 | 13.19 | 6.6E-05 | 1.1E-04 | 0.154326 | −1.61 |
| Frzb | 13.59 | 13.02 | 8.1E-05 | 1.2E-04 | 0.541255 | −1.48 |
| Fzd1 | 2.62 | 1.65 | 1.6E-01 | 3.2E-01 | 0.537557 | −1.96 |
| Fzd2 | 3.83 | 2.76 | 7.0E-02 | 1.5E-01 | 0.200846 | −2.09 |
| Fzd3 | 6.90 | 6.93 | 8.4E-03 | 8.2E-03 | 0.843733 | 1.02 |
| Fzd4 | 6.28 | 6.94 | 1.3E-02 | 8.2E-03 | 0.405254 | 1.58 |
| Fzd5 | 8.85 | 8.44 | 2.2E-03 | 2.9E-03 | 0.233498 | −1.32 |
| Fzd6 | 6.30 | 6.26 | 1.3E-02 | 1.3E-02 | 0.744854 | −1.03 |
| Fzd7 | 6.83 | 5.46 | 8.8E-03 | 2.3E-02 | 0.155697 | −2.59 |
| Fzd9 | 14.13 | 13.93 | 5.6E-05 | 6.4E-05 | 0.941213 | −1.15 |
| Gsk3a | 3.11 | 3.08 | 1.2E-01 | 1.2E-01 | 0.947278 | −1.02 |
| Gsk3b | 3.89 | 3.59 | 6.8E-02 | 8.3E-02 | 0.388341 | −1.23 |
| Jun | 5.31 | 4.74 | 2.5E-02 | 3.7E-02 | 0.579975 | −1.48 |
| Kremen1 | 5.85 | 5.63 | 1.7E-02 | 2.0E-02 | 0.540391 | −1.17 |
| Lef1 | 10.74 | 10.72 | 5.8E-04 | 5.9E-04 | 0.832187 | −1.02 |
| Lrp5 | 6.78 | 7.04 | 9.1E-03 | 7.6E-03 | 0.489102 | 1.19 |
| Lrp6 | 3.81 | 3.37 | 7.1E-02 | 9.7E-02 | 0.734114 | −1.36 |
| Mitf | 6.57 | 7.00 | 1.1E-02 | 7.8E-03 | 0.318318 | 1.35 |
| Myc | 4.65 | 5.27 | 4.0E-02 | 2.6E-02 | 0.311155 | 1.53 |
| Nkd1 | 7.24 | 6.07 | 6.6E-03 | 1.5E-02 | 0.145820 | −2.25 |
| Nkd2 | 4.78 | 4.29 | 3.6E-02 | 5.1E-02 | 0.451828 | −1.40 |
| RGD1561440 | 6.47 | 6.24 | 1.1E-02 | 1.3E-02 | 0.437063 | −1.17 |
| Pitx2 | 10.00 | 10.03 | 9.8E-04 | 9.6E-04 | 0.857792 | 1.02 |
| Porcn | 4.75 | 5.34 | 3.7E-02 | 2.5E-02 | 0.415860 | 1.50 |
| Ppp2ca | 3.52 | 3.35 | 8.7E-02 | 9.8E-02 | 0.652950 | −1.12 |
| Ppp2r1a | 2.99 | 3.10 | 1.3E-01 | 1.2E-01 | 0.611666 | 1.08 |
| Pygo2 | 6.82 | 6.55 | 8.9E-03 | 1.1E-02 | 0.917801 | −1.20 |
| Rhoa | 0.82 | 0.98 | 5.7E-01 | 5.1E-01 | 0.451892 | 1.12 |
| Senp2 | 5.13 | 4.95 | 2.9E-02 | 3.2E-02 | 0.606892 | −1.13 |
| Sfrp1 | 4.83 | 3.51 | 3.5E-02 | 8.8E-02 | 0.434107 | −2.49 |
| Sfrp2 | 6.31 | 6.01 | 1.3E-02 | 1.6E-02 | 0.470746 | −1.23 |
| Sfrp4 | 7.43 | 7.00 | 5.8E-03 | 7.8E-03 | 0.885189 | −1.34 |
| Sfrp5 | 11.47 | 11.58 | 3.5E-04 | 3.3E-04 | 0.786232 | 1.08 |
| Tcf3 | 6.38 | 6.24 | 1.2E-02 | 1.3E-02 | 0.618723 | −1.10 |
| Tcf4 | 4.21 | 4.13 | 5.4E-02 | 5.7E-02 | 0.699509 | −1.06 |
| Tcf7 | 11.27 | 10.70 | 4.1E-04 | 6.0E-04 | 0.216594 | −1.48 |
| Tcfe2a | 6.00 | 5.55 | 1.6E-02 | 2.1E-02 | 0.505454 | −1.36 |
| Tle1 | 7.21 | 6.69 | 6.8E-03 | 9.7E-03 | 0.770935 | −1.43 |
| Tle2 | 7.22 | 6.54 | 6.7E-03 | 1.1E-02 | 0.529451 | −1.60 |
| Wif1 | 7.16 | 7.56 | 7.0E-03 | 5.3E-03 | 0.328635 | 1.32 |
| Wisp1 | 2.52 | 1.95 | 1.7E-01 | 2.6E-01 | 0.586035 | −1.49 |
| Wnt1 | 13.16 | 12.49 | 1.1E-04 | 1.7E-04 | 0.374245 | −1.59 |
| Wnt10a | 13.75 | 13.27 | 7.2E-05 | 1.0E-04 | 0.948650 | −1.40 |
| Wnt10b | 13.09 | 12.90 | 1.1E-04 | 1.3E-04 | 0.748260 | −1.14 |
| Wnt11 | 8.51 | 7.61 | 2.8E-03 | 5.1E-03 | 0.287261 | −1.86 |
| Wnt2 | 11.62 | 10.83 | 3.2E-04 | 5.5E-04 | 0.373755 | −1.73 |
| Wnt2b | 10.97 | 10.77 | 5.0E-04 | 5.7E-04 | 0.634100 | −1.15 |
| Wnt3 | 10.48 | 10.23 | 7.0E-04 | 8.3E-04 | 0.564868 | −1.19 |
| Wnt3a | 6.61 | 6.06 | 1.0E-02 | 1.5E-02 | 0.540905 | −1.46 |
| Wnt4 | 8.59 | 8.88 | 2.6E-03 | 2.1E-03 | 0.869003 | 1.23 |
| Wnt5a | 3.42 | 3.69 | 9.4E-02 | 7.8E-02 | 0.588542 | 1.21 |
| Wnt5b | 5.54 | 5.31 | 2.1E-02 | 2.5E-02 | 0.402236 | −1.17 |
| Wnt6 | 15.60 | 16.84 | 2.0E-05 | 8.5E-06 | 0.332610 | 2.35 |
| Wnt7a | 17.12 | 17.92 | 7.0E-06 | 4.0E-06 | 0.415976 | 1.74 |
| Wnt7b | 7.46 | 6.70 | 5.7E-03 | 9.6E-03 | 0.243376 | −1.70 |
| Wnt8a | 14.74 | 13.88 | 3.6E-05 | 6.6E-05 | 0.276427 | −1.82 |
| Wnt8b | 16.05 | 16.15 | 1.5E-05 | 1.4E-05 | 0.502530 | 1.07 |
| Wnt9a | 8.94 | 8.93 | 2.0E-03 | 2.1E-03 | 0.497268 | −1.01 |
| Wnt9b | 9.38 | 9.38 | 1.5E-03 | 1.5E-03 | 0.642663 | 1.00 |
| Rplp1 | −2.04 | −2.43 | 4.1E+00 | 5.4E+00 | 0.058664 | −1.31 |
| Hprt1 | 4.43 | 4.61 | 4.6E-02 | 4.1E-02 | 0.526594 | 1.13 |
| Rpl13a | −2.39 | −2.18 | 5.2E+00 | 4.5E+00 | 0.372639 | 1.16 |
| Ldha | 2.88 | 2.93 | 1.4E-01 | 1.3E-01 | 0.431255 | 1.03 |
| Actb | −3.08 | −2.86 | 8.5E+00 | 7.3E+00 | 0.536666 | 1.16 |
WB and RT-qPCR analysis demonstrated that DKK3
expression was increased in a time-dependent manner in the control
groups but decreased in a time-dependent manner in
mineral-induction medium (Fig.
1E-G). After 2 weeks, DKK3 mRNA expression was decreased in
DFCs grown in mineral-induction medium compared with those grown in
routine medium (Fig. 1F;
P<0.05). After 4 weeks, DKK3 mRNA and protein expression levels
were significantly downregulated in the mineral-induction medium
group (Fig. 1F-G; P<0.01).
Expression of osteogenic markers in
DKK3-shRNA DFCs
ALP activity, mineral deposits, RT-qPCR and WB
analysis were used to analyze osteogenic markers. ALP activity was
upregulated in DKK3-shRNA DFCs cultured for 7, 14 or 21 days,
compared with in the vector-infected DFCs (Fig. 2A). In addition, following mineral
induction for 3 weeks, DKK3-shRNA DFCs exhibited greater mineral
deposition by Alizarin Red staining (Fig. 2B-E). RT-qPCR results demonstrated
that Col-I and ALP levels were upregulated in DKK3-shRNA DFCs grown
in mineral-induction medium for 1 or 3 weeks (Fig. 2F and G). The WB analysis results
indicated that nuclear β-catenin, RUNX2, OCN and total β-catenin
expression were increased, while DKK3 expression was inhibited in
DKK3-shRNA DFCs following growth in mineral-induction medium for 1
or 3 weeks (Fig. 2H-N). These
findings indicated that DKK3 suppression may promote osteogenic
differentiation-marker expression in DFCs.
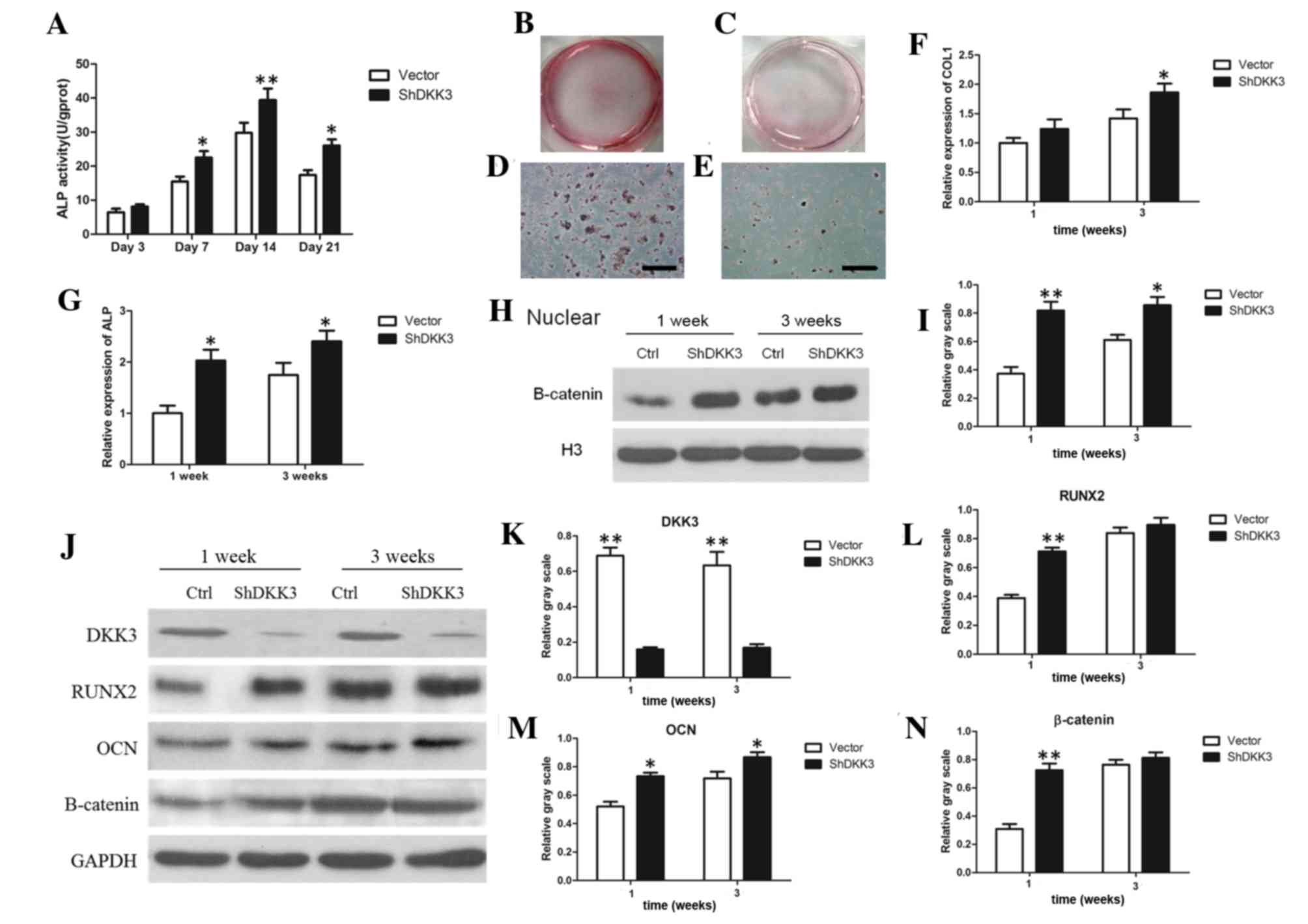 | Figure 2.Osteogenic differentiation markers
analysis in DKK3-shRNA DFCs in vitro. (A) ALP activity of
DKK3-shRNA DFCs was enhanced compared with in vector-infected DFCs
between days 7 and 21. (B) General view of mineralized nodules of
DKK3-shRNADFCs following growth in mineral-induction medium for 3
weeks. (C) General view of mineralized nodules of vector-infected
DFCs following mineral induction for 3 weeks. (D) Calcified nodules
of DKK3-shRNADFCs under microscopy. Scale bar, 200 µm. (E)
Calcified nodules of vector-infected DFCs under microscopy. Scale
bar, 200 µm. (F) mRNA expression of Col-I was upregulated in
DKK3-shRNA DFCs grown in mineral-induction medium. (G) mRNA
expression of ALP was upregulated in DKK3-shRNA DFCs grown in
mineral-induction medium. (H) WB analysis demonstrating nuclear
β-catenin expression inDKK3-shRNADFCs grown in mineral-induction
medium. (I) Relative gray scale of WB analysis results in (H). (J)
WB analysis of DKK3, RUNX2, OCN and β-catenin expression in
DKK3-shRNADFCs grown in mineral-induction medium. (K-N) Relative
gray scale of WB analysis results in (J). *P<0.05, **P<0.01
vs. vector-infected DFCs. ALP, alkaline phosphatase; DKK3,
Dickkopf-related protein 3; ShRNA/sh, short hairpin RNA; DFCs,
dental follicle cells; Col-I, collagen-type-I; WB, western blot;
RUNX2, runt-related transcription factor 2; OCN, osteocalcin. |
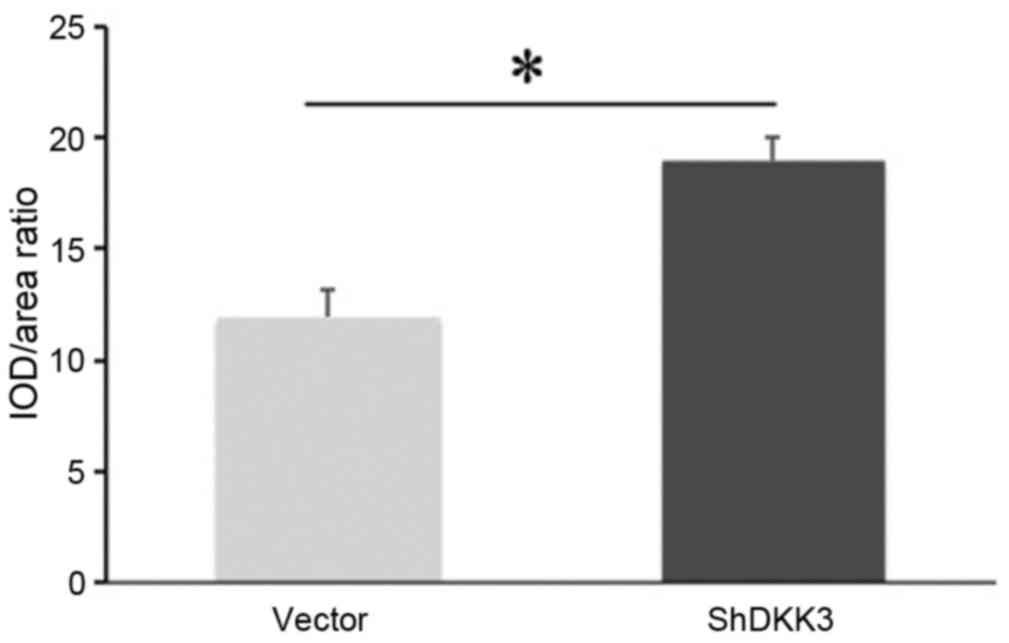
Mineralized capability of DKK3-shRNA
DFCs in vivo
The DKK3-shRNA DFCs and vector-infected DFCs were
successfully implanted in SCID mice. At 5 weeks post-implantation,
H&E staining demonstrated that DKK3-shRNA DFCs with HA/TCP
scaffolds formed new collagen fibers and vessels (Fig. 3A-D). Similarly, the Masson staining
demonstrated that DKK3-shRNA DFCs with HA/TCP formed more
mineralized matrices and collagen compared with the control group
(Fig. 3E-H), indicating that low
expression of DKK3 enhanced osteogenic differentiation in
vivo. The quantitative analysis also indicated that the
mineralization capabilities of the DKK3-shRNA DFCs with HA/TCP were
higher than those of the control group (Fig. 4).
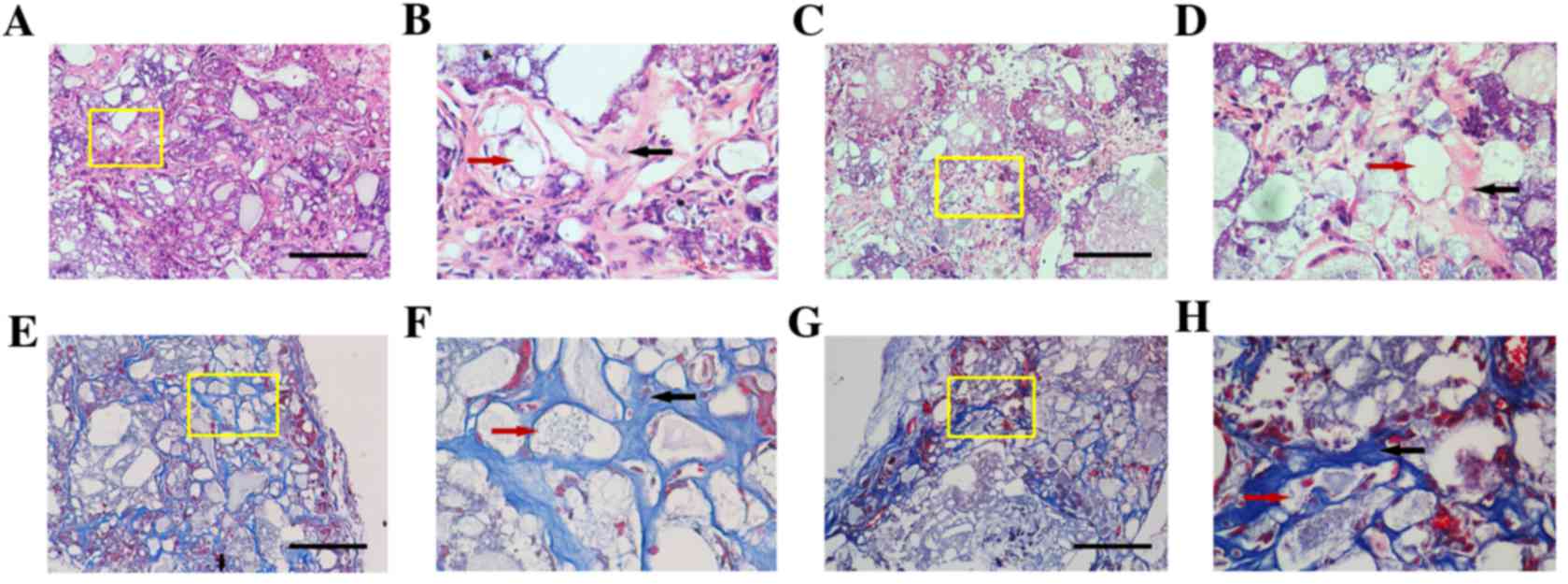 | Figure 3.H&E and Masson staining of
DKK3-shRNA DFCs and vector-infected DFCs. Representative images
obtained following (A-D) H&E and (E-H) Masson staining. (A) A
marked amount of mineralized matrices were deposited on the implant
of DKK3-shRNA DFCs. Scale bar, 100 µm. (B) Local magnification of
yellow box in A indicating limited space occurring between the
HA/TCP (red arrow) and matrices. Collagen fibers and vasculogenesis
(black arrow) are apparent. (C) Vector-infected DFCs presented the
formation of numerous semi-bone matrices. Scale bar, 100 µm. (D)
Local magnification of yellow box in C demonstrated that
vasculogenesis and collagen fiber (black arrow) formation around
the cells and HA/TCP (red arrow) were weak. (E) Newly formed blue
osteoid matrices and collagen in DKK3-shRNA DFCs. Scale bar, 100
µm. (F) Local magnification of yellow box in E indicating
mineralized matrices, collagen, newly formed vessels (black arrow)
and HA/TCP (red arrow) constituted a network in the implants. (G)
Vector-infected DFCs formed loose osteoid matrices and collagen.
Scale bar, 100 µm. (H) Local magnification of yellow box in G
indicating numerous intervals were formed in the osteoid matrix
(black arrow) around HA/TCP (red arrow). H&E, hematoxylin and
eosin; DKK3, Dickkopf-related protein 3; shRNA, short hairpin RNA;
DFCs, dental follicle cells; HA/TCP, hydroxyapatite/β-tricalcium
phosphate. |
Discussion
Wnt pathway activation serves an essential function
in tooth development and regeneration. The roles of specific Wnt
genes have been studied by monitoring their expression levels and
subcellular localization. Numerous Wnt-associated genes have been
implicated in early tooth formation. For example, at the initiation
stage, Wnt10b is expressed specifically in molar and incisor dental
epithelial thickenings, whereasWnt5a is expressed in mesenchymal
cells (31). Dental epithelial and
mesenchymal Wnt/β-catenin signaling is suppressed by forced
epithelial expression of the secreted Wnt inhibitor DKK1, followed
by arrested tooth development at the early bud stage (32). At the early cap stage, lymphoid
enhancer binding factor 1, Wnt3, Wnt6, Wnt10b and MFz6 are
expressed in the primary enamel knot, whereas Wnt5a and MFrzb1 are
detected in the dental papilla mesenchyme (31). Our previous studies demonstrated
that, in DFCs, β-catenin expression increased in a time-dependent
manner, andWnt5a is expressed in DFCs in postnatal rats (9,12).
These observations suggested that the effects of Wnt family
proteins on tooth formation should be focused on; however, the
associated Wnt-associated gene has yet to be identified, therefore
total genes or proteins should be screened in future studies.
At present, proteomics and DNA microarrays
facilitate the study of the genome-wide transcriptome of human
cells. Several studies have investigated signaling molecule
activation during the osteogenic differentiation of dental-derived
cells (33–36). A DNA microarray study previously
reported that Wnt-associated genes are differentially expressed in
differentiated DFCs (36). Since
Wnt-associated gene expression may be limited during DFC
differentiation, it suggests that the expression of certain genes
may not be high enough to be detected in a total gene microarray
assay. Therefore, a Wnt signaling pathway PCR array profile was
used in the present study to investigate a panel of genes
associated with Wnt-mediated signal transduction. It was
demonstrated that the DKK3 gene was downregulated in DFCs upon
mineral induction, supplying a novel target to control the
osteogenic differentiation of DFCs. Notably, a recent microarray
study of in vivo-implanted C3H10T1/2 cells expressing BMP2
also implicated DKK3 as a pivotal gene in endochondral bone
formation (37). Consequently, the
osteogenic differentiation of DFCs by controlling the expression of
DKK3 was focused on in subsequent experiments.
As a secreted protein in the Dickkopf family, DKK3
serves an essential function in early embryonic development.
Amphioxus DKK3 may regulate head formation by inhibiting
Wnt/β-catenin and Nodal/Vg1 signaling, and these functions may have
been partitioned among various vertebrate lineages during the
evolution of DKK3 proteins (38).
DKK3 expression has also been demonstrated o occur in the dental
mesenchyme of embryonic day12 mouse embryos (39). Another report claimed that the
DKK1-3 genes exhibited distinct spatiotemporal expression. During
molar morphogenesis, DKK3 was specifically expressed in the primary
and secondary enamel knots, whereas postnatal DKK3 mRNA expression
was transiently observed in preameloblasts prior to the onset of
enamel matrix secretion (29). In
the present study, DKK3 expression in DFCs cultured in 10% DMEM or
mineral-induction medium for 1, 2 or 4 weeks was explored. DKK3
mRNA expression was decreased at 2 weeks, whereasDKK3 protein
decreased after 4 weeks in DFCs cultured in mineral-induction
medium. In a previous study, DFCs were collected from postnatal
rats, during which period DKK3 expression was not detected in the
mesenchyme (29). Therefore, it
may be concluded that low DKK3 expression may be associated with
the osteogenic differentiation of DFCs in a time-dependent
manner.
Based on the shRNA experiments in the present study,
silencing DKK3 expression in DFCs led to enhanced formation of
calcified nodules, ALP activity and osteogenic marker expression.
WB analysis of DKK3-shRNA DFCs following mineral induction revealed
that total β-catenin was upregulated at 1 week, whereas nuclear
β-catenin was increased at 1 and 3 weeks. Since the Wnt canonical
pathway is activated by nuclear β-catenin accumulation, it is
possible that low DKK3 expression activated the canonical
Wnt/β-catenin pathway, thereby regulating downstream target genes,
including RUNX2 and OCN. In a previous study,
osteoblast/cementoblast differentiation could be promoted by adding
a canonical Wnt/β-catenin activator to DFCs (9). However, in murine SVF4 DFCs,
Wnt3a-dependent β-catenin activation inhibited BMP2-mediated
induction of cementoblast/osteoblast differentiation (40), which indicated that canonical Wnt
signaling may inhibit the osteogenic differentiation of DFCs. It
was hypothesized that this discrepancy may be caused by the source
and type of DFCs, since the SVF4 cell line is an immortalized cell
line, whereas rat DFCs were collected from primary cultures in the
present study. Furthermore, DKK3 is an effective inhibitor of the
canonical Wnt pathway; therefore, the positive effect of
Wnt/β-catenin signaling on the osteogenic differentiation of DFCs
was demonstrated.
To further confirm the aforementioned hypothesis,
the osteogenic differentiation of DKK3-shRNA DFCs was completed in
a SCID mouse model. Biphasic calcium phosphate bioceramics have
been developed for tissue engineering applications, with various
ratios of β-TCP and HA biphasic calcium phosphates (41). In the present study, the HA/TCP
scaffold (HA/TCP=1:8; 1 mm) was loaded on DFCs in order to observe
their osteogenic differentiation in vivo. Previous studies
have demonstrated that DFCs acquired cementoblast or
osteoblast-like features under BMP2 stimulation (4,5).
However, DKK3 and BMP2 co-expression in implanted C3H10T1/2 cells
significantly impaired bone formation, compared with cells
expressing only BMP2 (37). These
findings indicated that DKK3 may inhibit osteogenesis. In a similar
manner, the shRNA-DKK3 DFCs treated with HA/TCP injected into the
SCID mice presented increased osteoid matrices and collagen
compared with control DFCs, also indicating that DKK3 is a negative
regulator of osteogenesis. However, the small number of samples
used was a limitation of the present study, since the expression of
osteoblast markers, including RUNX2, could not be analyzed
quantitatively.
In conclusion, the results presented in the current
study indicated that DKK3 is a negative regulator during the
osteogenic differentiation of DFCs and, conversely, that DKK3
downregulation may enhance DFC osteogenesis.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant nos. 81170932 and 81300846), the
Science and Technology Research Fund of Guangdong Province (grant
no. B2013153) and the Science and Technology Planning Project of
Guangdong Province (grant no. 2013B021800058).
References
|
1
|
Ten Cate AR: The development of the
periodontium-a largely ectomesenchymally derived unit.
Periodontology 2000. 13:9–19. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wise GE, Lin F and Fan W: Culture and
characterization of dental follicle cells from rat molars. Cell
Tissue Res. 267:483–492. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morsczeck C, Götz W, Schierholz J,
Zeilhofer F, Kühn U, Möhl C, Sippel C and Hoffmann KH: Isolation of
precursor cells (PCs) from human dental follicle of wisdom teeth.
Matrix Biol. 24:155–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kemoun P, Laurencin-Dalicieux S, Rue J,
Farges JC, Gennero I, Conte-Auriol F, Briand-Mesange F, Gadelorge
M, Arzate H, Narayanan AS, et al: Human dental follicle cells
acquire cementoblast features under stimulation by BMP-2/−7 and
enamel matrix derivatives (EMD) in vitro. Cell Tissue Res.
329:283–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao M, Xiao G, Berry JE, Franceschi RT,
Reddi A and Somerman MJ: Bone morphogenetic protein 2 induces
dental follicle cells to differentiate toward a
cementoblast/osteoblast phenotype. J Bone Miner Res. 17:1441–1451.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Viale-Bouroncle S, Bey B, Reichert TE,
Schmalz G and Morsczeck C: β-tricalcium-phosphate stimulates the
differentiation of dental follicle cells. J Mater Sci Mater Med.
22:1719–1724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo W, Gong K, Shi H, Zhu G, He Y, Ding B,
Wen L and Jin Y: Dental follicle cells and treated dentin matrix
scaffold for tissue engineering the tooth root. Biomaterials.
33:1291–1302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang B, Chen G, Li J, Zou Q, Xie D, Chen
Y, Wang H, Zheng X, Long J, Tang W, et al: Tooth root regeneration
using dental follicle cell sheets in combination with a dentin
matrix-based scaffold. Biomaterials. 33:2449–2461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du Y, Ling J, Wei X, Ning Y, Xie N, Gu H
and Yang F: Wnt/β-catenin signaling participates in
cementoblast/osteoblast differentiation of dental follicle cells.
Connect Tissue Res. 53:390–397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khan M, Seppala M, Zoupa M and Cobourne
MT: Hedgehog pathway gene expression during early development of
the molar tooth root in the mouse. Gene Expr Patterns. 7:239–243.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Viale-Bouroncle S, Gosau M and Morsczeck
C: NOTCH1 signaling regulates the BMP2/DLX-3 directed osteogenic
differentiation of dental follicle cells. Biochem Biophys Res
Commun. 443:500–504. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiang L, Chen M, He L, Cai B, Du Y, Zhang
X, Zhou C, Wang C, Mao JJ and Ling J: Wnt5a regulates dental
follicle stem/progenitor cells of the periodontium. Stem Cell Res
Ther. 5:1352014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones WM and Bejsovec A: Wingless
signaling: An axin to grind. Curr Biol. 13:R479–R481. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McEwen DG and Peifer M: Wnt signaling:
Moving in a new direction. Curr Biol. 10:R562–R564. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JA, Choi HK, Kim TM, Leem SH and Oh
IH: Regulation of mesenchymal stromal cells through fine tuning of
canonical Wnt signaling. Stem Cell Res. 14:356–368. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu F and Millar SE: Wnt/beta-catenin
signaling in oral tissue development and disease. J Dent Res.
89:318–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scheller EL, Chang J and Wang CY:
Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J
Dent Res. 87:126–130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian H, Lv P, Ma K, Zhou C and Gao X:
Beta-catenin/LEF1 activated enamelin expression in ameloblast-like
cells. Biochem Biophys Res Commun. 398:519–524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yokoyama N, Golebiewska U, Wang HY and
Malbon CC: Wnt-dependent assembly of supermolecular
Dishevelled-3-based complexes. J Cell Sci. 123:3693–3702. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moschovi M, Alexiou GA, Patereli A, Siozos
G, Sfakianos G, Prodromou N and Stefanaki K: Immunohistochemical
expression of cell-cycle regulators in pediatric embryonal brain
tumors. J Neurooncol. 109:529–534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krupnik VE, Sharp JD, Jiang C, Robison K,
Chickering TW, Amaravadi L, Brown DE, Guyot D, Mays G, Leiby K, et
al: Functional and structural diversity of the human Dickkopf gene
family. Gene. 238:301–313. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niehrs C: Function and biological roles of
the Dickkopf family of Wnt modulators. Oncogene. 25:7469–7481.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsuji T, Miyazaki M, Sakaguchi M, Inoue Y
and Namba M: A REIC gene shows down-regulation in human
immortalized cells and human tumor-derived cell lines. Biochem
Biophys Res Commun. 268:20–24. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsieh SY, Hsieh PS, Chiu CT and Chen WY:
Dickkopf-3/REIC functions as a suppressor gene of tumor growth.
Oncogene. 23:9183–9189. 2004.PubMed/NCBI
|
|
25
|
Kurose K, Sakaguchi M, Nasu Y, Ebara S,
Kaku H, Kariyama R, Arao Y, Miyazaki M, Tsushima T, Namba M, et al:
Decreased expression of REIC/Dkk-3 in human renal clear cell
carcinoma. J Urol. 171:1314–1318. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roman-Gomez J, Jimenez-Velasco A, Agirre
X, Castillejo JA, Navarro G, Barrios M, Andreu EJ, Prosper F,
Heiniger A and Torres A: Transcriptional silencing of the
Dickkopfs-3 (Dkk-3) gene by CpGhypermethylation in acute
lymphoblastic leukaemia. Br J Cancer. 91:707–713. 2004.PubMed/NCBI
|
|
27
|
Lodygin D, Epanchintsev A, Menssen A,
Diebold J and Hermeking H: Functional epigenomics identifies genes
frequently silenced in prostate cancer. Cancer Res. 65:4218–4227.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuphal S, Lodermeyer S, Bataille F,
Schuierer M, Hoang BH and Bosserhoff AK: Expression of Dickkopf
genes is strongly reduced in malignant melanoma. Oncogene.
25:5027–5036. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fjeld K, Kettunen P, Furmanek T,
Kvinnsland IH and Luukko K: Dynamic expression of Wnt
signaling-related Dickkopf1, −2 and −3 mRNAs in the developing
mouse tooth. Dev Dyn. 233:161–166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C (T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sarkar L and Sharpe PT: Expression of Wnt
signalling pathway genes during tooth development. Mech Dev.
85:197–200. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu F, Chu EY, Watt B, Zhang Y, Gallant
NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, et al:
Wnt/beta-catenin signaling directs multiple stages of tooth
morphogenesis. Dev Biol. 313:210–224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei X, Wu L, Ling J, Liu L, Liu S, Liu W,
Li M and Xiao Y: Differentially expressed protein profile of human
dental pulp cells in the early process of odontoblast-like
differentiation in vitro. J Endod. 34:1077–1084. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu L, Wei X, Ling J, Liu L, Liu S, Li M
and Xiao Y: Early osteogenic differential protein profile detected
by proteomic analysis in human periodontal ligament cells. J
Periodontal Res. 44:645–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Morsczeck C, Petersen J, Völlner F,
Driemel O, Reichert T and Beck HC: Proteomic analysis of osteogenic
differentiation of dental follicle precursor cells.
Electrophoresis. 30:1175–1184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morsczeck C, Schmalz G, Reichert TE,
Völlner F, Saugspier M, Viale-Bouroncle S and Driemel O: Gene
expression profiles of dental follicle cells before and after
osteogenic differentiation in vitro. Clin Oral Investig.
13:383–391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aslan H, Ravid-Amir O, Clancy BM,
Rezvankhah S, Pittman D, Pelled G, Turgeman G, Zilberman Y, Gazit
Z, Hoffmann A, et al: Advanced molecular profiling in vivo detects
novel function of dickkopf-3 in the regulation of bone formation. J
Bone Miner Res. 21:1935–1945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Onai T, Takai A, Setiamarga DH and Holland
LZ: Essential role of Dkk3 for head formation by inhibiting
Wnt/β-catenin and Nodal/Vg1 signaling pathways in the basal
chordate amphioxus. Evol Dev. 14:338–350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Monaghan AP, Kioschis P, Wu W, Zuniga A,
Bock D, Poustka A, Delius H and Niehrs C: Dickkopf genes are
co-ordinately expressed in mesodermal lineages. Mech Dev. 87:45–56.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Silvério KG, Davidson KC, James RG, Adams
AM, Foster BL, Nociti FH Jr, Somerman MJ and Moon RT: Wnt/β-catenin
pathway regulates bone morphogenetic protein (BMP2)-mediated
differentiation of dental follicle cells. J Periodontal Res.
47:309–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jensen SS, Bornstein MM, Dard M, Bosshardt
DD and Buser D: Comparative study of biphasic calcium phosphates
with different HA/TCP ratios in mandibular bone defects. A
long-term histomorphometric study in minipigs. J Biomed Mater Res B
Appl Biomater. 90:171–181. 2009.PubMed/NCBI
|