Introduction
Cancer is the second leading cause of mortality in
developing countries (1). Despite
the advances in cancer biology and cancer therapeutics, most of the
currently available anticancer drugs are characterized by
immunosuppressive and cytotoxic adverse effects (2). Doxorubicin (DOX) is widely used in
the treatment of solid tumors and hematological malignancies.
Despite its extensive and longstanding clinical applications, its
use is associated with serious adverse effects, which include
cardiotoxicity and hematopoietic suppression (3,4).
However, the mechanism of DOX-induced cytotoxicity remains to be
elucidated. Reactive oxygen species (ROS) are involved in various
processes of DOX metabolism, including the redox cycling of its
quinone moiety, the disturbance of iron metabolism and the
production of DOX metabolites in myocardial tissue (5). DOX can directly induce platelet
cytotoxicity by stimulating the generation of ROS, decreasing
glutathione levels, and depleting protein thiol groups (6). The potentiation of ROS production and
the depletion of endogenous antioxidants can trigger the intrinsic
apoptotic pathway in hematopoietic cells and cardiomyocytes
(7). Therefore, oxidative stress
appears to hold a key role in DOX-induced myelosuppression and
cardiotoxicity.
The deleterious effects associated with ROS
production could be counteracted with antioxidants (7). Antioxidants and free radical
scavengers can prevent oxidative tissue damage by directly
neutralizing reactive hydroxyl and peroxy radicals, or through
regulating ROS signaling involved in gene expression and enzymatic
cascades (8). Various antioxidants
have been identified, including preventative antioxidants, such as
superoxide dismutase and catalase, and lipid peroxidation blockers,
such as vitamins C and E (9). In
addition, some herbs, such as ginseng, astragalus, poria and
notoginseng, have exhibited significant antioxidant potential
(10). Nevertheless, to the best
of our knowledge, the use of antioxidants in counteracting the
deleterious actions of DOX has yet to be investigated. Therefore,
the identification of an alternative, safe and widely available
treatment is required to mitigate the pathophysiological effects of
DOX.
Previous studies have investigated the potential of
natural products in minimizing the toxic effects of
chemotherapeutic agents on healthy cells, without compromising
their antineoplastic activity (11). SYKT is a natural medicine
originating from an ancient prescription of the Dai nationality in
Southwest China. SYKT is composed of eight primary ingredients,
including sanguis draconis, radix et rhizoma notoginseng, radix et
rhizoma glycyrrhizae, radix angelicae sinensis, ginger, Rhizoma
Dioscoreae, Poria cocos, and Fructus Amomi. SYKT has been
demonstrated to significantly improve the condition of anemic
patients (12). Previous
pharmacological and toxicological studies, as well as clinical
trials, have revealed that SYKT has no toxicity or negative adverse
effects (13). It has previously
been demonstrated that SYKT can potentiate hematopoiesis and the
function of the immune system; in addition, it can decrease
chemotherapy- and radiotherapy-induced toxicity (13). Therefore, it may be hypothesized
that SYKT can prevent chemotherapy-induced toxicity due to its
antioxidant and cytoprotective properties. The present study
evaluated the potential of SYKT administration as a protective
strategy against DOX-induced myelosuppression and cardiotoxicity,
and investigated the underlying mechanism using mouse models.
Materials and methods
Materials
DOX was purchased from Shenzhen Main Luck
Pharmaceuticals Inc. (Shenzhen, China) and dissolved in PBS. SYKT
(1.02 g/ml) containing sanguis draconis, radix et rhizoma
notoginseng, radix et rhizoma glycyrrhizae, radix angelicae
sinensis, ginger, Rhizoma Dioscoreae, Poria cocos and Fructus
Amomi., was purchased from Great Tao Pharmaceutical Co., Ltd
(Yunnan, China). A mixture of Cremophor RH40 (BASF SE,
Ludwigshafen, Germany) and dehydrated alcohol (1:1, w/w) was used
to dissolve vitamin E (d, l-a-tocopherol; BASF SE) to a stock
concentration. Further dilutions were made with water prior to
injection at a concentration corresponding to 100 IU/kg for each
animal. All drugs were prepared immediately prior to use, with
sterile solvents and under sterile conditions. Mouse lymphoma (EL4)
cells were obtained from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China). Anti-CD34 (catalogue no. 551387) and
CD44 antibodies (catalogue no. 561859) were purchased from BD
Biosciences (Franklin Lakes, NJ, USA). A terminal deoxynucleotidyl
transferase dUTP nick-end labeling (TUNEL) apoptosis assay kit was
purchased from Merck KGaA (Darmstadt, Germany). ROS detection
reagents were purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA).
Animals
Inbred female C57BL/6J mice (age, 8–9 weeks; weight
20–25 g) were purchased from Dashuo Laboratory Animal Technology
(Chengdu, China). The mice were housed in well-ventilated cages (5
mice per cage) under standard room temperature, pressure and
humidity conditions. The animals were provided with free access to
normal mouse chow and water. All experiments and procedures
involving animals and their care were conducted in conformity with
NIH guidelines (NIH Pub. No. 85–23, revised 1996) and were approved
by Animal Care and Use Committee of Kunming Medical College
(Kunming, China).
Mortality analysis
DOX was dissolved in normal saline prior to
injection in mice. A total of 24 mice were randomly assigned into
four groups (n=6/group). Mice in the SYKT group received SYKT (1.2
ml/kg/d) for 5 days, in a cumulative dosing scheme resembling its
clinical use. Mice in the DOX group received a single dose of DOX
(20 mg/kg, subclinical lethal dose) on day 5. Mice in the SYKT/DOX
group received both SYKT and DOX as aforementioned. Control mice
received normal saline. Mice in the control group were gavaged with
normal saline at an equal volume to the SYKT solution during day 1
to day 5, and treated with normal saline at an equal volume to the
DOX solution on day 5 via peritoneal perfusion. Mortality was
monitored daily.
Subgrouping and drug
administration
A total of 24 mice were randomly assigned into four
groups (n=6/group) and were treated as follows. The control group
received normal saline at an equal volume to the SYKT solution on
day 1, 3 and 5; and treated with normal saline at an equal volume
to DOX solution on day 2, 4 and 6 via peritoneal perfusion. The
SYKT group received SYKT via gavage at a dose of 1.2 ml/kg/d on
days 1, 3 and 5. In the DOX group, the mice were injected
intraperitoneally with DOX at a dose of 3 mg/kg (14) on days 2, 4 and 6. This cumulative
dose (9 mg/kg) was equivalent to 630 mg for a 70-kg human male,
just above the threshold at which DOX cardiomyopathy is expected to
occur in a clinical setting (15).
The SYKT/DOX group received SYKT via gavage at a dose of 1.2
ml/kg/d on days 1, 3 and 5, and DOX at a dose of 3 mg/kg/d (i.p.)
on days 2, 4 and 6. The mice were sacrificed at different times
according to the experimental plan. Whole blood samples were
collected, and the femur bone was isolated to obtain bone marrow
cells. The cardiac tissue was fixed in 10% formalin for
histopathological examination or stored at −80°C for further
analysis.
Blood cell test
A 10 µl blood sample was drawn from the tail vein of
each mouse into an EDTA-coated capillary tube prior to
administration (day 0), and day 5, 10, 15, 20 and 30 following
administration. The samples were mixed with 10 ml PBS. Peripheral
white blood cells (WBCs), red blood cells (RBCs) and platelets
(PLTs) were counted using a hematometer with an optical
microscope.
Bone marrow cells analysis
Bone marrow cells were flushed from the bilateral
femurs after mice were sacrificed. Following two washes with PBS,
bone marrow cells were collected. After erythrocytes were lysed
with RBC lysis buffer (BioLegend, Inc., San Diego, CA, USA), total
bone marrow cell viability was assessed using trypan blue. Bone
marrow cells were labeled with anti-CD34 and anti-CD44 antibodies
(1:1,000), which are markers of hematopoietic stem cells,
mesenchymal stem cells and other cell types in the bone marrow, by
incubating the mixture at 4°C for 30 min. Results were analyzed
using a BD FACSCalibur (BD Biosciences). All antibodies and dyes
were purchased from BD Biosciences.
Myocardial enzymes
Biochemical tests were used to detect aspartate
aminotransferase (AST), creatine kinase (CK), CK-MB and lactate
dehydrogenase (LDH) levels. Blood sampling was performed via
orbital sinus puncture while the animals were under light
diethylether anesthesia (common concentration of diethylether:
2–4%) on day 15 following drug delivery, then placed in safe-lock
centrifuge tubes (1.5 ml) and maintained for 1 h at room
temperature to allow spontaneous coagulation. The segregated sera
were extracted via collection in a 1.5 ml test tube, separated by
centrifugation at 1,006 × g for 15 min, stored as 50 µl aliquots at
−20°C and assayed within 72 h. The serum parameters were analyzed
using an Aeroset Clinical Chemistry Analyzer (Abbott Pharmaceutical
Co., Ltd, Lake Bluff, IL, USA). The activity measurements were
expressed in international units of enzyme activity per liter
(IU/L) of serum. The reference change limits were obtained from the
sera of 30 untreated mice.
Myocardial histological analysis
The mice were sacrificed with an overdose of sodium
pentobarbital (45 mg/kg) and hearts were removed. The right and
left ventricles were transected into sections parallel to the
atrioventricular sulcus according to the method of heart
preparation (16) and placed in
10% buffered formalin for 24 h at room temperature. Further
fixation of the tissue and paraffin embedding were carried out
according to standard procedures (17). The obtained specimens were stained
with hematoxylin-eosin and observed with an optimal microscope. The
degree of myocardial damage was scored according to rules described
by Billingham et al (18)
as follows: Grade 0, cells show no anthracycline (DOX) damage;
grade 0.5, the myocardium is not completely normal but no
anthracycline-specific changes are evident; grade 1.0, a few cells
(<5%) have myofibrillar loss or distended sarcoplasmic
reticulum, or both; grade 1.5, small groups of cells (5–15%)
exhibit anthracycline effects consisting of marked myofibrillar
loss or cytoplasmic vacuolization, or both; grade 2.0, 16–25% of
cells demonstrate the aforementioned alterations; grade 2.5, 26–35%
of cells demonstrate the aforementioned alterations; grade 3.0,
specimens exhibit diffuse cell damage with >35% of cells
exhibiting pathologic changes, the loss of contractile elements and
organelles, and mitochondrial and nuclear degeneration. The tissue
specimens were analyzed by three pathologists in a blind manner.
The results were recorded as the median of the three independent
scores for each animal. A total of six animals per group were
included. A statistical evaluation was performed using the
Kruskal-Wallis test.
Measurement of intracellular ROS
A different group of mice (6 mice in each group)
were treated with DOX combined with d, l-a-Tocopherol (vitamin E).
Vitamin E was used as a ROS scavenger positive control (19). Vitamin E was orally administered at
a 0.2 ml bolus dose on days 1, 3 and 5, and DOX was administered at
a dose of 3 mg/kg (i.p.) on days 2, 4 and 6. Day 15 post-treatment
was selected as the most appropriate time point for further
investigation due to myelotoxicity and cardiotoxicity being the
most significant at this time following treatment with DOX. The
heart and bilateral femur bones were removed after sacrificing the
animals; a single-cell suspension of bone marrow and myocardial
cells was obtained. The mice were immersed in 75% ethanol for 3–5
min following sacrifice. The four limbs were isolated while keeping
the humerus and femurs intact. The two ends of the epiphysis were
broken to expose the bone marrow cavity. The bone marrow cavity was
rinsed with DMEM culture medium to suspend the bone marrow. The
myocardial cell suspension was then prepared with neonatal mice,
with procedures briefly described as follows. The isolated heart
was placed on a petri dish containing serum-free DMEM culture
medium, to clean the stained blood and remove the base of the heart
and epicardial connective tissue. The ventricular muscle was cut
into 0.5–1 mm3 sized pieces and digested with PBS containing 0.125%
Trypsin. The medium was mixed and the culture flask was placed in a
CO2-enriched incubator at 37°C for 5–7 min, followed by
mixing with suction tubes. The supernatant was moved into a
centrifugation tube and an equal volume of 15% NBC-DMEM culture
medium was added to prevent digestion. Undigested tissue blocks
were further treated by addition of 0.06% Trypsin. Tissue blocks
were generally digested following a repeated digestion of 3–4
times. The digested solution and cell suspension were filtered
using stainless steel 100-mesh and then collected, followed by
centrifugation at 335 × g for 5–7 min. The supernatant was removed
and the precipitated section was added to a DMEM culture medium
containing 15% BSA. The myocardial cell suspension was ready
following gentle mixing. The cells were incubated with
2′,7′-dichlorofluorescin diacetate (DCFDA; 10 mM) for 1 h at 37°C
in the dark, followed by an immediate wash in PBS. Intracellular
ROS production was detected using the intensity of fluorescence of
the oxidant sensitive probe DCFDA. The intensity of emitted
fluorescence was measured using flow cytometry at a wavelength of
525 nm.
Assessment of bone marrow and
myocardial cell apoptosis
Cells (1–2×106) were harvested and fixed in 1%
paraformaldehyde at room temperature for 30 min. The cells were
washed in PBS containing 0.1 M glycine, resuspended in ice-cold 70%
ethanol, and stored overnight at −20°C. To detect apoptosis, the
cells were washed in PBS and resuspended in a 50 µl reaction
mixture containing 0.1 mM dithiothreitol, 0.05 mg/ml bovine serum
albumin (BSA) (Roche Diagnostics GmbH, Mannheim, Germany), 2.5 mM
CoCl2, 0.4 mM biotin-16-dUTP, and 0.1 U/ml terminal
deoxynucleotidyl transferase in 0.1 M sodium cacodylate buffer (pH
7.0). The mixture was incubated at 37°C for 30 min. The cells were
then washed in PBS and resuspended in 100 µl staining buffer
containing 2.5 mg/ml fluoresceinated avidin, 4X concentrated
saline-sodium citrate buffer, 0.1% Triton X-100 and 5% (w/v)
low-fat dried milk. The cells were incubated for 30 min at room
temperature in the dark and then rinsed twice in PBS prior to flow
cytometry (FACScan; BD Biosciences). An excitation wavelength of
488 nm was obtained using a 15-mW air-cooled argon ion laser.
Fluorescence emission was collected through a 530/30 band-pass
filter for fluorescein isothiocyanate (FITC) and a 585/42 band-pass
filter for phycoerythrin (PE). Both FITC and PE fluorescence data
were collected on a linear scale (FlowCytomixPro Software, Version
2.1, Bender MedSystems GmbH, Vienna, Austria).
Measurement of tumor volume and
apoptosis in mouse solid tumor xenograft model
The cell suspension was cultured in 1640 medium
containing 10% BSA and 10% penicillin and streptomycin. Mouse
lymphoma (EL4) cells (1×106) were then subcutaneously injected into
the right hind limb of each animal. Mice bearing tumors of similar
sizes were randomly divided into four groups (n=6/group). Mice in
the SYKT group received SYKT (1.2 ml/kg/d on days 1, 3 and 5,
p.o.). Mice in the DOX group received DOX (3 mg/kg/d on days 2, 4
and 6, i.p.). Mice in the SYKT/DOX group received both SYKT and DOX
as aforementioned. Control mice were gavaged with normal saline at
an equal volume to SYKT solution on day 1, 3, and 5; and normal
saline at an equal volume to DOX solution on day 2, 4 and 6 via
peritoneal perfusion. The radii of the developing tumors were
measured using Vernier calipers on days 3, 5, 7, 10 and 15, and the
tumor volume was calculated using the formula
V=4/3πr12r2, where r1 and
r2 denote the radii of the tumor in two different
planes. For the tumor cell apoptosis assay, the animals were
sacrificed and xenografts were isolated on days 7, 10 and 15. The
tumors were homogenated after thoroughly rinsing with ice-cold PBS.
The tumor stroma was then fully degraded with collagenase
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C in an
incubator for 1.5 h. After the tumor tissue was dispersed with
ice-cold PBS containing 1% BSA, a single-cell suspension was
acquired via filtration through a 400 mm filter. The cells were
stained with Fluorescein-12-dUTP and propidium iodide (PI)-PE for
TUNEL apoptosis assay and analyzed using flow cytometry. Procedures
for preparing the sample ready for flow cytometry detection were
briefly described as follows: Formaldehyde-fixed cells (15 min,
4°C) were centrifuged at 755 × g for 5 min, followed by rinsing
twice with PBS. The supernatant was then discarded and 70% pre-cold
ethanol was added to fix the cells for 15 min at 4°C, followed by
centrifugation at 755 × g for 5 min, and a rinse twice with PBS.
Double distilled water (30 µl), bio-dNTP (1 µl), TdT (terminal
deoxynucleotidyl transferase) (5 µl) and TdT buffer solution (10
µl) were added in proper sequence, mixed and cultured at 37°C for
30 min. Then, 100 µl of FITC-avidin was added and the mixture was
kept away from light, at room temperature for 30 min, followed by
centrifugation at 1500 rpm at 4°C for 5 min. The product was washed
twice with PBS containing 0.1% tritonX-100 and then 50 µl of PI was
added. The mixture was kept away from light, at room temperature
for 20 min prior to testing with flow cytometry to evaluate the
apoptotic rate. All samples were analyzed in triplicate using
FlowCytomixPro Software (Version 2.1).
Statistical analysis
Statistical analysis was performed using SPSS
software version 10.0 (SPSS, Inc., Chicago, IL, USA). Data were
expressed as the mean ± standard deviation (n=6). Statistical
significance was assessed using one-way analysis of variance, and
the group means were compared using Duncan's Multiple Range Test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
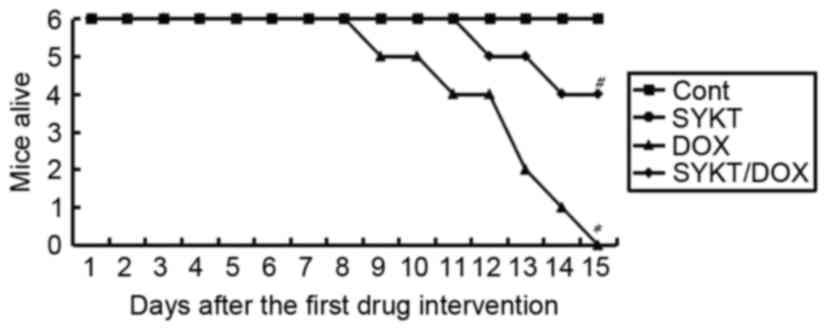
SYKT reduces DOX-induced mortality in
mice
In order to explore the potential of SYKT in
counteracting DOX toxicity, mice survival was monitored daily.
Under the experimental conditions, 100% of the mice succumbed
within 15 days following treatment with DOX alone, whereas only 33%
of the animals treated with a combination of SYKT and DOX succumbed
within 15 days following treatment (Fig. 1). The surviving animals were
monitored for 20 more days and did not exhibit any adverse effects.
There were no cases of mortality in the control or SYKT groups.
These results suggested that SYKT may reduce DOX-associated
mortality.
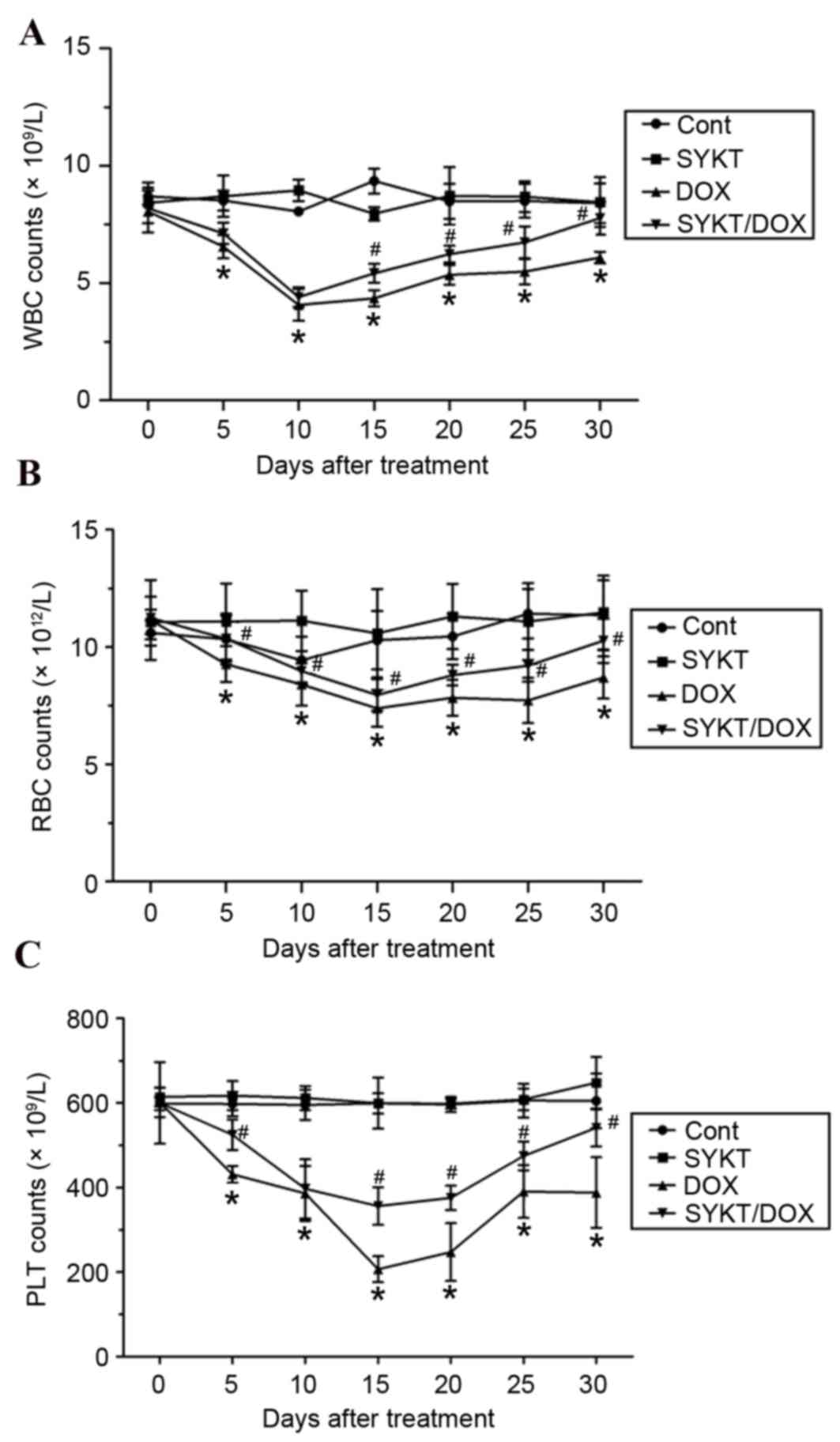
SYKT mitigates the DOX-induced
reduction in peripheral blood cell counts
To determine the effects of SYKT on DOX-induced
myelosuppression, WBC, RBC and PLT counts were performed in
peripheral blood samples. Mice treated with DOX alone significantly
decreased the WBC count to minimum on day 10 of treatment
(4.067±0.677×109/l), which persisted until day 30. In mice treated
with DOX plus SYKT, WBC count appeared to be initially reduced
(4.4±0.42×109/l on day 10); however, after day 15 WBC count
gradually increased and reached a count similar to that of the
control group by day 30 (Fig. 2A).
The control and SYKT groups exhibited no significant alterations in
WBC count. Similarly, the number of RBCs was markedly decreased
following treatment with DOX, reaching a minimum
(7.383±0.788×1012/l) on day 15, and remained at 8.7±0.901×1012/l on
day 30. SYKT significantly attenuated the effects of DOX on RBC
count, particularly at later time points (Fig. 2B). In addition, PLT counts in mice
receiving chemotherapy were markedly decreased, reaching a minimum
(206.667±30.722×109/l) on day 15; PLT count remained at
388±83.465×109/l on day 30. SYKT significantly mitigated the
effects of DOX on PLT count throughout the experiment (Fig. 2C). The present results indicated
that SYKT may significantly attenuate the DOX-induced reduction in
peripheral blood cell counts.
SYKT improves DOX-induced
myelotoxicity
CD34 and CD44 are transmembrane phosphoglycoproteins
expressed in bone marrow cells. To evaluate the myelotoxic effects
of DOX, CD34+ and CD44+ cells were assessed using flow cytometry on
post-treatment day 15 (the optimal time points for bone marrow
sampling were established in a pilot experiment). The results
indicated that DOX reduced the expression of CD34 and CD44, whereas
SYKT co-administration counteracted this effect (Fig. 3A). Although CD34 and CD44 are not
specific markers, both are usually expressed in hematopoietic and
mesenchymal stem cells, and other types of bone marrow cells. The
improved CD34 and CD44 expression following SYKT co-administration
may reflect a recovery in bone marrow function. To further assess
the effects of SYKT on DOX-induced myelotoxicity in mice, the
apoptotic rate of bone marrow cells was evaluated on post-treatment
day 15 via TUNEL staining coupled with flow cytometry. The
apoptotic rate in the control group was 5.8% compared with 28.4% in
the DOX group. However, the apoptotic rate in the SYKT/DOX group
was reduced to 17.7%, suggesting that SYKT may prevent DOX-induced
apoptosis of bone marrow cells (Fig.
3B).
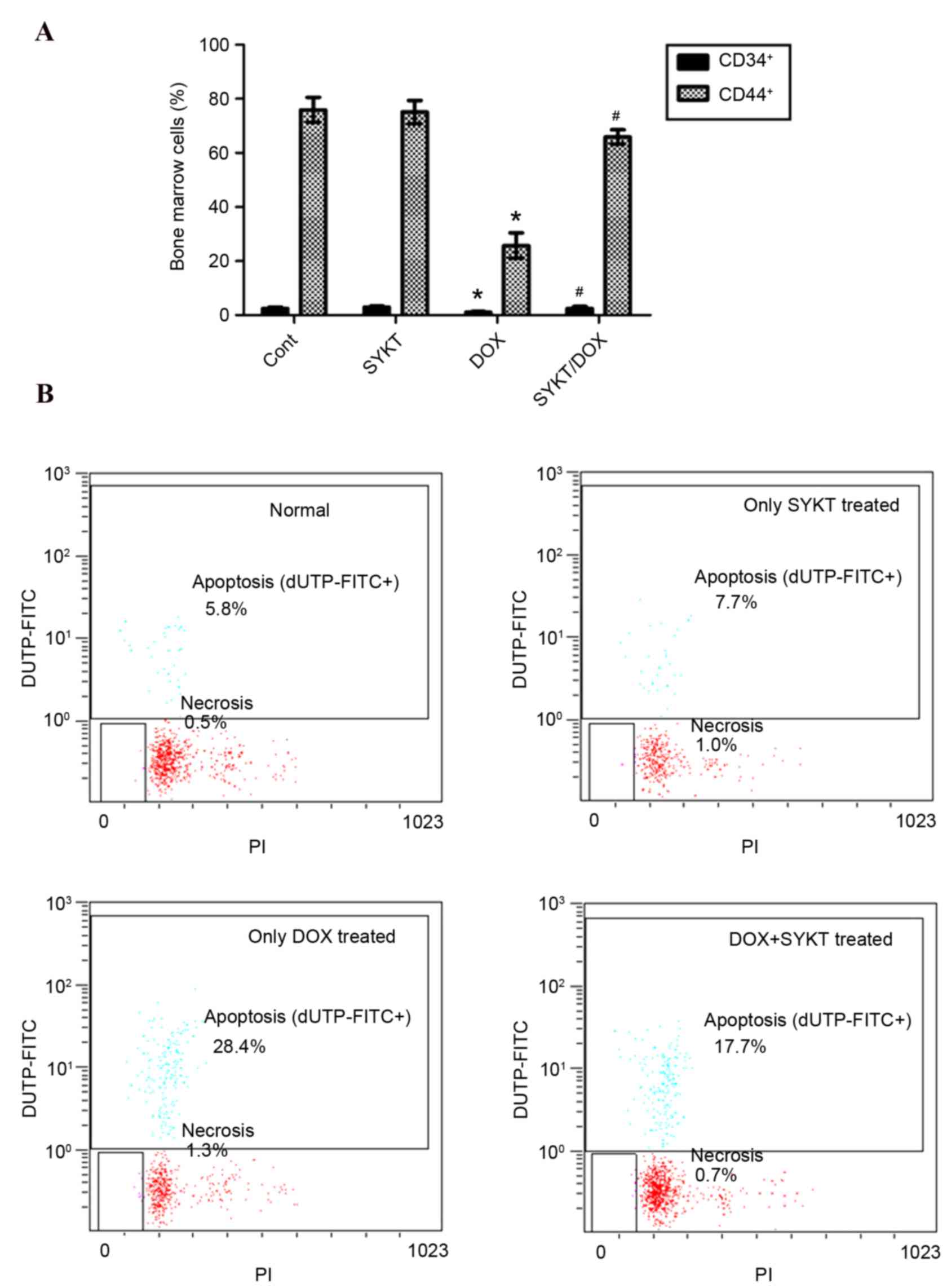 | Figure 3.SYKT reduces DOX-induced
myelotoxicity. Mice were treated with DOX (3 mg/kg/d on days 2, 4
and 6) and SYKT (1.2 ml/kg/d on days 1, 3 and 5) alone or combined,
and the number and apoptotic rate of bone marrow cells were
determined. (A) Flow cytometric analysis of CD34+ and
CD44+ cells in bone marrow on day 15. (B) Apoptotic rate
of bone marrow cells. The cell distribution was analyzed using dUTP
binding and PI uptake. Representative dot plots are shown for one
of the six independent experiments. Data are expressed as the mean
± standard deviation. *P<0.05 vs. control group;
#P<0.05 vs. DOX group. DOX, doxorubicin; CD, cluster
of differentiation; dUTP, 2′-deoxyuridine, 5′-triphosphate; PI,
propidium iodide; Cont, control group; FITC, fluorescein
isothiocyanate. |
SYKT ameliorates DOX-induced
cardiotoxicity
To assess the cardiotoxic effect of DOX and to
investigate whether SYKT can prevent this toxicity, myocardial
enzyme levels were determined by analyzing the activity of AST,
LDH, CK and CK-MB in serum samples. The optimal time points for
blood and heart tissue sampling were established in a pilot
experiment. Blood samples were collected from mice on days 7, 10,
15, 22 and 30 post-treatment with DOX (9 mg/kg, i.p.). The
elevation of myocardial enzymes was the greatest on day 15
post-treatment, and this was selected as the most appropriate time
point for further investigation. DOX induced a significant
elevation in all measured serum parameters compared with in the
control group. The observed changes were similar to those following
acute myocardial infarction (20),
which may indicate that DOX induced extensive myocardial injury.
However, in mice in the SYKT/DOX group, all biochemical parameters
appeared significantly decreased compared with in the DOX alone
group (Fig. 4A).
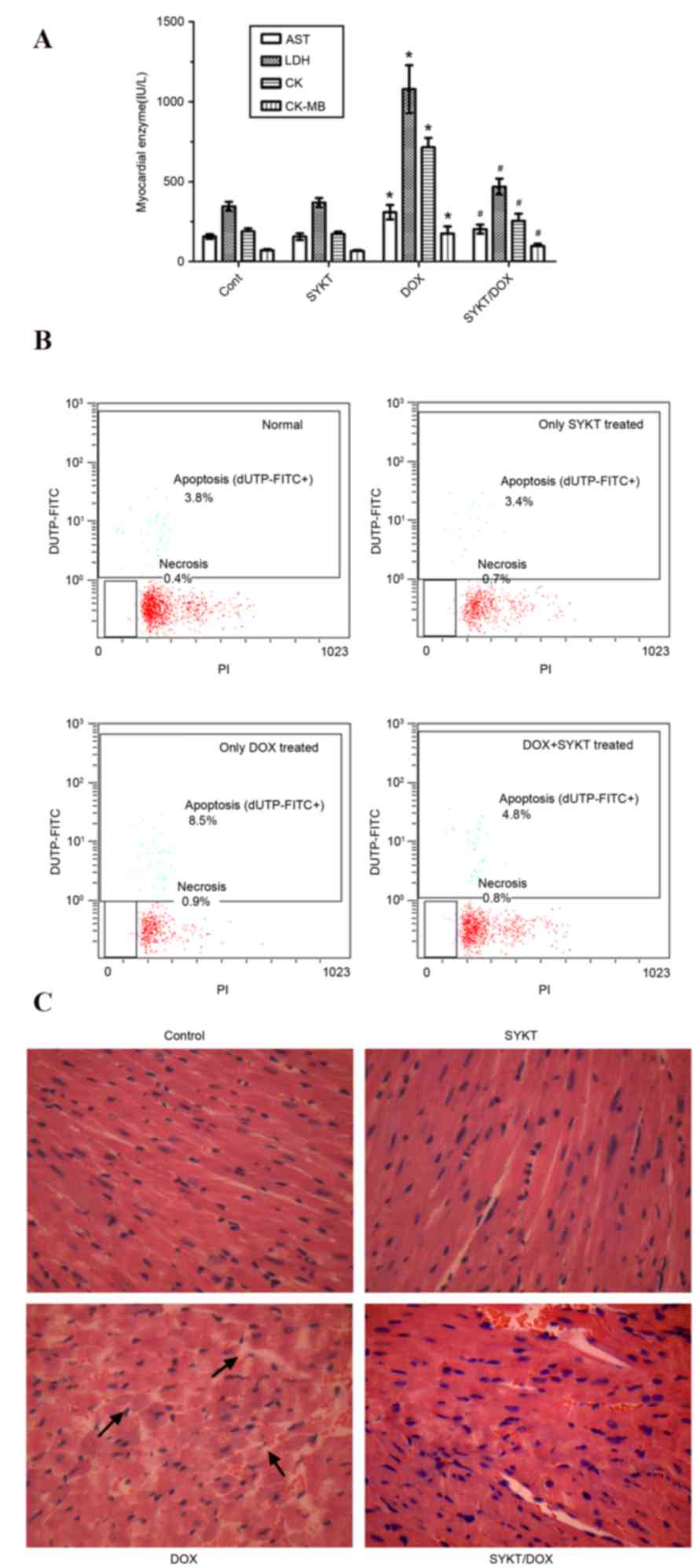 | Figure 4.SYKT reduces DOX-induced
cardiotoxicity. Mice were treated with DOX (3 mg/kg/d on days 2, 4
and 6) and SYKT (1.2 ml/kg/d on days 1, 3 and 5) alone or combined.
(A) Serum activity of myocardial enzymes was determined using an
automatic biochemical analyzer on day 15. (B) Apoptotic rate of
cardiomyocytes. Cell distribution was analyzed using dUTP binding
and PI uptake. The results are expressed as dot plots, as
represented in one of the six independent experiments. (C)
Hematoxylin-eosin staining pattern in cardiac sections. Arrows
indicate abnormal ultrastructural changes (as indicated by the loss
of the normal radiating pattern of the cell plates) (magnification,
×200). Data are expressed as the mean ± standard deviation.
*P<0.05 vs. control group; #P<0.05 vs. DOX group.
DOX, doxorubicin; AST, aspartate aminotransferase; LDH, lactate
dehydrogenase; CK, creatine kinase; dUTP, 2′-deoxyuridine,
5′-triphosphate; PI, propidium iodide; Cont, control group. |
In order to further investigate the effects of DOX
and SYKT on cardiomyocytes, cell apoptosis was assessed via flow
cytometry on day 15 post-treatment. Compared with the control
(3.8%) and SYKT (3.4%) groups, cardiomyocytes from mice in the DOX
group exhibited maximum dUTP-FITC binding (8.5%) but very little PI
staining (0.9%), indicating that the majority of cells were
apoptotic but not necrotic. In mice receiving DOX and SYKT, the
percentage of apoptotic cardiomyocytes was low (4.8%), indicating
that SYKT protected cardiomyocytes from DOX-induced apoptosis
(Fig. 4B).
Histological analysis revealed that DOX
administration disturbed the normal radiating pattern of cell
plates in the heart; however, SYKT co-administration mitigated the
DOX-induced alterations, so that the observed organ pattern
remained similar to in the control group (Fig. 4C). Furthermore, the extent of
typical histopathological changes on day 15 post-treatment with DOX
was ~20%, scored as grade 2 according to Billingham et al
(18). Evaluation of Billingham
scores in mice receiving a combination of DOX and SYKT revealed a
median score of 1.5, which was substantially improved compared with
in the DOX group (Table I). The
strong correlation between histopathology changes scored≥2 and
presence of congestive heart failure has been established (21). The present results suggested that
SYKT may mitigate DOX-induced cardiotoxicity in mice.
 | Table I.Billingham scores of cardiomyopathy
in mice from different intervention groups. |
Table I.
Billingham scores of cardiomyopathy
in mice from different intervention groups.
| Billingham
scores |
|---|
|
|---|
| Group | 0 | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 | Median score |
|---|
| Control | 6 |
|
|
|
|
|
| 0 |
| SYKT | 6 |
|
|
|
|
|
| 0 |
| DOX |
|
|
|
| 4 | 2 |
| 2a |
| DOX + SYKT |
|
| 2 | 3 | 1 |
|
| 1.5b |
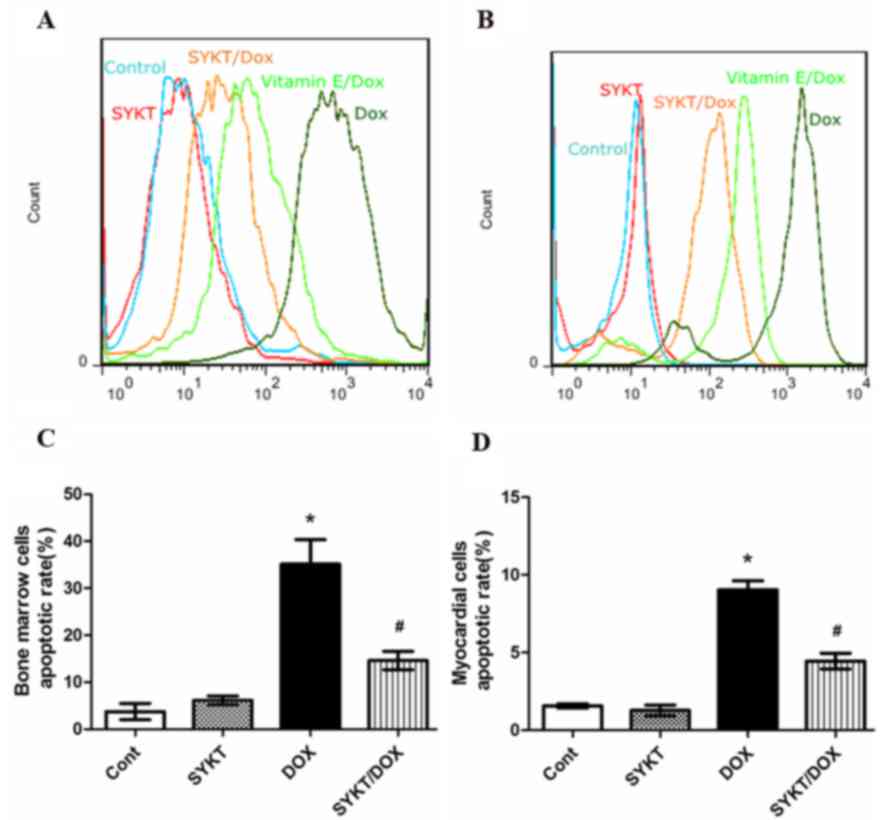
SYKT reverses DOX-induced
myelosuppression and cardiotoxicity by inhibiting ROS-mediated
apoptosis
Mounting evidence indicates the involvement of ROS
in DOX-induced pathophysiology (22,23).
The redox cycling of DOX is generally attributed to the production
of ROS (24). In order to
investigate the potential of SYKT in inhibiting ROS production,
bone marrow and myocardial cells were treated with
CM-H2DCFDA on day 15 post-treatment. Flow cytometric
analysis revealed that treatment with DOX caused a rightward shift
in the intensity of fluorescence of DCFDA, representing the ROS
content in bone marrow and myocardial cells. This shift was
markedly decreased when vitamin E (positive control) or SYKT were
co-administered with DOX (Fig. 5A and
B), indicating that SYKT co-administration reduced ROS
production. These results suggested that ROS production
participates in DOX-induced toxicity in bone marrow and myocardial
cells. In addition, DOX induced apoptosis in bone marrow (Fig. 5C) and myocardial cells (Fig. 5D), which was significantly
inhibited by SYKT co-administration. The present results suggested
that SYKT may reverse DOX-induced myelosuppression and
cardiotoxicity by inhibiting ROS-mediated apoptosis.
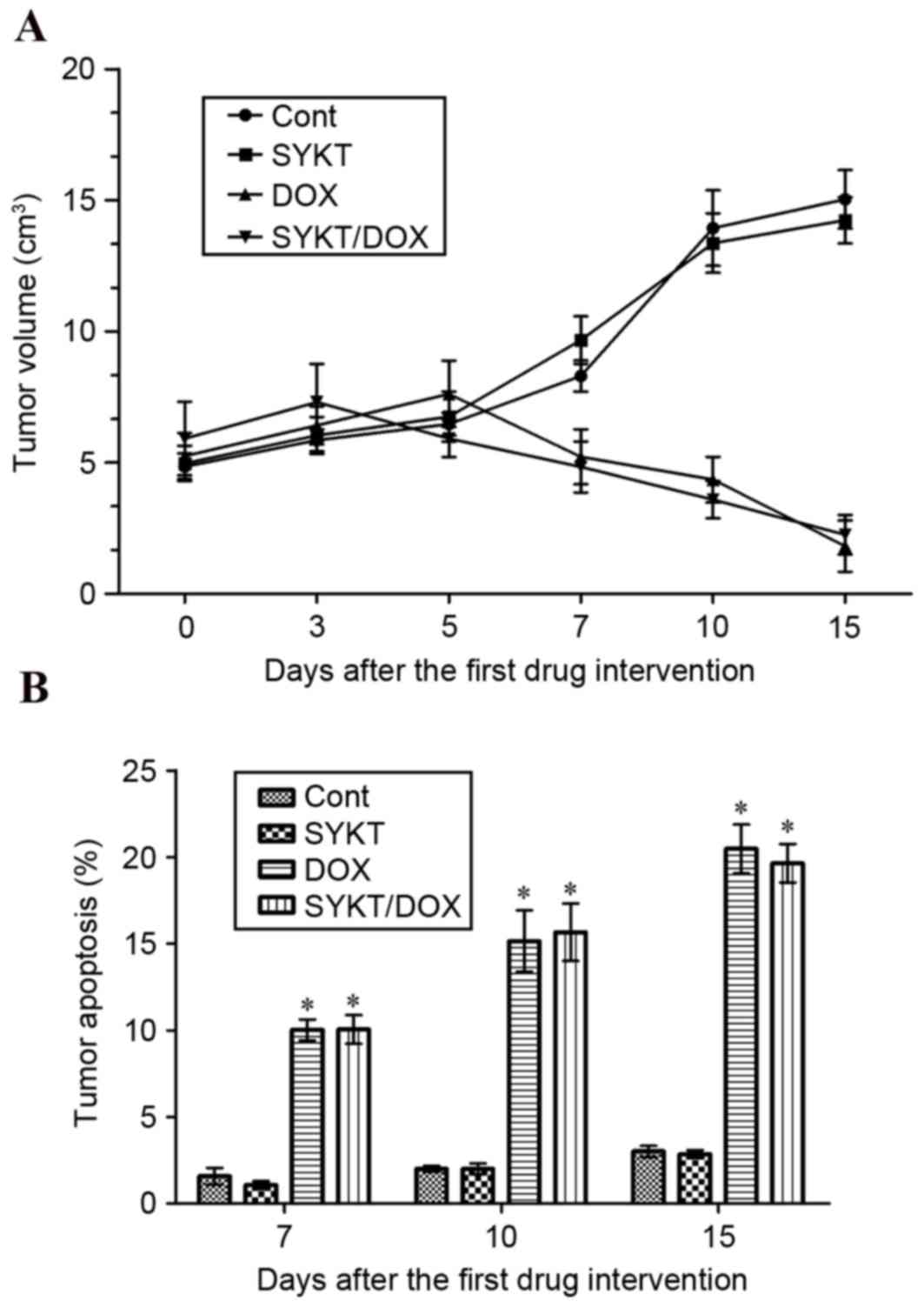
SYKT does not interfere with the
antitumor efficacy of DOX
In order to investigate whether SYKT interferes with
the antitumor effects of DOX, a mouse tumor xenograft was developed
via subcutaneous injection of EL4 cells. Mice were treated with DOX
and SYKT alone or in combination when xenograft tumors reached a
volume of ~5.25±0.876 cm3 (range 3.9–6.3 cm3). Treatment with DOX
reduced the tumor volume to ~1.817±0.975 cm3 (range, 0.6–3.2 cm3)
on day 15 post-treatment. No significant differences in tumor
volume were observed between mice treated with DOX alone and mice
receiving a combination of DOX and SYKT (Fig. 6A). Tumor volumes in control mice
were significantly larger compared with mice treated with DOX alone
or combined with SYKT. Furthermore, tumor cell apoptosis was
evaluated via TUNEL staining coupled with flow cytometry. Compared
with the control and SYKT alone groups, on days 7, 10 and 15,
treatment with DOX markedly increased the apoptotic rate. No
differences were observed between groups receiving DOX alone and
DOX combined with SYKT (Fig. 6B).
The present results demonstrated that SYKT does not interfere with
the antitumor efficacy of DOX.
Discussion
DOX is an anthracycline compound that is widely used
in the treatment of several types of solid tumor and leukemia in
humans. Despite its effectiveness, the clinical application of DOX
has been limited due to its dose-dependent and cumulative
myelosuppressive and cardiotoxic effects (25,26).
However, the mechanism of DOX-induced acute and chronic toxicity
remains to be elucidated. DOX induces the generation of ROS during
the redox cycling of its quinone moiety; it also disturbs iron
metabolism, whereas toxic DOX metabolites are produced in the heart
and hematopoietic tissue (6,27).
Oxidative stress triggers the intrinsic mitochondria-dependent
apoptotic pathway in cardiomyocytes and hematopoietic cells
(7). Molecules with antioxidant
properties can prevent ROS-mediated cytotoxicity. Numerous
traditional Chinese medicines, such as ginseng, astragalus
membranaceus, poria cocos and notoginseng, possess significant
antioxidant activity (28). The
present study demonstrated that the traditional herbal formula SYKT
may reverse DOX-induced myelosuppression and cardiotoxicity by
inhibiting ROS-mediated apoptosis.
DOX damages bone marrow cells and
cardiomyocytes via ROS production, which can be inhibited by
SYKT
To determine the mechanism underlying DOX-induced
myelosuppression and cardiotoxicity, as well as to investigate the
potential of SYKT in preventing these actions, myocardial function
was assessed via myocardial enzyme spectrum and histological
analysis, whereas hematopoietic function was assessed via
peripheral blood cell and bone marrow cell counts. The present
results revealed that DOX induced cardiotoxicity and
myelosuppression, whereas SYKT effectively protected the myocardial
and hematopoietic tissues. Numerous lines of evidence converge to
suggest a role for ROS in DOX-induced toxicity (22–24).
The present study demonstrated that treatment with DOX induced an
increase in ROS production, which was attenuated with the addition
of SYKT to the therapeutic scheme. These results suggested that ROS
may serve an important role in the mechanism of DOX-induced
toxicity in cardiomyocytes and bone marrow cells. The protective
effect of SYKT may be attributed to the antioxidant potential of
some of its components, which are able to scavenge superoxide,
hydroxyl, hydrogen peroxide and nitric oxide free radicals.
Therefore, it is possible that some of the beneficial effects
associated with SYKT co-administration may result from its
neutralizing action on ROS produced by DOX.
SYKT reverses DOX-induced
myelosuppression and cardiotoxicity by inhibiting ROS-mediated
apoptosis
Under physiological conditions, low levels of ROS
function as ‘redox messengers’ in intracellular signaling; however,
aberrant ROS production results in oxidative damage of cellular
macromolecules and promotes cell death. Apoptosis can be initiated
by extracellular and intracellular signals via death receptor- and
mitochondria-mediated pathways (29). Disequilibrium in intracellular
redox homeostasis, due to an increase in ROS production or a
dysfunction in antioxidant mechanisms, can cause irreversible
oxidative modifications to lipids, proteins and DNA, which can
result in oxidative stress-induced apoptotic signaling (30). The present study demonstrated that
cardiomyocytes and bone marrow cells from mice treated with DOX
exhibited a significantly increased apoptotic rate. SYKT
co-administration significantly reduced the number of apoptotic
cells, indicating that SYKT can protect cardiomyocytes and bone
marrow cells from DOX-induced apoptosis.
The intrinsic mitochondrial apoptotic pathway,
initiated by ROS and mitochondrial DNA damage, triggers
permeabilization of the outer mitochondrial membrane and the
translocation of cytochrome c, apoptosis-inducing factor and
the mitochondrial protein Smac/DIABLO from the mitochondria to the
cytosol, where they activate caspase-dependent or -independent
cytosolic signaling events (31).
Mitogen-activated protein kinases (MAPKs) serve an important role
in apoptotic signaling (32).
Oxidative stress can activate members of the MAPK protein family
(33). Among these, p38 and c-Jun
N-terminal kinases (JNK) serve critical roles in the mechanism of
DOX-induced cell death (34).
Activated p38 and JNK MAPKs phosphorylate the apoptosis regulator
B-cell lymphoma 2-associated X protein (Bax) and promote its
translocation to the mitochondria (33,35).
Once there, Bax triggers the opening of mitochondrial permeability
transition pores and subsequent release of cytochrome c. The
maintenance of the mitochondrial membrane potential
(Δψm) is fundamental to cell survival, and the loss of
Δψm activates a cascade leading to cellular apoptosis
(36,37). Following its release into the
cytosol, cytochrome c initiates the formation of apoptosomes
and subsequently activates caspases (37). Further studies are required to
investigate whether SYKT inhibits DOX-induced apoptosis of
myocardial and bone marrow cells via preventing the MAPK-mediated
Bax mitochondrial translocation, thus promoting the preservation of
Δψm and inhibiting cytochrome c release into the
cytosol.
SYKT does not affect the antitumor
efficacy of DOX
Chemoprotectants have relatively limited clinical
application, due to concerns regarding the potential for a negative
interaction with antineoplastic agents, resulting in reduced
chemotherapeutic efficacy. The present study demonstrated that SYKT
did not impair the antitumor efficacy of DOX in tumor-bearing mice.
DOX can induce apoptosis in healthy and tumor cells via various
mechanisms (38). In endothelial
cells and cardiomyocytes, DOX has been demonstrated to induce
apoptosis through a ROS-mediated mechanism independent of p53
activation. Conversely, the tumor suppressor p53 has a crucial role
in the mechanism of DOX-induced apoptosis in cancer cells (38). Therefore, it may be hypothesized
that SYKT reduces the damage induced by DOX to myocardial and bone
marrow cells by inhibiting ROS-mediated apoptosis, while having no
effect on the p53-mediated apoptotic pathway.
In conclusion, the present results demonstrated that
SYKT may prevent DOX-induced myelosuppression and cardiotoxicity
without impairing its antitumor efficacy, through a mechanism
involving the inhibition of ROS-mediated apoptosis. Therefore, the
present study suggested that SYKT co-administration may be
considered a potential solution to counteract the myelosuppressive
and cardiotoxic action of DOX.
Acknowledgements
The present study was supported by grants from the
Chinese National Natural Science Foundation (grant nos. 81260322,
81372322 and 81460440) and the project of Yunnan Provincial
Technology Department (grant nos. 2011HA013 and 2012FB003).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. Cancer J Clin. 61:69–90.
2011. View Article : Google Scholar
|
|
2
|
Emadi A, Jones RJ and Brodsky RA:
Cyclophosphamide and cancer: Golden anniversary. Nat Rev Clin
Oncol. 6:638–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Swain SM: Doxorubicin-induced
cardiomyopathy. N Engl J Med. 340:6541999.PubMed/NCBI
|
|
4
|
Vadhan-Raj S, Patel S, Bueso-Ramos C,
Folloder J, Papadopolous N, Burgess A, Broemeling LD, Broxmeyer HE
and Benjamin RS: Importance of predosing of recombinant human
thrombopoietin to reduce chemotherapy-induced early
thrombocytopenia. J Clin Oncol. 21:3158–3167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen Y, Jungsuwadee P, Vore M, Butterfield
DA and St Clair DK: Collateral damage in cancer chemotherapy:
Oxidative stress in nontargeted tissues. Mol Interv. 7:147–156.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim EJ, Lim KM, Kim KY, Bae ON, Noh JY,
Chung SM, Shin S, Yun YP and Chung JH: Doxorubicin-induced platelet
cytotoxicity: A new contributory factor for doxorubicin-mediated
thrombocytopenia. J Thromb Haemost. 7:1172–1183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kluza J, Marchetti P, Gallego MA, Lancel
S, Fournier C, Loyens A, Beauvillain JC and Bailly C: Mitochondrial
proliferation during apoptosis induced by anticancer agents:
Effects of doxorubicin and mitoxantrone on cancer and cardiac
cells. Oncogene. 23:7018–7030. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Afanasev I: Detection of superoxide in
cells, tissues and whole organisms. Front Biosci (Elite Ed).
1:153–160. 2009.PubMed/NCBI
|
|
9
|
Ingold KU and Pratt DA: Advances in
radical-trapping antioxidant chemistry in the 21st century: A
kinetics and mechanisms perspective. Chem Rev. 114:9022–9046. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guimarães R, Barreira JC, Barros L,
Carvalho AM and Ferreira IC: Effects of oral dosage form and
storage period on the antioxidant properties of four species used
in traditional herbal medicine. Phytother Res. 25:484–492. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ohnishi S and Takeda H: Herbal medicines
for the treatment of cancer chemotherapy-induced side effects.
Front Pharmacol. 6:142015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hua Hou, Hong-Wen Sun, Pei-Zhu Zhao, et
al: Effect of Neoadjuvant radio-chemotherapy of sanyang xuedai
mixture on Myelosuppression in patients with squamous carcinoma.
Chinese J Experimental Traditional Med Formulae. 21:173–176.
2015.
|
|
13
|
Ji-Ma Lv, Lu-Hua Wang, Li-Jun LI, et al:
Concurrent radiotherapy and SYKT for local advanced non-small cell
lung cancer. China Cancer. 13:743–746. 2004.
|
|
14
|
Jahnukainen K, Jahnukainen T, Salmi TT,
Svechnikov K, Eksborg S and Söder O: Amifostine protects against
early but not late toxic effects of doxorubicin in infant rats.
Cancer Res. 61:6423–6427. 2001.PubMed/NCBI
|
|
15
|
Liu TJ, Yeh YC, Ting CT, Lee WL, Wang LC,
Lee HW, Wang KY and Lai HC and Lai HC: Ginkgo biloba extract 761
reduces doxorubicin-induced apoptotic damage in rat hearts and
neonatal cardiomyocytes. Cardiovasc Res. 80:227–235. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huffmyer J and Raphael J: Physiology and
pharmacology of myocardial preconditioning and postconditioning.
Semin Cardiothorac Vasc Anesth. 13:5–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Angermann CE and Ertl G: Natriuretic
peptides-new diagnostic markers in heart disease. Herz. 29:609–617.
2004.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Billingham ME, Mason JW, Bristow MR and
Daniels JR: Anthracycline cardiomyopathy monitored by morphologic
changes. Cancer Treat Rep. 62:865–872. 1978.PubMed/NCBI
|
|
19
|
Bjelogrlic SK, Radic J, Jovic V and
Radulovic S: Activity of d,l-alpha-tocopherol (vitamin E) against
cardiotoxicity induced by doxorubicin and doxorubicin with
cyclophosphamide in mice. Basic Clin Pharmacol Toxicol. 97:311–319.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kitsis RN and Jialal I: Limiting
myocardial damage during acute myocardial infarction by inhibiting
C-reactive protein. N Engl J Med. 355:513–515. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferrans V, Sanchez J and Herman E: The
role of myocardial biopsy in the diagnosis of anthracycline
cardiotoxicityCancer treatment and the heart. Muggia F..Green
M..Speyer J: John Hopkins University; Baltimore, MD: pp. 198–216.
1992
|
|
22
|
Keizer HG, Pinedo HM, Schuurhuis GJ and
Joenje H: Doxorubicin (adriamycin): A critical review of free
radical-dependent mechanisms of cytotoxicity. Pharmacol Ther.
47:219–231. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Olson RD and Mushlin PS: Doxorubicin
cardiotoxicity: Analysis of prevailing hypotheses. FASEB J.
4:3076–3086. 1990.PubMed/NCBI
|
|
24
|
van Acker FA, Boven E, Kramer K, Haenen
GR, Bast A and van der Vijgh WJ: Frederine, a new and promising
protector against doxorubicin-induced cardiotoxicity. Clin Cancer
Res. 7:1378–1384. 2001.PubMed/NCBI
|
|
25
|
Keefe DL: Anthracycline-induced
cardiomyopathy. Semin Oncol. 28(4 Suppl 12): S2–S7. 2001.
View Article : Google Scholar
|
|
26
|
Bally MB, Nayar R, Masin D, Cullis PR and
Mayer LD: Studies on the myelosuppressive activity of doxorubicin
entrapped in liposomes. Cancer Chemother Pharmacol. 27:13–19. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu
YL, Liu LF and Yeh ET: Identification of the molecular basis of
doxorubicin-induced cardiotoxicity. Nat Med. 18:1639–1642. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo KJ, Xu SF, Yin P, Wang W, Song XZ, Liu
FH, Xu JQ and Zoccarato I: Active components of common traditional
Chinese medicine decoctions have antioxidantfunctions. J Anim Sci.
89:3107–3115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao J, Zhang L, Li J, Wu T, Wang M, Xu G,
Zhang F, Liu L, Yang J and Sun S: A novel pyrazolone-based
derivative induces apoptosis in human esophageal cells via reactive
oxygen species (ROS) generation and caspase-dependent
mitochondria-mediated pathway. Chem Biol Interact. 231:1–9. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ryter SW, Kim HP, Hoetzel A, Park JW,
Nakahira K, Wang X and Choi AM: Mechanisms of cell death in
oxidative stress. Antioxid Redox Signal. 9:49–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Turrens JF: Mitochondrial formation of
reactive oxygen species. J Physiol. 552:335–344. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Venkatesan B, Prabhu SD, Venkatachalam K,
Mummidi S, Valente AJ, Clark RA, Delafontaine P and Chandrasekar B:
WNT1-inducible signaling pathway protein-1 activates diverse cell
survival pathways and blocks doxorubicin-induced cardiomyocyte
death. Cell Signal. 22:809–820. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lou H, Danelisen I and Singal PK:
Involvement of mitogen-activated protein kinases in
adriamycin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol.
288:H1925–H1930. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim BJ, Ryu SW and Song BJ: JNK- and p38
kinase-mediated phosphorylation of Bax leads to its activation and
mitochondrial translocation and to apoptosis of human hepatoma
HepG2 cells. J Biol Chem. 281:21256–21265. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang GW, Klein JB and Kang YJ:
Metallothionein inhibits doxorubicin-induced mitochondrial
cytochrome c release and caspase-3 activation in cardiomyocytes. J
Pharmacol Exp Ther. 298:461–468. 2001.PubMed/NCBI
|
|
37
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase-9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang S, Konorev EA, Kotamraju S, Joseph J,
Kalivendi S and Kalyanaraman B: Doxorubicin induces apoptosis in
normal and tumor cells via distinctly different mechanisms.
Intermediacy of H(2)O(2)- and p53-dependent pathways. J Biol Chem.
279:25535–25543. 2004. View Article : Google Scholar : PubMed/NCBI
|