Introduction
Peritoneal dialysis (PD) is one of the most common
renal replacement therapies for end stage renal disease (ESRD).
Long-term PD is limited due to the morphological and functional
changes of the peritoneum induced by PD fluids, which contain high
concentrations of glucose that eventually lead to ultrafiltration
failure (1).
Epithelial-mesenchymal transition (EMT) is involved in the fibrosis
of various organs, including renal fibrosis, liver fibrosis and
peritoneal fibrosis (2–4). High glucose (HG)-induced EMT of human
peritoneal mesothelial cells (HPMCs) may be a key process in the
fibrosis and dysfunction of the peritoneal membrane (5). EMT is a pathological phenomenon where
normal epithelial cells lose their characteristics, such as cell
polarity and adhesion and gain characteristics of mesenchymal
cells, such as migration and invasion (6). Biomarkers for the occurrence of EMT
may include the loss of the epithelial adhesion protein E-cadherin
and upregulation of the mesenchymal markers α-smooth muscle actin
(α-SMA) and fibronectin (FN) (7).
Preventing EMT may ameliorate peritoneal fibrosis, preserving the
mesothelial cells during PD (8).
Additionally, high glucose treatment was demonstrated to induce
EMT-mediated inflammation in peritoneal mesothelial cells and the
kidney fibroblasts (9,10).
Vitamin D, beyond its role in the regulation of
calcium, phosphorus and the bone metabolism, has been demonstrated
to serve an important role in the regulation of cell
differentiation, cell proliferation and immunomodulation (11). 1,25(OH)2D3 is
the active form of vitamin D and regulates bone, calcium and
phosphate metabolism via the vitamin D receptor (VDR). The VDR
forms a heterodimer with the retinoid X receptor and regulates gene
expression in the nucleus. Vitamin D has received more attention in
EMT and inflammation. Previous studies have determined that vitamin
D attenuated renal tubular cell injury by suppressing EMT process
and inflammation through inhibition of the nuclear factor-κB
(NF-κB), transforming growth factor β (TGFβ)/SMAD family member 3
(Smad3) and β-catenin signaling pathways (12), vitamin D also inhibited migration,
invasion and EMT induced by TGFβ in human airway epithelial cells
(13). However, the effect of
1,25(OH)2D3 on HG-induced EMT and
inflammation in HPMCs and the underlying molecular mechanism remain
to be elucidated.
The present study determined whether
1,25(OH)2D3 protected HPMCs from HG-induced
EMT and inflammation and whether this effect occurs through the
modulation of TGFβ/Smad signaling pathway by binding to its
receptor VDR.
Materials and methods
Reagents
Fetal bovine serum (FBS), RPMI 1640 and penicillin
streptomycin were obtained from Gibco; Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). 1,25(OH)2D3 and
bovine serum albumin (BSA) were purchased from Sigma-Aldrich; Merck
Millipore (Darmstadt, Germany). Rabbit monoclonal VDR (cat. no.
ab109234), rabbit monoclonal α-SMA (cat. no. ab32575) and rabbit
monoclonal E-cadherin (cat. no. ab133597) antibodies were purchased
from Abcam (Cambridge, UK), rabbit monoclonal Smad3 (cat. no. 9523)
and rabbit monoclonal phospho-Smad3 (cat. no. 9520) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA), mouse
monoclonal FN (cat. no. sc- 53285) and mouse monoclonal β-actin
(cat. no. sc-47778) antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The dilution used for all
primary antibodies was 1:1,000 for western blot analysis and 1:200
for α-SMA in immunofluorescence staining. An enhanced
chemiluminescence (ECL) kit was obtained from Pierce; Thermo Fisher
Scientific, Inc. Human TGFβ and IL-6 ELISA kits were purchased from
R&D Systems, Inc. (Minneapolis, MN, USA).
HPMC culture
HPMCs were provided by Professor Di Na and Professor
Huimian Xu (First Affiliated Hospital of China Medical University,
Shenyang, China) and were routinely grown in RPMI 1640 (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS, 100
UI/ml penicillin and 100 µg/ml streptomycin. HPMCs were incubated
in an atmosphere of 5% CO2 at 37°C and every 2–3 days
the culture medium was changed. HPMCs were harvested with
trypsin-EDTA at a subcultivation ratio of 1:3 to 1:4. Cells at
passages 5–10 were used for all subsequent experiments. Cells were
treated with 4 different concentrations of HG (control, 1.5%, 76
mM; 2.5%, 126 mM; 4.25%, 214 mM) for 24 h, and for 5 different
durations (0, 6, 12, 24 and 48 h) of 2.5% HG (126 mM).
Additionally, cells were pretreated with 10−7 mol/l
1,25(OH)2D3 (14) for 2 h and followed by 2.5% HG (126
mM) for the 24 h.
Transfection
The VDR-short hairpin RNA (shRNA)-GV248 plasmids
that recognize human VDR and negative control plasmids were
purchased from GeneChem Co., Ltd. (Shanghai, China) were used for
the transient transfections. The transfections were performed using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol.
Treatment groups
HPMCs were exposed to 7 different treatments:
Control, cells were untreated; D, treatment with 10−7
mol/l 1,25(OH)2D3; Negative control plasmids;
VDR-shRNA; HG, treatment with 126 mM HG; HG+D, pretreatment with
10−7 mol/l 1,25(OH)2D3 followed by
126 mM HG; and HG+D+shVDR pretreatment with 10−7 mol/l
1,25(OH)2D3 followed by 126 mM HG and
VDR-shRNA.
Western blot analysis
Western blot analysis was performed as previously
described (9). All of the
experiments were repeated at least 3 times. The dilution used for
primary antibodies was 1:1,000, and 1:5,000 for the goat
anti-rabbit/mouse horseradish peroxidase-conjugated secondary
antibodies (cat. no. A0208 and A0216; Beyotime Institute of
Biotechnology, Haimen, China). The blots were developed using a ECL
kit and the images were captured with UVP (G:BOX EF, Chemi HR16;
Syngene, Frederick, MD USA). The intensity of each band was
measured with Image J software (Java v1.6.0_20; https://imagej.nih.gov/ij/) and the results were
normalized against the reference gene β-actin.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HPMCs using TRIzol
reagent in accordance with the manufacturer's protocol (Invitrogen;
Thermo Fisher Scientific, Inc.). Subsequently, first-strand cDNA
synthesis was performed using a Reverse Transcription kit in
accordance with the manufacturer's protocol (Takara Biotechnology
Co., Ltd., Dalian, China). qPCR was performed using ABI 7500
Real-Time PCR system (Thermo Fisher Scientific, Inc.) and the SYBR
Premix Ex Taq II kit (Takara Biotechnology Co., Ltd.). Briefly,
RT-qPCR was prepared in triplicates at a volume of 25 µl reaction
mixture as follows: 12.5 µl SYBR Premix Ex Taq II, 1.0 µl forward
and reverse specific primers and 2 µl cDNA template and 8.5 µl
RNase-free water. The mRNA expression of the respective genes was
calculated after normalizing to GAPDH. The specific primers were as
follows: TGFβ sense 5′-CCCTTCATGGTGGCTTTCTT-3′ and antisense
5′-CTGGTTCTTGGGCGTCTTG-3′; IL-6 sense 5′-GCCAGAGCTGTGCAGATGAG-3′
and antisense 5′-TCAGCAGGCTGGCATTTG-3′; VDR sense
5′-ATGCCATCTGCATCGTCTC-3′ and antisense 5′-GCACCGCACAGGCTGTCCTA-3′;
and GAPDH sense 5′-GCACCGTCAAGGCTGAGAAC-3′ and antisense
5′-TGGTGAACACGCCAGTGGA-3′.
Immunofluorescence staining with
α-SMA
HPMCs were fixed in 4% paraformaldehyde at room
temperature for 15 min, permeabilized with 0.25% Triton X-100 for
10 min, then washed with phosphate buffered saline (PBS), and
blocked for 30 min at room temperature with 5% BSA (Sigma-Aldrich;
Merck Millipore). The primary antibody rabbit anti-α-SMA (dilution,
1:200) was incubated with the cells overnight at 4°C in a
humidified chamber. Following 3 washes with PBS for 5 min,
fluorescein isothiocyanate-conjugated secondary antibody
anti-rabbit IgG (dilution, 1:50; cat. no. A0562; Beyotime Institute
of Biotechnology) was incubated for 1 h at room temperature. In
order to identify nuclei, cells were counterstained with DAPI
(Invitrogen; Thermo Fisher Scientific, Inc.) for 1 min. Stained
cells were visualized using a fluorescence microscope (Nikon
ECLIPSE Ti; Nikon Corporation, Tokyo, Japan).
ELISA
HPMCs were seeded at a density of 105
cells/well into 12-well plates, subsequently the cells were
cultured under the conditions aforementioned, the supernatants were
collected by centrifugation for 10 min at 1,500 × g, 4°C, and the
TGFβ and IL-6 proteins from the supernatants was detected using
human TGFβ and human IL-6 ELISA kits according to the
manufacturer's protocol. All procedures were performed at room
temperature. The TGFβ and IL-6 levels were then expressed as
pg/ml.
Statistical analysis
Statistical analysis was performed using SPSS
version 18 (SPSS, Inc., Chicago, IL, USA). Data were expressed as
the mean ± standard error of the mean. Multiple comparisons were
performed using analysis of variance. Differences between two
variables were assessed using an unpaired Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Influence of
1,25(OH)2D3 on VDR and HG-induced EMT in
HPMCs
The present study analyzed the expression levels of
VDR and EMT biomarkers, including α-SMA, FN and E-cadherin in order
to determine the effect of 1,25(OH)2D3 on
HG-treated HPMCs. As presented in Fig.
1A and B, it was determined that HG significantly downregulated
the mRNA expression of VDR in a dose- and time-dependent manner
(P<0.05). Fig. 1C-F indicated
that HG significantly upregulated the expression of α-SMA
(P<0.05) and FN (P<0.01) and downregulated the expression
levels of E-cadherin in HPMCs (P<0.01), which indicated that EMT
may have occurred following HG treatment. Additionally, protein and
mRNA expression of VDR were downregulated following HG treatment
(Fig. 1C, G and H). However,
HG-induced EMT was attenuated by 10−7 mol/l
1,25(OH)2D3 pretreatment in HPMCs (Fig. 1C-F) which led to increased
expression levels of VDR (Fig. 1C, G
and H). In addition, immunofluorescence staining demonstrated
that 1,25(OH)2D3 attenuated HG-induced α-SMA
in HPMCs (Fig. 1I).
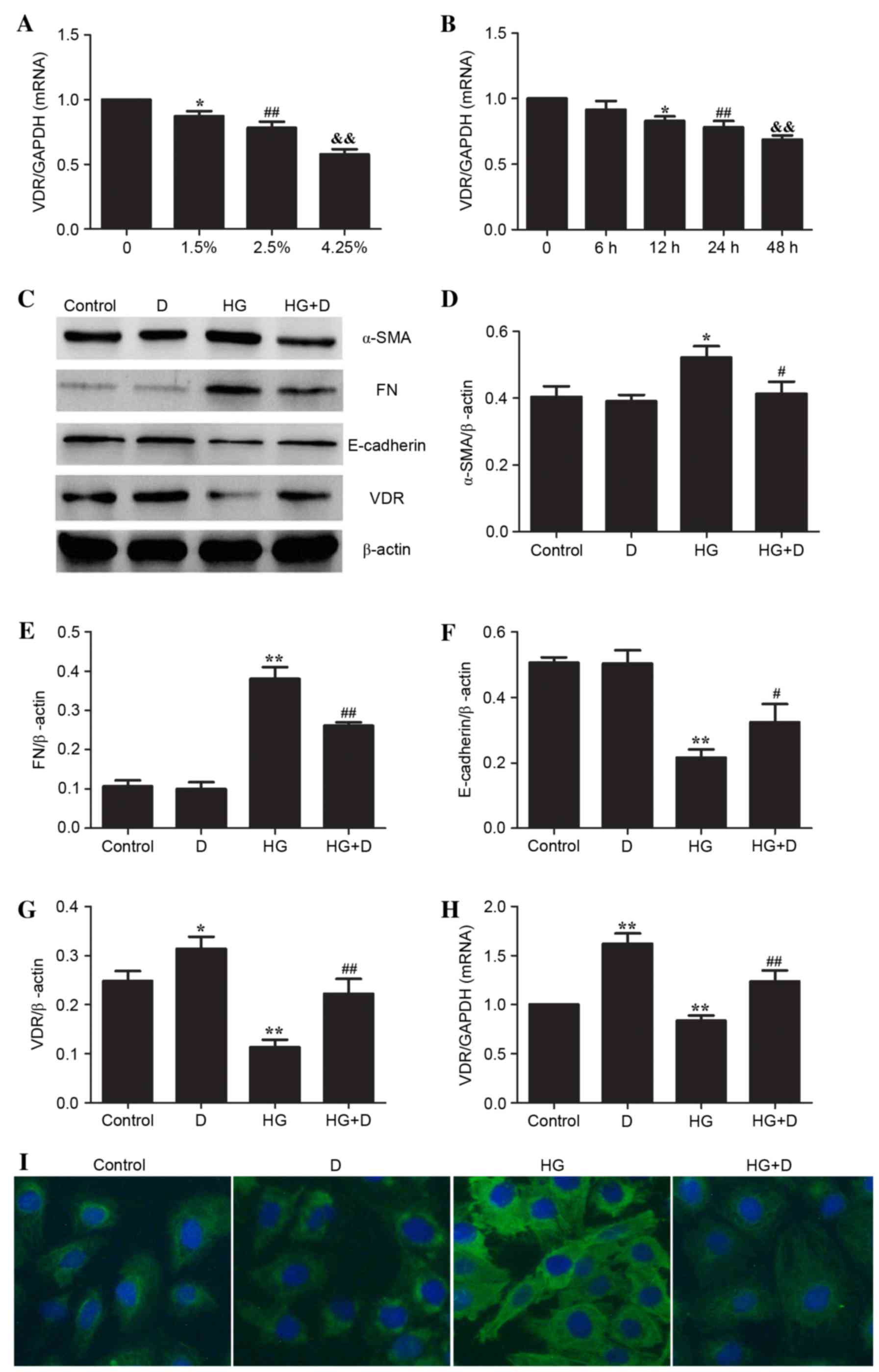 | Figure 1.Effect of
1,25(OH)2D3 on EMT biomarkers and VDR in
HG-treated HPMCs. HPMCs were treated with (A) 4 different
concentrations of HG (control; 1.5%, 76 mM; 2.5%, 126 mM; 4.25%,
214 mM) for 24 h and for (B) 5 different durations (0, 6, 12, 24
and 48 h) of 2.5% HG (126 mM). Cells were pretreated with
10−7mol/l 1,25(OH)2D3 and followed
by 2.5% HG (126 mM) for 24 h. Reverse transcription-quantitative
polymerase chain reaction was performed to detect the relative gene
expression of VDR in the different HPMCs treatment groups. Data are
presented as the mean ± standard error (n=3). *P<0.05;
##P<0.01; &&P<0.01 vs. control.
(C) Western blotting was used to determine protein expression
levels. Protein levels of (D) α-SMA, (E) FN, (F) E-cadherin and (G)
VDR were assessed using densitometry and were expressed as relative
intensities. Data are presented as the mean ± standard error (n=3).
*P<0.05 and **P<0.01 vs. control; #P<0.05 and
##P<0.01 vs. HG group. (H) Relative gene expression
of VDR in HPMCs following 24 h of HG stimulation in the presence or
absence of 1,25(OH)2D3. **P<0.01 vs.
control; ##P<0.01 vs. HG group. (I)
Immunofluorescence staining of α-SMA demonstrated that
1,25(OH)2D3 prevented expression of α-SMA in
HPMCs. Magnification, ×200. EMT, epithelial-mesenchymal transition;
VDR, vitamin D receptor; HG, high glucose; HPMCs, human peritoneal
mesothelial cells; α-SMA, α-smooth muscle actin; FN, fibronectin;
D, 1,25(OH)2D3. |
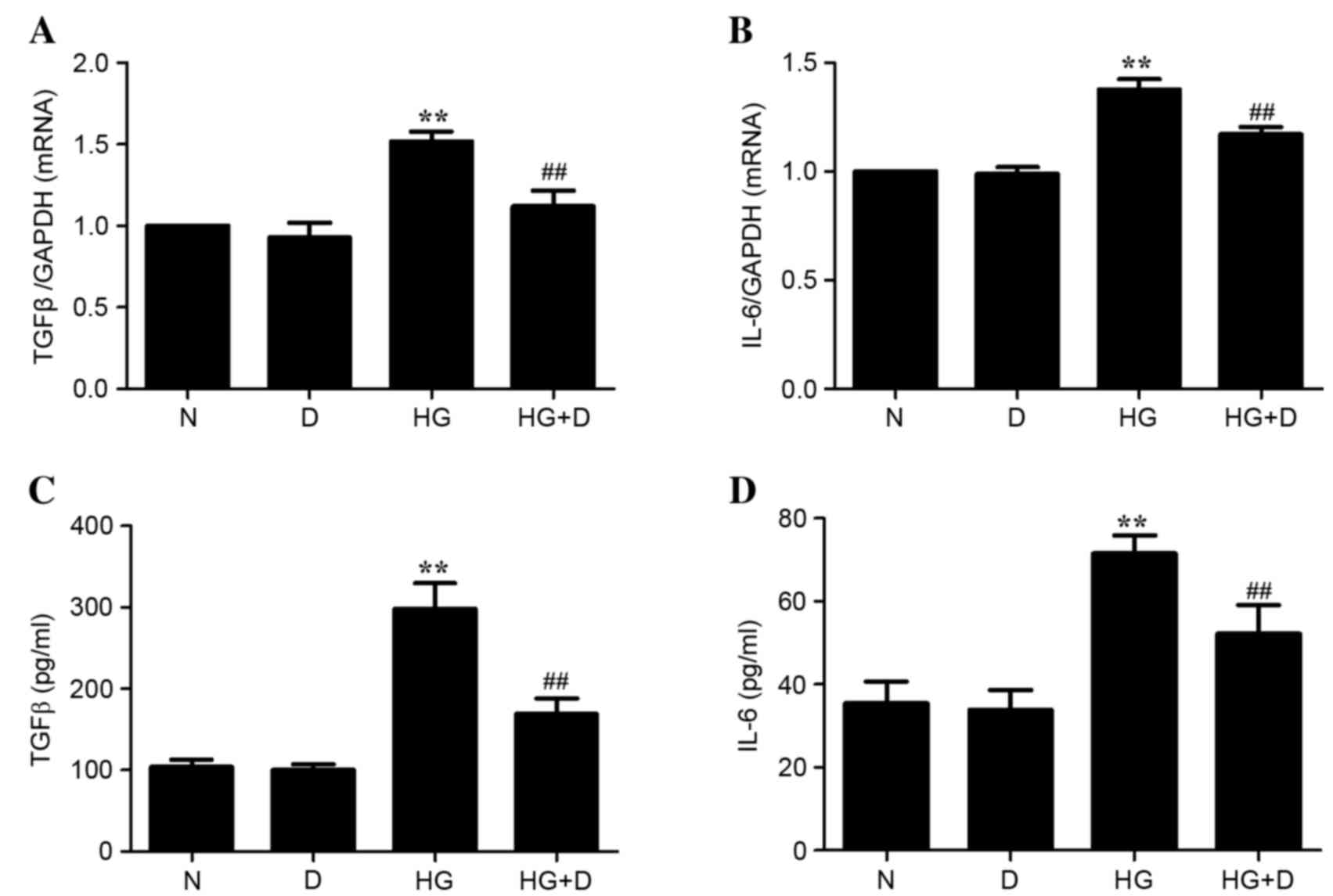
Effect of
1,25(OH)2D3 on inflammatory cytokines in
HG-treated HPMCs
The mRNA and protein expression levels of
inflammatory cytokines TGFβ and IL-6 were examined to determine the
effect of 1,25(OH)2D3 on inflammation in
HG-treated HPMCs. As presented in Fig.
2, HG treatment significantly upregulated the mRNA and protein
expression of inflammation cytokines TGFβ and IL-6 (P<0.01).
However, this effect was prevented by 10−7 mol/l
1,25(OH)2D3 pretreatment in HPMCs
(P<0.01). Therefore, it is possible that
1,25(OH)2D3 attenuated the HG-induced TGFβ
and IL-6 expression in HPMCs.
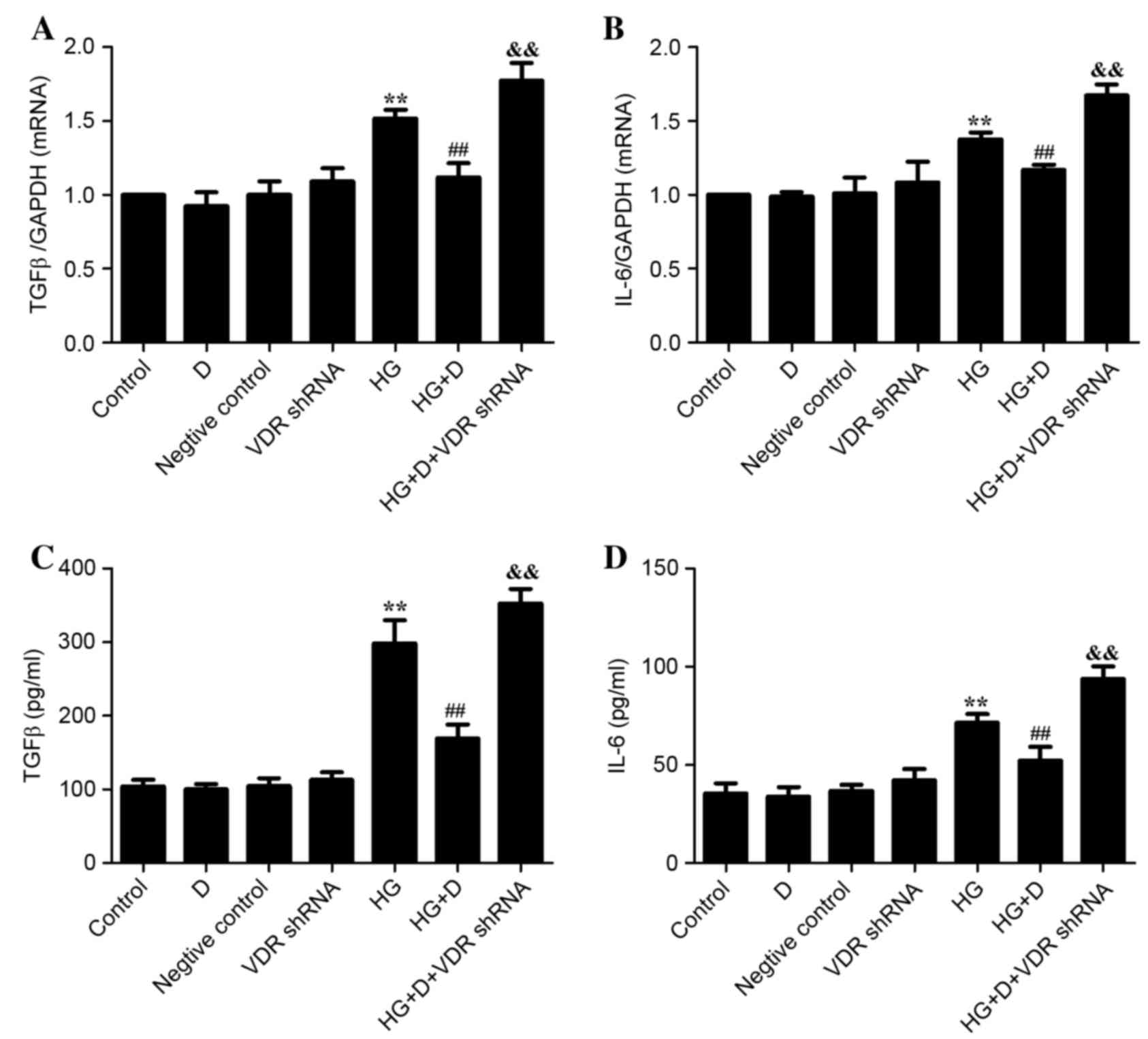
Effect of VDR on HG-induced EMT and
inflammation in HPMCs
The VDR-shRNA plasmid was used to observe the
association of VDR, EMT markers and inflammatory cytokines in order
to determine the effect of VDR on HG-induced EMT and inflammation.
As presented in Fig. 3, it was
determined that 126 mM HG treatment upregulated the expression
levels of α-SMA and FN and downregulated the expression levels of
E-cadherin and VDR (P<0.01). The expression levels were reversed
by 1,25(OH)2D3 pretreatment in HPMCs.
Additionally, these effects were diminished when cells were
transfected with VDR-shRNA. As presented in Fig. 4, it was demonstrated that 126 mM HG
treatment upregulated the expression of TGFβ and IL-6, whereas
1,25(OH)2D3 pretreatment was able to prevent
the expression of inflammatory cytokines in HPMCs. The protective
effect of 1,25(OH)2D3 was diminished when
transfected with VDR-shRNA. Therefore, the present study indicated
that 1,25(OH)2D3 may attenuate HG-induced EMT
and inflammation in HPMCs by binding to its receptor.
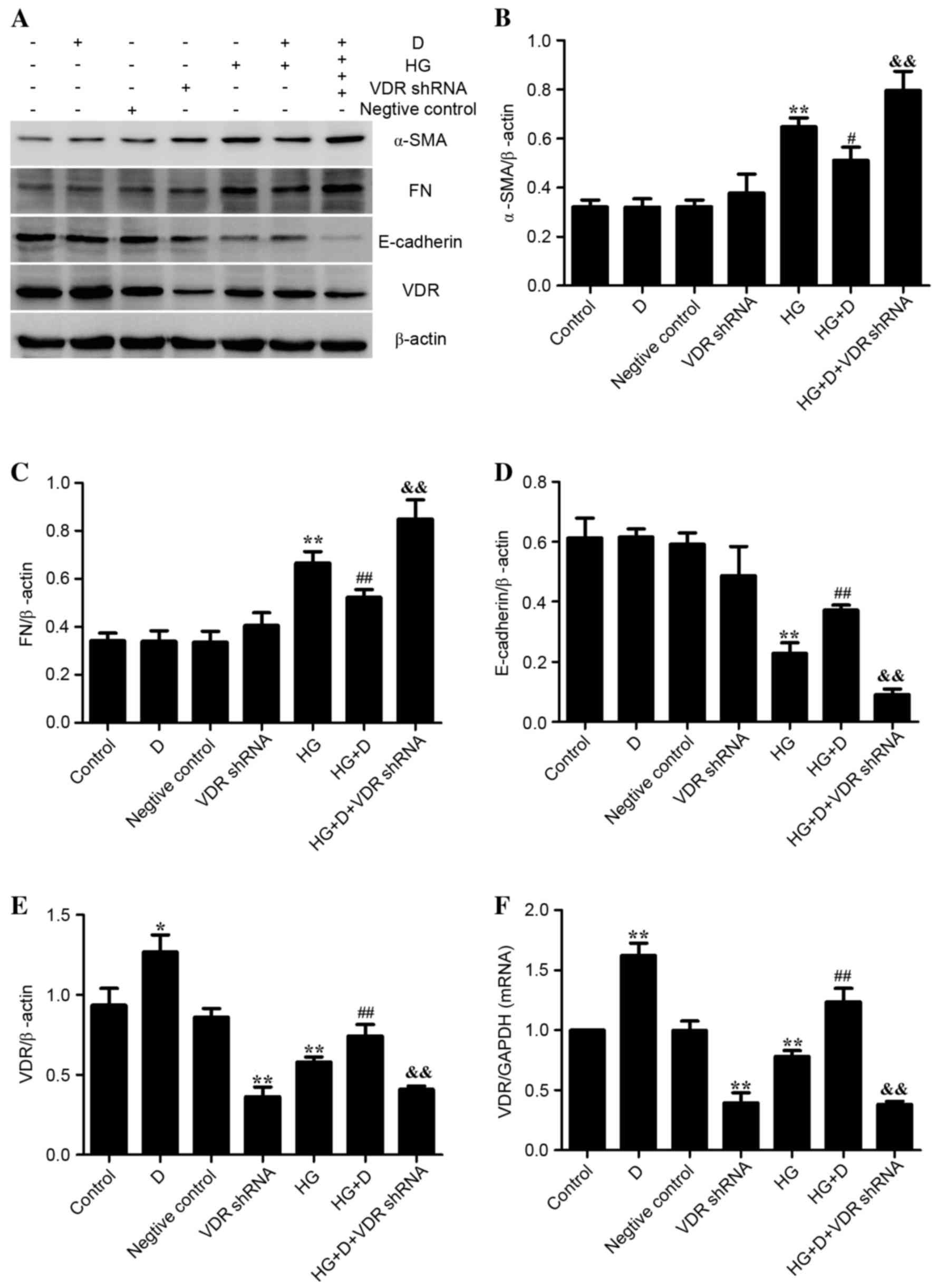 | Figure 3.Effect of VDR on
epithelial-mesenchymal transition biomarkers in HG-treated HPMCs.
(A) Western blotting was used to determine protein expression
levels. Relative expression levels of (B) α-SMA, (C) FN, (D)
E-cadherin and (E) VDR were calculated and normalized to the
loading control. Corresponding protein levels were assessed using
densitometry and are expressed as relative intensities. Data are
presented as the mean ± standard error (n=3). (F) Reverse
transcription-quantitative polymerase chain reaction was performed
to detect the relative gene expression of VDR in HPMCs. Data are
presented as the mean ± standard error (n=6). *P<0.05 and
**P<0.01 vs. control; #P<0.05 and
##P<0.01 vs. HG group; &&P<0.01
vs. HG+D group. D, 1,25(OH)2D3; HG, high
glucose; shRNA, short hairpin RNA; VDR, vitamin D receptor; α-SMA,
α-smooth muscle actin; FN, fibronectin; HPMCs, human peritoneal
mesothelial cells. |
Effects of
1,25(OH)2D3 on the TGFβ/Smad pathway in
HG-treated HPMCs
TGFβ/Smad signaling pathway has been previously
reported to be involved in EMT (15). However, it remains to be elucidated
whether 1,25(OH)2D3 attenuated EMT in HPMCs
through TGFβ/Smad pathway. Therefore, the present study examined
the effect of 1,25(OH)2D3 supplementation on
the TGFβ/Smad pathway in the HPMCs. It was indicated that HG
treatment increased the expression of TGFβ. Smad activation was
further analyzed by western blotting with a p-Smad3 antibody. As
presented in Fig. 5A and B, when
the cells were exposed to HG alone, Smad3 phosphorylation was
increased compared with the control (P<0.01), whereas it was
significantly decreased when 1,25(OH)2D3
pretreatment was administered (P<0.01). These observations
suggest that 1,25(OH)2D3 protects
HG-stimulated HPMCs via the TGFβ/Smad pathway.
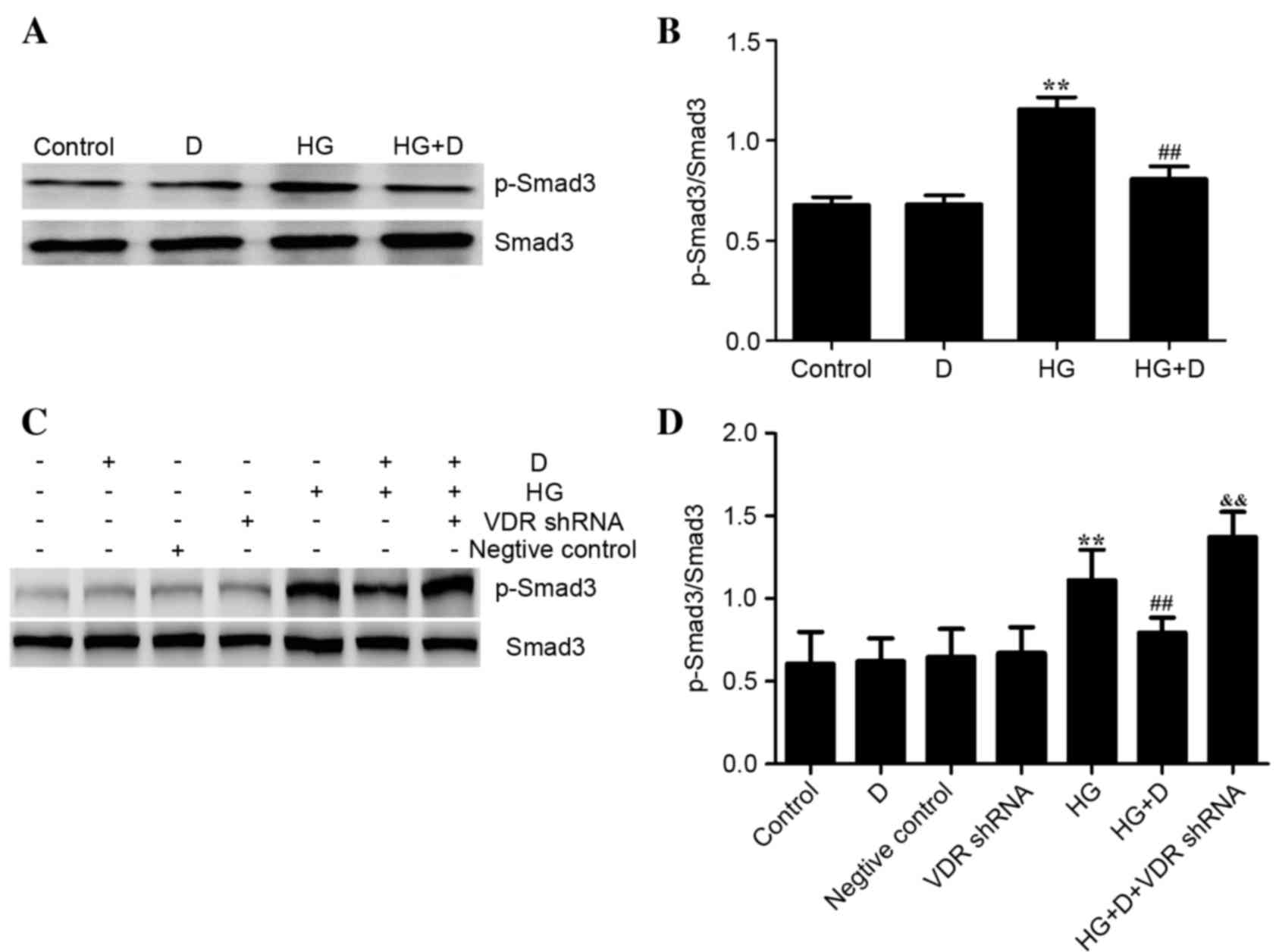 | Figure 5.Effects of
1,25(OH)2D3/VDR on the TGFβ/Smad pathway in
HG-treated HPMCs. HPMCs were exposed to 126 mM HG following
pretreatment with 10−7 mol/l
1,25(OH)2D3. (A) Western blotting was used to
determine protein expression. (B) p-Smad3/Smad3 protein expression
levels were assessed using densitometry and were expressed as
relative intensities. Data are presented as the mean ± standard
error (n=3). **P<0.01 vs. control; ##P<0.01 vs. HG
group. (C) Western blotting was used to determine protein
expression levels following VDR downregulation. (D) Protein
expression levels of p-Smad3/Smad3 were assessed using densitometry
and were expressed as relative intensities. Data are presented as
the mean ± standard error (n=3). **P<0.01 vs. control;
##P<0.01 vs. HG group, &&P<0.01
vs. HG+D group. VDR, vitamin D receptor; TGFβ, transforming growth
factor β; Smad3, SMAD family member 3; HG, high glucose; HPMCs,
human peritoneal mesothelial cells; D,
1,25(OH)2D3; p-Smad3, phosphorylated-Smad3;
shRNA, short hairpin RNA. |
The VDR-shRNA plasmid was used to observe the
association of VDR and the TGFβ/Smad pathway. As presented in
Fig. 5C and D, it was determined
that 126 mM HG upregulated the expression level of p-Smad3, which
was reversed by 1,25(OH)2D3 pretreatment.
These effects were diminished when cells were transfected with
VDR-shRNA.
Discussion
Peritoneal fibrosis is a serious complication in
patients with ESRD, especially those undergoing long-term PD
therapy. Vitamin D deficiency is highly prevalent in patients
undergoing dialysis. Previous studies suggested that
1,25(OH)2D3 may affect organ fibrosis and
exhibit anti-inflammatory capabilities (16–18).
EMT is a key process leading to the subsequent development of
peritoneal fibrosis and peritoneal failure associated with PD
(19). A previous study indicated
that the low expression of VDR in chronic kidney diseases was
likely mediated by proinflammatory TNF-α, and late administration
of active vitamin D was effective in restoring VDR expression and
inhibited EMT in the mouse unilateral ureter obstruction model
(20). Additionally,
1,25(OH)2D3 has been identified to prevent
lung and pancreatic cancer progression by inhibiting EMT (21,22).
Previous studies demonstrated that EMT in HPMCs was associated with
the recurrent use of HG treatment and has been linked to a decline
of peritoneal function due to peritoneal fibrosis (23,24).
Therefore, it is possible that 1,25(OH)2D3
may affect fibrosis via inhibition of EMT in HPMCs. The present
study used HG stimuli to reproduce the damage of peritoneal EMT
in vitro and examined the effect of
1,25(OH)2D3 on the EMT of HPMCs. The findings
of the current study indicated that HG reduced VDR expression,
altered HPMC morphology and EMT markers, decreased E-cadherin
expression levels and increased of α-SMA and FN expression.
Additionally, it was determined that
1,25(OH)2D3 served an important role in
protecting HG-treated HPMCs against EMT by binding to VDR.
Previous studies suggested that prolonged and
chronic inflammation may lead to the occurrence of peritoneal
fibrosis (25,26). However, how to prevent peritoneal
inflammation and EMT remains to be elucidated. The present study
determined that HG treatment promoted EMT, increased the expression
levels of inflammatory cytokines such as TGFβ and IL-6.
Additionally, it was identified that
1,25(OH)2D3 decreased TGFβ and IL-6
expression levels and reversed HG-induced EMT.
The TGFβ/Smad pathway has been thoroughly studied in
terms of EMT and inflammation. A previous study demonstrated that
vitamin D attenuated renal tubular cell injury by suppressing
inflammation and EMT processes through inhibition of the NF-κB,
TGFβ/Smad and β-catenin signaling pathways (12). Lee et al (27) determined that vitamin D reduced
fibrosis, which may be due to its modulation of the
anti-inflammatory potentials. The present study determined the
association between TGFβ/Smad signaling, EMT and inflammation of
HPMCs following HG treatment. The current observations demonstrated
that treatment with HG activated the TGFβ/Smad signaling, and these
changes were attenuated by 1,25(OH)2D3
pretreatment.
In conclusion, the present study provided novel
evidence on the association between
1,25(OH)2D3/VDR and EMT in HPMCs. It was
determined that 1,25(OH)2D3 inhibited
HG-induced EMT and inflammatory cytokines in HPMCs by binding to
its receptor, VDR. In addition, 1,25(OH)2D3
may exert its function via the TGFβ/Smad pathway. Understanding the
role of 1,25(OH)2D3/VDR in EMT and
inflammation may improve the understanding of the subsequent
EMT-mediated fibrosis and peritoneal injury in the development of
PD. However, future studies should use animal models in vivo
in order to determine efficacy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81300636).
References
|
1
|
Williams JD, Craig KJ, Topley N, Von
Ruhland C, Fallon M, Newman GR, Mackenzie RK and Williams GT:
Peritoneal Biopsy Study Group: Morphologic changes in the
peritoneal membrane of patients with renal disease. J Am Soc
Nephrol. 13:470–479. 2002.PubMed/NCBI
|
|
2
|
Ding X, Ma M, Teng J, Shao F, Teng RK,
Zhou S, Zhang Y, Wu E and Wang X: Numb induces E-cadherin adhesion
dissolution, cytoskeleton reorganization, and migration in tubular
epithelial cells contributing to renal fibrosis. Curr Mol Med.
15:368–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao S, Zhang Y, Zheng X, Tu X, Li H, Chen
J, Zang Y and Zhang J: Loss of microRNA-101 promotes epithelial to
mesenchymal transition in hepatocytes. J Cell Physiol.
230:2706–2717. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Strippoli R, Loureiro J, Moreno V,
Benedicto I, Lozano ML, Barreiro O, Pellinen T, Minguet S, Foronda
M, Osteso MT, et al: Caveolin-1 deficiency induces a
MEK-ERK1/2-Snail-1-dependent epithelial-mesenchymal transition and
fibrosis during peritoneal dialysis. EMBO Mol Med. 7:3572015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He L, Lou W, Ji L, Liang W, Zhou M, Xu G,
Zhao L, Huang C, Li R, Wang H, et al: Serum response factor
accelerates the high glucose-induced Epithelial-to-Mesenchymal
Transition (EMT) via snail signaling in human peritoneal
mesothelial cells. PLoS One. 9:e1085932014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Zeng L, Zhao Y, Zhu B, Ren W and Wu
C: Selenium suppresses lipopolysaccharide- induced fibrosis in
peritoneal mesothelial cells through inhibition of
epithelial-to-mesenchymal transition. Biol Trace Elem Res.
161:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu MA, Shin KS, Kim JH, Kim YI, Chung SS,
Park SH, Kim YL and Kang DH: Hgf and bmp-7 ameliorate high
glucose-induced epithelial-to-mesenchymal transition of peritoneal
mesothelium. J Am Soc Nephrol. 20:567–581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X, Wang J, Fan Y, Yang L, Wang L and
Ma J: Zinc supplementation attenuates high glucose-induced
epithelial-to-mesenchymal transition of peritoneal mesothelial
cells. Biol Trace Elem Res. 150:229–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanasaki K, Taduri G and Koya D: Diabetic
nephropathy: The role of inflammation in fibroblast activation and
kidney fibrosis. Front Endocrinol (Lausanne). 4:72013.PubMed/NCBI
|
|
11
|
Gocek E, Kiełbiński M, Wyłób P, Kutner A
and Marcinkowska E: Side-chain modified vitamin D analogs induce
rapid accumulation of VDR in the cell nuclei proportionately to
their differentiation-inducing potential. Steroids. 73:1359–1366.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim CS, Joo SY, Lee KE, Choi JS, Bae EH,
Ma SK, Kim SH, Lee J and Kim SW: Paricalcitol attenuates
4-hydroxy-2-hexenal-induced inflammation and epithelial-mesenchymal
transition in human renal proximal tubular epithelial cells. PLoS
One. 8:e631862013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fischer KD and Agrawal DK: Vitamin D
regulating TGF-β induced epithelial-mesenchymal transition. Respir
Res. 15:1462014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang L, Wang J, Fan Y, Chen S, Wang L and
Ma J: Effect of 1,25(OH)(2)D(3) on rat peritoneal mesothelial cells
treated with high glucose plus lipopolysaccharide. Cell Immunol.
271:173–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao Q, Pawlaczyk K, Ayala ER, Styszynski
A, Breborowicz A, Heimburger O, Qian JQ, Stenvinkel P, Lindholm B
and Axelsson J: The role of the TGF/Smad signaling pathway in
peritoneal fibrosis induced by peritoneal dialysis solutions.
Nephron Exp Nephrol. 109:e71–e78. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu L, Kong M, Han YP, Bai L, Zhang X,
Chen Y, Zheng S, Yuan H and Duan Z: Spontaneous liver fibrosis
induced by long term dietary vitamin D deficiency in adult mice is
related to chronic inflammation and enhanced apoptosis. Can J
Physiol Pharmacol. 93:385–394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Greń A: Effects of vitamin E, C and D
supplementation on inflammation and oxidative stress in
streptozotocin-induced diabetic mice. Int J Vitam Nutr Res.
83:168–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duggan C, de Dieu Tapsoba J, Mason C,
Imayama I, Korde L, Wang CY and McTiernan A: Effect of Vitamin D3
supplementation in combination with weight loss on inflammatory
biomarkers in postmenopausal women: a randomized controlled trial.
Cancer Prev Res (Phila). 8:628–635. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Q, Yang M, Lan H and Yu X: miR-30a
negatively regulates TGF-β1-induced epithelial-mesenchymal
transition and peritoneal fibrosis by targeting Snai1. Am J Pathol.
183:808–819. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiong M, Gong J, Liu Y, Xiang R and Tan X:
Loss of vitamin D receptor in chronic kidney disease: A potential
mechanism linking inflammation to epithelial-to-mesenchymal
transition. Am J Physiol Renal Physiol. 303:F1107–F1115. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Upadhyay SK, Verone A, Shoemaker S, Qin M,
Liu S, Campbell M and Hershberger PA: 1,25-dihydroxyvitamin D3
(1,25(OH)2D3) signaling capacity and the epithelial-mesenchymal
transition in non-small cell lung cancer (NSCLC): Implications for
Use of 1,25(OH)2D3 in NSCLC treatment. Cancers (Basel).
5:1504–1521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Z, Guo J, Xie K and Zheng S: Vitamin D
receptor signaling and pancreatic cancer cell EMT. Curr Pharm Des.
21:1262–1267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aroeira LS, Aguilera A, Sánchez-Tomero JA,
Bajo MA, del Peso G, Jiménez-Heffernan JA, Selgas R and
López-Cabrera M: Epithelial to mesenchymal transition and
peritoneal membrane failure in peritoneal dialysis patients:
Pathologic significance and potential therapeutic interventions. J
Am Soc Nephrol. 18:2004–2013. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Del Peso G, Jiménez-Heffernan JA, Bajo MA,
Aroeira LS, Aguilera A, Fernández-Perpén A, Cirugeda A, Castro MJ,
De Gracia R, Sánchez-Villanueva R, et al: Epithelial-to-mesenchymal
transition of mesothelial cells is an early event during peritoneal
dialysis and is associated with high peritoneal transport. Kidney
Int Suppl. S26–S33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yokoi H, Kasahara M, Mori K, Ogawa Y,
Kuwabara T, Imamaki H, Kawanishi T, Koga K, Ishii A, Kato Y, et al:
Pleiotrophin triggers inflammation and increased peritoneal
permeability leading to peritoneal fibrosis. Kidney Int.
81:160–169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gangji AS, Brimble KS and Margetts PJ:
Association between markers of inflammation, fibrosis and
hypervolemia in peritoneal dialysis patients. Blood Purif.
28:354–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee TW, Kao YH, Lee TI, Chang CJ, Lien GS
and Chen YJ: Calcitriol modulates receptor for advanced glycation
end products (RAGE) in diabetic hearts. Int J Cardiol. 173:236–241.
2014. View Article : Google Scholar : PubMed/NCBI
|