Introduction
The human heart loses most of its regenerative
capacity during postnatal development, and is not able to replace
any defects after damage with functional myocardium (1). An increasing number of general
treatments have been reported to be unable to aid complete cardiac
regeneration and repair (2). Since
stem cells can be induced for specific differentiation into
cardiomyocytes under certain conditions, therapies based on stem
cells have generated interest among researchers in recent years
(3,4). Mesenchymal stem cells (MSCs) have the
characteristics of autologous transplantation, as they are easy to
isolate and are characterized by a strong ability for
amplification, excellent gene stability and low immunogenicity
(1,5). A large number of studies have shown
that cardiovascular regeneration based on stem cells may cure
cardiovascular diseases with cardiomyocyte damage (6). However, the specific molecular
mechanism underlying this cure remains elusive.
Insulin gene enhancer binding protein ISL-1
(Islet-1), a subtype of the LIM-homeodomain (LIM-HD) transcription
factor subfamily, contains one DNA binding site and two LIM domains
(7–9). Several studies have demonstrated that
Islet-1 is crucial to cardiac development and cardiomyocyte
differentiation (10,11). Islet1-null mice completely lack the
outflow tract, right ventricle and much of the atria (10,12).
Lineage tracing of Islet1-expressing progenitors demonstrate that
Islet-1 is a marker for a distinct population of undifferentiated
cardiac progenitors (12).
Previous studies from this group indicated that Islet-1 serves a
critical role in the differentiation of MSCs into cardiomyocytes
and promotes the expression of heart development-related genes in
MSCs.
Islet-1 may be able to affect the acetylation levels
of the cardiomyocyte-specific early transcription factors NK2
homeobox 5 (Nkx2.5) and GATA binding protein 4 (GATA4) to regulate
their expression levels and promote their differentiation into
cardiomyocytes (13). Although
previous studies have demonstrated that histone acetyltransferases
(HATs) serve critical roles in the regulation of
cardiomyocyte-specific gene expression (14,15),
the specific HATs involved in this process are unknown. In addition
to histone acetylation, DNA methylation is significant in the
regulation of gene expression (16). For example, DNA methyltransferase
(DNMT)-1, DNMT-3a and DNMT-3b participate as the major DNMTs in the
methylation of different genes to regulate their DNMTs in the
methylation of different genes to regulate their expression
(17–20). Therefore, it was hypothesized that
DNA methylation participated in the regulation of relevant genes
during the complex process of MSCs differentiation into
cardiomyocytes. However, few investigations have been conducted to
identify the major DNMTs involved in this process.
In the current study, C3H10T1/2 MSCs were infected
with lentiviruses overexpressing Islet-1, in order to promote their
specific differentiation into cardiomyocytes. Alterations over time
in histone H3K9 acetylation and DNA methylation levels on the
promoter regions of GATA4 and Nkx2.5 were assessed during the
process of MSC differentiation into cardiomyocytes. In addition,
HATs and DNMTs that bound to the GATA4 and Nkx2.5 promoter regions
were evaluated. The expression trends of the early-stage
cardiomyocyte-specific genes GATA4 and Nkx2.5 were examined and the
relationship between the changing trends in histone H3K9
acetylation levels and DNA methylation levels during the MSCs
differentiation into cardiomyocyte-like cells promoted by Islet-1.
Finally, a mechanism underlying the involvement of these two
epigenetic modifications in the regulation of differentiation was
preliminarily proposed.
Materials and methods
Cell culture and lentiviral vector
infection
C3H10T1/2 cells, obtained from University of Chicago
Molecular Oncology Laboratory (Chicago, IL, USA), were grown in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS, EMD Millipore, Billerica, MA, USA). Lentiviruses
(Lv) overexpressing GFP or Islet-1/GFP (multiplicity of
infection=20) (GeneChem Co., Ltd., Shanghai, China), and 5 µg/ml
polybrene (GeneChem Co., Ltd.) were mixed together and added to the
culture medium of cells when they reached 30% confluence. The
culture medium was replaced following culturing at 37°C in 5%
CO2 for 24 h. Fluorescence microscopy (Eclipse Ti-s;
Nikon Corporation, Tokyo, Japan) was used to observe the GFP
expression after 3 days. The infection efficiency was assessed with
flow cytometry (BD FACSCanto II, BD Biosciences, San Jose, CA,
USA), and prepared by BD FACS Diva version 3.0. The experiment was
divided into 3 groups: The blank group, the Lv-GFP group (GFP
cells) and the Lv-islet-1 group (cells infected with a plasmid
overexpressing Islet-1/GFP). The Lv-islet-1 group was further
divided into the following subgroups: Islet-1-1 week, Islet-1-2
weeks, Islet-1-3 weeks and Islet-1-4 weeks based on the lentiviral
infection time.
Immunofluorescence
C3H10T1/2 cells (1×105 cells/well) were
plated in 24-well plates on 1×1 cm2 glass coverslips.
Then, the cells were fixed in absolute acetone for 15 min at 4°C.
Following 3 washes with PBS, the cells on the glass coverslips were
blocked with goat serum (dilution, 1:20, ZSGB-BIO, Beijing, China),
washed again, and incubated with the primary anti-cardiac troponin
T(cTnT) monclonal antibody (ab209813; 1:400; Abcam, Cambridge, MA,
USA) overnight at 4°C. Then, the cells were washed with PBS and
incubated with a Cy3-conjugated secondary antibody (CW0159S; 1:150;
Beijing Cowin Bioscience Co., Ltd., Beijing, China) for 1 h at
37°C. Following washing with PBS, 4′,6-diamidino-2-phenylindole was
added for 3 min. Following the final wash, images were acquired
under a fluorescence microscope (BX51; Olympus Corporation, Tokyo,
Japan) and prepared by Nikon NIS-element AR 4.0 software. A total
of six fields of view were assessed, and three replicates were
performed.
Total RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Cellular RNA was extracted from the blank group, the
Lv-GFP group and the Lv-islet-1 groups (Islet-1-1, Islet-1-2,
Islet-1-3 and Islet-1-4 weeks) according to the instructions of the
RNA extraction reagent kit (RP120; BioTeke Corporation, Beijing,
China) and subjected to reverse transcription by PrimeScript™ RT
Master Mix kit (RR047A; Takara Biotechnology Co., Ltd., Dalian,
China). The cDNA was amplified (RR047A; Takara Biotechnology Co.,
Ltd.), using the reaction conditions of: 40 cycles of 95°C for 30
sec, 95°C for 5 sec and 60°C for 40 sec. Each reaction contained
one blank well, and the samples of each group included three
replicate wells. β-actin was used as the internal control. The
relative expression levels of the genes were calculated using the
2-ΔΔCq method (21). The changes
in the gene expression levels of GATA4, Nkx2.5 and Mef2c were
assessed at all time points. The primer sequences of the genes are
provided in Table I.
 | Table I.Primer sequences used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used in reverse
transcription-quantitative polymerase chain reaction.
| Target | Sequence
(5′-3′) |
|---|
| Nkx2.5 |
F-GAGCCTGGTAGGGAAAGAGC |
|
|
R-GGTGGGTGTGAAATCTGAGG |
| GATA4 |
F-GACTACCACCACCACGCTGT |
|
|
R-ATTCAGGTTCTTGGGCTTCC |
| Mef2c |
F-ATCCCAGTGTCCAGCCATAA |
|
|
R-AGACCGCCTGTGTTACCTG |
| β-actin |
F-GGAGATTACTGCCCTGGCTCCTA |
|
|
R-GACTCATCGTACTCCTGCTTGCTG |
Chromatin immunoprecipitation
(ChIP)-qPCR assay
Formaldehyde (1%) was added to the samples to
cross-link the protein-DNA complexes. The ChIP trials were
conducted using a ChIP assay kit (Merck KGaA Darmstadt, Germany).
Following cross-linking, the DNA was fragmented by sonication
(Bioruptor UCD-200; Diagenode, Liège, Belgium) consisting of 25
cycles of 30 sec each, with an interval of 30 sec to cool down.
Then, the protein-DNA complexes were precipitated with the
following antibodies: Histone H3 (acetyl K9; ab10812; 3 µg/µl),
general control of amino acid biosynthesis protein 5 (Gcn5;
ab18381; 7 µg/µl), P300 (ab14984; 5 µg/µl), DNMT1 (ab87656; 5
µg/µl), DNMT3a (ab2850; 9 µg/µl) or DNMT3b (ab2851; 9 µg/µl), All
antibodies purchased from Abcam and incubated overnight on a shaker
at 4°C. DNA was extracted using the ChIP assay kit. The experiment
included both a positive control (DNA precipitated by the RNA
polymerase II antibody) and a negative control (DNA precipitated by
normal mouse IgG), these antibodies all part of the assay kit noted
above and used according to the manufacturer's protocols. The
amount of extracted DNA was determined by qPCR (RR420A; Takara
Biotechnology Co., Ltd., Dalian, China), the thermocycler
conditions and the method of quantification strictly followed the
ChIP assay kit protocols. The primer sequences and annealing
temperatures of the ChIP-qPCR reaction are presented in Table II.
 | Table II.Primer sequences and annealing
temperatures used in chromatin immunoprecipitation-quantitative
polymerase chain reaction. |
Table II.
Primer sequences and annealing
temperatures used in chromatin immunoprecipitation-quantitative
polymerase chain reaction.
| Target | Sequence
(5′-3′) | Tm
(°C) |
|---|
| Nkx2.5 |
F-ACCGCCTGGGTGATAGAC | 58.37 |
|
|
R-CCCTCCCGAGATTGAAGAT | 55.87 |
| GATA4 |
F-GCTACAGGGAGTGATGAGAAG | 53.90 |
|
|
R-CACCAGCCCAGGAGTTTAT | 54.70 |
Methylation-specific (MS-)PCR
Cellular DNA was extracted from the blank group, the
negative control group and the Lv-islet-1 groups (Islet-1-1,
Islet-1-2, Islet-1-3, and Islet-1-4 weeks) according to the
instructions of the DNA extraction kit (Tiangen Biotech Co., Ltd.,
Beijing, China). The DNA concentrations were determined to ensure
that the amount of DNA treated with bisulfite was 350 ng. The
volume of the DNA required for each group was calculated based on
the concentration. The DNA bisulfite treatment reagent kit (Zymo
Research, Irvine, CA, USA) was used. The primers for MS-PCR were
designed using the MethPrimer software according to the previously
described method (22). The primer
sequences for GATA4 and Nkx2.5 are provided in Table III.
 | Table III.Primer sequences for GATA4 and Nkx2.5
used in methylation-specific-polymerase chain reaction. |
Table III.
Primer sequences for GATA4 and Nkx2.5
used in methylation-specific-polymerase chain reaction.
| Target | Primer | Upstream primer
(5′-3′) | Downstream primer
(5′-3′) | Product length
(bp) |
|---|
| GATA4 | Methylation |
GGGTTTATAGGTATTGACGTCGA |
GATAAAAACTACAAAACGCCGAA | 291 |
| GATA4 |
Non-methylation |
AGGGTTTATAGGTATTGATGTTGA |
CCAATAAAAACTACAAAACACCAAA | 294 |
| Nkx2.5 | Methylation |
ATTTTTTAAATTGTTATCGCGATTC |
AACCTAACTTAAAACCCTCCCG | 203 |
| Nkx2.5 |
Non-methylation |
TTTTTAAATTGTTATTGTGATTTGT |
ACCTAACTTAAAACCCTCCCAAA | 200 |
A 2% agarose gel was prepared, and the loading
volume of the DNA ladder marker and the MS-PCR products was 5 µl.
The electrophoresis was run for 45 min at 120 V in 0.25X TAE
agarose gel electrophoresis buffer. The agarose electrophoresis
results were observed using the chemiluminescence gel imaging
system (G:box; Syngene, Cambridge, UK). The gray value of the
electrophoresis band was determined using the Quantity One software
(version 4.6.2; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Protein extraction and western
blotting
Proteins were extracted from the cells using the
radioimmunoprecipitation assay reagent (P0013B; Beyotime Institute
of Biotechnology, Shanghai, China) containing 1%
phenylmethanesulfonyl fluoride (cat. no. ST506; Beyotime Institute
of Biotechnology) to prevent protein degradation. The protein
concentrations were measured with the bicinchoninic acid method.
The protein samples (40 µg protein each well) were mixed with 5X
SDS-PAGE buffer (Beyotime Institute of Biotechnology). The sample
loading buffer was boiled for 5 min prior to loading onto a 10%
SDS-PAGE gel. Following electrophoresis, the proteins were
transferred to polyvinylidene fluoride membranes (EMD Millipore).
The membranes were cut according to the marker and incubated in 5%
non-fat milk with PBS and Tween-20 (PBST, 0.05% Tween-20) for 1 h
on a shaker at room temperature to block non-specific protein
binding. The primary antibodies used in the present study were as
follows: Anti-Islet-1 (EP4182; 1:2,000; Epitomics, Burlingame, CA,
USA), anti-Gcn5 (ab18381; 1:1,000; Abcam), anti-P300 (ab14984;
1:1,000; Abcam), anti-DNMT1 (ab87656; 1:1,000; Abcam), anti-DNMT3a
(ab2850; 1:1,000; Abcam) and anti-DNMT3b (ab2851; 1:1,000; Abcam).
The β-actin antibody (A5441; 1:2,000; Sigma-Aldrich; Merck KGaA)
was used as a control. The membranes were incubated with the
primary antibodies overnight at 4°C and then washed in PBST 3 times
for 10 min and incubated with the corresponding secondary antibody
(ZB-2301 and ZB-2305, 1:2,000; ZSGB-BIO) on a shaker at room
temperature. Positive bands were detected using a chemiluminescent
reaction (EMD Millipore). The image collection and densitometry
analyses were performed with the Quantity One analysis software
(version 4.6.2; Bio-Rad Laboratories, Inc.).
Statistical analysis
Each experiment was repeated at least three times.
All data were expressed as the mean ± standard deviation. The
statistical evaluations were performed using independent samples by
using Student's paired t-tests, continuity correction chi-square
test and one-way analysis of variance and Dunnett's as a post hoc
test. SPSS software (version 17.0; SPSS Inc., Chicago, IL, USA) was
used for the statistical analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Islet-1 promotes the differentiation
of MSCs into cardiomyocyte-like cells
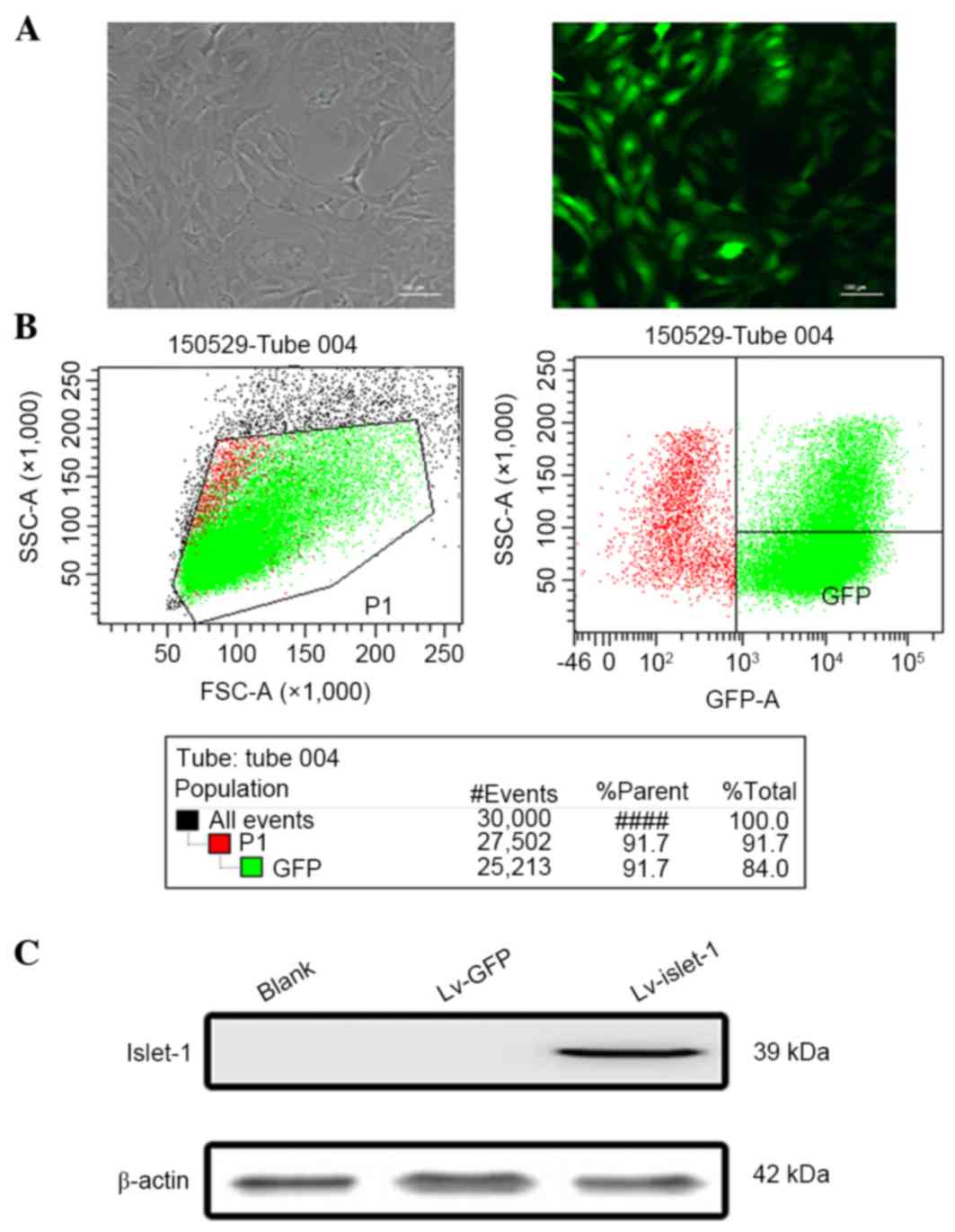
The GFP fluorescence results demonstrated that the
GFP was stably expressed, indicating that the lentiviral infection
was successful (Fig. 1A). The flow
cytometry results demonstrated that the infection efficiency
reached 91.7% (Fig. 1B). These
results ensured the reliability of subsequent experiments. The
western blotting results indicated that the C3H10 T1/2 cells had a
high level of Islet-1 expression following lentiviral infection
compared with the blank group and the control group (Fig. 1C). No visible difference in
morphology was observed in untransfected MSCs and the Lv-GFP group
(Fig. 2A). However, following
Islet-1 transfection, the MSCs became fibroblast-like cells
arranged in the same direction, exhibiting a short rod-shaped
morphology and had a homogenous direction, a tight arrangement and
a strong refraction (Fig. 2A).
cTnT immunofluorescence was visibly higher in the Lv-islet-1 group
compared with the blank and Lv-GFP groups, indicating that the MSCs
expressed the cardiomyocyte-specific protein in the cytoplasm at 4
weeks following Islet-1 infection (Fig. 2B). The detection of
cardiomyocyte-specific early-stage transcription factors indicated
that the expression of Nkx2.5, GATA4 and myocyte enhancer factor 2C
(Mef2c) gradually increased with time, and was highest in the
Islet-1-3W group (Fig. 2C). These
results suggested that Islet-1 promoted the differentiation of MSCs
into cardiomyocyte-like cells.
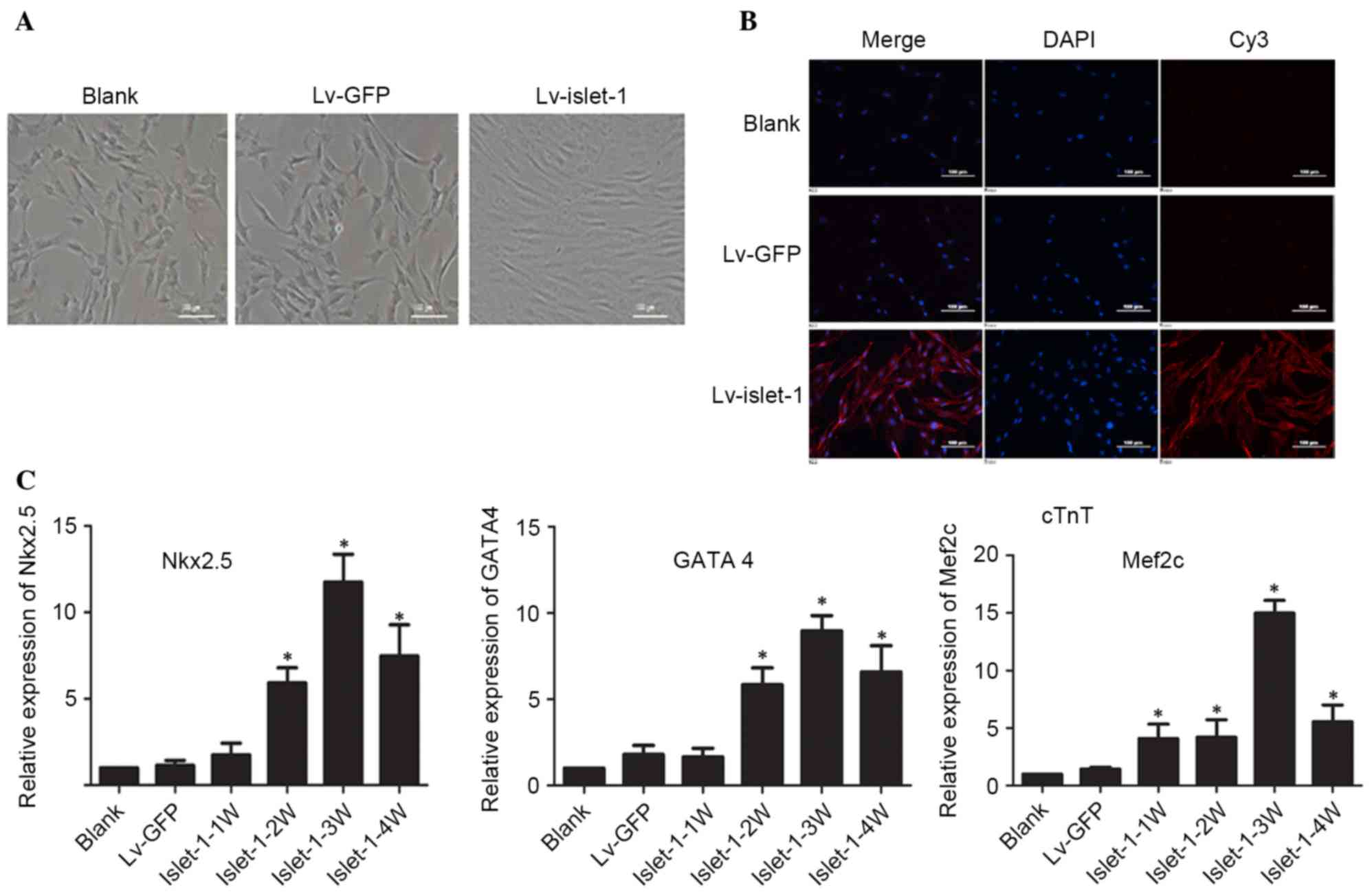 | Figure 2.Islet-1 induces the differentiation
of C3H10T1/2 cells into cardiomyocytes. (A) The morphological
alterations in C3H10T1/2 cells transfected with Lv-GFP or
Lv-islet-1 were observed under a microscope. Scale bar=100 µm. (B)
Expression of cTnT detected by immunofluorescence microscopy. Scale
bar=100 µm. (C) Reverse transcription-quantitative polymerase chain
reaction detected variations in mRNA expression levels of
cardiac-specific transcription factors in C3H10T1/2 cells infected
with lentiviral vectors containing Islet-1. *P<0.05 vs. blank
group. Lv-GFP, lentiviral vector containing green fluorescent
protein; Lv-islet-1, lentiviral vector containing Islet-1; cTnT,
troponin T2 cardiac type; Nkx2.5, NK2 homeobox 5; GATA4, GATA
binding protein 4; Mef2c, myocyte enhancer factor 2C; 1 W, 1 week;
2 W, 2 weeks; 3 W, 3 weeks; 4 W, 4 weeks. |
Histone acetylation and DNA
methylation participate in the regulation of early-stage
transcription factors involved in cardiomyocyte development during
MSC differentiation into cardiomyocyte-like cells
The MS-PCR results indicated that the methylation
level of the CpG sites on the GATA4 promoter gradually decreased
following Islet-1 transfection; the decrease was most significant
at week 3 (P<0.05; Fig. 3A).
The methylation levels of the CpG sites on the Nkx2.5 gene promoter
were higher but did not exhibit significant differences compared
with the blank group and Lv-GFP group (Fig. 3B).
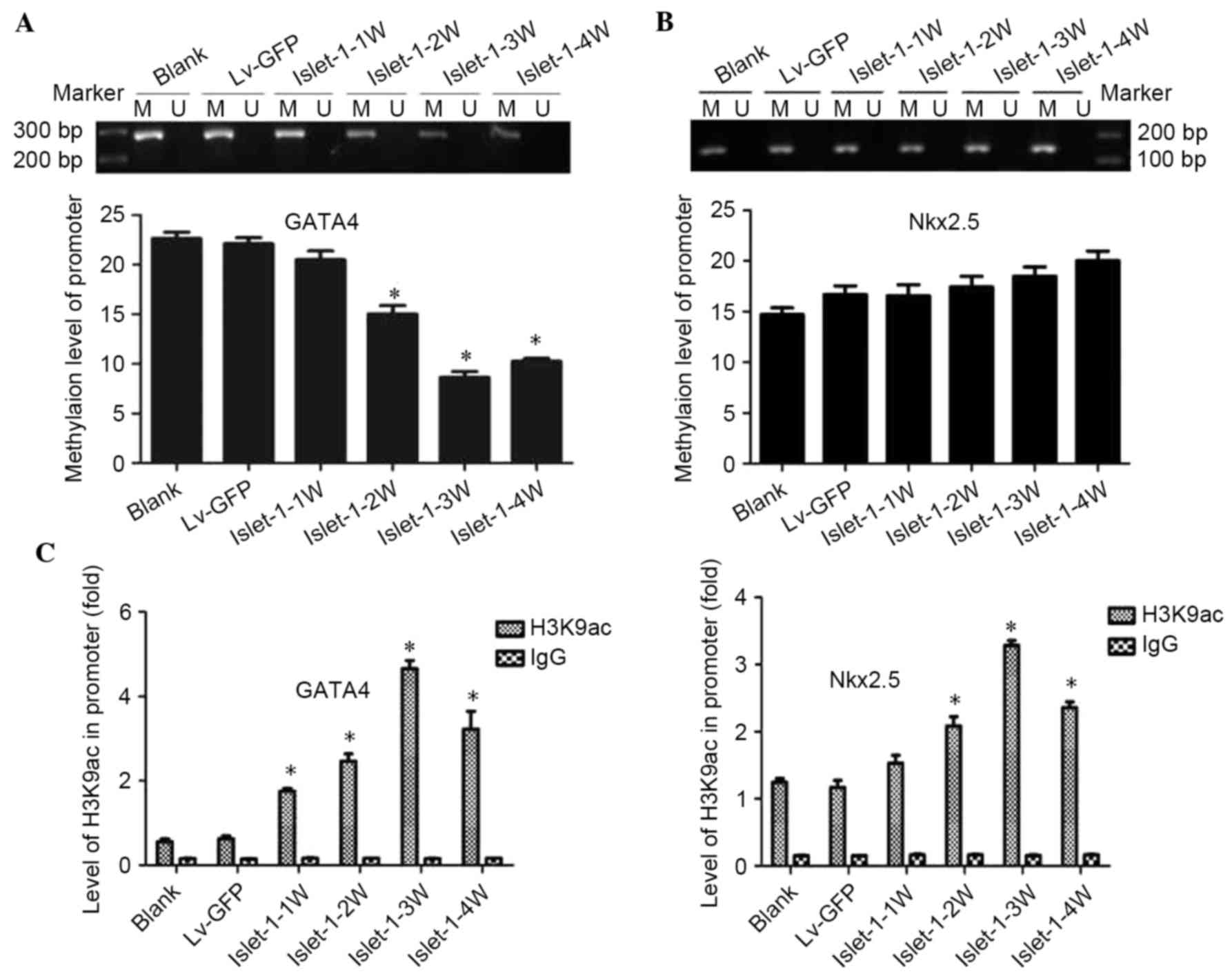 | Figure 3.DNA methylation levels and
acetylation levels of the histone H3K9 site in the GATA4 and Nkx2.5
promoter regions during the differentiation process promoted by
Islet-1. (A) The detection of methylation levels on the GATA4
promoter (1329–1489 bp) by MSP assay. (B) The detection of the
methylation levels at the Nkx2.5 promoter (51–219 bp) by MSP assay.
(C) ChIP results demonstrated the levels of histone acetylation on
the promoter regions of GATA4 and Nkx2.5. *P<0.05 vs. blank
group. GATA4, GATA binding protein 4; Nkx2.5, NK2 homeobox 5; MSP,
methylation-specific polymerase chain reaction; Lv-GFP, lentiviral
vector containing green fluorescent protein; Lv-islet-1, lentiviral
vector containing Islet-1; M, methylated; U, unmethylated; 1 W, 1
week; 2 W, 2 weeks; 3 W, 3 weeks; 4 W, 4 weeks. |
The ChIP-qPCR results demonstrated that the levels
of histone acetylation on the promoter regions of GATA4 and Nkx2.5
in the Lv-islet-1 group were gradually increased with time; the
expression of GATA4 and Nkx2.5 combined with H3K9ac in the
C3H10T1/2 cells infected with Lv-Islet-1 gradually increased, with
the peak time of binding at week 3 (P<0.05; Fig. 3C). These results indicated that
histone acetylation participated in the regulation of GATA4 and
Nkx2.5; by contrast, Nkx2.5 may not be affected by DNA
methylation.
Islet-1 alters the histone acetylation
levels of GATA4 and Nkx2.5 through the regulation of Gcn5
To elucidate the mechanism underlying the
involvement of histones in the regulation of early-stage
transcription factors in cardiomyocytes, protein expression of the
major HATs, Gcn5 and P300, was assessed. The western blotting
results indicated that the expression level of Gcn5 gradually
increased following Islet-1 infection: The expression levels at all
time points in the Lv-islet-1 group were higher than those in the
blank group and Lv-GFP group, and the Islet-1-3 W was highest
(Fig. 4A). The expression levels
of P300 gradually decreased and the expression levels at all time
points in the Lv-islet-1 group were significantly lower than those
in the blank group and the negative control group (P<0.05;
Fig. 4A).
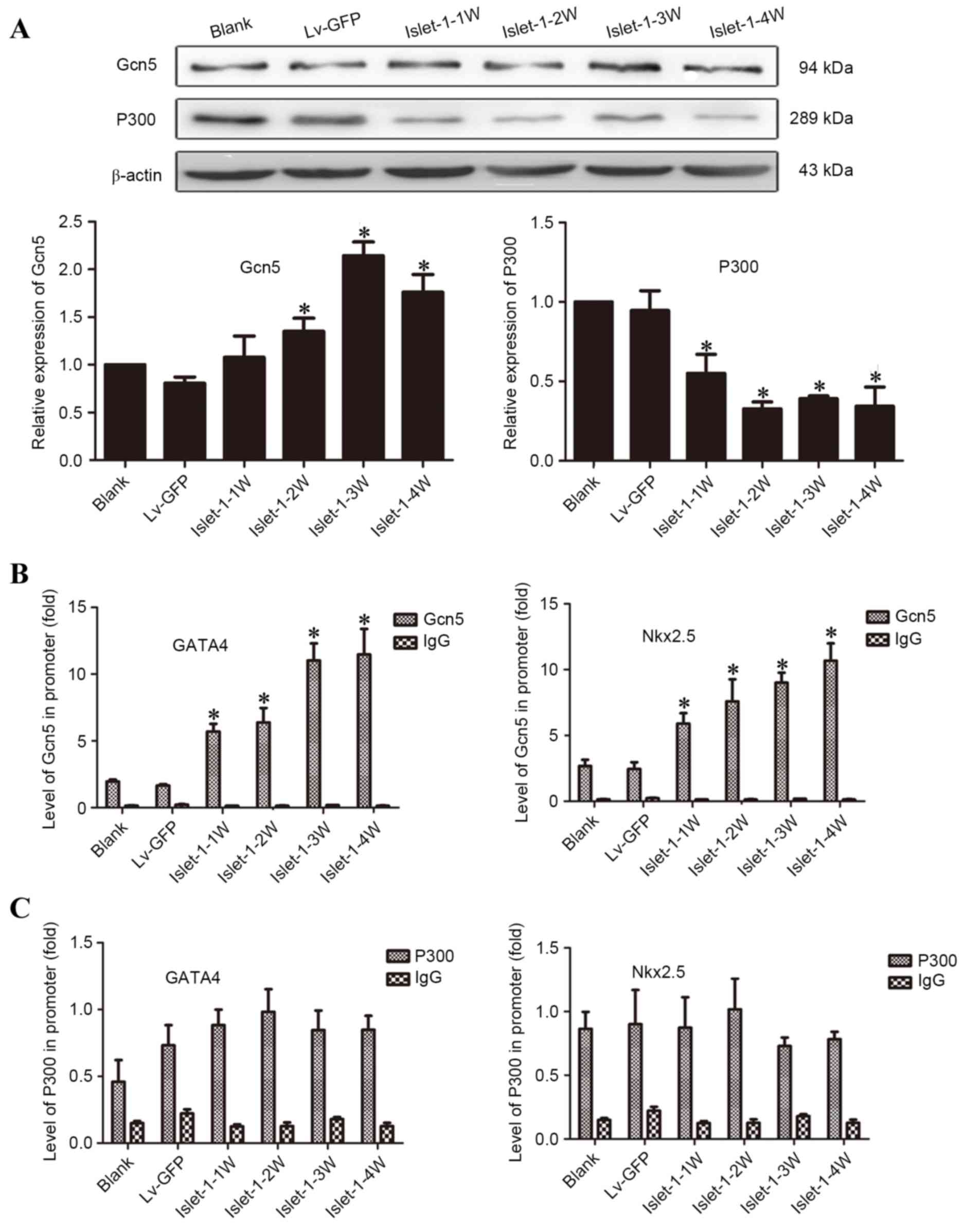 | Figure 4.Detection of HATs on the histone H3K9
site that regulate the promoter regions of GATA4 and Nkx2.5. (A)
Western blot analysis of Gcn5 and P300 HATs, with quantification
relative to β-actin. (B) ChIP analysis of Gcn5 bound to the GATA4
and Nkx2.5 promoter regions. (C) ChIP analysis of P300 bound to the
GATA4 and Nkx2.5 promoter regions. *P<0.05 vs. blank control.
HATS, histone acetyltransferases; GATA4, GATA binding protein 4;
Nkx2.5, NK2 homeobox 5; Lv-GFP, lentiviral vector containing green
fluorescent protein; Lv-islet-1, lentiviral vector containing
Islet-1; 1 W, 1 week; 2 W, 2 weeks; 3 W, 3 weeks; 4 W, 4 weeks;
Gcn5, general control of amino acid biosynthesis protein 5; ChIP,
chromatin immunoprecipitation. |
The ChIP-qPCR results demonstrated that the levels
of GATA4 and Nkx2.5 bound with Gcn5 gradually increased following
Islet-1 infection, which was consistent with the increased
expression of Gcn5 (Fig. 4B). The
binding levels at all time points in the Lv-islet-1 group were
higher than those in the blank group and the Lv-GFP group
(P<0.05; Fig. 4B). The
expression of the GATA4 and Nkx2.5 binding with P300 did not
significantly change following Islet-1 infection compared with
those in the blank group and the Lv-GFP group (P>0.05; Fig. 4C). These results indicated that
Islet-1 enhanced the binding level of Gcn5 to the GATA4 and Nkx2.5
promoter regions through the increase in Gcn5 expression.
Islet-1 alters the DNA methylation
levels of the GATA4 promoter region through the regulation of
DNMT-1
Previous studies indicated that DNA methylation
participated in the Islet-1-induced MSCs differentiation into
cardiomyocyte-like cells (23).
Therefore, the present study further investigated the underlying
mechanism. The western blotting results indicated that the DNMT-1
expression level gradually decreased following Islet-1 infection
and that the expression levels at all time points in the Lv-islet-1
group were lower than those in the blank group and the Lv-GFP group
(Fig. 5A). The expression level of
DNMT-3a in the Lv-islet-1 group gradually increased; the expression
levels at all time points in the Lv-islet-1 group were
significantly higher than those in the blank group and the Lv-GFP
group (P<0.05; Fig. 5A). By
contrast, DNMT-3b expression was almost undetectable (data not
shown).
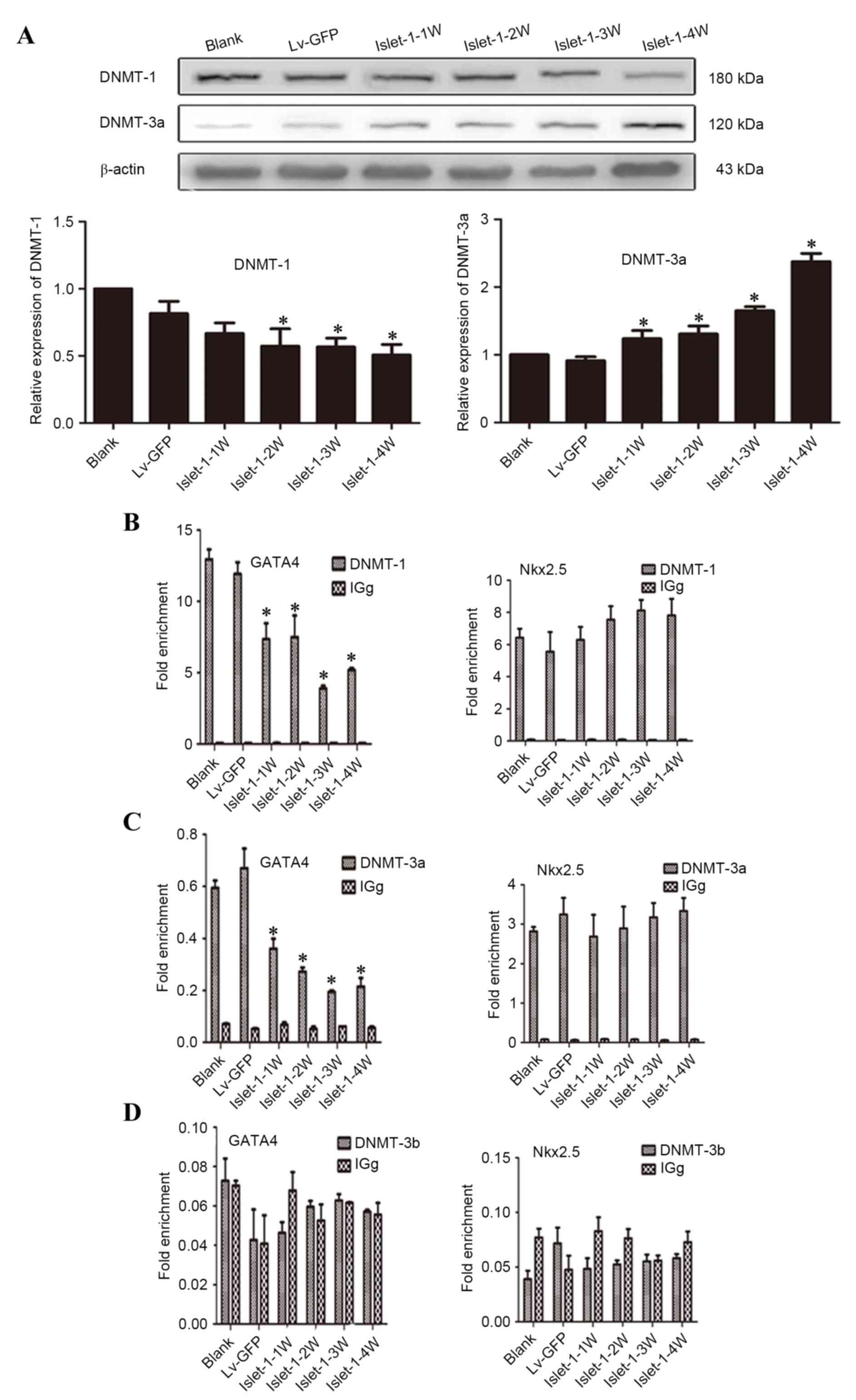 | Figure 5.Detection of DNMTs that regulate the
GATA4 promoter region. (A) Western blot analysis of DNMT-1 and
DNMT-3a expression, with quantification relative to β-actin. (B)
ChIP analysis of DNMT-1 bound to the GATA4 and Nkx2.5 promoter
regions. (C) ChIP analysis of DNMT-3a bound to the GATA4 and Nkx2.5
promoter regions. (D) ChIP analysis of DNMT-3b bound to the GATA4
and Nkx2.5 promoter regions. *P<0.05 vs. blank control. DNMT,
DNA methyltransferase; GATA4, GATA binding protein 4; Nkx2.5, NK2
homeobox 5; Lv-GFP, lentiviral vector containing green fluorescent
protein; Lv-islet-1, lentiviral vector containing Islet-1; 1 W, 1
week; 2 W, 2 weeks; 3 W, 3 weeks; 4 W, 4 weeks; ChIP, chromatin
immunoprecipitation. |
ChIP-qPCR analysis identified that the levels of
GATA4 bound with DNMT-1 gradually decreased following Islet-1
infection and the binding levels at all time points in the
experimental group were significantly lower than those in the blank
group and the Lv-GFP group (P<0.05; Fig. 5B, GATA4); the same trend was also
demonstrated for the GATA4 bound with DNMT-3a (P<0.05; Fig. 5C, GATA4). Almost no DNMT-3b binding
was detected on the GATA4 and Nkx2.5 promoter region (Fig. 5D). In addition, DNMT-1 and DNMT-3a
were demonstrated to bind to the Nkx2.5 promoter region, and the
level of binding following Islet-1 infection was not significantly
different compared with the blank group (P>0.05; Fig. 5B and C, Nkx2.5, respectively).
These results indicated that Islet-1 could reduce the DNMT-1
expression level and thus reduce its binding to the GATA4 promoter
region. Eventually, the DNA methylation levels in the GATA4
promoter region decreased and GATA4 expression was promoted.
However, DNMT-1 did not affect Nkx2.5 expression.
Discussion
The process of mesenchymal stem cell differentiation
into cardiomyocytes is regulated by many factors, including
intercellular interaction, signal pathway, epigenetics and
paracrine (24–26). Studies have demonstrated that
epigenetic modifications, such as histone acetylation and DNA
methylation serve important roles in this process (27). Histone acetylation is the process
by which the lysine residues within the N-terminal tail protruding
from the histone core of the nucleosome are acetylated to determine
the transcriptional activity of chromatin (28), while DNA methylation is a process
by which methylation modifications are added to alter the function
of the DNA that is critical in the regulation of gene expression
(29). A previous study from this
group suggested the differentiation of stem cells into
cardiomyocyte-like cells promoted by Islet-1 (13). The current study focused on two
epigenetic modification methods: Histone acetylation and DNA
methylation. The aim of the study was to elucidate which histone
acetyltransferases and DNA methyltransferases could regulate the
expression of specific early-stage transcription factors in
cardiomyocytes and promote the differentiation of MSCs into
cardiomyocyte-like cells.
The role of histone acetylation in early development
and differentiation is a current topic of interest (30,31).
Regulation by this modification primarily occurs through HATs. The
major function of HATs is to perform acetylation modification of
the lysine residue at the amino terminus of the chromatin core
histones, thereby loosening the chromatin structure and increasing
the gene transcription activities (32). The first discovered histone
acetyltransferase, Gcn5, primarily modifies nucleosomal histones
and the free histones H3 and H4 (33–35).
P300 is a coactivator and HAT that modifies 4 histones (H2A, H2B,
H3 and H4) (33,36,37).
The present study demonstrated that during the Islet-1-induced
differentiation of stem cells into cardiomyocyte-like cells, Gcn5
expression and its binding to the GATA4 and Nkx2.5 promoter regions
both gradually increased. Conversely, the expression of P300
gradually decreased during the process of Islet-1-induced
differentiation of stem cells into cardiomyocyte-like cells, and
only a low level of binding was detected at the GATA4 and Nkx2.5
promoter regions. These results suggested that Islet-1 increased
Gcn5 expression to increase its binding to the Nkx2.5 and GATA4
promoter regions, enhance Nkx2.5 and GATA4 expression, and finally
promote the differentiation of MSCs into cardiomyocyte-like
cells.
DNA methylation is an important process in
epigenetic modification, and is essential for normal development
and stem cell differentiation. In mammalian cells, DNA methylation
occurs mainly at the C5 position of CpG dinucleotides by DNA
methyltransferase, which is a key enzyme in DNA methylation
(38,39). The main function of DNMT1 is to
maintain the status and form of DNA methylation, whereas the main
functions of DNMT3a and DNMT3b are to catalyze new DNA methylation
sites and establish new methylation patterns (40). The results of the present study
demonstrated a decrease in the methylation of CpG sites on the
GATA4 promoter during the differentiation of C3H10T1/2 cells into
cardiomyocyte-like cells induced by Islet-1, while this process was
negatively associated with the GATA4 mRNA expression level. In
addition, DNMT-1 expression and its binding to GATA4 both gradually
decreased during the Islet-1 induced differentiation of stem cells
into cardiomyocyte-like cells. Although DNMT-3a expression
gradually increased, the binding level to the GATA4 promoter was
decreased. DNMT-3b expression and its binding to GATA4 and Nkx2.5
were almost undetectable. Furthermore, it was observed that,
although DNMT-1 bound to Nkx2.5, the level of binding did not
become altered during the differentiation process. The authors
speculated that Islet-1 decreased DNMT-1 expression to reduce its
binding to GATA4 and caused the gradual reduction of the
methylation level of the GATA4 gene, thereby increasing GATA4 gene
expression. There was no association between the binding level of
DNMT-1 in Nkx2.5 promoter and the expression of Nkx2.5, which
suggested that Nkx2.5 was not regulated by DNA methylation in the
process.
A previous study has identified links between DNA
methylation and histone hypoacetylation (41). In the present study, the histone
acetylation level on the GATA4 promoter presented a gradual
increasing trend that was positively correlated with the mRNA
level. In addition, the histone acetylation level on the Nkx2.5
promoter was consistent with its expression level and showed a
gradual increasing trend. However, the methylation level of CpG
sites on the Nkx2.5 promoter did not significantly alter during the
differentiation process. Therefore, it was concluded that DNA
methylation and histone acetylation concurrently participated in
the regulation of GATA4 expression during the Islet-1-induced
differentiation of C3H10T1/2 cells into cardiomyocyte-like cells.
In contrast, Nkx2.5 expression may not be affected by DNA
methylation. These results indicated that DNA methylation did not
regulate the expression of all genes and thus exhibited
selectivity. Furthermore, histone acetylation levels and DNA
methylation levels had opposing trends with GATA4 expression.
Previous studies have reported that epigenetic modifications
influenced one another during the regulation of gene expression
(42). Therefore, these two
modifications may have interactive functions during the regulation
of GATA4 expression. However, this hypothesis requires further
study for validation.
In summary, the present study confirmed that histone
acetylation and DNA methylation participated in the regulation of
the early specific gene GATA4 in cardiomyocytes through Gcn5 and
DNMT-1 during the Islet-1-induced differentiation of MSCs into
cardiomyocytes. However, the Nkx2.5 expression appeared to be
regulated by Gcn5 instead of DNA methylation. Furthermore, it was
observed that these two epigenetic modifications had a specific
relationship. Future studies are required to clarify whether there
is association between them and to elucidate the mechanism
underlying their interaction. The current study preliminarily
proposed the mechanism underlying the promotion of MSCs
differentiation into cardiomyocyte-like cells based on the histone
acetylation and DNA methylation induced by Islet-1. These results
provided an important experimental basis for future studies on the
function of epigenetic modifications in MSCs differentiation and
novel insights into the study of the specific differentiation of
MSCs.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81370261).
Glossary
Abbreviations
Abbreviations:
|
MSCs
|
mesenchymal stem cells
|
|
LIM-HD
|
LIM-homeodomain
|
|
DNMT
|
DNA methyltransferase
|
|
HATs
|
histone acetyltransferases
|
|
cTnT
|
troponin T2 cardiac type
|
|
ChIP
|
chromatin immunoprecipitation
|
|
MSP
|
methylation-specific PCR
|
|
Gcn5
|
general control of amino acid
biosynthesis protein 5
|
|
Islet-1
|
insulin gene enhancer binding protein
ISL-1
|
|
H3K9
|
histone H3 at lysine 9
|
|
GATA4
|
GATA binding protein 4
|
|
Nkx2.5
|
NK2 homeobox 5
|
|
Mef2c
|
myocyte enhancer factor 2C
|
|
Lv
|
lentivirus
|
|
SDS-PAGE
|
sodium dodecyl sulfate polyacrylamide
gel electrophoresis
|
References
|
1
|
Chou SH, Lin SZ, Kuo WW, Pai P, Lin JY,
Lai CH, Kuo CH, Lin KH, Tsai FJ and Huang CY: Mesenchymal stem cell
insights: Prospects in cardiovascular therapy. Cell Transplant.
23:513–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuraitis D, Ruel M and Suuronen EJ:
Mesenchymal stem cells for cardiovascular regeneration. Cardiovasc
Drugs Ther. 25:349–362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bianco P, Robey PG and Simmons PJ:
Mesenchymal stem cells: Revisiting history, concepts and assays.
Cell Stem Cell. 2:313–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aldahmash A, Zaher W, Al-Nbaheen M and
Kassem M: Human stromal (mesenchymal) stem cells: Basic biology and
current clinical use for tissue regeneration. Ann Saudi Med.
32:68–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang L, Ma W, Ma Y, Feng D, Chen H and
Cai B: Exosomes in mesenchymal stem cells, a new therapeutic
strategy for cardiovascular diseases? Int J Biol Sci. 11:238–245.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sheng CC, Zhou L and Hao J: Current stem
cell delivery methods for myocardial repair. Biomed Res Int.
2013:5479022013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brade T, Gessert S, Kühl M and Pandur P:
The amphibian second heart field: Xenopus islet-1 is required for
cardiovascular development. Dev Biol. 311:297–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bu L, Jiang X, Martin-Puig S, Caron L, Zhu
S, Shao Y, Roberts DJ, Huang PL, Domian IJ and Chien KR: Human ISL1
heart progenitors generate diverse multipotent cardiovascular cell
lineages. Nature. 460:113–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang L, Cai CL, Lin L, Qyang Y, Chung C,
Monteiro RM, Mummery CL, Fishman GI, Cogen A and Evans S: Isl1Cre
reveals a common Bmp pathway in heart and limb development.
Development. 133:1575–1585. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Laugwitz KL, Moretti A, Caron L, Nakano A
and Chien KR: Islet1 cardiovascular progenitors: A single source
for heart lineages? Development. 135:193–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakano A, Nakano H and Chien KR:
Multipotent islet-1 cardiovascular progenitors in development and
disease. Cold Spring Harb Symp Quant Biol. 73:297–306. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL,
Chen J and Evans S: Isl1 identifies a cardiac progenitor population
that proliferates prior to differentiation and contributes a
majority of cells to the heart. Dev Cell. 5:877–889. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yin N, Lu R, Lin J, Zhi S, Tian J and Zhu
J: Islet-1 promotes the cardiac-specific differentiation of
mesenchymal stem cells through the regulation of histone
acetylation. Int J Mol Med. 33:1075–1082. 2014.PubMed/NCBI
|
|
14
|
Li L, Zhu J, Tian J, Liu X and Feng C: A
role for Gcn5 in cardiomyocyte differentiation of rat mesenchymal
stem cells. Mol Cell Biochem. 345:309–316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng C, Zhu J, Sun HC, Huang XP, Zhao WA,
Zheng M, Liu LJ and Tian J: Inhibition of histone H3K9 acetylation
by anacardic acid can correct the over-expression of Gata4 in the
hearts of fetal mice exposed to alcohol during pregnancy. PLoS One.
9:e1041352014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bird A: DNA methylation patterns and
epigenetic memory. Genes Dev. 16:6–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ting AH, Jair KW, Suzuki H, Yen RW, Baylin
SB and Schuebel KE: Mammalian DNA methyltransferase 1: Inspiration
for new directions. Cell Cycle. 3:1024–1026. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, Strawn E, Basir Z, Halverson G and
Guo SW: Aberrant expression of deoxyribonucleic acid
methyltransferases DNMT1, DNMT3A and DNMT3B in women with
endometriosis. Fertil Steril. 87:24–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luczak MW, Roszak A, Pawlik P, Kędzia H,
Kędzia W, Malkowska-Walczak B, Lianeri M and Jagodziński PP:
Transcriptional analysis of CXCR4, DNMT3A, DNMT3B and DNMT1 gene
expression in primary advanced uterine cervical carcinoma. Int J
Oncol. 40:860–866. 2012.PubMed/NCBI
|
|
20
|
Liao J, Karnik R, Gu H, Ziller MJ, Clement
K, Tsankov AM, Akopian V, Gifford CA, Donaghey J, Galonska C, et
al: Targeted disruption of DNMT1, DNMT3A and DNMT3B in human
embryonic stem cells. Nat Genet. 47:469–478. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
reltive gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu L, Xia Y, He J, Liu X, Chen X, Wang Y
and Ding Y: Data analysis and its analytical softs application on
DNA methylation in tumor research. Zhong Qing Yi Xue Bian Ji Bu.
41:1719–1721, 1726. 2012.(In Chinese).
|
|
23
|
Xu H, Yi Q, Yang C, Wang Y, Tian J and Zhu
J: Histone modifications interact with DNA methylation at the GATA4
promoter during differentiation of mesenchymal stem cells into
cardiomyocyte-like cells. Cell Prolif. 49:315–329. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawamura T, Ono K, Morimoto T, Wada H,
Hirai M, Hidaka K, Morisaki T, Heike T, Nakahata T, Kita T and
Hasegawa K: Acetylation of GATA-4 is involved in the
differentiation of embryonic stem cells into cardiac myocytes. J
Biol Chem. 280:19682–19688. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pasini A, Bonafè F, Govoni M, Guarnieri C,
Morselli PG, Sharma HS, Caldarera CM, Muscari C and Giordano E:
Epigenetic signature of early cardiac regulatory genes in native
human adipose-derived stem cells. Cell Biochem Biophys. 67:255–262.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang H and Wang ZZ: Mechanisms that
mediate stem cell self-renewal and differentiation. J Cell Biochem.
103:709–718. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohtani K and Dimmeler S: Epigenetic
regulation of cardiovascular differentiation. Cardiovasc Res.
90:404–412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Spivakov M and Fisher AG: Epigenetic
signatures of stem-cell identity. Nat Rev Genet. 8:263–271. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hawkins RD, Hon GC, Lee LK, Ngo Q, Lister
R, Pelizzola M, Edsall LE, Kuan S, Luu Y, Klugman S, et al:
Distinct epigenomic landscapes of pluripotent and lineage-committed
human cells. Cell Stem Cell. 6:479–491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Horikoshi M: Histone acetylation: From
code to web and router via intrinsically disordered regions. Curr
Pharm Des. 19:5019–5042. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oligny LL: Human molecular embryogenesis:
An overview. Pediatr Dev Pathol. 4:324–343. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sadoul K, Boyault C, Pabion M and Khochbin
S: Regulation of protein turnover by acetyltransferases and
deacetylases. Biochimie. 90:306–312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kurdistani SK and Grunstein M: Histone
acetylation and deacetylation in yeast. Nat Rev Mol Cell Biol.
4:276–284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu Z, Song N, Zheng M, Liu X, Liu Z, Xing
J, Ma J, Guo W, Yao Y, Peng H, et al: Histone acetyltransferase
GCN5 is essential for heat stress-responsive gene activation and
thermotolerance in arabidopsis. Plant J. 84:1178–1191. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kuo YM and Andrews AJ: Quantitating the
specificity and selectivity of Gcn5-mediated acetylation of histone
H3. PLoS One. 8:e548962013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kornacki JR, Stuparu AD and Mrksich M:
Acetyltransferase p300/CBP associated factor (PCAF) regulates
crosstalk-dependent acetylation of histone H3 by distal site
recognition. ACS Chem Biol. 10:157–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng M, Zhu J, Lu T, Liu L, Sun H, Liu Z
and Tian J: P300-mediated histone acetylation is essential for the
regulation of GATA4 and MEF2C by BMP2 in H9c2 cells. Cardiovasc
Toxicol. 13:316–322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nagre NN, Subbanna S, Shivakumar M,
Psychoyos D and Basavarajappa BS: CB1-receptor knockout neonatal
mice are protected against ethanol-induced impairments of DNMT1,
DNMT3A and DNA methylation. J Neurochem. 132:429–442. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arakawa Y, Watanabe M, Inoue N, Sarumaru
M, Hidaka Y and Iwatani Y: Association of polymorphisms in DNMT1,
DNMT3A, DNMT3B, MTHFR and MTRR genes with global DNA methylation
levels and prognosis of autoimmune thyroid disease. Clin Exp
Immunol. 170:194–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Subramaniam D, Thombre R, Dhar A and Anant
S: DNA methyltransferases: A novel target for prevention and
therapy. Front Oncol. 4:802014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Minardi D, Lucarini G, Filosa A, Zizzi A,
Milanese G, Polito M Jr, Polito M, Di Primio R, Montironi R and
Muzzonigro G: Do DNA-methylation and histone acetylation play a
role in clear cell renal carcinoma? Analysis of radical nephrectomy
specimens in a long-term follow-up. Int J Immunopathol Pharmacol.
24:149–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu LP, Wang X, Li L, Zhao Y, Lu S, Yu Y,
Zhou W, Liu X, Yang J, Zheng Z, et al: Histone deacetylase
inhibitor depsipeptide activates silenced genes through decreasing
both CpG and H3K9 methylation on the promoter. Mol Cell Biol.
28:3219–3235. 2008. View Article : Google Scholar : PubMed/NCBI
|