Introduction
Hepatic fibrosis is a primary consequence of liver
injury that results from chronic liver diseases, including chronic
viral hepatitis, alcohol and metabolic liver disease (1,2). The
ultimate aim is to treat the cause of the liver disease, which may
lead to the reversal of fibrosis (3,4).
With emerging novel technology and refined methodologies, knowledge
concerning the mechanisms underlying liver fibrosis and its
pathogenesis are continuously expanding (5). However, the development of
anti-fibrotic compounds represents a major therapeutic challenge
(6).
Vascular endothelial growth factor (VEGF) is an
important mediator of angiogenesis (7). In addition, VEGF and its receptors
serve critical roles in chronic inflammation and liver fibrosis
(8,9). Angiogenesis is involved in chronic
inflammatory liver disease. The fact that these diseases respond
poorly to conventional anti-inflammatory therapy suggests that
angiogenesis may be an effective therapeutic target in reversing
persistent, stable inflammation in chronic liver diseases (10). Intra-hepatic angiogenesis and
sinusoidal remodeling occur in many chronic liver diseases
(11). Anti-angiogenesis treatment
may be a therapeutic approach in liver fibrosis and portal
hypertension (11). It has been
previously reported that anti-angiogenesis drugs, including
sorafenib, sunitinib, and Endostar, which are used in the treatment
of carcinoma, inhibit hepatocellular carcinoma and liver fibrosis
(12–15).
Vatalanib, also known as PTK/ZK, is an orally
active, small molecule tyrosine kinase inhibitor that is effective
against all VEGF receptors (16,17).
It has been demonstrated that the combination of vatalanib and
intravenous doxorubicin administration has encouraging activity in
treating advanced hepatocellular carcinoma (HCC) patients (18). In China, the majority of HCC
patients have co-existing hepatitis B and liver cirrhosis (19). A previous study indicated that
vatalanib had anti-fibrotic effects in experimental liver fibrosis
in vivo (20). However, the
underlying mechanism by which this occurs remains unclear. The aim
of the current study was to determine the anti-fibrogenic effects
of vatalanib in terms of pathological alterations of the liver and
hepatic sinusoidal endothelial cell (SEC) phenotypes in
CCl4-induced fibrotic mice.
Materials and methods
Mouse model of CCl4-induced
liver fibrosis
Vatalanib was purchased from LC Laboratories
(Woburn, MA, USA; cat. no. V-8303), and dissolved in 100 mg/ml
distilled water. A total of 32 male BALB/c mice weighing 18–20 g
were obtained from Beijing Vital River Laboratory Animal Technology
(Beijing, China). The Animal Care and Use Committee of Harbin
Medical University approved all protocols and procedures (Harbin,
China). Animals were housed in an air-conditioned room at 23–25°C
with a 12-h dark/light cycle for one week prior to the initiation
of experiments. All animals received appropriate care during the
study with unlimited access to food and water.
Liver fibrosis was induced in mice by
intraperitoneal injection of CCl4 (40% CCl4
in olive oil, 0.2 ml/100 g body weight, twice weekly) for 6 weeks
as previously reported (21). A
total of four groups were studied: Group 1, normal control mice;
group 2, CCl4 alone; group 3, CCl4 +
vatalanib (50 mg/kg/d by gavage for 6 weeks, vatalanib was
administered simultaneously with a CCl4 injection for 6
weeks); and group 4, CCl4 + vatalanib (50 mg/kg/d by
gavage for 4 weeks, CCl4 alone was administered to mice
for 2 weeks, following which vatalanib was administered to mice
simultaneously with CCl4 injection for another 4 weeks).
In the previous study, vatalanib (50 and 100 mg/kg/d) was well
tolerated by the mice, and had no significant effects on body
weight (22). Therefore, 50
mg/kg/d was used in the present study. Previous studies identified
that CCl4-induced hepatic fibrosis is present after 2
weeks (20). Group 1 received
olive oil intraperitoneally at the same dose and conditions as the
CCl4-treated mice.
Mice were euthanized (via 2% pentobarbital i.p. at
0.3 ml/ 100 g body weight) and liver samples were immediately
snap-frozen in liquid nitrogen and stored at −80°C until further
use. An additional section was embedded in paraffin and sliced into
4–5 µm sections.
Histological examination
Sections were stained with hematoxylin and eosin and
Sirius Red (Solarbio Life Sciences, Beijing, China) for
histopathological analysis and liver fibrosis evaluation. Each
sample was independently assessed and scored by two pathologists,
blinded to the study protocol, according to a fibrosis score system
(16). Liver fibrosis was divided
into seven stages: 0, no fibrosis; 1, short fibrous tissue in
central venule (C); 2, fibrous between central venule and central
venule (C-C) septa appearance; 3, C-C fibrous septa incompletely
developed; 4, C-C septa completely connected (pseudo-lobule); 5,
C-P (portal tract) bridging fibrosis, nodular appearance ≤50%; and
6, nodular appearance >50%.
Hepatic hydroxyproline analyses
Hepatic hydroxyproline was measured using a
hydroxyproline detection kit (Jiancheng Institute of Biotechnology,
Nanjing, China) according to the manufacturer's protocol. The
hydroxyproline content is expressed as mg/g wet liver.
Transmission electron microscopy
(TEM)
Samples were processed for TEM as described
previously (23). Fresh specimens
were fixed in 3% glutaraldehyde (Beijing Brilliance Biochemical
Company, Beijing, China), washed with PBS, and fixed in 1% osmic
acid for 60 min. The samples were dehydrated through an alcohol
series, embedded in EPON 812 epoxy resin (Hede Biotechnology Co.,
Ltd., Beijing, China), and then cut into 50 nm sections with an
ultrathin microtome. Following staining with uranyl acetate and
lead citrate for 30 min, the sections were examined using a
transmission electron microscope (cat. no. H-7650; Hitachi, Ltd.,
Tokyo, Japan). A total of 10 hepatic sinusoids with a diameter of
2–3 µm were randomly selected from each group and subgroup, and the
mean number of fenestrae per hepatic sinusoid was determined. Each
sample was independently assessed and scored by two pathologists,
both blinded to the study protocol.
Biochemical analyses
Blood was collected from the inferior vena cava.
Serum alanine aminotransferase (ALT), aspartate aminotransferase
(AST), total bilirubin (TBIL) and albumin were assayed using an
automatic biochemical analyzer (cat. no. 7600; Hitachi, Tokyo,
Japan).
Immunohistochemistry staining for
α-SMA and CD34
CD34 is a marker of newly-formed blood vessels. The
vascular density in portal and peri-portal areas was assessed by
determining the number of CD34-labeled vessel sections (24). Liver tissue samples were fixed in
10% formalin for 1 week at room temperature, sliced into 4–6 mm
pieces, dehydrated in ethanol and embedded in paraffin wax. α-SMA
and CD34 were detected in paraffin-embedded liver sections (4–5 µm
thick) using the following specific primary antibodies: α-SMA (cat.
no. A2547; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) and
CD34 (cat no. ab81289; Abcam, Cambridge, MA, USA) and an
avidin-biotin complex immunoperoxidase method (15). Following antigen retrieval by
immersion in EDTA (1 mmol/l, pH 8.0) and boiling for 15 min,
sections were pre-blocked with normal goat serum for 10 min, and
incubated for 2 h at room temperature with specific antibodies
(α-SMA, dilution 1:200; CD34, dilution, 1:200). PBS was used in
place of the primary antibodies for the negative controls.
Following rinsing, the biotinylated secondary antibody (goat
anti-rabbit IgG, cat no. ZDR-5307, goat anti-mouse IgG, cat. no.
ZDR-5306, OriGene Technologies, Inc., Beijing, China),
avidin-biotin complex and horseradish peroxidase (Strept ABComplex;
Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) were
applied. Finally, the sections were washed with PBS, developed with
diaminobenzidine tetrahydrochloride substrate (Strept ABComplex;
Dako; Agilent Technologies, Inc.) for 3 min, and counterstained
with hematoxylin.
Computer-assisted semi-quantitative analysis was
used to evaluate the areas of positive α-SMA and CD34 staining
using Image-ProPlus software (version 4.5; Media Cybernetics, Inc.,
Rockville, MD, USA). The data for the α-SMA and CD34 staining are
expressed as the mean percentage of the positively stained area
relative to the total tissue section area (17).
Measurement of mRNA levels of collagen
I, α-SMA, TGF-β1, VEGFR1 and VEGFR2 by RT-qPCR
Total RNA was extracted from liver tissues and
HSC-T6 cells using a TRIzol® Reagent kit (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. The quality of total RNA was verified by
ultraviolet absorbance spectrophotometry at 260 and 280 nm. cDNA
was reversed transcribed using the High-Capacity cDNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. The thermocycling
parameters were as follows: 25°C for 10 min, 37°C for 150 min, 85°C
for 5 sec, and 4°C for 5 min for one cycle, before chilling on ice.
cDNA was stored at −20°C until further required. Collagen I, α-SMA,
TGF-β1, VEGFR1 and VEGFR2 mRNA levels were quantified using the
primers presented in Tables I and
II. GAPDH, a housekeeping gene,
was used as an internal control primer for target genes. All
primers were obtained from Invitrogen; Thermo Fisher Scientific,
Inc. The expression of mRNA was measured by SYBR Green Real-Time
PCR using an ABI 7500 instrument (Applied Biosystems; Thermo Fisher
Scientific, Inc.). PCR was performed in 20 µl buffer that contained
2 µg cDNA, 1 µl each primer and 10 µl SYBR Green PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Comparative
quantitation threshold (Cq) calculations were all relative to the
control group. The expression of mRNA relative to the control was
derived using the equation 2−∆∆Cq (25).
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Direction | Primer sequence
(5′-3′) | Accession
number |
|---|
| GAPDH (mouse) | F |
GCACAGTCAAGGCCGAGAAT | XM_001476707.3 |
|
| R |
GCCTTCTCCATGGTGGTGAA |
|
| Col I a1
(mouse) | F |
TTCACCTACAGCACGCTTGTG | NM_007742.3 |
|
| R |
GATGACTGTCTTGCCCCAAGTT |
|
| α-SMA (mouse) | F |
TCAGCGCCTCCAGTTCCT | NM_007392.2 |
|
| R |
AAAAAAAACCACGAGTAACAAATCAA |
|
| TGF-β1 (mouse) | F |
CCCGAACCCCCATTGCTGTCC | NM_011577.1 |
|
| R |
AGGCGTATCAGTGGGGGTCAG |
|
| VEGFR1 (mouse) | F |
CCACCTCTCTATCCGCTGG | NM_010228.3 |
|
| R |
ACCAATGTGCTAACCGTCTTATT |
|
| VEGFR2 (mouse) | F |
CAAACCTCAATGTGTCTCTTTGC | NM_010612.2 |
|
| R |
AGAGTAAAGCCTATCTCGCTGT |
|
 | Table II.Effects of vatalanib on
CCl4-induced liver fibrosis. |
Table II.
Effects of vatalanib on
CCl4-induced liver fibrosis.
|
| Liver fibrosis
score |
|
|---|
|
|
|
|
|---|
| Group | 0 | 1 | 2 | 3 | 4 | 5 | 6 | Mean ± standard
deviation |
|---|
| 1 (n=7) | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 (n=8) | 0 | 0 | 0 | 0 | 2 | 5 | 1 | 4.88±0.64 |
| 3 (n=8) | 0 | 0 | 4 | 3 | 1 | 0 | 0 |
2.6±0.74a |
| 4 (n=8) | 0 | 0 | 3 | 3 | 1 | 1 | 0 |
3.0±1.07a |
Statistical analysis
The results are expressed as the mean ± standard
deviation. Statistical analysis was performed by analysis of one
way analysis of variance and unpaired Student's t-test as
appropriate. Non-parametric data were analyzed by the Mann-Whitney
U-test. P<0.05 was considered to indicate a statistically
significant difference. Statistical analyses were performed using
SPSS (version 17.0; SPSS Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation.
Results
Vatalanib inhibits liver fibrosis in
CCl4-induced mice
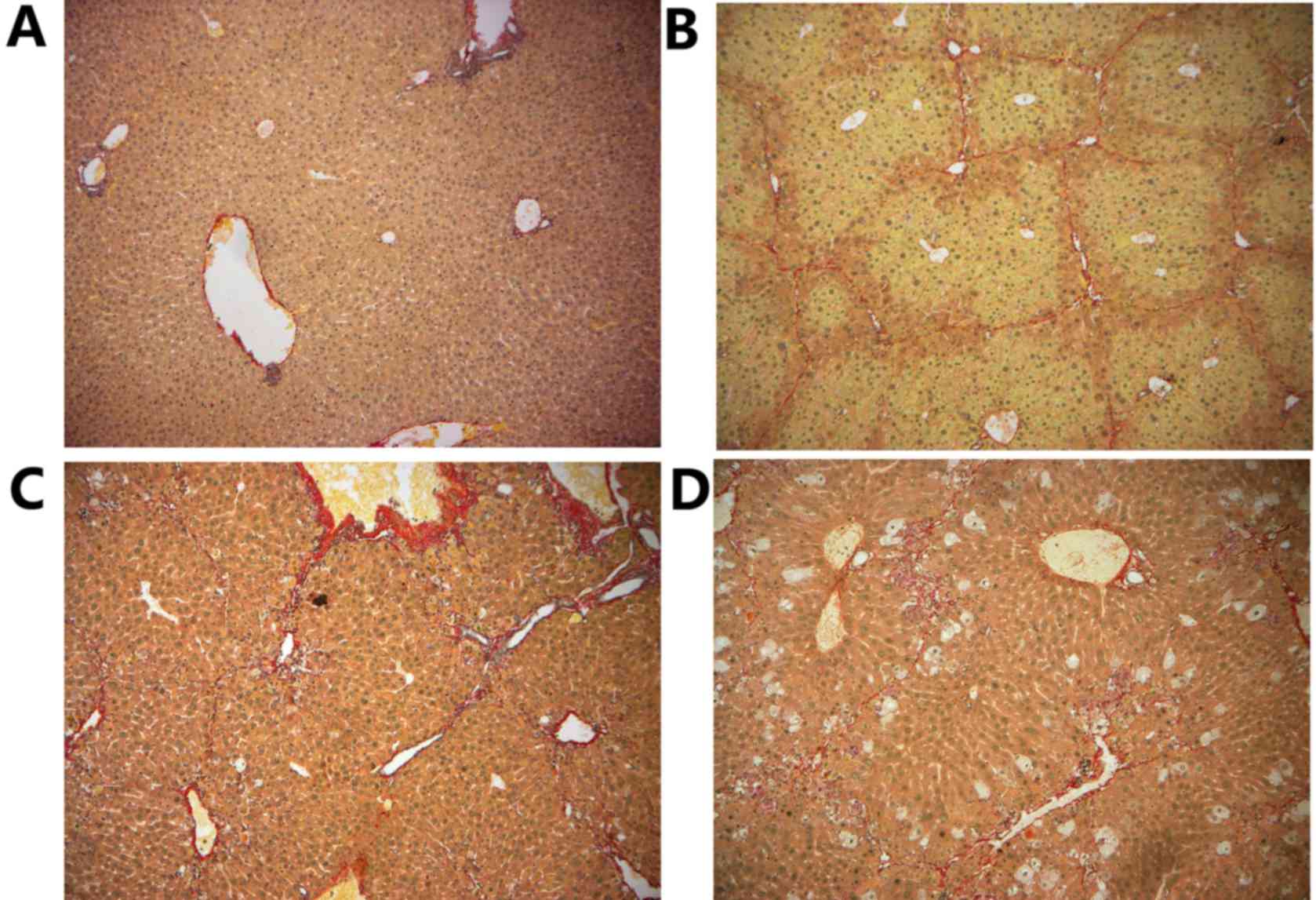
There were no collagen fibers along the central vein
in control group 1 (Fig. 1A). In
group 2, collagenous fibers extended from the central vein and the
portal area to the hepatic lobules with pseudo-lobule formation
(Fig. 1B). With administration of
vatalanib (50 mg/kg/d, for 4 or 6 weeks) in groups 3 (Fig. 1C) and 4 (Fig. 1D), collagenous fibers were
decreased compared with group 2. The fibrosis scores following
vatalanib administration in groups 3 and 4 were significantly
reduced when compared with those in group 2 (P<0.05; Table II). In addition, vatalanib reduced
hydroxyproline concentrations in liver tissue, reflecting decreased
total hepatic collagen content (P<0.01; Table III).
 | Table III.Effects of vatalanib on biochemical
markers in CCl4-treated mice. |
Table III.
Effects of vatalanib on biochemical
markers in CCl4-treated mice.
|
| Group 1 (n=7) | Group 2 (n=8) | Group 3 (n=8) | Group 4 (n=8) |
|---|
| Hydroxyproline
(mg/g liver) | 0.1±0.04 | 92.0±4.6 |
54.2±5.4a |
49.8±6.9a |
| ALT (IU/ml) | 38.4±5.9 | 149.5±19.4 |
98.8±14.6a |
109.2±10.4a |
| AST (IU/ml) | 41.0±8.3 | 235.5±16.8 |
149.0±14.8a |
153.9±19.0a |
| TBIL (mg/dl) | 1.0±0.1 | 1.9±0.2 | 1.8±0.3 | 1.8±0.2 |
| Albumin (g/l) | 34.8±1.5 | 26.2±1.9 | 28.6±1.6 | 29.2±1.8 |
Vatalanib reduces liver
inflammation
Serum ALT and AST levels were significantly
decreased following vatalanib treatment (P<0.01), whereas no
significant alterations in serum TBIL and albumin levels were
observed (P>0.05; Table
III).
Vatalanib inhibits α-SMA and CD34
protein levels
Levels of α-SMA, a typical marker of activated HSCs,
were assessed by immunohistochemistry to evaluate the effect of
vatalanib on HSC activation during hepatic fibrosis. There was no
positive staining in control group 1 (Fig. 2A). CCl4 alone led to
considerable increases in the amount of α-SMA positive cells, which
were distributed throughout the fibrotic septa (Fig. 2B). Computer-assisted
semi-quantitative analysis revealed that vatalanib-treated groups
(groups 3 and 4) presented significantly decreased α-SMA-positive
areas compared with group 2 (P<0.05; Fig. 2C-E). These results demonstrated
that vatalanib decreased the number of activated HSCs.
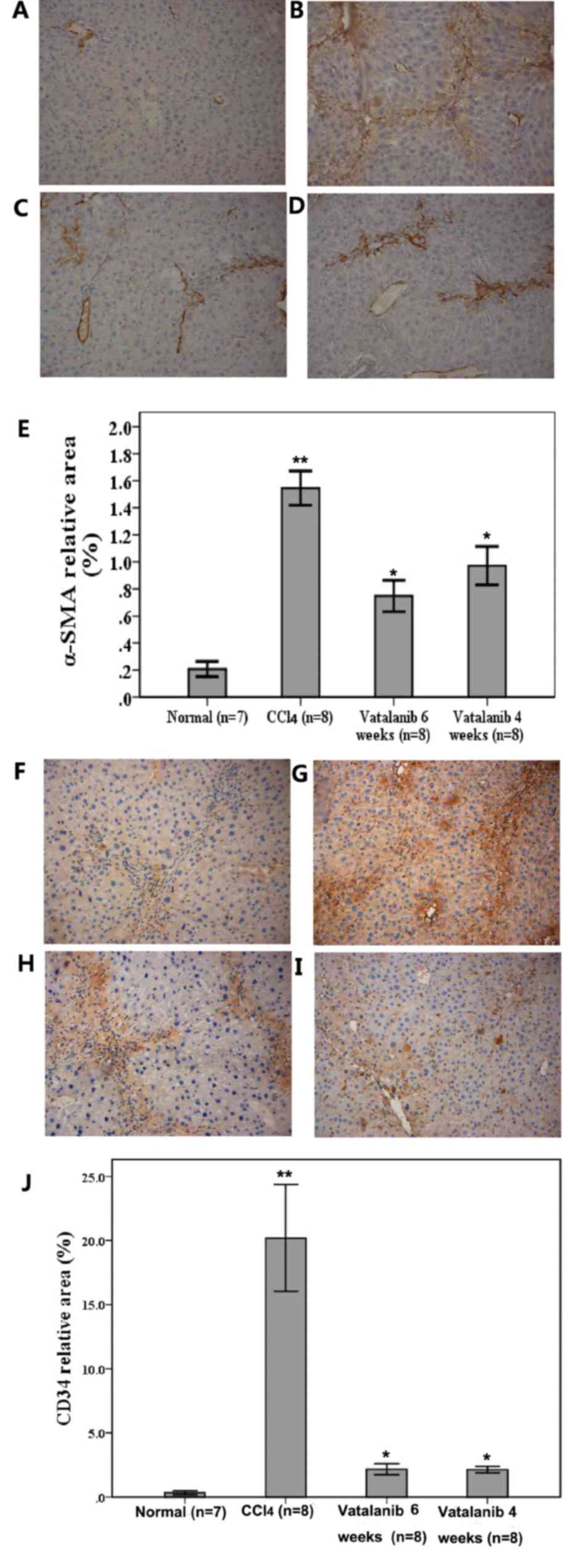 | Figure 2.Vatalanib suppresses α-SMA and CD34
expression in CCl4-induced fibrotic mouse livers.
Representative photomicrographs of immunohistochemistry staining of
α-SMA expression in the following groups: (A) 1, normal control;
(B) 2, CCl4 model; (C) 3, CCl4 + vatalanib (6
weeks, 50 mg/kg) and (D) 4, CCl4 + vatalanib (4 weeks,
50 mg/kg). (E) Semi-quantitative analysis of the
immunohistochemical staining. (F-J) Representative photomicrographs
of immunohistochemical staining of CD34 expression in groups (F) 1,
(G) 2, (H) 3 and (I) 4. (J) Semi-quantitative analysis of the
immunohistochemical staining. Magnification, ×100. α-SMA, α-smooth
muscle actin; CCl4, carbon tetrachloride; CD34, cluster
of differentiation 34. Data are presented as the mean ± standard
deviation. *P<0.05 vatalanib vs. CCl4 group.
**P<0.05 CCl4 vs. normal group. |
There was little positive CD34 staining in the
control group 1 (Fig. 2F).
Following six weeks of CCl4 induction, this led to
significant increases in the number of CD34 positive cells, while
groups 3 and 4 presented significantly decreased CD34 positive
areas compared with group 2 (P<0.05; Fig. 2G-J).
Ultrastructural changes in fibrotic
mice after vatalanib treatment
There were significantly increased fenestrae per SEC
in group 1 (Fig. 3A) compared with
group 2 (Fig. 3B; P<0.01;
Table IV). In group 2, the
microvilli of hepatocytes in the perisinusoidal space and the
intra-lobular bile ducts (cholangioles) disappeared (Fig. 3C), and the junctions between
hepatocytes were destroyed (Fig.
3D). In group 3, the number of fenestrae was significantly
increased compared with group 2 (P<0.01; Fig. 3E; Table IV), and cholangioles appeared
morphologically healthy (Fig. 3F).
Group 4 additionally had an increased number of fenestrae compared
with group 2 (Fig. 3G; Table IV), and presented with cell
junctions similar to normal control mice (Fig. 3H). There were no significant
differences in the numbers of fenestrae between groups 3 and 4
(P>0.05). The extent of hepatocyte necrosis and inflammation was
less so in groups 3 and 4 compared with group 2.
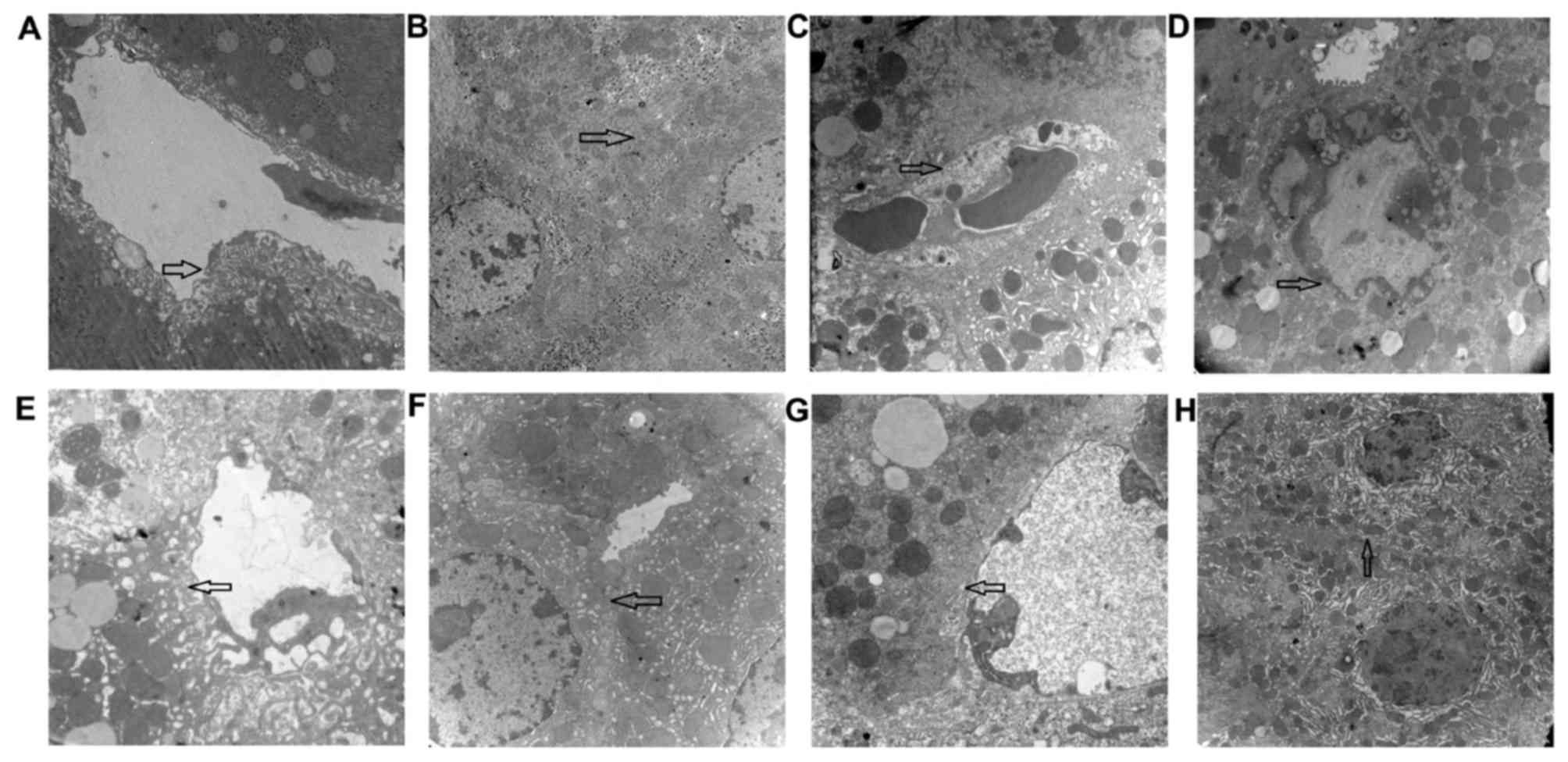 | Figure 3.CCl4-stimulates SEC
capillarization, cholangiole extension and decreased hepatocyte
microvilli. Vatalanib attenuated SEC capillarization and decreased
the damage in cholangioles, microvilli and cell junctions between
hepatocytes. Representative images representing different fields of
views of the four groups. Group 1 exhibiting (A) normal SECs,
fenestrae and (B) cell junctions between hepatocytes. Group 2 was
in a fibrotic state and exhibited (C) disappearance of SEC
fenestrae and the appearance of a basement membrane, and (D)
disappearance of cell junctions with cholangiole extension, and
karyopyknosis in hepatocytes. Group 3, treated with vatalanib (6
weeks, 50 mg/kg) presented with (E) fenestrae of SECs similar to
those of normal control mice and (F) cell junctions between
hepatocytes similar to normal control mice. Group 4 treated with
vatalanib (4 weeks, 50 mg/kg) demonstrated (G) fenestrae of SECs
similar to normal control mice and (H) cell junctions between
hepatocytes similar to normal control mice. Magnification, ×12,000.
CCl4, carbon tetrachloride; SEC, sinusoidal endothelial
cell. |
 | Table IV.Quantitation of hepatic sinusoidal
endothelial cell fenestrae. |
Table IV.
Quantitation of hepatic sinusoidal
endothelial cell fenestrae.
| Group | Number of
fenestrae |
|---|
| 1 (n=7) | 7.43±0.98 |
| 2 (n=8) | 2.38±0.91 |
| 3 (n=8) |
4.75±0.71a |
| 4 (n=8) |
3.88±0.64a |
Vatalanib was associated with
decreased collagen I, α-SMA, TGF-β1, VEGFR1 and VEGFR2 mRNA
levels
mRNA expression levels of collagen I, α-SMA, TGF-β1,
VEGFR1 and VEGFR2 were increased in group 2 compared with group 1
(P<0.01; Fig. 4). mRNA levels
were reduced in groups 3 and 4 compared with group 2
(P<0.01).
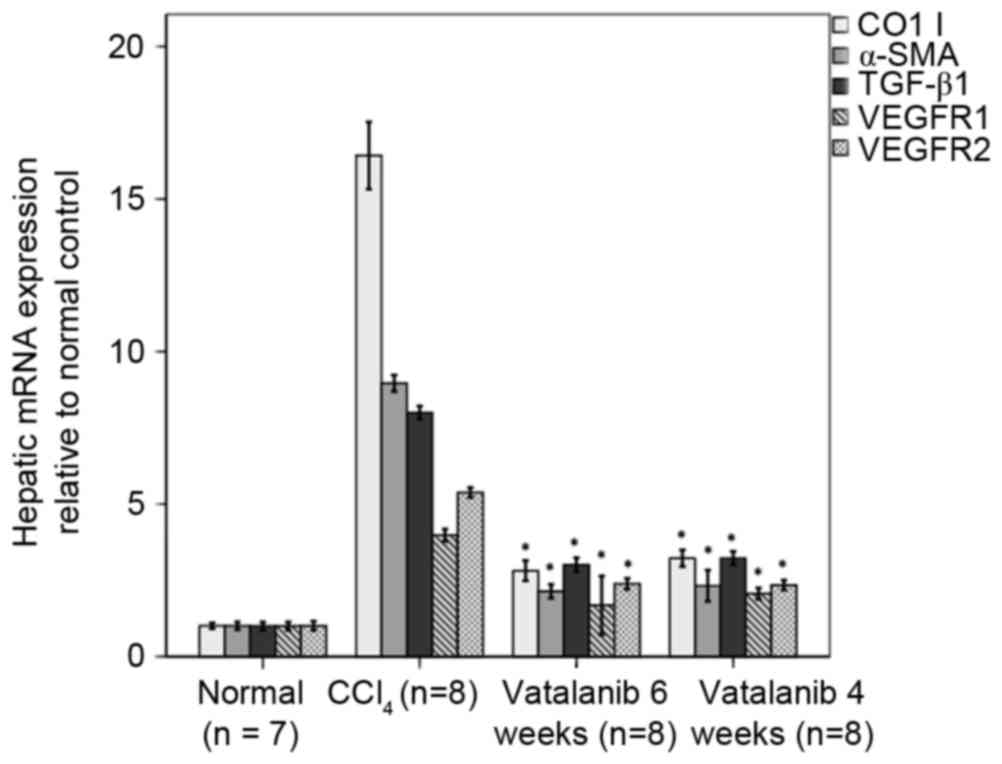 | Figure 4.Vatalanib inhibits mRNA expression
levels of collagen I, α-SMA, TGF-β1, VEGFR1 and VEGFR2 in fibrotic
mice. mRNA expression of collagen I, α-SMA, TGF-β1, VEGFR1 and
VEGFR2 were determined by reverse transcription-quantitative
polymerase chain reaction in the livers treated with or without
vatalanib. Data were normalized to the internal control (GAPDH) and
are expressed as the mean ± standard deviation. *P<0.01 vs.
CCl4 group. α-SMA, α-smooth muscle actin; TGF-β1,
transforming growth factor-β1; VEGFR, vascular endothelial growth
factor receptor; CCl4, carbon tetrachloride. |
Discussion
Intrahepatic hypoxia may occur during the liver
inflammatory and fibrotic processes that characterize several
chronic liver diseases (26). As a
consequence, intrahepatic vascular angiogenesis and sinusoidal
remodeling may occur in these chronic liver diseases (8,26).
In previous years, numerous studies have examined the expression of
various anti-angiogenic molecules in chronic liver disease
(26). Among these molecules, VEGF
and its receptors are of the most important, and have been fully
studied in terms of their roles in liver inflammation and fibrosis
(26,27). Anti-VEGF treatment may represent a
potential therapeutic approach in liver fibrosis and portal
hypertension (10,28).
Vatalanib has been previously identified to
specifically block VEGF-induced phosphorylation of VEGFR-1, −2 and
−3 and inhibit endothelial cell proliferation, differentiation and
tumor angiogenesis (29). It is
considered safe for patients in previous cancer studies (30,31).
The most common grade 3 and 4 non-hematological toxicities are
mucositis and alopecia (17,18,32).
In addition, Liu et al (20) investigated the role of vatalanib in
CCl4-induced mice. They identified that vatalanib
decreases liver fibrosis in a CCl4-induced liver
fibrosis model. This result is consistent with current findings.
However, they examined the effect of vatalanib in liver fibrosis
and HSCs, not SECs. The present study examined the effects of
vatalanib on the number of hepatic SEC fenestrae, and levels of
VEGFR in liver tissues. The results suggested that vatalanib may
exert its effects via the VEGF signaling pathway. The present
results reinforced the concept that angiogenesis has a major role
in liver fibrogenesis (8,10,33,34).
The underlying molecular mechanism by which
vatalanib decreases liver injury remains unknown. It has been
demonstrated that, in primary HSCs, vatalanib decreases the levels
of α-SMA, collagen, tissue inhibitor of metalloproteinase-1, and
reduces cell proliferation, migration and actin filament formation.
This effect was associated with inhibition of VEGF, PDGF and TGF-β1
signaling and their downstream target, protein kinase B (35). In the current study, vatalanib was
identified to inhibit TGF-β1, VEGFR1 and VEGFR2.
Dysfunction of SECs is likely to be one of the
initial events in liver injury (36). Defenestration and capillarization
of the sinusoidal endothelium may be major contributors to hepatic
failure in hepatic cirrhosis (36). Two studies from 2010 suggested that
intrahepatic angiogenesis and sinusoidal remodeling may be involved
in sinusoidal resistance, fibrosis and portal hypertension
(11,37). In the present study, vatalanib
altered the SEC phenotype, leading to decreased sinusoidal
capillarization. Microvilli disappearance, inflammation, hepatocyte
necrosis and bile duct alterations were decreased in all
vatalanib-treated groups. These results indicated that
anti-angiogenic agents may inhibit SECs, and that they may be
involved in sinusoidal capillarization and hepatocyte damage. SECs
may be potential target cells in the inhibition of this process.
Furthermore, it is understood that paracrine signaling between SECs
and HSCs regulates fibrogenesis, angiogenesis and portal
hypertension in chronic liver disease (8,9,14,38).
In the present study, the underlying mechanism of vatalanib on SECs
was not addressed. Therefore, the nature of the interactions
between SECs and HSCs in chronic liver disease following vatalanib
treatment requires further investigation.
In conclusion, vatalanib treatment was associated
with decreased hepatic sinusoidal capillarization, hepatocyte
damage and liver fibrosis in CCl4-induced fibrotic mice.
Vatalanib may represent a potential therapeutic agent for
counteracting hepatic fibrosis.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Heilongjiang Province of China (grant no.
H2015099).
Glossary
Abbreviations
Abbreviations:
|
TGF-β
|
transforming growth factor-β
|
|
HSC
|
hepatic stellate cell
|
|
α-SMA
|
α-smooth muscle actin
|
|
VEGF
|
vascular endothelial growth factor
|
|
VEGFR
|
vascular endothelial growth factor
receptor
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
TBIL
|
total bilirubin
|
|
TEM
|
transmission electron microscopy
|
References
|
1
|
Friedman SL: Cellular networks in hepatic
fibrosis. Digestion. 59:368–371. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedman SL: Hepatic fibrosis-overview.
Toxicology. 254:120–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ghiassi-Nejad Z and Friedman SL: Advances
in antifibrotic therapy. Expert Rev Gastroenterol Hepatol.
2:803–816. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiao J, Friedman SL and Aloman C: Hepatic
fibrosis. Curr Opin Gastroenterol. 25:223–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Friedman SL: Liver fibrosis: From
mechanisms to treatment. Gastroenterol Clin Biol. 31:812–814. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Friedman SL and Bansal MB: Reversal of
hepatic fibrosis-fact or fantasy? Hepatology. 43:(2 Suppl 1).
S82–S88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor. Endocr Rev. 18:4–25. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coulon S, Heindryckx F, Geerts A, Van
Steenkiste C, Colle I and Van Vlierberghe H: Angiogenesis in
chronic liver disease and its complications. Liver Int. 31:146–162.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fernández M, Semela D, Bruix J, Colle I,
Pinzani M and Bosch J: Angiogenesis in liver disease. J Hepatol.
50:604–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai WK and Adams DH: Angiogenesis and
chronic inflammation; The potential for novel therapeutic
approaches in chronic liver disease. J Hepatol. 42:7–11. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thabut D and Shah V: Intrahepatic
angiogenesis and sinusoidal remodeling in chronic liver disease:
New targets for the treatment of portal hypertension? J Hepatol.
53:976–980. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mejias M, Garcia-Pras E, Tiani C, Miquel
R, Bosch J and Fernandez M: Beneficial effects of sorafenib on
splanchnic, intrahepatic and portocollateral circulations in portal
hypertensive and cirrhotic rats. Hepatology. 49:1245–1256. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Majumder S, Piguet AC, Dufour JF and
Chatterjee S: Study of the cellular mechanism of Sunitinib mediated
inactivation of activated hepatic stellate cells and its
implications in angiogenesis. Eur J Pharmacol. 705:86–95. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thabut D, Routray C, Lomberk G, Shergill
U, Glaser K, Huebert R, Patel L, Masyuk T, Blechacz B, Vercnocke A,
et al: Complementary vascular and matrix regulatory pathways
underlie the beneficial mechanism of action of sorafenib in liver
fibrosis. Hepatology. 54:573–585. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Liu DG, Yang G, Kong LJ, Du YJ,
Wang HY, Li FD, Pei FH, Song JT, Fan YJ, et al: Endostar, a novel
human recombinant endostatin, attenuates liver fibrosis in
CCl4-induced mice. Exp Biol Med (Maywood). 239:998–1006. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jones SF, Spigel DR, Yardley DA, Thompson
DF and Burris HA III: A phase I trial of vatalanib (PTK/ZK) in
combination with bevacizumab in patients with refractory and/or
advanced malignancies. Clin Adv Hematol Oncol. 9:845–852.
2011.PubMed/NCBI
|
|
17
|
Thomas AL, Morgan B, Drevs J, Unger C,
Wiedenmann B, Vanhoefer U, Laurent D, Dugan M and Steward WP:
Vascular endothelial growth factor receptor tyrosine kinase
inhibitors: PTK787/ZK 222584. Semin Oncol. 30:(3 Suppl 6). S32–S38.
2003. View Article : Google Scholar
|
|
18
|
Yau T, Chan P, Pang R, Ng K, Fan ST and
Poon RT: Phase 1–2 trial of PTK787/ZK222584 combined with
intravenous doxorubicin for treatment of patients with advanced
hepatocellular carcinoma: Implication for antiangiogenic approach
to hepatocellular carcinoma. Cancer. 116:5022–5029. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Y, Jin L, He YL, Wang K, Ma XH, Wang
J, Yan Z, Feng YL, Li YQ, Chen TY, et al: Hepatitis B virus
infection in clustering of infection in families with unfavorable
prognoses in northwest China. J Med Virol. 85:1893–1899. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Lui EL, Friedman SL, Li L, Ye T,
Chen Y, Poon RT, Wo J, Kok TW and Fan ST: PTK787/ZK22258 attenuates
stellate cell activation and hepatic fibrosis in vivo by inhibiting
VEGF signaling. Lab Invest. 89:209–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Zhang Y, Wang T, You H and Jia J:
N-methyl-4-isoleucine cyclosporine attenuates CCl-induced liver
fibrosis in rats by interacting with cyclophilin B and D. J
Gastroenterol Hepatol. 26:558–567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Poon RT, Li Q, Kok TW, Lau C and
Fan ST: Both antiangiogenesis- and angiogenesis-independent effects
are responsible for hepatocellular carcinoma growth arrest by
tyrosine kinase inhibitor PTK787/ZK222584. Cancer Res.
65:3691–3699. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu H, Shi BM, Lu XF, Liang F, Jin X, Wu TH
and Xu J: Vascular endothelial growth factor attenuates hepatic
sinusoidal capillarization in thioacetamide-induced cirrhotic rats.
World J Gastroenterol. 14:2349–2357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elpek GÖ, Unal B and Bozova S: H-ras
oncogene expression and angiogenesis in experimental liver
cirrhosis. Gastroenterol Res Pract. 2013:8689162013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Medina J, Arroyo AG, Sánchez-Madrid F and
Moreno-Otero R: Angiogenesis in chronic inflammatory liver disease.
Hepatology. 39:1185–1195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Calderone V, Gallego J, Fernandez-Miranda
G, Garcia-Pras E, Maillo C, Berzigotti A, Mejias M, Bava FA,
Angulo-Urarte A, Graupera M, et al: Sequential functions of CPEB1
and CPEB4 regulate pathologic expression of vascular endothelial
growth factor and angiogenesis in chronic liver disease.
Gastroenterology. 150:982–997.e30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fernandez M, Vizzutti F, Garcia-Pagan JC,
Rodes J and Bosch J: Anti-VEGF receptor-2 monoclonal antibody
prevents portal-systemic collateral vessel formation in portal
hypertensive mice. Gastroenterology. 126:886–894. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schomber T, Zumsteg A, Strittmatter K,
Crnic I, Antoniadis H, Littlewood-Evans A, Wood J and Christofori
G: Differential effects of the vascular endothelial growth factor
receptor inhibitor PTK787/ZK222584 on tumor angiogenesis and tumor
lymphangiogenesis. Mol Cancer Ther. 8:55–63. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kircher SM, Nimeiri HS and Benson AB III:
Targeting angiogenesis in colorectal cancer: Tyrosine kinase
inhibitors. Cancer J. 22:182–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dragovich T, Laheru D, Dayyani F, Bolejack
V, Smith L, Seng J, Burris H, Rosen P, Hidalgo M, Ritch P, et al:
Phase II trial of vatalanib in patients with advanced or metastatic
pancreatic adenocarcinoma after first-line gemcitabine therapy
(PCRT O4-001). Cancer Chemother Pharmacol. 74:379–387. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Langenberg MH, Witteveen PO, Lankheet NA,
Roodhart JM, Rosing H, van den Heuvel IJ, Beijnen JH and Voest EE:
Phase 1 study of combination treatment with PTK 787/ZK 222584 and
cetuximab for patients with advanced solid tumors: Safety,
pharmacokinetics, pharmacodynamics analysis. Neoplasia. 12:206–213.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vanheule E, Geerts AM, Van Huysse J,
Schelfhout D, Praet M, Van Vlierberghe H, De Vos M and Colle I: An
intravital microscopic study of the hepatic microcirculation in
cirrhotic mice models: Relationship between fibrosis and
angiogenesis. Int J Exp Pathol. 89:419–432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Wen XM, Lui EL, Friedman SL, Cui W,
Ho NP, Li L, Ye T, Fan ST and Zhang H: Therapeutic targeting of the
PDGF and TGF-beta-signaling pathways in hepatic stellate cells by
PTK787/ZK22258. Lab Invest. 89:1152–1160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Braet F and Wisse E: Structural and
functional aspects of liver sinusoidal endothelial cell fenestrae:
A review. Comp Hepatol. 1:12002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huebert RC, Jagavelu K, Liebl AF, Huang
BQ, Splinter PL, LaRusso NF, Urrutia RA and Shah VH: Immortalized
liver endothelial cells: A cell culture model for studies of
motility and angiogenesis. Lab Invest. 90:1770–1781. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Friedman SL: Preface. Hepatic fibrosis:
Pathogenesis, diagnosis and emerging therapies. Clin Liver Dis.
12:xiii–xiv. 2008. View Article : Google Scholar : PubMed/NCBI
|