Introduction
Gliomas are the most common primary intracranial
tumors. In the Netherlands, their incidence rate has gradually
increased from 4.9 per 100,000 in 1989 to 5.9 in 2010 (1). With multiple psychiatric, cognitive
and neurological symptoms, gliomas may seriously affect the daily
lives of patients. Despite great efforts to improve diagnosis and
treatment over the past decades, the prognosis for glioma patients
remains poor. According to World Health Organization statistics,
the median survival of grade IV glioma patients is 12–14 months
(2). Currently, the only effective
treatment for patients is neurosurgery followed by chemo- or
radiotherapy. However, due to the invasive ability of gliomas, it
is unfeasible to remove all glioma cells by surgery, resulting in a
high recurrence rate.
Glioma cell invasion is a complex process, involving
receptor-mediated matrix adhesion, degradation of extracellular
matrix (ECM) and active cell migration into the newly created
space. To invade the narrow extracellular space, cells undergo
dramatic morphological alterations by reducing their water content
and secreting Cl−, K+ or Na+
(3,4). Therefore, ion channels serve an
important role in cell invasion. In China, dried whole scorpion
(Quan Xie) has been used to treat apoplexy, epilepsy, migraine and
other channelopathies for ~1,000 years. Previous studies suggested
that the primary active ingredient of Quan Xie may be associated
with its toxicity to ion channels (5,6).
Chlorotoxin (CTX) is a neurotoxin isolated from the venom of
Leiurus quinquestriatus in 1991 and is an established
blocker of small-conductance Cl− channels (7,8). It
has previously been demonstrated that CTX may specifically bind to
glioma Cl− channels and thus reduce the invasive ability
of glioma cells (9–11). Previous studies have indicated that
CTX exerted an anti-invasive effect on glioma cells by reducing the
surface expression of matrix metalloproteinase (MMP)-2 and
suppressing its enzymatic activity, which was associated with and
indirectly regulated the function of the glioma-specific
Cl− channels (3,12).
CTX radiolabeled with radionuclide 125I or
131I has been revealed to selectively accumulate in the
brains of glioma-bearing mice (13–15).
Additionally, 131I-CTX has been used in patients with
recurrent high-grade gliomas to determine its biodistribution,
toxicity and therapeutic effect (16).
However, the isolation and purification of CTX
remains costly. Therefore, alternatives, and methods for the
synthesis of chlorotoxin-like peptides, have been investigated;
>70 different toxins have been isolated and identified.
Buthus martensii Karsch (BmK) chlorotoxin-like toxin (BmK
CT) was the first chlorotoxin-like peptide and was isolated from
BmK, a scorpion widely distributed throughout Asia (17). The amino acid sequence identity of
BmK CT reveals ~68% homology with CTX (18,19).
Previous studies determined that BmK CT may specifically bind to
glioma Cl− channels and alter their functional
properties, as for CTX (20,21).
Fu et al (22) demonstrated
that BmK CT may additionally suppress the enzymatic activity of
MMP-2 in glioma cells.
Previous studies indicated that BmK CT may induce
apoptosis of cultured malignant glioma cells in vitro
(19,20,23).
As CTX had previously been radiolabeled with 131I or
125I for glioma Single-Photon Emission Computed
Tomography (SPECT) imaging and therapy, our previous study
radiolabeled BmK CT with 131I, which revealed that it
was superior to BmK CT in inhibiting the growth of glioma cells
(24). The present study used
various experiments to further evaluate the inhibitory ability of
BmK CT and 125I-BmK CT on the invasion of glioma
cells.
Materials and methods
Cell cultures and reagents
C6 rat glioma cells were purchased from the Shanghai
Institute of Biochemistry and Cell Biology (Shanghai, China). The
cells were cultured in Ham's F12K medium (Applichem GmbH,
Darmstadt, Germany) supplemented with 15% horse serum and 2.5%
fetal calf serum (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at 37°C in a 5% CO2 humidified atmosphere.
Logarithmic phase cells were used in the experiments. BmK CT was
synthesized by the Institute of Protein Research, Shanghai Tongji
University (Shanghai, China).
Radiolabeling of BmK CT
BmK CT was labeled with 125I using the
indirect labeling method according to the protocols described in
our previous study (24).
Following the complicated radiolabeling process, the initial mass
concentration of 125I-BmK CT was hard to calculate, and
it was <2 µg/ml. The initial radioactivity concentration of it
was 50 µCi/ml. Briefly, 5–10 mCi (0.1–0.2 ml) sterile
Na125I solution (Shanghai GMS Pharmaceutical Co., Ltd.,
Shanghai, China) was mixed with 100 µg chloramine-T (J&K
Scientific Ltd., Beijing, China; dissolved in 200 µl PBS) and 200
µg Bolton-Hunter reagent (J&K Scientific Ltd.; dissolved in
benzene) and stirred constantly. Subsequently, the reaction mixture
was incubated for 1 min at room temperature. Next, 100 µg
Na2S2O5 (J&K Scientific Ltd.)
and 100 µg KI (J&K Scientific Ltd.) were dissolved in PBS, and
added to terminate the aforementioned reaction and obtain
125I-labeled Bolton-Hunter reagent (125I-BH).
Subsequently, 5 ml N, N-dimethylformamide (J&K Scientific Ltd.)
and 200 ml benzene (J&K Scientific Ltd.) were added to extract
the 125I-BH from the reaction mixture. Next, 200 µg BmK
CT was added to the extracted dry 125I-BH in a glass
tube and incubated in an ice-bath for 30 min with constant
stirring. Following this, 50 µg glycine (J&K Scientific Ltd.)
was added to the aforementioned mixture dropwise to combine with
the remaining 125I-BH. The final reaction mixture was
eluted with a PD-10 desalting column (GE Healthcare Life Sciences,
Shanghai, China) and 1 ml liquid was collected in each tube. The
radioactivity of 30 tubes was determined with a CRC-15R
radioisotope dose calibrator (Capintec, Inc., Ramsey, NJ, USA).
Cell invasion assays
Cell invasion assays were performed using Transwell
inserts (Corning, Inc., Corning, NY, USA) with polycarbonate
membrane filters containing 8-µm pores, according to the
manufacturer's protocol. The upper side of the membrane was coated
with Matrigel matrix (BD Biosciences, Franklin Lakes, NJ, USA).
Cell culture media was replaced with serum-free Ham's F12K medium
and logarithmic phase C6 cells were treated with 0, 0.15, 0.3 or
1.5 µmol/l BmK CT. The cells were incubated at 37°C for 24 h and
seeded into the upper chambers at a density of 4×105
cells/filter in 200 µl serum-free medium. The lower chambers were
filled with Ham's F12K medium supplemented with 30% horse serum and
10% fetal calf serum as the chemoattractant. Following a 24 h
incubation at 37°C, the cells that remained in the upper chamber
were scraped off the inserts and the invaded cells were fixed in 4%
paraformaldehyde and stained with 0.5% crystal violet. The entire
membrane was observed under a light microscope (Olympus
Corporation, Tokyo, Japan). The relative inhibition of invasion was
expressed as the number of invaded cells/well in the presence of
different concentrations of BmK CT. The experiments were performed
in triplicate and repeated three times.
Following evaluation of the inhibitory effect of BmK
CT on the invasive ability of C6 cells, the effect of
125I-radiolabeled BmK CT on C6 cells was investigated.
The initial radioactivity concentration of 125I-BmK CT
was 50 µCi/ml (<2 µg/ml). The cell culture media was replaced
with Ham's F12 K medium and C6 cells in the logarithmic phase were
treated with 125I-NaI (0.5, 5 or 50 µCi/ml), BmK CT
(0.02, 0.2 or 2 µg/ml) or 125I-BmK CT (0.5, 5 or 50
µCi/ml). The control group was treated with the same volume of
Ham's F12K medium. Following a 24 h incubation, the cells were
seeded, further incubated, fixed and stained as aforementioned. The
experiments were performed in triplicate and repeated three
times.
ELISA analysis
Logarithmic phase C6 cells were cultured in
serum-free Ham's F12K medium and treated with 125I-NaI
(0.5, 5 or 50 µCi/ml), BmK CT (0.02, 0.2 or 2 µg/ml) or
125I-BmK CT (0.5, 5 or 50 µCi/ml). Following a 24 h
incubation at 37°C, the supernatants were collected and centrifuged
(1,006 × g, 4°C, 15 min) for ELISA analysis. The levels of
MMP-2 in the culture supernatants were detected using Rat MMP-2
ELISA kit (cat. no. KB18320; Shanghai Pucheng Biotechnology
Company, Shanghai, China) according to the manufacturer's protocol.
Each assay was performed in triplicate and repeated three
times.
RNA isolation, cDNA synthesis and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
In a previous study, BmK CT ~0.2 µg/ml demonstrated
obvious inhibition of the invasion of glioma cells (22). To elucidate the underlying
mechanism, logarithmic phase C6 cells were treated with BmK CT (0.2
µg/ml), 125I-NaI (5 µCi/ml) or 125I-BmK CT (5
µCi/ml) and incubated at 37°C for 24 h. The control group was
treated with the same volume of Ham's F12K medium. Subsequently,
total RNA was extracted from the cells using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The purity of the RNA was examined by
spectrophotometry (Bio-Rad Laboratories, Inc., Hercules, CA, USA),
and had an optical density value (A260/A280) between 1.8 and 2.0.
The extracted RNA was reverse-transcribed into cDNA using Prime
Script™ RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian, China).
GAPDH served as an internal control. The sequences of the primers
for MMP-2 and GAPDH (Shanghai Sangon Biological Engineering
Technology & Services Co., Ltd., Shanghai, China) are presented
in Table I. The cDNA products were
amplified using Taq polymerase (Takara Biotechnology Co., Ltd.)
under the following thermocycling conditions: Denaturation at 95°C
for 2 min, annealing at 95°C for 15 sec and extension at 58°C for 1
min, for 38 cycles. All samples were run in triplicate in each
experiment. Data were analyzed using the 2−∆∆Cq method
(25).
 | Table I.Nucleotide sequences of primers and
product size. |
Table I.
Nucleotide sequences of primers and
product size.
| Gene | Primer sequence
(5′-3′) | Product size
(bp) |
|---|
| MMP-2 | F:
ACCGGGATAAGAAGTATGGATT | 182 |
|
| R:
GTCATCATCGTAGTTGGTTGTG |
|
| GAPDH | F:
ACAGCAACAGGGTGGTGGAC | 252 |
|
| R:
TTTGAGGGTGCAGCGAACTT |
|
Statistical analysis
All data were analyzed by one-way analysis of
variance with Scheffe's Method using SPSS software version 18.0
(SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ±
standard error. P<0.05 was considered to indicate a
statistically significant difference. Three independent replicates
of each experiment were conducted.
Results
BmK CT inhibits the migration and
invasion of glioma cells
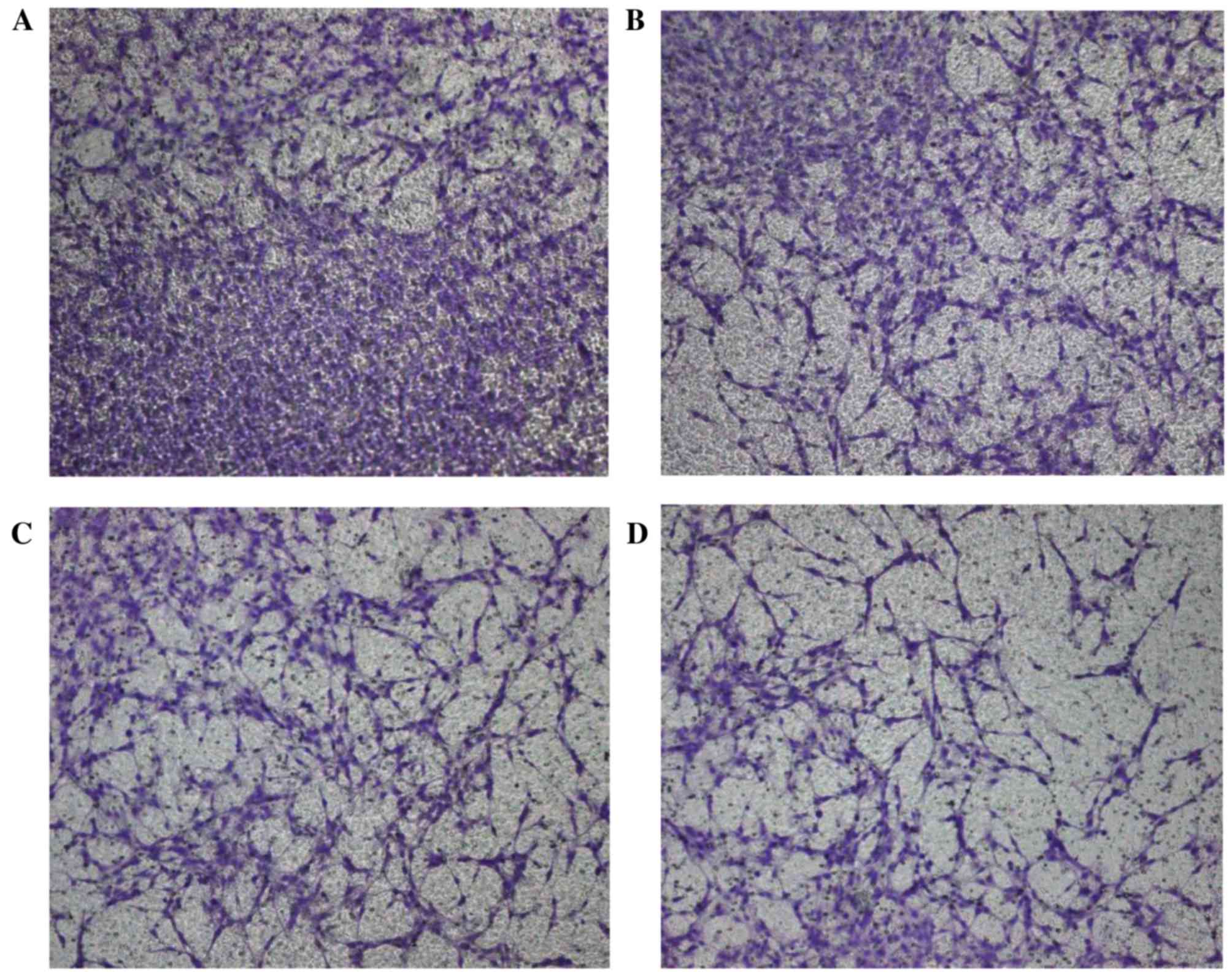
A Transwell assay was conducted to evaluate the
inhibitory ability of BmK CT on the invasion of C6 cells. Following
staining with crystal violet, 4 different fields (magnification,
×100) were examined. The numbers of invaded cells in the four
groups were as follows: Control group, 125±8.9; 0.15 µmol/l BmK CT,
91±7.7; 0.3 µmol/l BmK CT, 67±5.9; 1.5 µmol/l BmK CT, 35±7.9. The
total numbers of invaded C6 rat glioma cells were significantly
reduced following treatment with all concentrations of BmK CT
compared with the control group (Fig.
1; P<0.05). These data suggested that BmK CT may inhibit the
invasion of glioma cells (P<0.05).
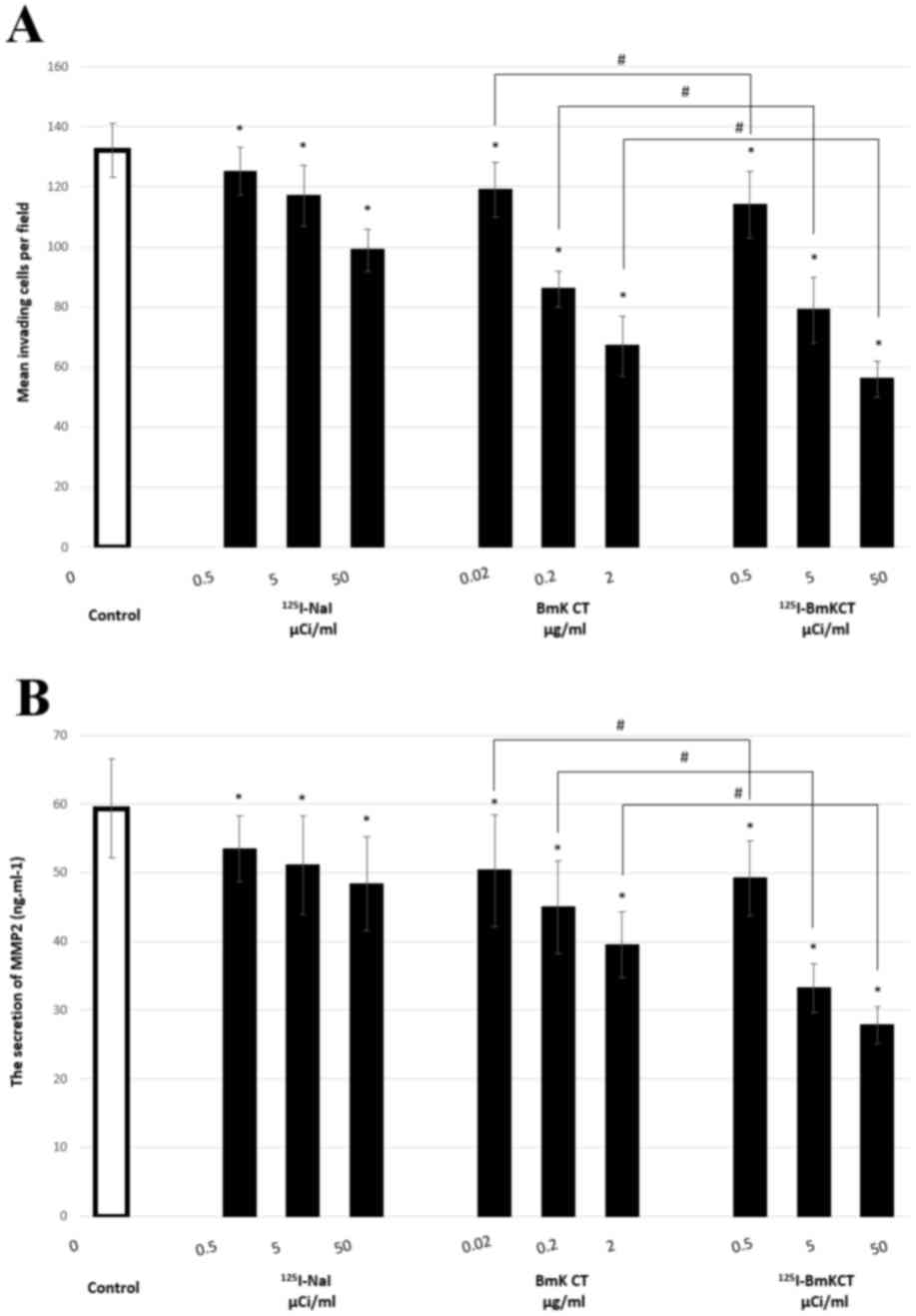
125I-BmK CT is superior to BmK CT in
inhibiting the migration and invasion of glioma cells.
Following the validation of the inhibitory ability of BmK CT,
Transwell assays were conducted following 125I-BmK CT
treatment. As presented in Table
II, the number of invaded cells in the BmK CT,
125I-NaI and 125I-BmK CT groups was
significantly reduced compared with the control group (P<0.05).
Additionally, the inhibition of invasion was greater in the
125I-Bmk CT group compared with the BmK CT group
(P<0.05; Table II, Fig. 2A). These findings revealed that
125I-BmK CT may inhibit the invasion of glioma cells to
a greater extent than BmK CT.
 | Table II.Transwell and ELISA assays were
performed to determine the effect of 125I-NaI, BmK CT
and 125I-BmK CT on C6 cells. |
Table II.
Transwell and ELISA assays were
performed to determine the effect of 125I-NaI, BmK CT
and 125I-BmK CT on C6 cells.
| Group | Concentration | No. of invaded
cells/well | MMP-2 levels
(ng/ml) |
|---|
| Control | 0 | 132.0±9.0 | 59.4±7.2 |
|
125I-NaI | 0.50 µCi/ml |
125.0±8.0a |
53.5±4.8c |
|
| 5.00 µCi/ml |
117.0±10.0a |
51.1±7.2c |
|
| 50.00 µCi/ml |
99.0±7.0a |
48.4±6.8c |
| BmK CT | 0.02 µg/ml |
119.0±9.0a,b |
50.3±8.2c,d |
|
| 0.20 µg/ml |
86.0±6.0a,b |
45.0±6.7c,d |
|
| 2.00 µg/ml |
67.0±10.0a,b |
39.5±4.8c,d |
| 125I-BmK
CT | 0.50 µCi/ml |
114.0±11.0a,b |
49.2±5.4c,d |
|
| 5.00 µCi/ml |
79.0±11.0a,b |
33.2±3.5c,d |
|
| 50.00 µCi/ml |
56.0±6.0a,b |
27.8±2.7c,d |
BmK CT and 125I-BmK CT
downregulate MMP-2 expression
MMP-2 protein levels in cell culture supernatants of
control, BmK CT, 125I-NaI and 125I-BmK CT
groups were quantified. ELISA revealed that MMP-2 secretion in the
BmK CT, 125I-NaI and 125I-BmK CT groups was
significantly reduced compared with the control group (P<0.05).
Additionally, MMP-2 levels were decreased to a greater extent in
the 125I-Bmk CT group compared with the BmK CT group
(P<0.05; Table II, Fig. 2B).
BmK CT and 125I-BmK CT have
no effect on MMP-2 mRNA expression levels
RT-qPCR was performed to identify the effect of BmK
CT and 125I-BmK CT on MMP-2 mRNA expression levels in C6
cells. The 2−∆∆Cq value of the control group was set as
1.0, and the values of the 125I-NaI, BmK CT and
125I-BmK CT groups were 0.85±0.14, 1.21±0.13 and
0.79±0.05, respectively. No statistically significant differences
were identified between the groups.
Discussion
Gliomas comprise the majority of primary intrinsic
brain tumors. As gliomas are fast-growing and highly invasive, they
preclude traditional treatments and are associated with high
mortality. Therefore, inhibiting proliferation and invasion of
glioma cells has become the key target of glioma therapy.
Our previous study determined that BmK CT and
131I-BmK CT may inhibit the growth of glioma cells in
vitro (24). The present study
used Matrigel to simulate the ECM and a Transwell assay to detect
the ability of BmK CT and 125I-BmK CT to inhibit the
invasion of glioma cells. It was determined that BmK CT and
125I-BmK CT may inhibit the invasion of glioma cells.
Additionally, 125I-BmK CT was more effective compared
with BmK CT.
The mechanism underlying glioma cell invasion is
complex and the degradation of the ECM is a key step. MMPs are a
group of zinc-dependent proteolytic enzymes that degrade the
majority of ECM and promote tumor invasion (26). MMP-2 is a core member of the MMP
protease family. It has been established that abnormally elevated
MMP-2 expression and activity may be directly associated with cell
invasion (27,28). The present study used ELISA to
evaluate the effect of BmK CT and 125I-BmK CT on the
secretion of MMP-2 by C6 cells. It was determined that the
secretion of MMP-2 by C6 cells was significantly reduced following
treatment with BmK CT or 125I-BmK CT. Additionally, the
inhibitory effect of 125I-BmK CT on C6 cells was greater
compared with that of BmK CT.
Previous studies have focused on the inhibitory
effect of BmK CT on the protein expression levels of MMP-2
(22,29). The present study used RT-qPCR to
investigate whether BmK CT affected the invasive ability of glioma
cells at the mRNA level. However, no statistically significant
differences were identified between the groups.
The present study additionally aimed to identify
novel therapeutic agents for patients with gliomas. As
aforementioned, BmK CT was able to inhibit the growth and invasion
of glioma cells. Therefore, BmK CT may be a potential novel
therapeutic agent for the treatment of glioma. For the clinical
application of BmK CT, Fu et al (30) combined BmK CT with LiCl and
demonstrated synergistic inhibition of proliferation of high-grade
glioma cells. Our previous study suggested that BmK CT radiolabeled
with the 131I radionuclide may be used as a radiotracer
for glioma SPECT imaging to localize the lesion range. The present
study radiolabeled BmK CT with the 125I radionuclide,
which should kill tumor cells by issuing β rays and thereby
strengthen the therapeutic effect of BmK CT on glioma cells.
In conclusion, BmK CT and 125I-BmK CT may
suppress the invasion of glioma cells via downregulation of MMP-2
secretion; 125I-BmK CT was superior compared with BmK
CT. However, no alterations were observed in the mRNA expression
levels of MMP-2 in glioma cells.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China (grant nos. 81171368, 81301245
and 81401440).
References
|
1
|
Ho VK, Reijneveld JC, Enting RH, Bienfait
HP, Robe P, Baumert BG and Visser O: Dutch Society for
Neuro-Oncology (LWNO): Changing incidence and improved survival of
gliomas. Eur J Cancer. 50:2309–2318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, Van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soroceanu L, Manning TJ Jr and Sontheimer
H: Modulation of glioma cell migration and invasion using Cl(−) and
K(+) ion channel blockers. J Neurosci. 19:5942–5954.
1999.PubMed/NCBI
|
|
4
|
Mao J, Chen L, Xu B and Wang L, Li H, Guo
J, Li W, Nie S, Jacob TJ and Wang L: Suppression of ClC-3 channel
expression reduces migration of nasopharyngeal carcinomacells.
Biochem Pharmacol. 75:1706–1716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goudet C, Chi CW and Tytgat J: An overview
of toxins and genes from the venom of the Asian scorpion Buthus
martensi Karsch. Toxicon. 40:1239–1258. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhijian C, Feng L, Yingliang W, Xin M and
Wenxin L: Genetic mechanisms of scorpion venom peptide
diversification. Toxicon. 47:348–355. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
DeBin JA and Strichartz GR: Chloride
channel inhibition by the venom of the scorpion Leiurus
quinquestriatus. Toxicon. 29:1403–1408. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
DeBin JA, Maggio JE and Strichartz GR:
Purification and characterization of chlorotoxin, a chloride
channel ligand from the venom of the scorpion. Am J Physiol.
264:C361–C369. 1993.PubMed/NCBI
|
|
9
|
Ullrich N, Bordey A, Gillespie GY and
Sontheimer H: Expression of voltage-activated chloride currents in
acute slices of human gliomas. Neuroscience. 83:1161–1173. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ullrich N, Gillespie GY and Sontheimer H:
Human astrocytoma cells express a unique chloride current.
Neuroreport. 7:1020–1024. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olsen ML, Schade S, Lyons SA, Amaral MD
and Sontheimer H: Expression of voltage-gated chloride channels in
human glioma cells. J Neurosci. 23:5572–5582. 2003.PubMed/NCBI
|
|
12
|
Deshane J, Garner CC and Sontheimer H:
Chlorotoxin inhibits glioma cell invasion via matrix
metalloproteinase-2. J Biol Chem. 278:4135–4144. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soroceanu L, Gillespie Y, Khazaeli MB and
Sontheimer H: Use of chlorotoxin for targeting of primary brain
tumors. Cancer Res. 58:4871–4879. 1998.PubMed/NCBI
|
|
14
|
Shen S, Khazaeli MB, Gillespie GY and
Alvarez VL: Radiation dosimetry of 131I-chlorotoxin for targeted
radiotherapy in glioma-bearing mice. J Neurooncol. 71:113–119.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hockaday DC, Shen S, Fiveash J,
Raubitschek A, Colcher D, Liu A, Alvarez V and Mamelak AN: Imaging
glioma extent with 131I-TM-601. J Nucl Med. 46:580–586.
2005.PubMed/NCBI
|
|
16
|
Mamelak AN, Rosenfeld S, Bucholz R,
Raubitschek A, Nabors LB, Fiveash JB, Shen S, Khazaeli MB, Colcher
D, Liu A, et al: Phase I single-dose study of
intracavitary-administered iodine-131-TM-601 in adults with
recurrent high-grade glioma. J Clin Oncol. 24:3644–3650. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng XC, Li WX, Zhu SY, Peng F, Zhu ZH, Wu
KL and Yiang FH: Cloning and characterization of a cDNA sequence
encoding the precursor of a chlorotoxin-like peptide from the
Chinese scorpion Buthus martensii Karsch. Toxicon.
38:1009–1014. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu JJ, Dai L, Lan ZD and Chi CW: The gene
cloning and sequencing of Bm-12, a chlorotoxin-like peptide from
the scorpion Buthus martensi Karsch. Toxicon. 38:661–668.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang R, Peng F, Liu H, Cao ZJ, LI WX, Mao
X and Jiang DH: Functional analysis of a gene encoding a
Chlorotoxin-like peptide derived from scorpion toxin. Chinese J
Biochemistry Mol Biol. 21:19–23. 2005.
|
|
20
|
Wang WX and Ji YH: Scorpion venom induces
glioma cell apoptosis in vivo and inhibits glioma tumor growth in
vitro. J Neurooncol. 73:1–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan S, Sun Z, Jiang D, Dai C, Ma Y, Zhao
Z, Liu H, Wu Y, Cao Z and Li W: BmKCT toxin inhibits glioma
proliferation and tumor metastasis. Cancer Lett. 291:158–166. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu YJ, An N, Chan KG, Wu YB, Zheng SH and
Liang AH: A model of BmK CT in inhibiting glioma cell migration via
matrix metalloproteinase-2 from experimental and molecular dynamics
simulation study. Biotechnol Lett. 33:1309–1317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu YJ, Yin LT, Liang AH, Zhang CF, Wang W,
Chai BF, Yang JY and Fan XJ: Therapeutic potential of
chlorotoxin-like neurotoxin from the Chinese scorpion for human
gliomas. Neurosci Lett. 412:62–67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao J, Qiao W, Zhang Y and Shao X:
Preparation and in vitro evaluation of 131I-BmK CT as a
glioma-targeted agent. Cancer Biother Radiopharm. 25:353–359. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kondo S, Shukunami C, Morioka Y, Matsumoto
N, Takahashi R, Oh J, Atsumi T, Umezawa A, Kudo A, Kitayama H, et
al: Dual effects of the membrane-anchored MMP regulator RECK on
chondrogenic differentiation of ATDC5 cells. J Cell Sci.
120:849–857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kargiotis O, Chetty C, Gondi CS, Tsung AJ,
Dinh DH, Gujrati M, Lakka SS, Kyritsis AP and Rao JS:
Adenovirus-mediated transfer of siRNA against MMP-2 mRNA results in
impaired invasion and tumor-induced angiogenesis, induces apoptosis
in vitro and inhibits tumor growth in vivo in glioblastoma.
Oncogene. 27:4830–4840. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rahme GJ and Israel MA: Id4 suppresses
MMP2-mediated invasion of glioblastoma-derived cells by direct
inactivation of Twist1 function. Oncogene. 34:53–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fu Y, Jiao Y, An N and Liang A:
pEGFP-N1-mediated BmK CT expression suppresses the migration of
glioma. Cytotechnology. 65:533–539. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fu Y, Zheng S, Huang R, An N, Zheng Y,
Zhang Z and Liang A: A potential strategy for high-grade gliomas:
Combination treatment with lithium chloride and BmK CT. Biotechnol
Lett. 34:9–17. 2012. View Article : Google Scholar : PubMed/NCBI
|