Introduction
Cervical cancer is a common gynecological malignant
disease with the second highest morbidity and the third-highest
mortality rates among female patients with malignant tumors
(1). Chemotherapy combined with
surgery is a common method of treatment, with paclitaxel (PTX) as
the primary chemotherapeutic agent used to treat patients with
cervical cancer. However, a side effect of PTX is severe toxicity,
and drug resistance may occur. Therefore, increasing PTX
sensitivity and efficacy is an important aim for the treatment of
patients with cervical cancer.
The age of onset of cervical cancer is becoming
increasingly younger (2,3). The prognosis of patients in the
advanced stages of the disease is very poor, with a low five-year
survival rate (2,3). Thus, the early diagnosis of cervical
cancer based on specific high-performance biomarkers may be an
effective approach to reduce morbidity and mortality and improve
prognosis. MicroRNAs (miRNAs) are endogenous non-coding RNAs with
highly-conserved sequences. miRNAs bind to the 3′-untranslated
region of target mRNA sequences and subsequently repress their
translation and expression (4). In
addition, miRNAs are closely correlated with the development and
progression of cervical cancer (5,6).
They serve important roles in regulating the proliferation,
apoptosis, invasion and metastasis of cervical cancer cells.
Previous studies have demonstrated that miRNAs such as miR-21,
miR-218, miR-10a, miR-196a, miR-132, and miR-148a increase the
sensitivity of cervical cancer cells to chemotherapeutic agents,
such as PTX (5–8). Therefore, miRNAs may provide an
alternative strategy for the early diagnosis and prognosis of
cervical cancer (5,6).
miR-21 is the only miRNA that is highly expressed in
540 samples of human solid tumors including those of the lung,
breast, stomach, prostate, colon and pancreas (9). It has been confirmed to function as
an oncogene during cancer pathogenesis (9). Zeng et al (5) demonstrated that miR-21 was
overexpressed in cervical intraepithelial neoplasia tissues and in
cervical cancer tissues through reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay analysis, which was
~6-fold higher when compared to normal tissues. Deftereos et
al (10) reported that miR-21
was highly expressed in invasive cervical carcinomas when compared
with cervical intraepithelial neoplasia tissues. Lui et al
(11) determined the expression of
166 miRNAs in normal cervical cells and cervical cancer cells,
which indicated that miR-21 demonstrated the most significant
upregulation in cervical cancer cells. These results indicate that
miR-21 may be extensively involved in the development and
progression of cervical cancer. In the current study, the effects
of silenced miR-21 expression on the proliferation, colony
formation and apoptosis of cervical cancer cells were explored.
Furthermore, the sensitivity of cervical cancer cells to PTX
following forced miR-21 downregulation was investigated.
Materials and methods
Materials and cell culture
The Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis detection kit, Hoechst 33258
staining kit, radioimmunoprecipitation assay buffer, enhanced
chemiluminescence solution, and the bicinchoninic acid (BCA) assay
kit were purchased from Beyotime Institute of Biotechnology
(Haimen, China). Lipofectamine™ 2000, TRIzol reagent, and the
SuperScript III One-Step RT-PCR kit were purchased from Invitrogen;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). MTT reagent and
trypsin were purchased from Gibco; Thermo Fisher Scientific, Inc.
PTX (lot no. 10382-201102; purity 99.6%) was purchased from the
National Institute for the Control of Pharmaceutical and Biological
Products (Beijing, China). Rabbit anti-B-cell lymphoma-2 (Bcl-2),
anti-Bcl-2-associated X (Bax), anti-survivin, and anti-c-myc
monoclonal antibodies were obtained from Epitomics (cat. nos.
1017-1, 1063-1, 2463-1 and 1472-1, respectively; Burlingame, CA,
USA). Rabbit anti-programmed cell death 4 (PDCD4), anti-phosphate
and phosphatase and tensin homolog (PTEN), anti-Ser/Thr protein
kinase (AKT) and anti-phosphorylated (p)-AKT monoclonal antibodies
were purchased from Cell Signaling Technology, Inc. (cat. nos.
9535, 5384, 4685, and 12178, respectively, Danvers, MA, USA).
Cervical cell lines, C-33A, CaSki, SiHa, HeLa and
ME-180 (cat. nos. TCHu176, TCHu137, TCHu113, TCHu187 and TCHu180,
respectively) were obtained from The Cell Bank of Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
The normal cervical epithelial squamous cell line H8 was purchased
from the Institute of Basic Medical Sciences of the Chinese Academy
of Medical Sciences, (Beijing, China). Cells were cultured in
Dulbecco's Modified Eagle's medium (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% (v/v) fetal bovine
serum (Hyclone; GE Healthcare Life Sciences).
Preparation of miRNA
miR-21 antisense oligonucleotide (asOGC);
(5′-UCAACAUCAGUCUGAUAAGCU-3′) and the negative control (NC;
5′-GAUGUUGAAACAUCAGUCUGA-3′) were synthesized by Shanghai
GenePharma Co., Ltd., (Shanghai, China). The quantity of miRNA and
Lipofectamine™ 2000 used for gene transfection was established
according to the manufacturer's instructions (Invitrogen; Thermo
Fisher Scientific, Inc.). HeLa cells were seeded in 96-well plates
at a density of 5×103 cells/well. When cells reached 50%
confluence, they were transfected with miR-21 asOGC or NC using
Lipofectamine™ 2000. Cells were harvested for analysis at 48 h
following transfection.
miR-21 levels in normal cervical cells
and cervical cancer cells
Total RNA from normal and cancer cells was extracted
using TRIzol reagent according to the manufacturer's instructions
(Invitrogen; Thermo Fisher Scientific, Inc.) in an RNase-free
environment. RNA was reverse transcribed into cDNA and subsequently
amplified by PCR using the One-Step RT-PCR kit (Invitrogen; Thermo
Fisher Scientific, Inc.). Primers (20 µM; Sangon Biotechnology Co.,
Ltd., Shanghai, China; Table I)
were added into the PCR reaction mixture (total volume, 25 µl). The
thermal cycling parameters included 35 cycles of denaturation for
45 sec at 94°C, annealing for 45 sec at 59°C and elongation for 60
sec at 72°C. The PCR product (5 µl each lane) was separated by gel
electrophoresis on a 2% (w/v) agarose gel. Electrophoresis strips
were examined with Quantity One software v.4.6.2 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) using a gel imaging system
(ChemiDoc™ XRS; Bio-Rad Laboratories, Inc.).
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer (5′-3′) |
|---|
| miR-21 | F:
GCCGCTAGCTTATCAGACTGATGT |
|
| R:
GTGCAGGGTCCGAGGT |
| GADPH | F:
AGCCACATCGCTCAGACA |
|
| R:
TGGACTCCACGACGTACT |
Cell viability
At 48 h following transfection, 20 µl MTT (5 mg/ml)
was added to each well, and cells were cultured for a further 4 h.
The culture media was then discarded and 150 µl dimethyl sulfoxide
was added to each well. Plates were agitated to thoroughly dissolve
the crystals, before the absorbance was read at 560 nm using a
microplate reader (Infinite M200; Tecan Trading AG, Zurich,
Switzerland). The relative cell viability was calculated by
comparing the absorbance values of treated cells with the untreated
control cells.
Colony formation
At 48 h following transfection of HeLa cells with
miR-21 asOGC or NC, cells were fixed with 10% (w/v) formaldehyde
and stained with 0.1% (w/v) crystal violet for 30 min at room
temperature. The staining solution was then discarded carefully and
each well was washed with water. The plates were then inverted on
absorbent paper to dry. Finally, the cells were visualized in five
fields under a fluorescence microscope (AF6000, Leica Microsystems
GmbH, Wetzlar, Germany). Results were expressed as the average
number of cells in every visual field.
Cell apoptosis with annexin V-FITC/PI
staining
The Annexin V-FITC/PI apoptosis detection kit was
utilized to evaluate cell apoptosis according to the manufacturer's
instructions (Beyotime Institute of Biotechnology). At 48 h
following transfection of HeLa cells with miR-21 asOGC and NCs,
cells were digested with 0.25% (w/v) trypsin (without EDTA), washed
with phosphate-buffered saline (PBS), and collected by
centrifugation at 800 × g for 5 min at room temperature. Cells were
the resuspended in 500 µl binding buffer, 5 µl Annexin V-FITC stain
and 5 µl PI, before they were incubated for 10 min at room
temperature in the dark. Cell apoptosis was evaluated using a flow
cytometer with CellQuest Pro v.5.2 software (BD FACScan; BD
Biosciences, Franklin Lakes, NJ, USA).
Cell apoptosis with Hoechst 33258
staining
The Hoechst 33258 kit was employed to evaluate cell
apoptosis according to the manufacturer's instructions (Beyotime
Institute of Biotechnology). At 48 h following transfection, cells
were fixed with 4% (w/v) paraformaldehyde for 15 min at room
temperature, then washed three times with PBS, before they were
incubated with Hoechst 33258 (10 µg/ml) for 15 min at room
temperature in the dark. Finally, cells were washed three times
with PBS and observed in five fields of view under a fluorescence
microscope. Results were expressed as the average number of cells
in every visual field.
Western blot analysis
At 48 h following transfection, cells were collected
and lysed in radioimmunoprecipitation assay buffer. Lysates were
agitated using a vortex for 30 sec every 10 min. Following 40 min,
the supernatant was carefully separated from the mixture by
centrifugation at 7,000 × g for 10 min at 4°C to obtain total
protein. The protein concentration was determined using a BCA kit.
Proteins were loaded (20 µg per lane) to perform SDS-PAGE (10%
separation gel, 5% concentrated gel) electrophoresis and
transferred to a polyvinylidene difluoride membrane using a wet
transfer methodology. Membranes were blocked with 5% (w/v) non-fat
milk buffer at room temperature for 1 h, then incubated with
primary antibodies (dilution 1:100) overnight at 4°C, and then
rinsed with PBS and incubated with a secondary antibody buffer
(dilution 1:100) for 2 h at room temperature. Following a further
rinse with PBS, enhanced chemiluminescence solution was added to
the membrane, which was then exposed using a gel imaging system
(ChemiDoc™ XRS; Bio-Rad Laboratories, Inc.). Quantity One software
v4.6.2 (Bio-Rad Laboratories, Inc.) was employed to determine the
gray values of proteins. GADPH served as the internal control.
PTX sensitivity
At 48 h following transfection, cells were
trypsinized to produce single-cell suspensions and seeded in
96-well plates at a density of 5×103 cells/well. After
24 h, cells were incubated with 25, 50, and 100 µg/ml PTX for a
further 48 h. Cell viability was evaluated with an MTT assay using
the aforementioned procedures.
Statistical analysis
Data are expressed as the mean ± standard deviation
(n=6). Statistical analysis was performed using a Student's t-test
with SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-21 expression levels in normal
cervical cells and cervical cancer cells
miR-21 levels in normal cervical cells and cervical
cancer cells were evaluated by RT-qPCR analysis and the results are
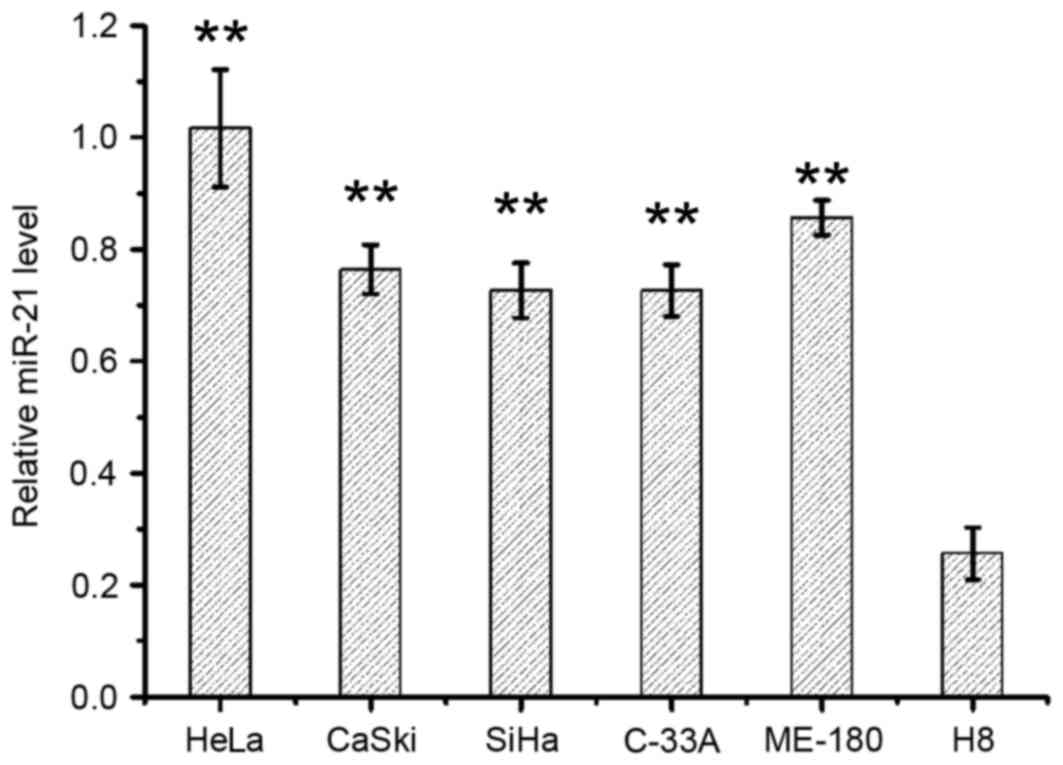
shown in Fig. 1. miR-21 levels in
cervical cancer cell lines HeLa, CaSki, SiHa, C-33A and ME-180 were
2.8–4.0-fold higher when compared with the normal cervical cell
line, H8 (P<0.01 for each comparison; Fig. 1). Among the five cervical cancer
cell lines, HeLa cells exhibited the highest expression levels of
miR-21 (Fig. 1).
Effect of miR-21 asOGC on the
viability of cervical cancer cells
The viability of HeLa cells transfected with the
miR-21 asOGC or the miR-21 NC was determined by MTT assay. As
demonstrated in Table II,
compared with the NC, miR-21 asOGC transfection reduced the
proliferation of HeLa cells (P=0.007).
 | Table II.Viability of HeLa cells transfected
with the miR-21 asOGC or the miR-21 NC. |
Table II.
Viability of HeLa cells transfected
with the miR-21 asOGC or the miR-21 NC.
| Group | Cell viability |
|---|
| miR-21 NC | 0.68±0.04 |
| miR-21 asOGC |
0.40±0.01a |
Effect of miR-21 asOGC on the colony
formation of cervical cancer cells
As demonstrated in Table III, miR-21 asOGC-transfected HeLa
cells exhibited a significant reduction in colony number when
compared with NC-transfected cells (P=0.002).
 | Table III.Colony formation in HeLa cells
transfected with the miR-21 asOGC or the miR-21 NC. |
Table III.
Colony formation in HeLa cells
transfected with the miR-21 asOGC or the miR-21 NC.
| Group | Cell colony
number |
|---|
| miR-21 NC | 152.4±114.7 |
| miR-21 asOGC |
58.4±5.7a |
Effect of an miR-21 asOGC on the
apoptosis of cervical cancer cells
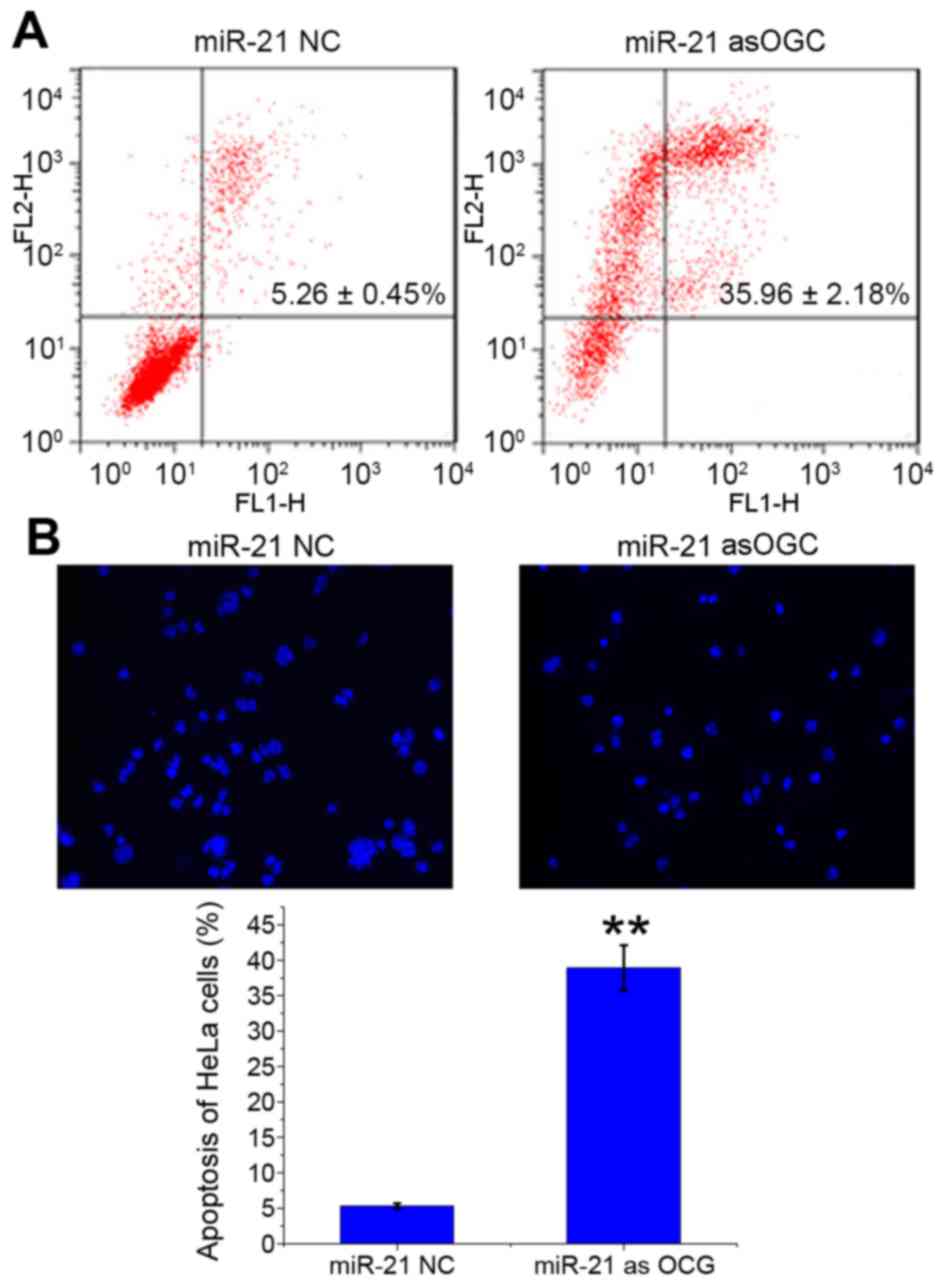
The number of apoptotic HeLa cells transfected with
miR-21 asOGC or NC was evaluated by Annexin V-FITC/PI staining
(Fig. 2A) and Hoechst 33258
staining (Fig. 2B). Following
transfection of cells with the miR-21 asOGC, the late apoptosis
ratio of HeLa cells was significantly enhanced when compared with
the NC group (P=0.001; Fig. 2A).
Similarly, Hoechst 33258 staining revealed significantly more
apoptotic HeLa cells with the miR-21 asOGC (38.89±3.22%; Fig. 2B) compared with the NC (5.29±0.42%;
P=0.001; Fig. 2B). The results of
the present study, therefore, suggest that suppression of miR-21
expression induces apoptosis in cervical cancer cells.
Effect of miR-21 asOGC on the
expression of PDCD4, Bax, Bcl-2, survivin, c-myc and PTEN/AKT in
cervical cancer cells
The expression of signaling molecules in HeLa cells
transfected with an miR-21 asOGC or a NC was assessed by western
blot analysis, as demonstrated in Fig.
3. As an internal control, similar GADPH protein band densities
were observed between the two groups, thus confirming the reliable
evaluation of the western blotting results.
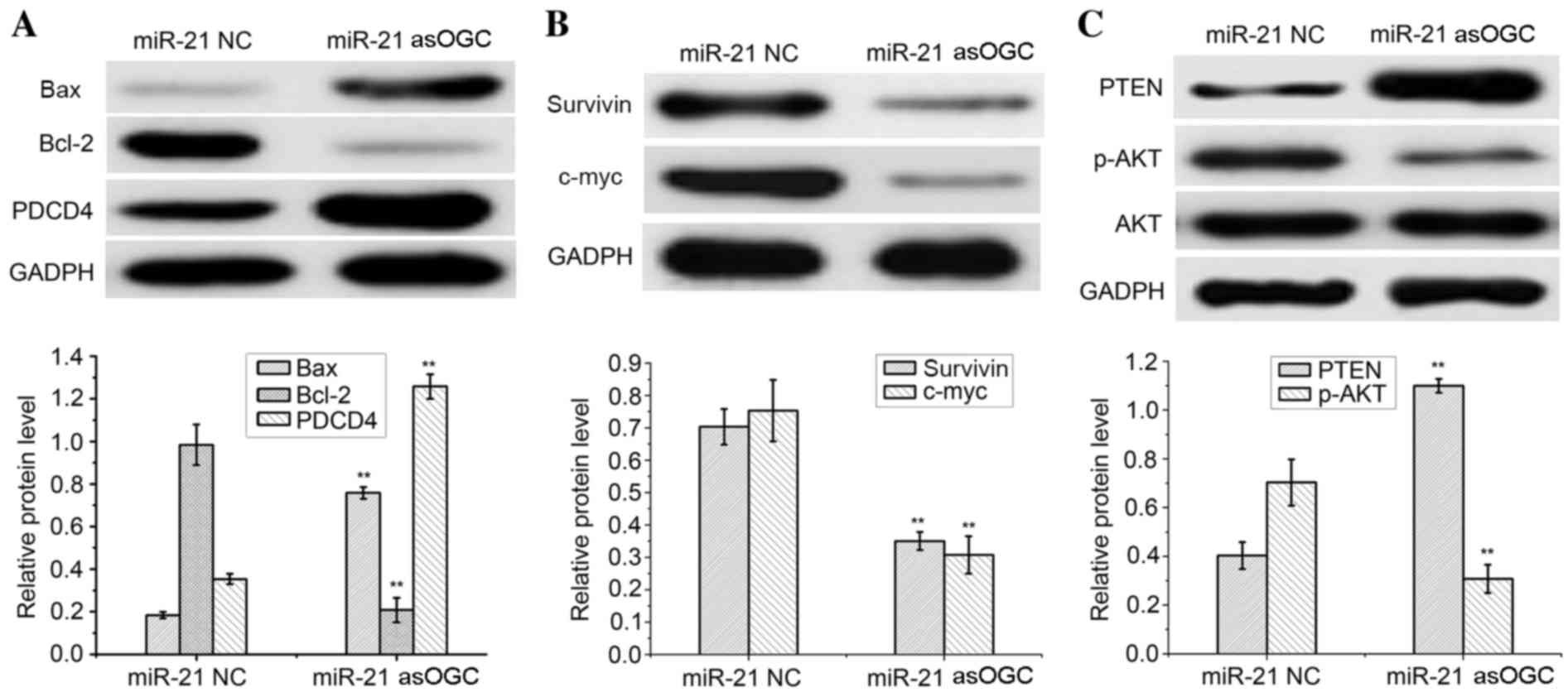 | Figure 3.Qualitative and quantitative western
blot analysis of (A) Bax, Bcl-2, PDCD4, (B) survivin, c-myc and (C)
PTEN, AKT and p-AKT in HeLa cells transfected with a miR-21
antisense oligonucleotide or NC. **P<0.01 vs. miR-21 NC. Bax,
Bcl-2-associated X; Bcl-2, B-cell lymphoma 2; PDCD4, programmed
cell death protein 4; PTEN, phosphatase and tensin homolog; p-AKT,
phosphorylated AKT; miR-21, microRNA-21; NC, negative control. |
Notably, a significant increase in the protein
expression levels of PDCD4 and Bax were observed in HeLa cells
transfected with the miR-21 asOGC when compared with cells
transfected with the NC (P=0.003 and P=0.002, respectively;
Fig. 3A). Conversely, a
significant reduction in Bcl-2 expression was observed in cells
transfected with the miR-21 asOGC when compared with the NC group
(P=0.002; Fig. 3A).
Compared with the NC group, HeLa cells expressed
significantly lower levels of survivin and c-myc following
transfection with the miR-21 asOGC (P=0.004 and P=0.003,
respectively; Fig. 3B), which
demonstrated that the miR-21 asOGC downregulated the expression of
survivin and c-myc in cervical cancer cells. Similar levels of AKT
protein expression were observed in the miR-21 asOGC-transfected
HeLa cells and the NC-transfected cells (Fig. 3C).
However, significantly increased levels of PTEN and
decreased levels of p-AKT were detected in HeLa cells transfected
with the miR-21 asOGC when compared with those transfected with the
NC (P=0.002 and P=0.004, respectively; Fig. 3C). Therefore, transfection of an
miR-21 asOGC into cervical cancer cells increased the levels of
PTEN and decreased the phosphorylation of AKT.
Effect of an miR-21 inhibitor on the
sensitivity of cervical cancer cells to PTX
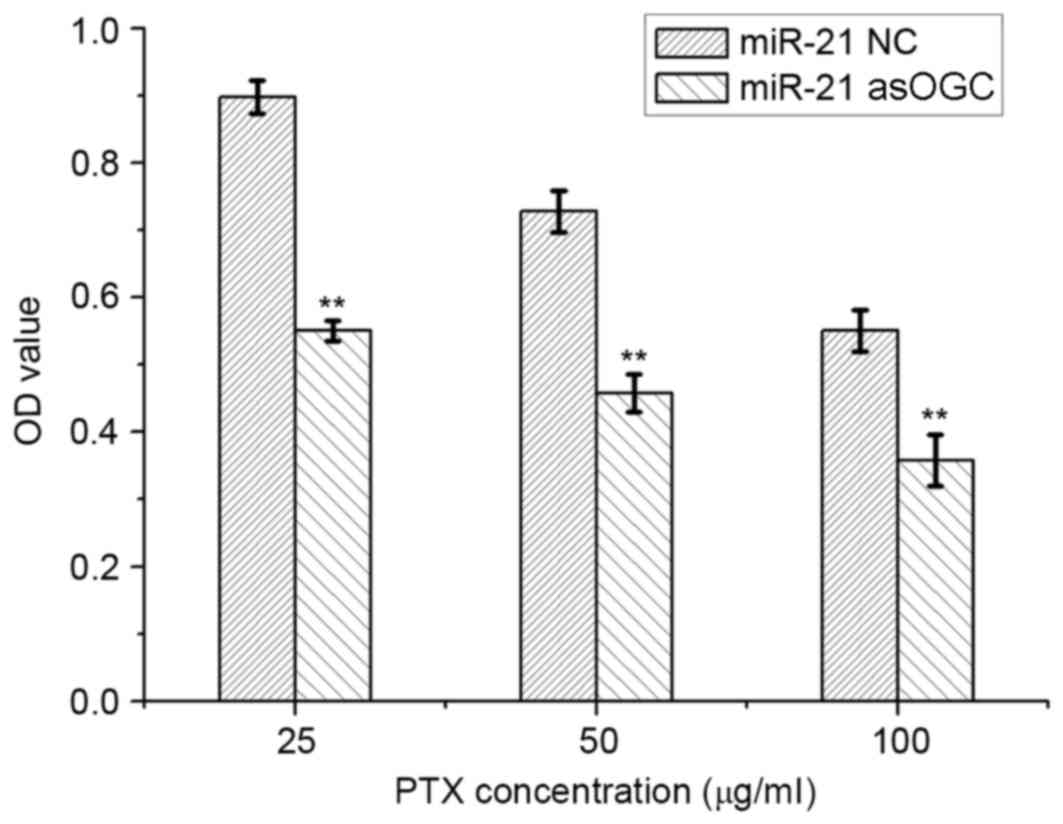
The sensitivity of HeLa cells to PTX was evaluated
using an MTT assay and the results are presented in Fig. 4. Compared with the NC group,
transfection of cells with the miR-21 asOGC significantly reduced
the viability of HeLa cells following incubation with 25, 50 and
100 µg/ml PTX by 54–63% (P=0.005, P=0.006 and P=0.007,
respectively; Fig. 4), which
suggests that the miR-21 asOGC was capable of enhancing the
sensitivity of cervical cancer cells to PTX.
Discussion
The human papillomavirus (HPV) is one of the most
important causes of cervical cancer, and chromosome 17q23.3 is the
most common integration site for the viral genome (7,12).
The subsequent genetic and epigenetic alterations exert significant
effects on tumorigenesis involving miRNA sequences localized near
the integration site (7,12). A previous study demonstrated that
miR-21, located in the FRA17B fragile region of 17q23.3, exhibits
the highest expression levels in HPV16-positive cervical cancer
tissues (12). In addition, it was
demonstrated to be overexpressed in cervical cancers when compared
with normal cervical tissues (5,10,11).
Furthermore, investigations involving miRNA chip screening of 363
tumor samples, including breast cancer, colon cancer, lung cancer,
pancreatic cancer, prostate cancer and gastric cancer, as well as
177 corresponding normal tissue samples, demonstrated that miR-21
was the only miRNA with upregulated expression in all tumor samples
(9). These results suggested that
miR-21 was overexpressed in cervical cancer, as well as a number of
additional cancer tissues. Therefore, miR-21 may be considered to
be an oncogenic miRNA sequence. In the present study, miR-21 was
demonstrated to be overexpressed in various cervical cancer cells
when compared with normal cervical cells, which was consistent with
the findings of a previous report (11). These results provided additional
evidence to suggest that miR-21 may be oncogenic in cervical
cancer, and may present a novel target for the diagnosis and
treatment of patients with cervical cancer. In addition, as the
highest expression of miR-21 was observed in the HeLa cell line,
these cells were therefore selected as a model cervical cancer cell
line for the subsequent investigations.
In the present study, an miR-21 asOGC was applied as
an miR-21 inhibitor to downregulate intracellular miR-21 levels in
cervical cancer cells. It was demonstrated that transfection of the
miR-21 asOGC suppressed the proliferation and colony formation, and
enhanced apoptosis of HeLa cells. This result was in accordance
with previous reports, where an miR-21 inhibitor was observed to
suppress the proliferation and promote apoptosis of cervical,
pancreatic, breast and prostate cancer cells, as well as tongue
squamous carcinoma cells (13–15).
The balance between cell proliferation and apoptosis
strongly influences tumor development and progression (16,17).
If cell apoptosis is inhibited, cell hyperplasia may occur. Mutant
cells that are unable to undergo apoptosis, may proliferate
uncontrollably, leading to tumorigenesis (16,17).
Therefore, the inhibition of cell proliferation and induction of
cell apoptosis may be an effective strategy for cancer therapy.
Cell proliferation and apoptosis are regulated by related genes,
such as the Bcl-2 family, which has been extensively investigated
(16,17). Bcl-2 promotes the proliferation of
cancer cells (16,17). In addition, Bcl-2 is overexpressed
in cervical cancer cells and is closely correlated with the
development and progression of cervical cancer (18). Bax belongs to the Bcl-2 family and
forms dimers with Bcl-2 to regulate the balance between cell
proliferation and apoptosis. Bax expression is downregulated, or
even completely absent in cervical cancer cells, and its gene
polymorphism [BAX-248G>A (rs4645878)] is intensively correlated
with the progression of cervical cancer (19). Therefore, the upregulation of Bax
expression and downregulation of Bcl-2 expression observed in
miR-21 asOGC-transfected cells in the present study, may have been
responsible for the increased apoptosis and reduction in cell
proliferation of cervical cancer cells. A previous report revealed
that altered regulation of miR-21 promoted cancer cell apoptosis by
influencing diverse target genes associated with cell apoptosis,
including Bcl-2, PDCD4 and PTEN (20). In the present study, a miR-21 asOGC
elevated Bax expression and reduced Bcl-2 expression in HeLa cells.
In previous studies, a downstream target gene of miR-21, PDCD4, was
determined to be a tumor suppressor gene (21,22).
Its expression is absent in multiple cancers, including cervical
cancer, and is closely associated with tumor development and
progression (22). In addition,
miR-21 has been demonstrated to promote the proliferation of
cervical cancer cells via downregulation of PDCD4 expression
(21). Therefore, suppression of
miR-21 expression may inhibit the proliferation of HeLa cervical
cancer cells (21), Colo206f
colorectal cancer cells (23) and
T89 G glioma cells (24), due to
elevation of PDCD4 expression. The present study demonstrated that,
following a reduction of intracellular miR-21 levels by
transfection with a miR-21 asOGC, PDCD4 levels and cancer cell
apoptosis was increased.
Survivin, a regulator of cell mitosis, is a member
of an apoptosis-suppressing protein family (25). To date, it is currently the most
effective apoptosis-suppressing protein (25). The positive expression of survivin
is correlated with clinical stage, lesion size, and degree of tumor
differentiation (26).
Overexpression of survivin has been demonstrated to elevate
intracellular levels of the proto-oncogene c-myc, which
subsequently accelerates tumorigenesis (26). Moreover, the expression of c-myc in
cervical cancer tissues is notably higher when compared with that
in normal cervical tissues, and c-myc is involved in the level of
pathological differentiation and clinical stage of cancer tissues
(27). Therefore, the decreased
level of survivin and c-myc observed in miR-21 asOGC-transfected
HeLa cells in the present study, may have led to inhibition of the
proliferation of HeLa cells and may prevent tumor progression. The
results of the current study demonstrated that miR-21 asOGC induced
apoptosis and inhibited the proliferation of cervical cancer cells
likely through the upregulation of Bax and PDCD4 and downregulation
of Bcl-2, survivin and c-myc.
As a novel tumor suppressor gene, PTEN is a
downstream target gene of miR-21, which is expressed at a low
level, deleted, or mutated in a number of cancers (28). It dephosphorylates phosphatidyl
inositol triphosphate into phosphatidyl inositol bisphosphate, and
subsequently negatively regulates the phosphatidylinositol-3-kinase
(PI3K)/AKT signaling pathway (29). Among the various signaling pathways
that regulate tumor cell proliferation and apoptosis, the PI3K/AKT
pathway is an anti-apoptotic pathway (29). This signaling pathway mediates
cancer cell apoptosis, tumor angiogenesis, invasion and metastasis
by regulating a variety of downstream target proteins. The positive
rate of PTEN in cervical cancer tissues is significantly lower than
that in normal cervical tissues, and its positive expression is
associated with low clinical stage and reduced lymphatic metastasis
(30). Numerous heterozygotes are
deleted in cervical cancer tissues, which are located in D10S198
and D10S192 sites (31). It has
been suggested that inactivation of the PTEN gene and inhibition of
protein expression may promote the progression of cervical cancer
(32,33). However, the PI3K/AKT signaling
pathway is abnormally activated in cervical cancer tissues
(34). Tao et al (35) utilized oligonucleotides to inhibit
the expression of miR-21 in HCT116 human colon cancer cells, and
demonstrated that the proliferation and metastasis of these cells
was suppressed by upregulation of PTEN expression. Therefore,
overexpression of miR-21 in cervical cancer cells may inhibit the
expression of PTEN and facilitate the proliferation and metastasis
of cervical cancer cells (36). In
the present study, when the expression of miR-21 was downregulated
by an miR-21 asOGC in HeLa cells, the expression of PTEN was
increased and the levels of p-AKT were significantly reduced. This
may have been responsible for the observed increase in apoptosis
and decrease in proliferation of miR-21 asOGC-transfected HeLa
cells.
In addition, the miR-21 asOGC was capable of
enhancing the sensitivity of HeLa cells to PTX at a series of
increasing concentrations. This indicated that the miR-21 asOGC
increased the therapeutic efficacy of PTX in vitro. PTX is a
major chemotherapeutic agent for the treatment of cervical cancer,
however it leads to severe side effects, including arrest of bone
marrow function, anaphylactic reaction and damage to the liver and
kidneys (37). Therefore, the dose
of PTX is strictly controlled to alleviate the painful side
effects, which may, however, compromise the efficacy (37). The results of the current study
demonstrated that a miR-21 asOGC significantly enhanced the
sensitivity of cervical cancer cells to PTX, and improved its
efficacy, even at a low dose. Therefore, a miR-21 inhibitor could
be applied as an adjuvant agent for the treatment of cervical
cancer. However, the detailed mechanisms of action require further
investigation.
In conclusion, miR-21 may serve an important role in
the development and progression of cervical cancer. In the present
study, cervical cancer cells exhibited high levels of miR-21
expression when compared with a normal cervical tissue cell line,
which may underlie the aggressive biological behaviors of cervical
tumors. Following the transfection of a miR-21 asOGC into cervical
cancer cells, apoptosis of cervical cancer cells was increased,
while their proliferation and colony formation was suppressed. This
may have been through regulation of the PTEN/AKT signaling pathway,
where Bcl-2, survivin and c-myc were negatively regulated, and Bax
and PDCD4 were positively regulated. In addition, the miR-21 asOGC
improved the sensitivity of cervical cancer cells to PTX. These
findings may serve as guidelines for the development of miRNA-based
agents and therapeutics for the treatment of cervical cancer.
References
|
1
|
Missaoui N, Hmissa S, Trabelsi A, Frappart
L, Mokni M and Korbi S: Cervix cancer in Tunisia: Clinical and
pathological study. Asian Pac J Cancer Prev. 11:235–238.
2010.PubMed/NCBI
|
|
2
|
Feng Y, Cao T, Wang Y, Huang H, Xie Y and
Liu J: Neoadjuvant chemotherapy followed by conization to spare
fertility in cases of locally advanced cervical cancer: A case
report and review of the literature. Mol Clin Oncol. 5:411–416.
2016.PubMed/NCBI
|
|
3
|
Qin AQ, Liang ZG, Ye JX, Li J, Wang JL,
Chen CX and Song HL: Significant efficacy of additional concurrent
chemotherapy with radiotherapy for postoperative cervical cancer
with risk factors: A systematic review and meta-analysis. Asian Pac
J Cancer Prev. 17:3945–3951. 2016.PubMed/NCBI
|
|
4
|
Gambari R, Brognara E, Spandidos DA and
Fabbri E: Targeting oncomiRNAs and mimicking tumor suppressor
miRNAs: New trends in the development of miRNA therapeutic
strategies in oncology (Review). Int J Oncol. 49:5–32.
2016.PubMed/NCBI
|
|
5
|
Zeng K, Zheng W, Mo X, Liu F, Li M, Liu Z,
Zhang W and Hu X: Dysregulated microRNAs involved in the
progression of cervical neoplasm. Arch Gynecol Obstet. 292:905–913.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen Y, Wang P, Li Y, Ye F, Wang F, Wan X,
Cheng X, Lu W and Xie X: miR-375 is upregulated in acquired
paclitaxel resistance in cervical cancer. Br J Cancer. 109:92–99.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pedroza-Torres A, López-Urrutia E,
Garcia-Castillo V, Jacobo-Herrera N, Herrera LA, Peralta-Zaragoza
O, López-Camarillo C, De Leon DC, Fernández-Retana J, Cerna-Cortés
JF and Pérez-Plasencia C: MicroRNAs in cervical cancer: Evidences
for a miRNA profile deregulated by HPV and its impact on
radio-resistance. Molecules. 19:6263–6281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu S, Cheng X, Zheng H and Xie R:
Clinical efficacy and safety of paclitaxel plus cisplatin
neoadjuvant treatment on locally advanced cervical cancer. Zhongguo
Linchuang Yaolixue Zazhi. 31:432–434. 2015.(In Chinese).
|
|
9
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deftereos G, Corrie SR, Feng Q, Morihara
J, Stern J, Hawes SE and Kiviat NB: Expression of mir-21 and
mir-143 in cervical specimens ranging from histologically normal
through to invasive cervical cancer. PLoS One. 6:e284232011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lui WO, Pourmand N, Patterson BK and Fire
A: Patterns of known and novel small RNAs in human cervical cancer.
Cancer Res. 67:6031–6043. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Tang S, Le SY, Lu R, Rader JS,
Meyers C and Zheng ZM: Aberrant expression of oncogenic and
tumor-suppressive microRNAs in cervical cancer is required for
cancer cell growth. PLoS One. 3:e25572008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang XM, Xu J, Cheng ZQ, Peng QZ, Hu JT,
Gao LK, Zhang SF and Jin HT: Study on effects of microRNA-21
antisense oligonucleotide in vivo and in vitro on bionomics of
human cervical squamous carcinoma cell lines SiHa. Zhonghua Bing Li
Xue Za Zhi. 41:254–259. 2012.(In Chinese). PubMed/NCBI
|
|
14
|
Zhu W and Xu B: MicroRNA-21 identified as
predictor of cancer outcome: A meta-analysis. PLoS One.
9:e1033732014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Zhu Y, Lv P and Li L: Targeting
miR-21 with AS-miR-21 suppresses aggressive growth of human tongue
squamous cell carcinoma in vivo. Int J Clin Exp Pathol.
8:4773–4781. 2015.PubMed/NCBI
|
|
16
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeestraten EC, Benard A, Reimers MS,
Schouten PC, Liefers GJ, van de Velde CJ and Kuppen PJ: The
prognostic value of the apoptosis pathway in colorectal cancer: A
review of the literature on biomarkers identified by
immunohistochemistry. Biomark Cancer. 5:13–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Yang H, Barnie PA, Yang P, Su Z,
Chen J, Jiao Z, Lu L, Wang S and Xu H: The expression of Toll-like
receptor 8 and its relationship with VEGF and Bcl-2 in cervical
cancer. Int J Med Sci. 11:608–613. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fernandes AT, Rocha NP, Vendrame E,
Russomano F, Grinsztejn BJ, Friedman RK, Pinto AC, Klumb EM, Avvad
E, Macedo J, et al: Polymorphism in apoptotic BAX (−248G>A) gene
but not in anti-apoptotic BCL2 (−938C>A) gene and its protein
and mRNA expression are associated with cervical intraepithelial
neoplasia. Apoptosis. 20:1347–1357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang Y, He Y and Li J: MicroRNA-21: A
central regulator of fibrotic diseases via various targets. Curr
Pharm Des. 21:2236–2242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao Q, Xu H, Zhang QQ, Zhou H and Qu LH:
MicroRNA-21 promotes cell proliferation and down-regulates the
expression of programmed cell death 4 (PDCD4) in HeLa cervical
carcinoma cells. Biochem Biophys Res Commun. 388:539–542. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lankat-Buttgereit B and Göke R: The tumour
suppressor Pdcd4: Recent advances in the elucidation of function
and regulation. Biol Cell. 101:309–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Liu W, Chao T, Zhang Y, Yan X,
Gong Y, Qiang B, Yuan J, Sun M and Peng X: MicroRNA-21
down-regulates the expression of tumor suppressor PDCD4 in human
glioblastoma cell T98G. Cancer Lett. 272:197–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roy K, Singh N, Kanwar RK and Kanwar JR:
Survivin modulators: An updated patent review (2011–2015). Recent
Pat Anticancer Drug Discov. 11:152–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu D, Yin X and Xiao Q: Expressions of
PTEN and Survivin in the progression of cervical neoplasia and
their clinical significances. Zhongguo Fuyou Baojian. 28:4721–4724.
2013.(In Chinese).
|
|
27
|
Hu Q and Liu K: The expression of CIP2A
and c-Myc and their correlation analysis in cervical carcinoma
tissues. Zhong Qing Yi Xue Bian Ji Bu. 44:1072–1074. 2015.(In
Chinese).
|
|
28
|
Wang Y and Dai B: PTEN genomic deletion
defines favorable prognostic biomarkers in localized prostate
cancer: A systematic review and meta-analysis. Int J Clin Exp Med.
8:5430–5437. 2015.PubMed/NCBI
|
|
29
|
Wang LL, Hao S, Zhang S, Guo LJ, Hu CY,
Zhang G, Gao B, Zhao JJ, Jiang Y, Tian WG, et al: PTEN/PI3K/AKT
protein expression is related to clinicopathologic features and
prognosis in breast cancer with axillary lymph node metastases. Hum
Pathol pii. S0046–8177. 2016.
|
|
30
|
Bu L, Ma Y and Shi S: Expressions of CD31,
CD105 and PTEN in cervical cancer and the clinical pathological
significance. Zhongguo Fuyou Baojian. 30:1446–1449. 2015.
|
|
31
|
Rizvi MM, Alam MS, Mehdi SJ, Ali A and
Batra S: Allelic loss of 10q23.3, the PTEN gene locus in cervical
carcinoma from Northern Indian population. Pathol Oncol Res.
18:309–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qi Q, Ling Y, Zhu M, Zhou L, Wan M, Bao Y
and Liu Y: Promoter region methylation and loss of protein
expression of PTEN and significance in cervical cancer. Biomed Rep.
2:653–658. 2014.PubMed/NCBI
|
|
33
|
Lu D, Qian J, Yin X, Xiao Q, Wang C and
Zeng Y: Expression of PTEN and survivin in cervical cancer:
Promising biological markers for early diagnosis and prognostic
evaluation. Br J Biomed Sci. 69:143–146. 2012.PubMed/NCBI
|
|
34
|
Schwarz JK, Payton JE, Rashmi R, Xiang T,
Jia Y, Huettner P, Rogers BE, Yang Q, Watson M, Rader JS and
Grigsby PW: Pathway-specific analysis of gene expression data
identifies the PI3K/Akt pathway as a novel therapeutic target in
cervical cancer. Clin Cancer Res. 18:1464–1471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tao YJ, Li YJ, Zheng W, Zhao JJ, Guo MM,
Zhou Y, Qin NL, Zheng J and Xu L: Antisense oligonucleotides
against microRNA-21 reduced the proliferation and migration of
human colon carcinoma cells. Cancer Cell Int. 15:772015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu J, Zhang W, Lv Q and Zhu D:
Overexpression of miR-21 promotes the proliferation and migration
of cervical cancer cells via the inhibition of PTEN. Oncol Rep.
33:3108–3116. 2015.PubMed/NCBI
|
|
37
|
Eskander RN and Tewari KS: Chemotherapy in
the treatment of metastatic, persistent, and recurrent cervical
cancer. Curr Opin Obstet Gynecol. 26:314–321. 2014. View Article : Google Scholar : PubMed/NCBI
|