Introduction
Pesticides are substances or mixture of substances
used for preventing, destroying, repelling or mitigating pests
which include insects, rodents, weeds, as well as other unwanted
organisms (1,2). Hence, worldwide use of pesticides,
especially in agriculture, has grown over the last years in rural
areas. Extensive agricultural production includes large use of
pesticides; their residues pollute the air, soil, water, plants,
harvested products, equipment, clothes, as well as human and animal
tissues. The applied pesticides entering the human body through
breathing, swallowing and skin absorption may cause poisoning
(2–4). The degree of generated damage depends
on the intrinsic toxicity of the substances and the individual
health status and sensitivity (2,4–6).
Exposure to pesticides represents a potential health risk for the
general population and for agricultural workers in particular
(7–12).
Many studies have focused on the association between
exposure to pesticides and cancer occurrence such as sarcoma,
multiple myeloma, bladder cancer, pancreatic cancer and leukemia
(13–21). Some researchers observed that
occupational exposure to pesticides has been associated with risk
of non-Hodgkins lymphoma (NHL) (22–24).
NHL is a heterogeneous group of lymphoproliferative malignancies
that can arise from B- or T-lymphocytes. Reciprocal rearrangements
of B-cell immunoglobulin or T-cell receptor genes occur with
oncogenes within immature lymphoid cells in the bone marrow or more
mature cells in the peripheral lymphoid organs (25,26).
These chromosomal translocations often result in the overexpression
of oncogenes and cause the cells to become malignant and
proliferate in an uncontrolled way (26).
The chromosomal translocation t(14;18)(q32;q21) is
one of the most common chromosomal abnormalities in NHL which
occurs in 70–90% of cases of follicular lymphoma (FL), 20–30% of
diffuse large B-cell lymphoma and 5–10% of other less common
subtypes (27). Furthermore, an
increased prevalence of the chromosomal translocation
t(14;18)(q32;q21) has been detected in peripheral blood lymphocytes
from individuals occupationally exposed to pesticides (28,29).
The aim of this study is to detect the effects of
pesticides on t(14;18) chromosome translocation in agricultural
workers after short-term exposure.
Materials and methods
Ethics statement
The research protocol was approved by the Ethics
Committee of Catania University Hospital (Catania, Italy) and the
written informed consent of all subjects was acquired prior to
their inclusion in the study.
Study design and pesticide
exposure
This was a case-control study conducted in the
province of Ragusa (Sicily, Italy) which has a population of
~320,000. This is a highly agricultural district with a large
proportion of the population employed in ~25,000 farms producing
fruits and vegetables in greenhouses and products derived from
animal husbandry. All these activities involve the use of large
quantities of pesticides. The main crops are carrots, potatoes and
zucchini in open-fields and tomatoes, eggplants, peppers and
zucchini in greenhouses (30).
Fifty-two workers occupationally exposed to
pesticide and 52 non-exposed were recruited. Exposed workers
participating in this study carry out their work with safety
protective devices (SPDs): gloves, masks, overalls and protective
glasses. Pesticide application was done 5–6 times/week and for 6–7
working hours. Non-exposed workers (control) did not have any
contact with pesticides. Exposed ones were recruited on a seasonal
basis (April to August), in the cultivation of greenhouse tomatoes.
Table I reports the pesticides
utilized by exposed workers. It was not possible to detect exposure
biological markers with regard to pesticides employed.
 | Table I.Pesticides applied in the greenhouse
in cultivation of tomatoes. |
Table I.
Pesticides applied in the greenhouse
in cultivation of tomatoes.
| Type of
pesticide | Active
ingredient | No. of
applications | Dose |
|---|
| Fungicides | Propamocarb | 1 | 1 l/ha |
|
| hydrochloride |
|
|
|
| Metalaxyl-M | 2 | 3 l/ha |
|
| Cyproconazole | 1 | 0.05 kg/ha |
| Insecticides | Thiamethoxam | 1 | 0.4 kg/ha |
|
| Deltamathrin | 3 | 0.5 l/ha |
|
| Acrinathrin | 1 | 0.5 l/ha |
|
| Abamectin | 1 | 1 l/ha |
A structured questionnaire investigating
environmental and occupational risks was administered by trained
interviewers to gather accurate data on demographics, medical
history, healthcare habits and pesticide and/or other chemical
exposures. Exclusion criteria were diabetes, hypertension, thyroid,
liver, kidney, lung and hematological diseases.
t(14;18)-(IgH;Bcl-2)
translocation
Peripheral blood samples (10 ml/subjects) were
collected in vacutainer EDTA (K2) tubes (BD Biosciences, Franklin
Lakes, NJ, USA). DNA was extracted from the PBMCs contained in the
buffy coat according to the manufacturers instructions.
t(14;18)-(IgH;Bcl-2) translocation, at the major
breakpoint region (MBR) and minor cluster region (mcr), was
assessed by the polymerase chain reaction (PCR) as previously
reported (31). AccuPrime™
SuperMix (Invitrogen Life Technologies, Carlsbad, CA, USA) was used
to increase the specificity and sensitivity of PCR analysis. The
sensitivity of our assay was 10−5.
DNA integrity of each sample was verified by
amplification of a 430 bp fragment of the growth hormone
(GH) gene by PCR. The forward and reverse primer sequences
used for GH amplification were: 5-CACCATTACATCCCACCT-3 and
5-GCTTCTTGCTTGAGTGA-3, respectively. PCR conditions used for
GH amplification were identical to that reported for the MBR
(31).
PCR products were separated by electrophoresis on
2.5% agarose gel. Positive and negative control samples were
included throughout all steps of the experimental procedures.
Single bands obtained by amplification of the MBR and MCR from
blood specimens were purified from the gel and then sequenced on an
ABI 310 Genetic Analyzer (Perkin-Elmer, Foster City, CA, USA) as
previously reported (31).
Statistical analysis
Data were summarized as mean ± SD for continuous
variables and frequencies for categorical variables. Normality was
checked by Kolmogrov-Smirnov test and homogeneity of variance by
Levenes test. Logistic regression was utilized to evaluate the
presence of translocation t(14;18) in workers exposed to
pesticides, smokers and alcohol consumers. Data analysis was
performed using GraphPad Prism version 7.0 (GraphPad Software,
Inc., La Jolla, CA, USA).
Results
The exposed group presented characteristics similar
to those non-exposed. In particular, all subjects were male and
there were no statistically significant differences as to age, BMI,
smoking habits, alcohol intake, working age and sunlight
exposure.
Farm workers were on average exposed to pesticides
for ~3.7 h a day for 5 years. Table
II reports the main sample characteristics; Fig. 1.
 | Table II.Characteristics of study population
expressed as frequency or mean ± SD. |
Table II.
Characteristics of study population
expressed as frequency or mean ± SD.
|
Characteristics | Exposed (n) | Non-exposed
(n) | P-value |
|---|
| Gender (male) | 52 (100%) | 52 (100%) | ns |
| Age (years) | 33.7±1.7 | 34.2±1.4 | ns |
| BMI
(kg/m2) | 21.8±2.1 | 22.5±1.8 | ns |
| Smokers | 16 (31%) | 17 (33%) | ns |
| Alcohol consumption
(g/day) | 17.6±8.5 | 18.7±7.7 | ns |
| Family history of
cancer | 9 (17%) | 13 (25%) | ns |
| Working age
(years) | 6.3±2.1 | 6.9±1.9 | ns |
| Sunlight exposure
(h/day) | 4.3±1.1 | 4.5±0.8 | ns |
| Exposure duration
(years) | 5.1±0.8 | 0 |
<0.0001 |
| Hours of spraying
(h/day) | 3.7±1.4 | 0 |
<0.0001 |
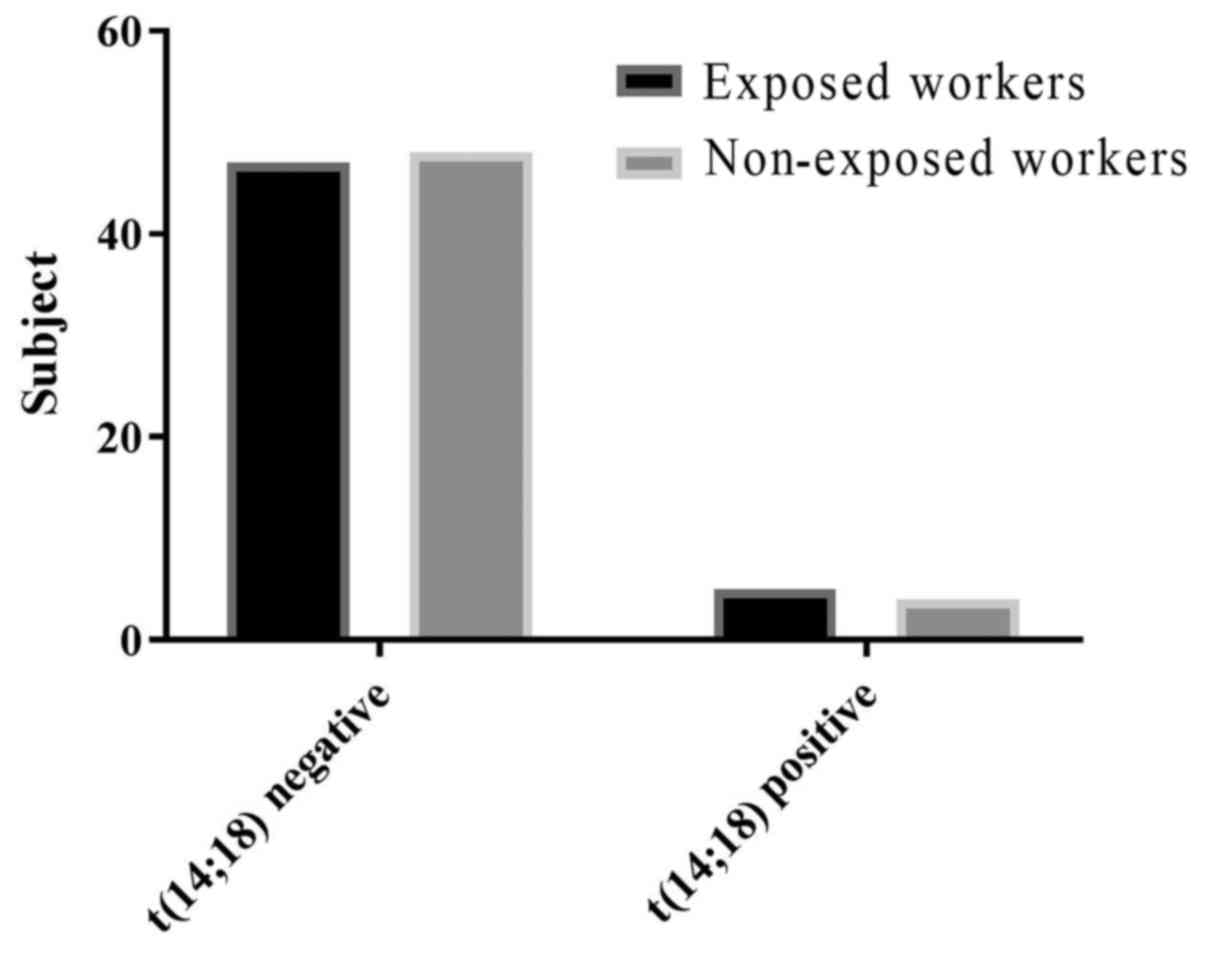
The frequency of BCL2-IGH t(14;18) translocation in
workers occupationally exposed to pesticides was 10% (5 of 52) vs.
8% (4 of 52) in the control group. These results showed no
significant association between occupational exposure to pesticides
and an increased frequency of the chromosomal translocation
BCL2-IGH t(14;18) in farmers (Table
III).
 | Table III.Logistic regression t(14;18)
chromosome trans-location. |
Table III.
Logistic regression t(14;18)
chromosome trans-location.
| Patients | OR (CI) | P-value |
|---|
| Pesticides |
| ns |
|
Non-exposed | 1 |
|
|
Exposed | 1.01
(0.94–0.08) |
|
| Alcohol
consumption |
| ns |
|
Non-consumers | 1 |
|
|
Consumers | 0.87
(0.71–1.03) |
|
| Smoke |
| ns |
|
Non-smokers | 1 |
|
|
Smokers | 0.93
(0.82–1.04) |
|
Moreover, it was not possible to assess the
frequency of t(14;18) in relation to the types of pesticide used,
because all subjects were exposed to insecticides and
fungicides.
Discussion
The chromosomal translocation t(14;18)(q32;q21) is
one of the most common chromosomal abnormalities in NHL. This
translocation involves 2 specific loci, the immunoglobulin heavy
chain (IgH) locus on chromosome 14q32 and the B-cell
leukemia/lymphoma 2 (BCL2) locus on chromosome 18q2l (32).
During the typical translocation process, the BCL2
gene located on chromosome 18 is juxtaposed to the
transcriptionally active IgH gene on chromosome 14, resulting in
overexpression of the former. Consequently, the heightened
anti-apoptotic function of BCL2 increases cell survival, which
represents an early step in the malignant process of NHL (32–34).
An increased incidence of NHL has been reported
among farmers and other occupational groups working with pesticides
(35). Furthermore, an increased
prevalence of the chromosomal translocation t(14;18)(q32;q21) has
been detected in peripheral blood lymphocytes from individuals
occupationally exposed to pesticides (29,36,37).
In a recent study conducted on 96 agricultural
workers, Qaqish et al (1)
found that occupational exposure to pesticides in open-field
farming and insecticides used on animals, increased the frequency
of the chromosomal translocation t(14;18). Farmers occupationally
exposed to pesticides and insecticides were 13.5 times more likely
to harbor t(14;18). Instead, 63.5% (61 of 96) of farmers compared
to 11.5% (11 of 96) of control ones carried the translocation [odds
ratio, 13.5; 95% confidence interval (CI) = 6.3–28.6].
In our study, the BCL2-IGH t(14;18) translocation
frequency in workers occupationally exposed to pesticides was 10%
(5 of 52) vs. 8% (4 of 52) in the control group, without
significantly statistical differences.
The discrepancy of results between ours and Qaqish
et al (1) can be attributed
to the lesser time of exposure (50%) compared to the latter
(10.9±7.9 vs. 5.1±0.8 years).
Besides, in our sample all workers availed
themselves of standard SPDs, whereas in Qaqish et al
(1) only 2.1% of farmers used
masks and 27.1% used masks and gloves.
As shown by Qaqish et al (1), using SPDs may help prevent t(14;18).
The risk of t(14;18) was significantly associated to the exposures
to different kinds of pesticides: insecticides, herbicides and
fumigants (28).
Chiu et al (28) observed that the use of insecticides
and herbicides was associated with a 2.6- to 3-fold higher risk of
t(14;18)-positive NHL. These results are consistent with findings
from previous studies in which pesticides were specifically
associated with follicular NHL (23,38–40),
which is usually positive for the t(14;18).
Chiu et al (28) and Schroeder et al (41) found that the risk of NHL associated
with farming and exposure to dieldrin, lindane, atrazine or
fungicides was associated with t(14;18).
In Italy, the use of pesticides like dieldrin,
lindane and atrazine was forbidden long ago. Moreover, exposed
subjects in our study were exposed both to fungicides (propamocarb
hydrochloride, metalaxyl-M, cyproconazole) and to insecticides
(thiamethoxam, deltamathrin, acrinathrin and abamectin) and so the
action of each could not be differentiated.
The results of our study are in line with that
observed by others (1,28), who detected an increased risk when
insecticides and herbicides are used for a longer time and in
relation with using SPDs.
We assessed the effects of potential confounding
factors on the frequency of BCL2-IGH t(14;18) translocation
detection. Firstly, alcohol consumption did not contribute to the
frequency of detection, potentially due to the low rate of alcohol
consumption in our study group (17.6±8.5 vs. 18.7±7.7 g/day for
exposed and non-exposed, respectively). Additionally, we did not
detect an association between the age of samples and the frequency
of BCL2-IGH t(14;18) translocation detection (1,29).
Our study as well as Roulland et al (29) and Qaqish et al (1) included subjects with median ages
<50 years, where an association with age was detected only in
samples older than 60 (42) and 70
years (43) of age. Moreover,
cigarette smoking did not increase the frequency of BCL2-IGH
t(14;18) translocation in our sample, consistent with a previous
study (28). Furthermore, sunlight
exposure did not show an effect on t(14:18) detection frequency,
consistent with other studies (29).
Therefore, from the results of our study it is
possible to conclude that a constant use of law prescribed SPDs and
time of exposure may impact on the translocation frequency in
pesticide exposed workers.
Our study shall be continued with a follow-up of
these workers, in order to better determine the role of ‘Time of
exposure’ factor on gene translocation.
References
|
1
|
Qaqish BM, Al-Dalahmah O, Al-Motassem Y,
Battah A and Ismail SS: Occupational exposure to pesticides and
occurrence of the chromosomal translocation t(14;18) among farmers
in Jordan. Toxicol Rep. 3:225–229. 2016. View Article : Google Scholar
|
|
2
|
Bolognesi C: Genotoxicity of pesticides: a
review of human biomonitoring studies. Mutat Res. 543:251–272.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martínez-Valenzuela C, Waliszewski SM,
Amador-Muñoz O, Meza E, Calderón-Segura ME, Zenteno E,
Huichapan-Martínez J, Caba M, Félix-Gastélum R and
Longoria-Espinoza R: Aerial pesticide application causes DNA damage
in pilots from Sinaloa, Mexico. Environ Sci Pollut Res Int. Nov
5–2016.(Epub ahead of print).
|
|
4
|
Gaikwad AS, Karunamoorthy P, Kondhalkar
SJ, Ambikapathy M and Beerappa R: Assessment of hematological,
biochemical effects and genotoxicity among pesticide sprayers in
grape garden. J Occup Med Toxicol. 10:112015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ismail AA, Rohlman DS, Rasoul Abdel GM,
Salem Abou ME and Hendy OM: Clinical and biochemical parameters of
children and adolescents applying pesticides. Int J Occup Environ
Med. 1:132–143. 2010.PubMed/NCBI
|
|
6
|
Patil JA, Patil AJ and Govindwar SP:
Biochemical effects of various pesticides on sprayers of grape
gardens. Indian J Clin Biochem. 18:16–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saldana TM, Basso O, Hoppin JA, Baird DD,
Knott C, Blair A, Alavanja MCR and Sandler DP: Pesticide exposure
and self-reported gestational diabetes mellitus in the Agricultural
Health Study. Diabetes Care. 30:529–534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ledda C, Fiore M, Santarelli L, Bracci M,
Mascali G, DAgati MG, Busà A, Ferrante M and Rapisarda V:
Gestational hypertension and organophosphorus pesticide exposure: a
cross-sectional study. Biomed Res Int. 2015:2808912015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malekirad AA, Faghih M, Mirabdollahi M,
Kiani M, Fathi A and Abdollahi M: Neurocognitive, mental health,
and glucose disorders in farmers exposed to organophosphorus
pesticides. Arh Hig Rada Toksikol. 64:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Costa C, Rapisarda V, Catania S, Di Nola
C, Ledda C and Fenga C: Cytokine patterns in greenhouse workers
occupationally exposed to α-cypermethrin: an observational study.
Environ Toxicol Pharmacol. 36:796–800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bolognesi C, Creus A, Ostrosky-Wegman P
and Marcos R: Micronuclei and pesticide exposure. Mutagenesis.
26:19–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sailaja N, Chandrasekhar M, Rekhadevi PV,
Mahboob M, Rahman MF, Vuyyuri SB, Danadevi K, Hussain SA and Grover
P: Genotoxic evaluation of workers employed in pesticide
production. Mutat Res. 609:74–80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
La Vecchia C, Negri E, DAvanzo B and
Franceschi S: Occupation and lymphoid neoplasms. Br J Cancer.
60:385–388. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brown LM, Blair A, Gibson R, Everett GD,
Cantor KP, Schuman LM, Burmeister LF, Van Lier SF and Dick F:
Pesticide exposures and other agricultural risk factors for
leukemia among men in Iowa and Minnesota. Cancer Res. 50:6585–6591.
1990.PubMed/NCBI
|
|
15
|
Hardell L and Eriksson M: A case-control
study of non-Hodgkin lymphoma and exposure to pesticides. Cancer.
85:1353–1360. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meinert R, Schüz J, Kaletsch U, Kaatsch P
and Michaelis J: Leukemia and non-Hodgkin's lymphoma in childhood
and exposure to pesticides: results of a register-based
case-control study in Germany. Am J Epidemiol. 151:639–646. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Petrelli G, Figà-Talamanca I, Tropeano R,
Tangucci M, Cini C, Aquilani S, Gasperini L and Meli P:
Reproductive male-mediated risk: spontaneous abortion among wives
of pesticide applicators. Eur J Epidemiol. 16:391–393. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lockwood AH: Pesticides and parkinsonism:
is there an etiological link? Curr Opin Neurol. 13:687–690. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ji BT, Silverman DT, Stewart PA, Blair A,
Swanson GM, Baris D, Greenberg RS, Hayes RB, Brown LM, Lillemoe KD,
et al: Occupational exposure to pesticides and pancreatic cancer.
Am J Ind Med. 39:92–99. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brody JG, Aschengrau A, McKelvey W, Rudel
RA, Swartz CH and Kennedy T: Breast cancer risk and historical
exposure to pesticides from wide-area applications assessed with
GIS. Environ Health Perspect. 112:889–897. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calvert GM, Plate DK, Das R, Rosales R,
Shafey O, Thomsen C, Male D, Beckman J, Arvizu E and Lackovic M:
Acute occupational pesticide-related illness in the US, 1998–1999:
surveillance findings from the SENSOR-pesticides program. Am J Ind
Med. 45:14–23. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blair A and Zahm SH: Agricultural
exposures and cancer. Environ Health Perspect. 103:(Suppl 8).
205–208. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chiu BC and Weisenburger DD: An update of
the epidemiology of non-Hodgkin's lymphoma. Clin Lymphoma.
4:161–168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dich J, Zahm SH, Hanberg A and Adami HO:
Pesticides and cancer. Cancer Causes Control. 8:420–443. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schwaenen C, Wessendorf S, Kestler HA,
Döhner H, Lichter P and Bentz M: DNA microarray analysis in
malignant lymphomas. Ann Hematol. 82:323–332. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Potter JD: Toward the last cohort. Cancer
Epidemiol Biomarkers Prev. 13:895–897. 2004.PubMed/NCBI
|
|
27
|
Janz S, Potter M and Rabkin CS: Lymphoma-
and leukemia-associated chromosomal translocations in healthy
individuals. Genes Chromosomes Cancer. 36:211–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiu BC, Dave BJ, Blair A, Gapstur SM,
Zahm SH and Weisenburger DD: Agricultural pesticide use and risk of
t(14;18)-defined subtypes of non-Hodgkin lymphoma. Blood.
108:1363–1369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roulland S, Lebailly P, Lecluse Y, Briand
M, Pottier D and Gauduchon P: Characterization of the t(14;18)
BCL2-IGH translocation in farmers occupationally exposed to
pesticides. Cancer Res. 64:2264–2269. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Licciardello F, Antoci ML, Brugaletta L
and Cirelli GL: Evaluation of groundwater contamination in a
coastal area of south-eastern Sicily. J Environ Sci Health B.
46:498–508. 2011.PubMed/NCBI
|
|
31
|
Libra M, Gloghini A, Malaponte G, Gangemi
P, De Re V, Cacopardo B, Spandidos DA, Nicoletti F, Stivala F,
Zignego AL, et al: Association of t(14;18) translocation with HCV
infection in gastrointestinal MALT lymphomas. J Hepatol.
49:170–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nadel B, Marculescu R, Le T, Rudnicki M,
Böcskör S and Jäger U: Novel insights into the mechanism of
t(14;18)(q32;q21) translocation in follicular lymphoma. Leuk
Lymphoma. 42:1181–1194. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hockenbery D, Nuñez G, Milliman C,
Schreiber RD and Korsmeyer SJ: Bcl-2 is an inner mitochondrial
membrane protein that blocks programmed cell death. Nature.
348:334–336. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ismail SI, Sughayer MA, Al-Quadan TF,
Qaqish BM and Tarawneh MS: Frequency of t(14;18) in follicular
lymphoma patients: geographical or technical variation. Int J Lab
Hematol. 31:535–543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chiu BC and Blair A: Pesticides,
chromosomal aberrations, and non-Hodgkins lymphoma. J Agromed.
14:250–255. 2009. View Article : Google Scholar
|
|
36
|
Garry VF, Tarone RE, Long L, Griffith J,
Kelly JT and Burroughs B: Pesticide appliers with mixed pesticide
exposure: G-banded analysis and possible relationship to
non-Hodgkins lymphoma. Cancer Epidemiol Biomarkers Prev. 5:11–16.
1996.PubMed/NCBI
|
|
37
|
Agopian J, Navarro JM, Gac AC, Lecluse Y,
Briand M, Grenot P, Gauduchon P, Ruminy P, Lebailly P, Nadel B, et
al: Agricultural pesticide exposure and the molecular connection to
lymphomagenesis. J Exp Med. 206:1473–1483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zahm SH, Weisenburger DD, Babbitt PA, Saal
RC, Vaught JB, Cantor KP and Blair A: A case-control study of
non-Hodgkins lymphoma and the herbicide 2,4-dichlorophenoxyacetic
acid (2,4-D) in eastern Nebraska. Epidemiology. 1:349–356. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cantor KP, Blair A, Everett G, Gibson R,
Burmeister LF, Brown LM, Schuman L and Dick FR: Pesticides and
other agricultural risk factors for non-Hodgkins lymphoma among men
in Iowa and Minnesota. Cancer Res. 52:2447–2455. 1992.PubMed/NCBI
|
|
40
|
Zahm SH and Blair A: Pesticides and
non-Hodgkins lymphoma. Cancer Res. 52:(Suppl 19). 5485s–5488s.
1992.PubMed/NCBI
|
|
41
|
Schroeder JC, Olshan AF, Baric R, Dent GA,
Weinberg CR, Yount B, Cerhan JR, Lynch CF, Schuman LM, Tolbert PE,
et al: Agricultural risk factors for t(14;18) subtypes of
non-Hodgkins lymphoma. Epidemiology. 12:701–709. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Y, Hernandez AM, Shibata D and
Cortopassi GA: BCL2 translocation frequency rises with age in
humans. Proc Natl Acad Sci USA. 91:8910–8914. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hirt C, Weitmann K, Schüler F, Kiefer T,
Rabkin CS, Hoffmann W and Dölken G: Circulating t(14;18)-positive
cells in healthy individuals: association with age and sex but not
with smoking. Leuk Lymphoma. 54:2678–2684. 2013. View Article : Google Scholar : PubMed/NCBI
|