Introduction
Apoptosis is a tightly regulated cell death program,
which is actively implemented to ensure proper development.
Insufficient or excessive apoptosis can provoke a number of
diseases, including autoimmune disease, cancer and disorders in
inflammation (1,2). Misregulated apoptosis often leads to
severe organ damage in various infectious diseases, including
sepsis. The pathogenesis of sepsis involves a complex process of
cellular activation at multiple levels resulting in the release of
pro-inflammatory cytokines, including tumor necrosis factor-α,
interleukin (IL)-6, interferon-γ, IL-1β and high-mobility group box
1, and anti-inflammatory cytokines, including IL-10 (3,4).
Other mechanisms include the activation of neutrophils, monocytes
and microvascular endothelial cells, in addition to the apoptosis
of these cells (3,4). Detailed studies have focused on
apoptotic signaling pathways (5),
however, the exact molecular mechanism remains to be fully
elucidated.
Cellular FLICE-inhibitory protein (c-FLIP) is a
catalytically inactive caspase-8 (CASP8) homologue, which
negatively interferes with apoptotic signaling (6). The triggering of death-receptors by
cognate ligands induces the recruitment of death adaptor proteins,
including Fas-associated death domain (FADD) or TRAIL
receptor-associated death domain by means of the receptors'
intracellular death domain. Death-receptors also possess a death
effect domain, which is necessary for the recruitment of the
initiator procaspase-8 to form the death inducing signaling complex
(DISC). c-FLIP interferes with efficient DISC formation in the
extrinsic pathway directly at the receptor level by competing with
procaspase-8 to bind FADD. Prevention of the proteolytic cleavage
and activation of the procaspase inhibits the transduction of
apoptotic signaling, and ultimately promotes cell survival
(7). There are three forms of
c-FLIP, c-FLIPL (55 KDa), c-FLIPS (26 KDa)
and c-FLIPR (24 KDa). All the three forms of c-FLIP (L,
S and R) are traditionally accepted as anti-apoptotic proteins. The
role of c-FLIPL remains to be elucidated as it has been
described to have pro- and anti-apoptotic effects in immune system
cells, and this dual role has been found to depend on a variety of
parameters, including cellular context and CASP8 to FLIP ratio
(6).
There is controversy surrounding the function of
c-FLIPL in endothelial cell apoptosis. Bannerman et
al found that c-FLIP protected against the apoptosis of human
dermal microvessel endothelial cells and suppressed the activation
of nuclear factor (NF)-κB induced by lipopolysaccharide (LPS)
(8), whereas Karahashi et
al found that c-FLIP was not involved in apoptosis induced by
LPS or cycloheximide (CHX) (9). As
endothelial cell apoptosis is critical in the pathogenesis of
sepsis, the aim of the present study was to detect the expression
of c-FLIPL in a rat model of sepsis, and examine the
association between the expression of c-FLIPL and
endothelial apoptosis.
Materials and methods
Materials
LPS was purchased from Sigma-Aldrich; Merck
Millipore (Darmstadt, Germany). CHX was purchased from Sangon
Biotech Co., Ltd. (Shanghai, China). Sprague-Dawley rats were
obtained from the Shanghai Animal Center of the Chinese Academy of
Science (Shanghai, China). The human umbilical vein endothelial
cells (HUVECs) were purchased from American Type Culture Collection
(Manassas, VA, USA).
Rat sepsis model establishment using
cecal ligation and puncture (CLP)
A total of 24 Sprague-Dawley rats, male, weighing
250–300 g, were housed under a 12 h daylight cycle at 23–25°C
temperature and fed with standard chow and water. The animals were
randomly divided into two groups: Sham surgery group and sepsis
model group. The sepsis model was induced by CLP (10). Briefly, the animals were deprived
of food, but allowed water, for 6 h prior to surgery. To prepare
for the surgical procedures, the animals were anesthetized with
chloral hydrate (350 mg/kg bodyweight) intraperitoneally. A
laparotomy was performed through a midline abdominal incision, and
the cecum was exteriorized and ligated halfway between the distal
pole and the base of the cecum. Subsequently, the cecum was
perforated by a single through-and-through puncture with a 2.5 mm
needle and gently compressed until fecal material was extruded. The
bowel was then relocated to the abdomen and the abdominal incision
was closed in layers. The animals were resuscitated via injection
of pre-warmed normal saline (37°C; 5 ml/100 g body weight)
subcutaneously. Animals in the sham group received sham surgery,
during which the cecum was neither ligated nor punctured. All
procedures performed involving animals were in accordance with the
guidelines of the Institutional Animal Care and Use Committee. The
study protocols were approved by the Research Ethics Committee of
Huashan Hospital, Fudan University (Shanghai, China).
Cell culture
The HUVECs were cultured in DMEM (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) enriched with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), bovine brain extract (12 µg/ml), L-glutamine (2 mmol/l) and
sodium pyruvate (1 mmol/l) in the presence of penicillin (100 U/ml)
and streptomycin (100 µg/ml; Sangon Biotech Co., Ltd).
Western blot analysis
The tissues and HUVECs were homogenized with
modified RIPA buffer and stationed on ice for 1 h, following which
they were centrifuged at 14,000 × g at 4°C for 5 min. The
supernatants were collected, mixed with loading buffer containing
0.1% bromophenol blue and boiled for 10 min, following
determination of protein concentration by bicinchoninic acid assay
(Beyotime Institute of Biotechnology, Haimen, China). Equal
quantities of protein (10 µg) were loaded into a 10% SDS-PAGE gel,
followed by electrophoresis, separation under denaturing conditions
and electroblotting onto PVDF membranes. The membranes were
incubated overnight in Tris-buffered saline containing 7% milk to
inhibit nonspecific antibody binding. The proteins of interest were
revealed via incubation with specific mouse anti-human monoclonal
antibody (anti-FLIPS/L; 1:500 dilution; cat. no.
sc-5276; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h
at room temperature, followed by incubation with a 1:1,000 dilution
of horseradish peroxidase-conjugated goat anti-mouse IgG antibody
(cat. no. A0216; Beyotime Insititute of Biotechnology) for 1 h at
room temperature. Signals were visualized using chemiluminescence.
β-actin antibody was used as a control. All western blots were
quantified using densitometry.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Tissues and HUVECs were homogenized using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and total RNA
was extracted according to the manufacturer's protocol. Total RNA
(1 µg) was then reverse transcribed with reverse transcriptase
(Promega Corporation, Madison, WI, USA) for 1 h at 37°C to
synthesize cDNA. The following primers, synthesized by Sangon
Biotech Co., Ltd., were used: c-FLIP, sense
5′-ATAGGGTGCTGCTGATGG-3′ and antisense 5′-TTGCTTCTTGGCTGGACT-3′.
GAPDH, sense 5′-ACCACAGTCCATGCCATCAC-3′ and antisense
5′-CCACCACCCTGTTGCTGTAG-3′. The reactions were performed in a 25-µl
volume comprising diluted c-DNA sample, primers and SYBR-Green
reagent (Takara Biotechnology Co., Ltd., Dalian, China), according
to the manufacturer's protocol. PCR was performed in triplicate as
follows: 95°C for 10 min, and 45 cycles of 95°C for 15 sec, 64°C
for 30 sec, and 72°C for 30 sec. The qPCR amplifications were
performed on an ABI 7500 PCR system (Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Relative
quantification of the mRNA expression of the target gene was
determined using the 2−ΔΔCq method, with GAPDH as an
internal reference control (11).
Overexpression of c-FLIP by
transfecting HUVECs with PEGFP-N1-CASP8 and FADD-like apoptosis
regulator (cflar)
The PEGFP-N1-cflar plasmid was constructed by
Shanghai Genechem Co., Ltd. (Shanghai, China) which contained the
gene of c-FLIP and kanamycin resistance, and was transfected into
HUVECs using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.)
(12). Briefly, the plasmid was
sequenced by Sangon Biotech Co. Ltd. to confirm that the c-FLIP
gene was expressed. Subsequently, 1 µg plasmid DNA and 2 µl
Lipofectamine 2000 were added to each well of 24-well plates and
incubated with HUVECs (105 cells/well) for 4 h at 37°C
in 5% CO2. After 4 h, the supernatants were removed and
fresh media was added, following which incubation continued for
another 32 h. The total transfection time was 36 h and the
transfection efficiencies were observed through a fluorescent
inverted microscope (Nikon TE2000; Nikon Corporation, Tokyo,
Japan).
Induction of HUVEC apoptosis by LPS
and CHX
Following transfection with plasmid DNA and
Lipofectamine 2000 for 36 h, HUVEC apoptosis was induced by adding
LPS (100 ng/ml) or LPS (100 ng/ml)+CHX (40 µg/ml) for 6, 9, 12 and
24 h at 37°C in 5% CO2. The cells were then stained with
Annexin V and propidium iodide (PI) using an apoptosis kit (BD
Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's protocol, and immediately analyzed using flow
cytometry.
Statistical analysis
The results are presented in graphs following
analysis using Prism 5.0 (GraphPad Software, Inc., La Jolla, CA,
USA). Comparisons between groups were made using unpaired t tests,
or Mann-Whitney U tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Physiological and pathological changes
in rats following CLP
In the present study, the survival rates of the rats
in the sham surgery and CLP groups were 100 and 40%, respectively
(Fig. 1A), which was consistent
with a previous report (10).
Significant decreases in white blood counts (WBCs;
6.4±2.3×109/l, vs. 1.9±1.5×109/l; P=0.0046),
pH (7.39±0.07, vs. 7.24±0.09; P=0.0297) and PaO2
(96.7±7.0, vs. 78.3±5.9 mm Hg; P=0.0129), and a significant
increase in PaCO2 (38.1±7.4, vs. 54.2±5.4 mm Hg;
P=0.0030) were observed in the CLP rats, compared to those in the
sham group at 24 h post-surgery (Fig.
1B). H&E staining of the organs, including the lungs,
liver, kidney, heart, vessel and intestines, revealed a marked
inflammatory response (Fig.
1Ca-g). These data confirmed establishment of the CLP-induced
rat model of polymicrobial sepsis for subsequent investigations of
the mechanism underlying sepsis.
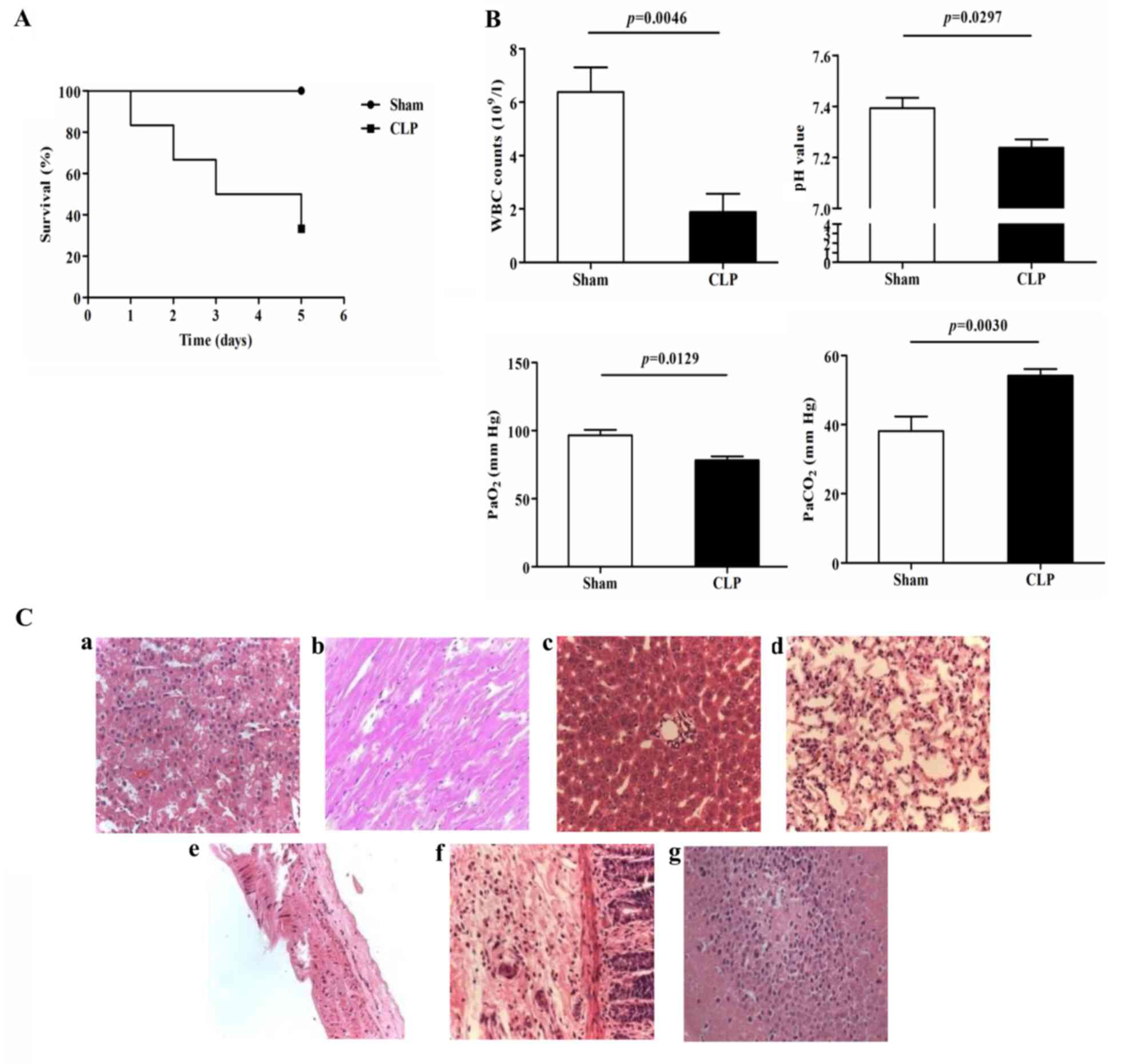 | Figure 1.Physiological and pathological changes
in the sepsis model induced by CLP. (A) Survival curves of the Sham
and CLP groups (n=6 in each group). (B) WBC counts, pH,
PaO2 and PaCO2 of the Sham and CLP groups
(n=6 in each group). (C) Pathological changes detected using
H&E staining in the (a) kidney, (b) heart, (c) liver, (d) lung,
(e) vessel, (f) intestine and (g) brain (magnification, ×200). CLP,
cecal ligation and puncture; WBC, white blood cell. |
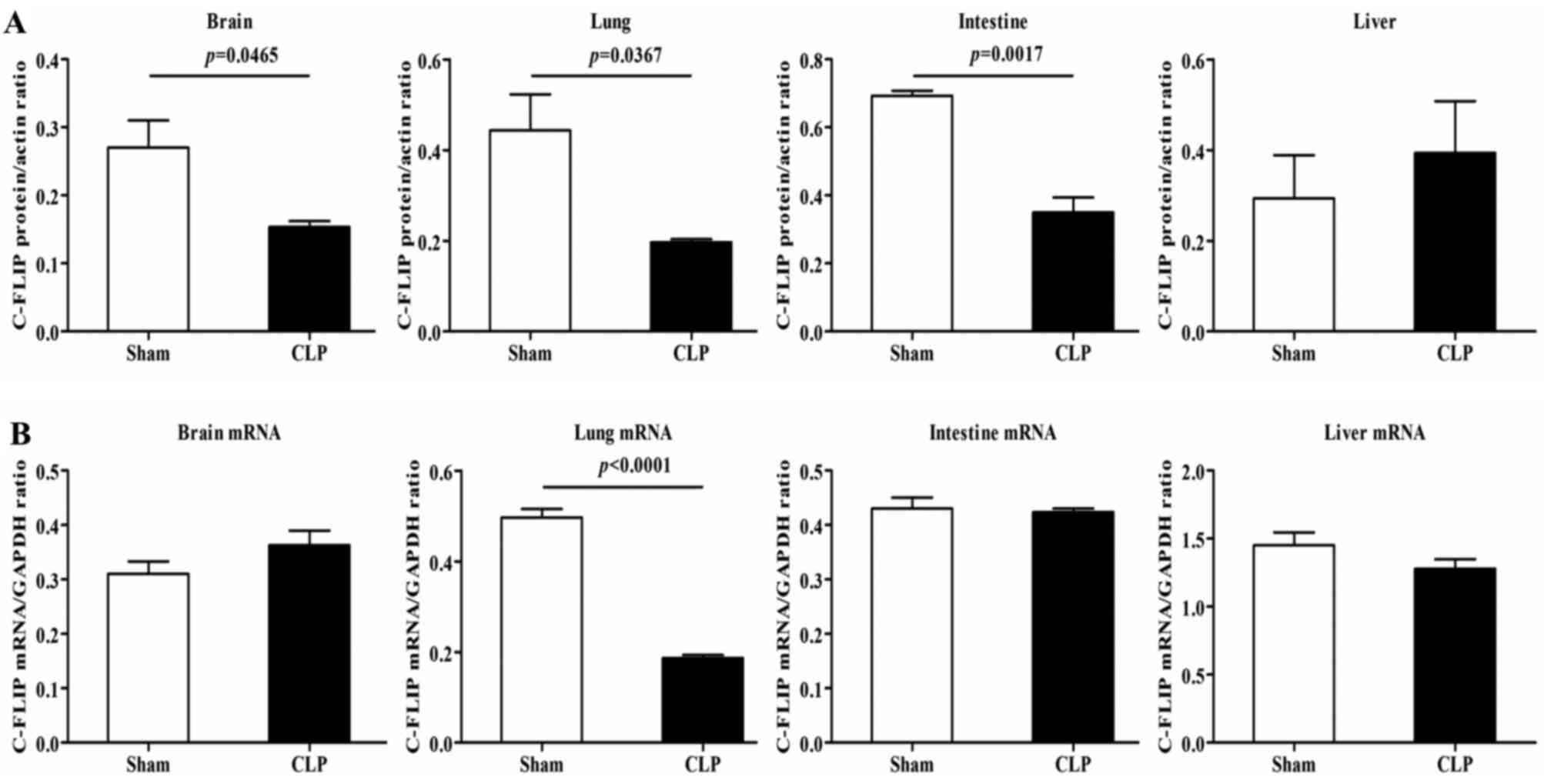
mRNA and protein expression levels of
c-FLIP in rats with CLP
The results of the western blot analysis revealed
that, compared with the rats in the sham group, the CLP rats showed
notable decreases in the protein expression of c-FLIP, primarily
c-FLIPL, in the brain, intestine and lungs, but not in
the liver (Fig. 2A). In terms of
the mRNA expression of c-FLIP, detected using RT-qPCR analysis, a
significant decrease was observed in the lung tissues of the CLP
rats, compared with the rats in the sham group, with no detectable
differences in other organs (Fig.
2B). As c-FLIP functions as an inhibitor of the activation of
CASP8 (6), the reduced protein
expression of c-FLIP suggested a high induction of apoptosis.
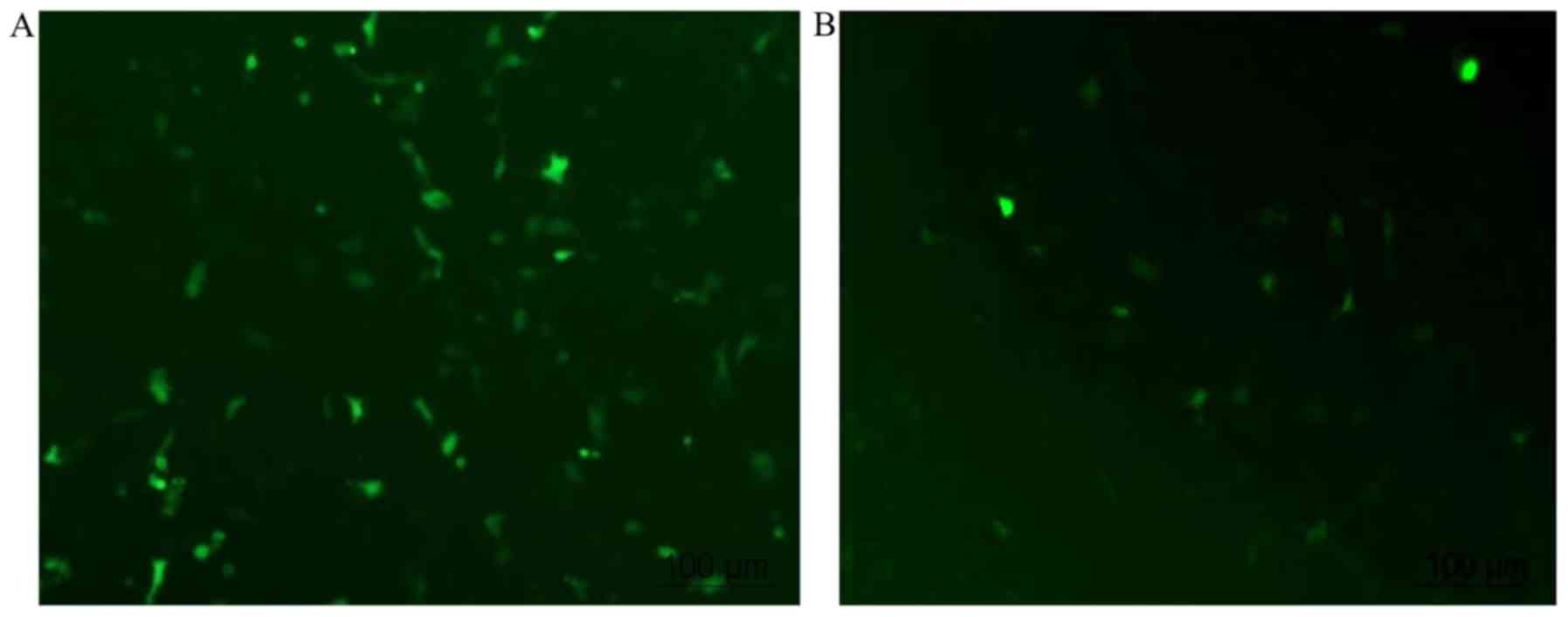
Transfection of cflar into HUVECs
It has been reported that the transfection
efficiency of HUVECs varies between 0.45 and 77% (12). When using Lipofectamine 2000, which
is recommended as one of the optimal transfection reagents in
HUVECs, the reproducible transfection efficiency was previously
reported to be 19±9% (12). In the
present study, low efficiency was also a problem. As shown in
Fig. 3, green signals represent
cells transfected with the plasmid. PEGFP-N1 alone was transfected
into cells with a transfection efficiency of ~50% (46.8±6.2%;
Fig. 3A). The transfection
efficiency decreased to <15% (13.7±4.6%) when the cells were
transfected with PEGFP-N1+ cflar (Fig. 3B).
LPS and CHX following cflar
transfection induce the protein expression of c-FLIP in HUVECs
Although the transfection efficiency in the HUVECs
was only ~15%, the western blot analysis of c-FLIP protein
indicated that the protein expression of c-FLIP in the transfected
HUVECs was significantly increased 36 h following cflar
transfection (Fig. 4A). Compared
with the initial time point (36+0 h), the expression of c-FLIP
decreased at 36+9 h (P<0.05), and then gradually increased with
increasing duration, reaching a significantly elevated level at
36+24 h (P<0.05), compared with the 36+6, 36+9 and 36+12 h time
points, respectively (Fig. 4B).
Additionally, previous studies have suggested that LPS induces the
expression of c-FLIP in HUVECs, but that CHX suppresses the
expression of c-FLIP (7,8). However, in the present study, LPS+CHX
exerted a biphasic effect on the expression of c-FLIP, with an
initial upregulation, followed by downregulation (Fig. 4C), which was opposite to the change
in the protein expression of c-FLIP following transfection. The
present study also examined the expression of c-FLIP following
sequential stimulation of cflar-transfected cells with LPS
and CHX combined. The results of the western blot analysis showed
that the overexpression of c-FLIP was only present at the 9 h point
following treatment with LPS and CHX (P<0.05, compared with the
36+0 h time point; Fig. 4D) and
there were significant differences among the control, LPS and
LPS+CHX groups (P<0.05; Fig. 4E and
F). At the remaining time points, no significant overexpression
of c-FLIP was observed among these three groups (Table I).
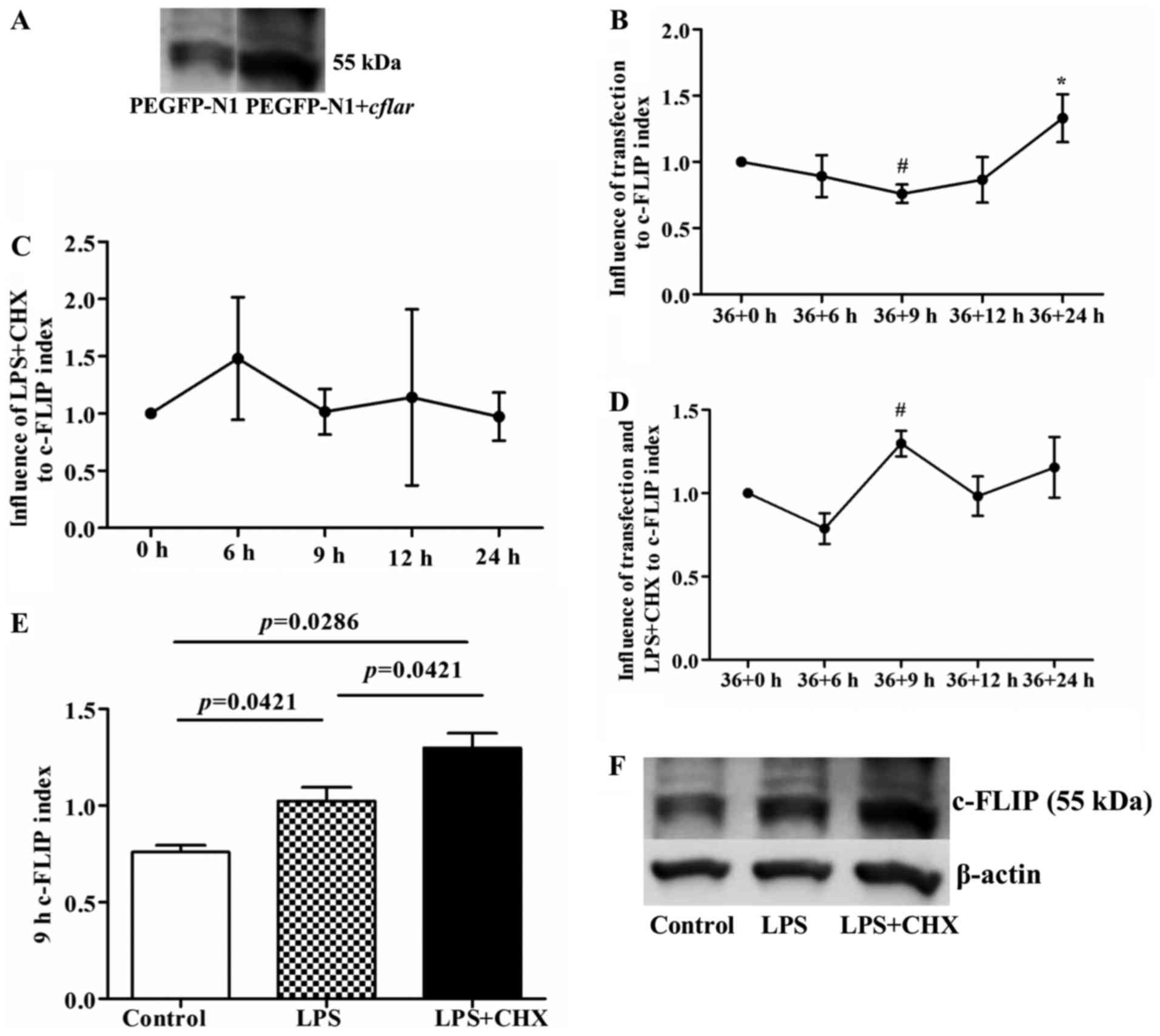 | Figure 4.Expression of c-FLIP following
cflar transfection and subsequent LPS and CHX induction. (A)
Expression of c-FLIP in the transfected cflar human
umbilical vein endothelial cells was higher, compared with that in
cells transfected with PEGFP-N1 alone, confirmed using western blot
analysis. (B) Following transfection only, the expression of c-FLIP
decreased at 36+9 h (#P<0.05, compared with 36+0 h),
and then gradually increased with increasing transfection duration,
particularly at 36+24 h (*P<0.05, compared with the 36+6, 36+9
and 36+12 h groups, respectively). (C) Expression of c-FLIP
increased, and then decreased following treatment of untransfected
cells with LPS+CHX, but without statistical significance. (D)
Expression of c-FLIP increased significantly at 9 h
post-transfection in the LPS+CHX group (#P<0.05,
compared with 36+0 h). (E) Expression of c-FLIP in cells 9 h
post-transfection with LPS or LPS+CHX. The LPS group showed higher
protein expression of c-FLIP, compared with the control group
(P=0.0421). The LPS+CHX group showed higher protein expression of
c-FLIP, compared with the control and LPS groups (P=0.0286 and
P=0.0421, respectively). (F) Representative images of western blot
analysis of protein expression of c-FLIP and β-actin in the three
groups. c-FLIP, cellular FLICE-inhibitory protein; LPS,
lipopolysaccharide; CHX, cycloheximide; cflar, caspase-8 and
Fas-associated death domain-like apoptosis regulator. |
 | Table I.Expression of cellular
FLICE-inhibitory protein following transfection with CASP8 and
Fas-associated death domain-like apoptosis regulator, and
subsequent LPS or LPS+CHX-induced apoptosis. |
Table I.
Expression of cellular
FLICE-inhibitory protein following transfection with CASP8 and
Fas-associated death domain-like apoptosis regulator, and
subsequent LPS or LPS+CHX-induced apoptosis.
| Time point (h) | Control | LPS | LPS+CHX | P-value |
|---|
| 36+6 | 0.89±0.16 | 0.80±0.44 | 0.79±0.19 | 0.857 |
| 36+9 | 0.76±0.07 | 1.02±0.14 | 1.30±0.15 | 0.001 |
| 36+12 | 0.87±0.17 | 1.56±1.10 | 0.98±0.24 | 0.324 |
| 36+24 | 1.94±1.23 | 1.16±0.49 | 1.16±0.36 | 0.317 |
c-FLIP protein protects HUVECs against
apoptosis induced by LPS+CHX
The present study used PI and Annexin V staining to
investigate the apoptosis of HUVECs following cflar
transfection, and following induction by LPS or LPS+CHX. Compared
with the cells transfected with PEGFP-N1 only, the HUVECs
transfected with PEGFP+ cflar exhibited a significant
decrease in the percentage of Annexin V-positive apoptotic cells
following LPS+CHX stimulation, which indicated that transfection
with the cflar gene protected the HUVECs against LPS and
CHX-induced apoptosis (Fig. 5A).
It was shown that LPS+CHX treatment led to the overexpression of
c-FLIP (P=0.0286, vs. control group; P=0.0421, vs. LPS group;
Fig. 4E). It was subsequently
demonstrated that the percentage apoptosis in the cells transfected
with cflar and treated with LPS+CHX was significantly lower,
compared with that in the control group and LPS group (P=0.0003 and
P=0.026, respectively; Fig. 5B).
No significant difference was observed between the control group or
LPS group (Fig. 5B). The
percentage apoptosis was inversely correlated with the expression
of c-FLIP (r=−0.0647; P=0.023; Fig.
5C). The representative results of flow cytometry are shown in
Fig. 5D. Taken together, these
data suggested that c-FLIP protected HUVECs against apoptosis
induced by LPS+CHX.
 | Figure 5.Apoptosis of human umbilical vein
endothelial cells is induced by LPS and CHX. (A) Percentage of
Annexin V-positive cells was lower following transfection with
PEGFP-N1+ cflar, compared with cells transfected with
PEGFP-N1 alone, when exposed to LPS+CHX at 9 h (P=0.0197). (B)
Percentage of Annexin V-positive cells transfected with
cflar induced by LPS+CHX was lower, compared with the cells
in the LPS alone and control groups (P=0.0003 and 0.026,
respectively). In all groups, cells were transfected with
cflar at 36 h. Subsequently, cells in the control group were
incubated in media for 9 h, cells in the LPS group were induced by
LPS for 9 h, and cells in the LPS+CHX group were induced by LPS+CHX
for 9 h. (C) Percentage of apoptotic cells was inversely correlated
with the expression of c-FLIP (r=−0.0647; P=0.023). (D)
Representative results of flow cytometric analysis. The percentage
of Annexin V-positive cells represents the level of apoptosis.
c-FLIP, cellular FLICE-inhibitory protein; LPS, lipopolysaccharide;
CHX, cycloheximide; PI, propidium iodide; cflar, caspase-8
and Fas-associated death domain-like apoptosis regulator. |
Discussion
CLP is considered to be the gold standard rodent
model for the investigations of sepsis and has become the most
widely used model for experimental sepsis. However, disease outcome
varies depending on several factors, including the length of
intestine tied off, the size of the needle and the quantity of
fecal material released (10). In
the present study, a 50% length ligation was performed, followed by
a single through-and-through puncture with a 2.5 mm needle. This
model mimicked the sepsis status successfully, resulting in rat
mortality, physiological changes in WBC counts and blood gas
analysis, and pathological changes in the majority of the rat
organs.
In the present study, the expression of
c-FLIPL was characterized in different organs of the rat
model of sepsis induced by CLP, and found that the expression of
c-FLIPL was decreased in the majority of vital organs,
including the brain, lungs and intestine. In terms of the mRNA
levels, no significant differences in the expression of
c-FLIPL were observed. c-FLIP has three forms,
c-FLIPL, c-FLIPS and c-FLIPR, and
all three forms are widely accepted anti-apoptotic proteins
(6). As the effects of
c-FLIPL have been described to be pro- and
anti-apoptotic, the present study primarily focused on the
expression and function of c-FLIPL. The expression of
c-FLIPL can be regulated at multiple levels. NF-κB and
activator protein 1 family proteins regulate the expression of
c-FLIPL at the transcriptional level. At the
translational level, regulation of the expression of
c-FLIPL also occurs by activation of the Akt-mammalian
target rapamycin-p70S6 kinase pathway (13). Therefore, the difference between
the protein and mRNA expression levels of c-FLIPL can be
interpretative. In the present in vitro study, the
expression of c-FLIPL increased following transfection
with the plasmid, whereas endothelial apoptosis was attenuated,
which indicated that the decreased expression of c-FLIPL
in rats with sepsis did not protect cells against apoptosis in
vital organs, which may be associated with the poor prognosis of
sepsis. Perlman et al (14)
found that the overexpression of c-FLIP protected monocytes from
Fas-mediated apoptosis, whereas acute c-FLIP inhibition in
macrophages induced apoptosis. The addition of an antagonistic Fas
ligand antibody to c-FLIP-antisense-treated macrophages rescued the
cultures from apoptosis. Therefore, the expression of c-FLIP in
macrophages conferred resistance to Fas-mediated apoptosis, which
may contribute to the development of inflammatory disease.
Bannerman et al (7,8) found that c-FLIP protected against
apoptosis and suppressed the activation of NF-κB induced by LPS.
These findings are in accordance with those of the present
study.
Bacterial LPS is a major component of Gram-negative
bacteria, which has been implicated in the pathogenesis of
Gram-negative sepsis. To ensure consistency with the rat sepsis
model using CLP, which induces abdominal infection, LPS was
selected in the present study as an inducer of inflammation in
HUVECs. LPS is known as a potent activator of proinflammatory
responses in various types of cells. The vascular endothelium is a
key host target of LPS and the first host tissue barrier
encountered by circulating LPS. Injury to and/or dysfunction of the
vascular endothelium has been implicated in the development of
sepsis-associated complications, including systemic vascular
collapse, disseminated intravascular coagulation, multi-organ
failure, and the development of vascular leak syndromes, including
acute respiratory distress syndrome (15,16).
Therefore, the present study focused on the changes in function of
the vascular endothelium, particularly on apoptosis, in the
pathogenesis of sepsis. It was described previously that LPS
induces apoptosis in human mammary epithelial cells (HMECs) only in
the presence of protein synthesis inhibitors, including CHX.
Bannerman et al found that c-FLIP protected against HMEC
apoptosis and suppressed the activation of NF-κB induced by LPS
(8), whereas Karahashi et
al found that c-FLIP was not involved in the induction of
apoptosis induced by LPS+CHX (9).
In order to elucidate the role of c-FLIP in endothelial cell
apoptosis, the present study cultured HUVECs with LPS in the
presence or absence of CHX, and showed that only LPS and CHX
together induced HUVEC apoptosis, and that the expression of
c-FLIPL was inversely correlated with apoptosis. Taken
together, these data suggested that c-FLIP, primarily
c-FLIPL, protected the HUVECs against the apoptosis
induced by LPS+CHX.
There were a number of limitations in the present
study. Although the effects of upregulated c-FLIPL were
addressed, the opposing arm was not investigated. Subsequent
investigations aim to examine the role of downregulated
c-FLIPL by c-FLIP-specific antisense oligonucleotides or
small interfering RNA lipocomplexes. In addition, the present study
did not detect the levels of apoptosis in cells of different organs
in the rats with sepsis.
Taken together, the present study demonstrated that
c-FLIP expression was significantly decreased in organs of septic
rats compared with control rats, and that c-FLIP overexpression
protected HUVECs from LPS+CHX-induced apoptosis in vitro.
These data indicated a protective role of c-FLIP in endothelial
cells and suggested that c-FLIP may have potential as a therapeutic
target in sepsis treatment in the future.
Acknowledgements
We would like to acknowledge the efforts of the
staff at the Central Laboratory, Huashan Hospital (Shanghai,
China). The present study was supported by grants from Huashan
Hospital (Initiation grants; grant no. 212).
References
|
1
|
Yu JW and Shi Y: FLIP and the death
effector domain family. Oncogene. 27:6216–6227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Longley DB, Wilson TR, McEwan M, Allen WL,
McDermott U, Galligan L and Johnston PG: c-FLIP inhibits
chemotherapy-induced colorectal cancer cell death. Oncogene.
25:838–848. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yan T, Li Q, Zhou H, Zhao Y, Yu S, Xu G,
Yin Z, Li Z and Zhao Z: Gu-4 suppresses affinity and avidity
modulation of CD11b and improves the outcome of mice with
endotoxemia and sepsis. PLoS One. 7:e301102012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang W, Zhao F, Fang Y, Li X, Shen L, Cao
T and Zhu H: Glycyrrhizin protects against porcine endotoxemia
through modulation of systemic inflammatory response. Crit Care.
17:R442013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krautwald S, Ziegler E, Tiede K, Pust R
and Kunzendorf U: Transduction of the TAT-FLIP fusion protein
results in transient resistance to Fas-induced apoptosis in vivo. J
Biol Chem. 279:44005–44011. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bagnoli M, Canevari S and Mezzanzanica D:
Cellular FLICE-inhibitory protein (c-FLIP) signalling: A key
regulator of receptor-mediated apoptosis in physiologic context and
in cancer. Int J Biochem Cell Biol. 42:210–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bannerman DD and Goldblum SE: Mechanisms
of bacterial lipopolysaccharide-induced endothelial apoptosis. Am J
Physiol Lung Cell Mol Physiol. 284:L899–L914. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bannerman DD, Eiting KT, Winn RK and
Harlan JM: FLICE-like inhibitory protein (FLIP) protects against
apoptosis and suppresses NF-kappaB activation induced by bacterial
lipopolysaccharide. Am J Pathol. 165:1423–1431. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karahashi H, Michelsen KS and Arditi M:
Lipopolysaccharide-induced apoptosis in transformed bovine brain
endothelial cells and human dermal microvessel endothelial cells:
The role of JNK. J Immunol. 182:7280–7286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hunt MA, Currie MJ, Robinson BA and Dachs
GU: Optimizing transfection of primary human umbilical vein
endothelial cells using commercially available chemical
transfection reagents. J Biomol Tech. 21:66–72. 2010.PubMed/NCBI
|
|
13
|
Safa AR, Day TW and Wu CH: Cellular
FLICE-like inhibitory protein (C-FLIP): A novel target for cancer
therapy. Curr Cancer Drug Targets. 8:37–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Perlman H, Pagliari LJ, Georganas C, Mano
T, Walsh K and Pope RM: FLICE-inhibitory protein expression during
macrophage differentiation confers resistance to fas-mediated
apoptosis. J Exp Med. 190:1679–1688. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coletta C, Módis K, Oláh G, Brunyánszki A,
Herzig DS, Sherwood ER, Ungvári Z and Szabo C: Endothelial
dysfunction is a potential contributor to multiple organ failure
and mortality in aged mice subjected to septic shock: Preclinical
studies in a murine model of cecal ligation and puncture. Crit
Care. 18:5112014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ostrowski SR, Haase N, Müller RB, Møller
MH, Pott FC, Perner A and Johansson PI: Association between
biomarkers of endothelial injury and hypocoagulability in patients
with severe sepsis: A prospective study. Crit Care. 19:1912015.
View Article : Google Scholar : PubMed/NCBI
|