Introduction
Drug-induced osteoporosis is an important adverse
effect in patients with various chronic debilitating diseases
requiring drug intervention (1),
with drug-induced osteoporosis having a significant effect on
patient morbidity and mortality rates (2). Treatment with anticoagulants,
including heparin, is associated with osteoporosis (3). Heparin can damage bones by reducing
bone formation and increasing bone resorption (4). Another anticoagulant, rivaroxaban
(XARELTO®), is a novel oral drug and a direct inhibitor
of factor Xa with, showing >10,000-fold higher selectivity for
factor Xa, compared with other serine proteases (5). The effects of rivaroxaban on bone
metabolism and fracture repair remain to be fully elucidated. A
previous study reported that rivaroxaban did not impair fracture
healing in a rat femur fracture model (6). By contrast, other studies have found
that long-term treatment with rivaroxaban had negative effects on
bone metabolism and microstructure in vitro and in
vivo (7–9), although rivaroxaban had fewer adverse
effects on bone health, compared with heparin (9). However, the mechanisms underlying the
negative effects of unfractionated heparin and rivaroxaban remain
to be fully elucidated.
Heparanase (HPSE) is an endoglucuronidase, which
degrades heparan sulfate proteoglycans (HSPGs) and releases
heparin-binding growth factors, including vascular endothelial
growth factor (10). HPSE is
expressed in osteoblastic cells (11), stimulating bone formation and
fracture repair (12). Fibroblast
growth factor (FGF) signaling is key in regulating chondrogenesis,
osteogenesis and bone homeostasis (13). The FGF family consists of 22
members, which can be classified into three groups: Canonical,
hormone-like and intracellular (14). FGF2 is a member of the FGF
polypeptide family, which is expressed in osteoblasts and stored in
the extracellular matrix (15).
Canonical FGFs, including FGF2, are crucial in bone formation
(16). As the effects of heparin
and rivaroxaban on HPSE and FGFs remain to be fully elucidated, the
present study was designed to investigate the effect of heparin and
rivaroxaban on the expression of HPSE and FGF2 in human
osteoblasts.
Materials and methods
Osteoblast culture and treatment
The human calvarial osteoblast cell line (4600) was
purchased from ScienCell Research Laboratories (Shanghai, China)
and characterized by the supplier as positive for alkaline
phosphatase and osteocalcin. These cells were routinely cultured in
osteoblast growth medium (PromoCell GmbH, Heidelberg, Germany) at
37°C in a humidified atmosphere of 95% O2 and 5%
CO2. The cells were seeded into 6-well plates at a
density of 1×105 cells/well for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analyses, or were seeded in 96-well plates at a
density of 5×104 cells/well for cell viability assays.
The cells were cultured to ~80% confluence prior to treatment. The
osteoblast cells were treated with 0 (negative control), 0.5, 5 and
50 IU/ml unfractionated heparin in osteoblast growth medium for 1,
3 and 5 days. For rivaroxaban treatment, the cells were treated
with rivaroxaban (cat. no. BAY 59-7939) obtained from Bayer AG
(Leverkusen, Germany), and dissolved in dimethyl sulfoxide (DMSO;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) at
concentrations of 0.13, 1.3 and 13 µg/ml in growth medium at 37°C
for 1, 3 and 5 days (17); as a
negative control, the cells were treated with medium containing
equivalent concentrations of DMSO.
Plasmid construction and
transfection
Plasmids encoding HPSE and FGF2, and the vector
control p-EGFP-N1 were obtained from Zoonbio Biotechnology Co.,
Ltd. (Nanjing, China), and transfected into osteoblasts using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), as described previously
(18). Briefly, the cells
(1×106 cells/well) were trypsinized and seeded 24 h
prior to transfection. The transfection mixture was dissolved in
Opti-MEM serum-free medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and added to the cells. After 24 h, the medium
was replaced with fresh medium with or without unfractionated
heparin or rivaroxaban at 37°C for 3 days. Protein and RNA were
subsequently extracted from the cells.
Cell viability assay
Osteoblast viability was assessed using MTT assays.
Briefly, the cells were incubated with 20 µl of MTT (0.5 mg/ml) at
37°C for 4 h. The culture medium was then removed, and 200 µl of
DMSO was added to each well, followed by agitation for 10 min at
room temperature. The absorbance of the wells at 490 nm was
measured using an Infinite M200 microplate reader (Tecan, Salzburg,
Austria). The viability of the treated cultures was calculated as a
percentage of control cultures, with reported data representative
of five independent experiments.
RNA isolation and RT-qPCR
analysis
Total RNA was extracted from the osteoblasts using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
concentration was examined by Nanodrop 2000 (Thermo, MA, USA). The
reverse-transcription PCR reaction was performed using a reverse
transcription kit (Thermo Fisher Scientific, Inc.) programmed to
25°C 10 min, 37°C 120 min, 85°C 5 min to complete cDNA synthesis.
Gene expression was analyzed using qPCR analysis with SYBR-Green
PCR Master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.)
in a FAST Real-time PCR system with the following protocol: 95°C 30
sec, 55°C 20 sec, 72°C 20 sec for 40 cycles with β-actin as an
internal control. The reaction mixture (20 µl) included: SYBR-Green
10 µl, cDNA 1 µl, forward prime 1 µl, reverse prime 1 µl, and DEPC
water 7 µl. The following specific primers were designed to assess
expression levels: HPSE, forward 5′-GCAGATGGCCCATACCTTCA-3′ and
reverse 5′-CACCTGGCTGCTCCCC-3′; HSPG, forward
5′-AAACGGACAGAAGTCCTAGCAG-3′ and reverse
5′-CTCATGCGATACACCAACAGC-3′); FGF2, forward
5′-ATGGCTCCCTTAGCCGAAGT-3′ and reverse 5′-AGGAAATGCGAACCCACCTG-3′;
and β-actin, forward 5′-CCGTTGCCCTGAGGCTCTTT-3′ and reverse
5′-ACTGTGTTGGCATACAGGTCTT-3′. The ΔΔCq value was calculated for
each sample (19), and expression
levels were expressed as 2−ΔΔCq.
Western blot analysis
Proteins from the osteoblasts were lysed with RIPA
buffer containing 300 mM NaCl, 50 mM Tris-HCl (pH 7.6), 0.5%
TritonX-100, 2 mM PMSF, 2 µg/ml aprotinin and 2 µg/ml leupeptin,
and incubated at 4°C for 1 h. The lysates were centrifuged at
13,600 × g for 15 min at 4°C. Protein concentration was examined by
BCA kit (Beyotime Institute of Biotechnology, Haimen, China). The
supernatants (~100 µg) were electrophoresed on 10% SDS-PAGE gels,
and the separated proteins were transferred onto nitrocellulose
membranes. These membranes were blocked in 5% non-fat milk
overnight at 4°C and incubated with primary antibodies against HPSE
(cat. no. PR-1645; 1:200; Zhenjiang Hope Biotechnology Co., Ltd.),
FGF2 (cat. no. PB0619; 1:200; Boster Biological Technology, Ltd.,
Wuhan, China) and β-actin (cat. no. 1854-s; 1:500; Hangzhou Huaan
Biotechnology Co., Ltd.) overnight at 4°C. Following washing with
PBS containing 0.5% Tween-20 (PBS-T), the membranes were incubated
with horseradish peroxidase-conjugated secondary antibodies
(1:2,000, goat-anti-rabbit, cat. no. HA1001; 1:2,000,
goat-anti-mouse, cat. no. HA1006; Shanghai Huabio Co., Ltd.) and
enhanced chemiluminescence reagents at 37°C for 1 h. The
immunoblots were scanned using a GS-800 densitometer, and protein
bands were quantified using QuantityOne version 4.62 software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All values are expressed as the mean ± standard
error of the mean, and compared using one-way analysis of variance
followed by Bonferroni's post hoc test. All statistical analyses
were performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference. Figures were constructed using GraphPad
Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA,
USA).
Results
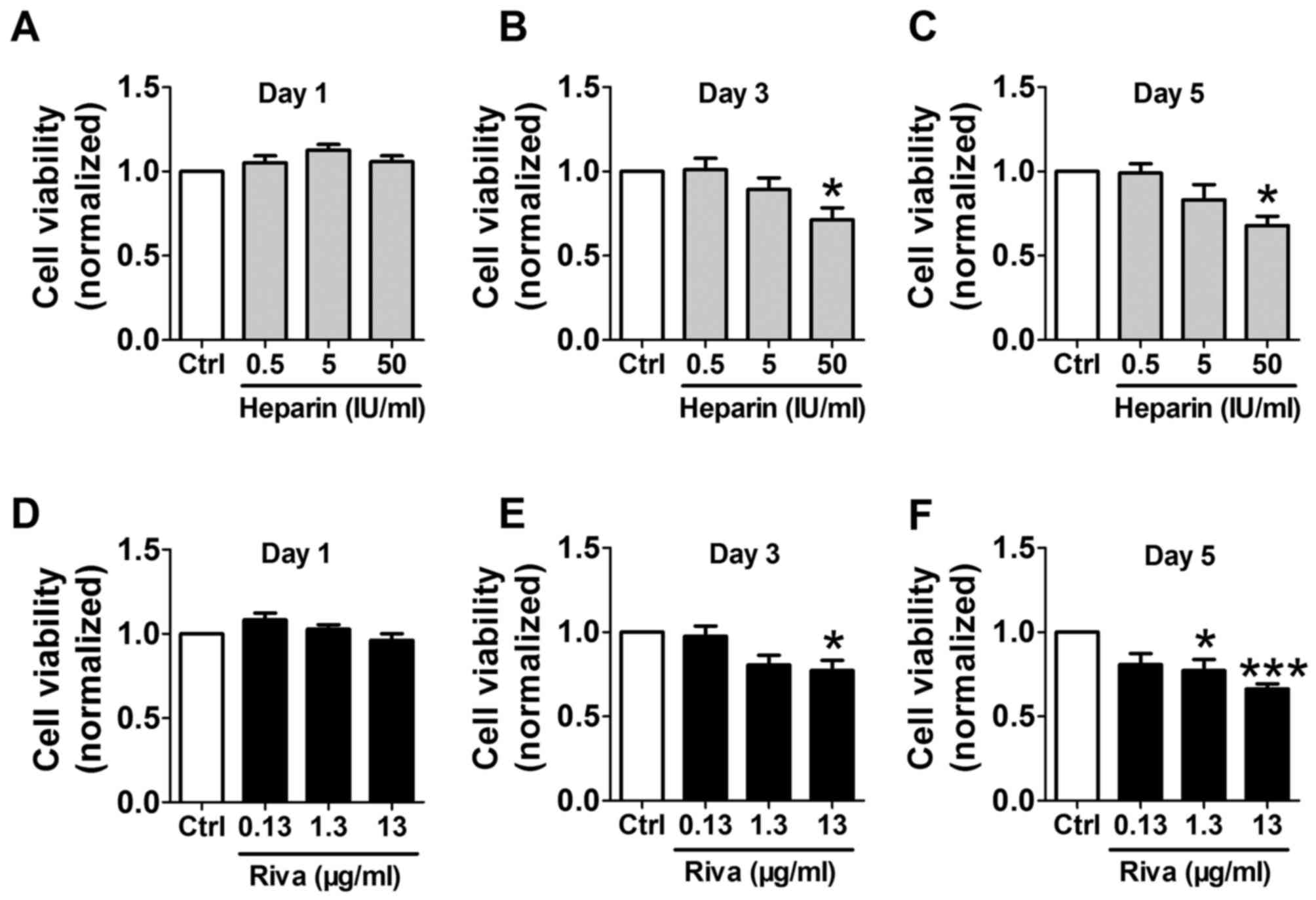
Effects of unfractionated heparin and
rivaroxaban on osteoblast viability
Osteoblasts were incubated with unfractionated
heparin (0.5, 5 and 50 IU/ml) or rivaroxaban (0.13, 1.3 and 13
µg/ml) for 1, 3 and 5 days, and cell viability was determined using
MTT assays. Although incubation with unfractionated heparin for 1
day did not significantly alter osteoblast viability (Fig. 1A), treatment for 3 and 5 days with
50 IU/ml unfractionated heparin significantly reduced cell
viability, however lower doses had no significant effect (Fig. 1B and C). Rivaroxaban treatment for
1 day did not affect cell viability (Fig. 1D). Cell viability was significantly
reduced by incubation with 13 µg/ml rivaroxaban for 3 and 5 days,
and by treatment with 1.3 µg/ml for 5 days (Fig. 1E and F). Based on these findings,
the osteoblasts were treated with 50 IU/ml unfractionated heparin
and 13 µg/ml rivaroxaban for 3 days in the subsequent
experiments.
Effects of unfractionated heparin and
rivaroxaban on expression levels of HPSE, HSPG and FGF2
Treatment of osteoblasts with unfractionated heparin
significantly reduced the mRNA level of HPSE and significantly
increased the mRNA level of HSPG, compared with levels in the
control cells (P<0.05; Fig. 2A and
B). By contrast, rivaroxaban did not significantly alter the
mRNA levels of HPSE or HSPG. Treatment with unfractionated heparin
or rivaroxaban significantly reduced the mRNA level of FGF2
(Fig. 2C). Consistent with the
results of RT-qPCR analysis, western blot analysis of the protein
expression of HPSE showed that the protein level of HPSE was
downregulated by unfractionated heparin, but not by rivaroxaban
(Fig. 2D). Incubation of the
osteoblasts with either unfractionated heparin or rivaroxaban
significantly reduced the protein levels of FGF2, with lower mRNA
and protein levels of FGF2 in the rivaroxaban-treated cells,
compared with the unfractionated heparin-treated cells (Fig. 2E).
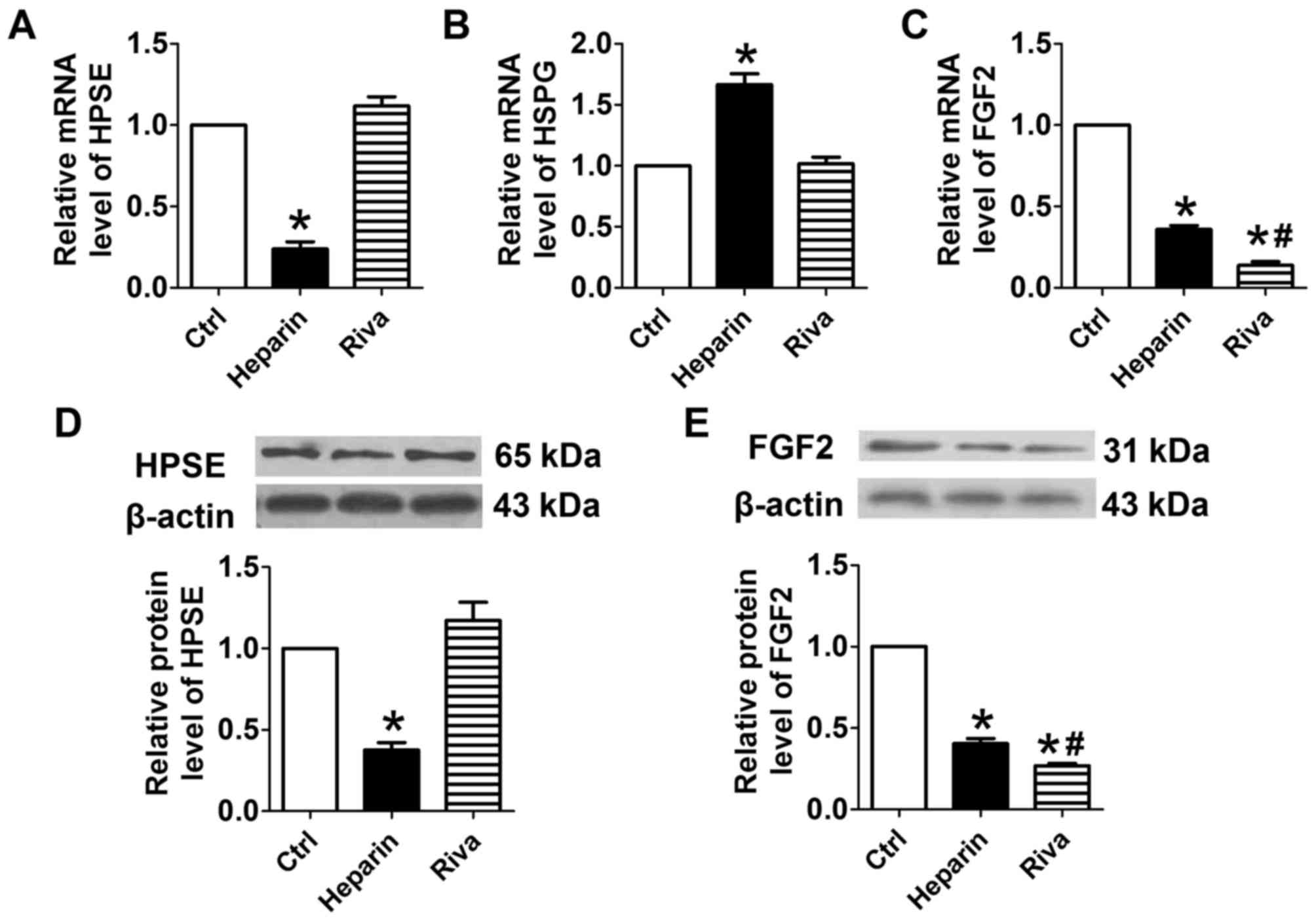 | Figure 2.Effects of heparin and rivaroxaban on
the mRNA expression of HPSE, HSPG and FGF2. Relative expression of
(A) HPSE, (B) HSPG and (C) FGF2. *P<0.05, vs. Ctrl;
#P<0.05, vs. heparin (n=5). Representative western
blots of the expression of (D) HPSE and (E) FGF2, and the relative
expression of each compared with β-actin. Data are presented as the
mean ± standard error of the mean, *P<0.05, vs. control;
#P<0.05, vs. heparin (n=3). HPSE, heparanase; HSPG,
heparan sulfate proteoglycan; FGF, fibroblast growth factor; Riva,
rivaroxaban; Ctrl, control. |
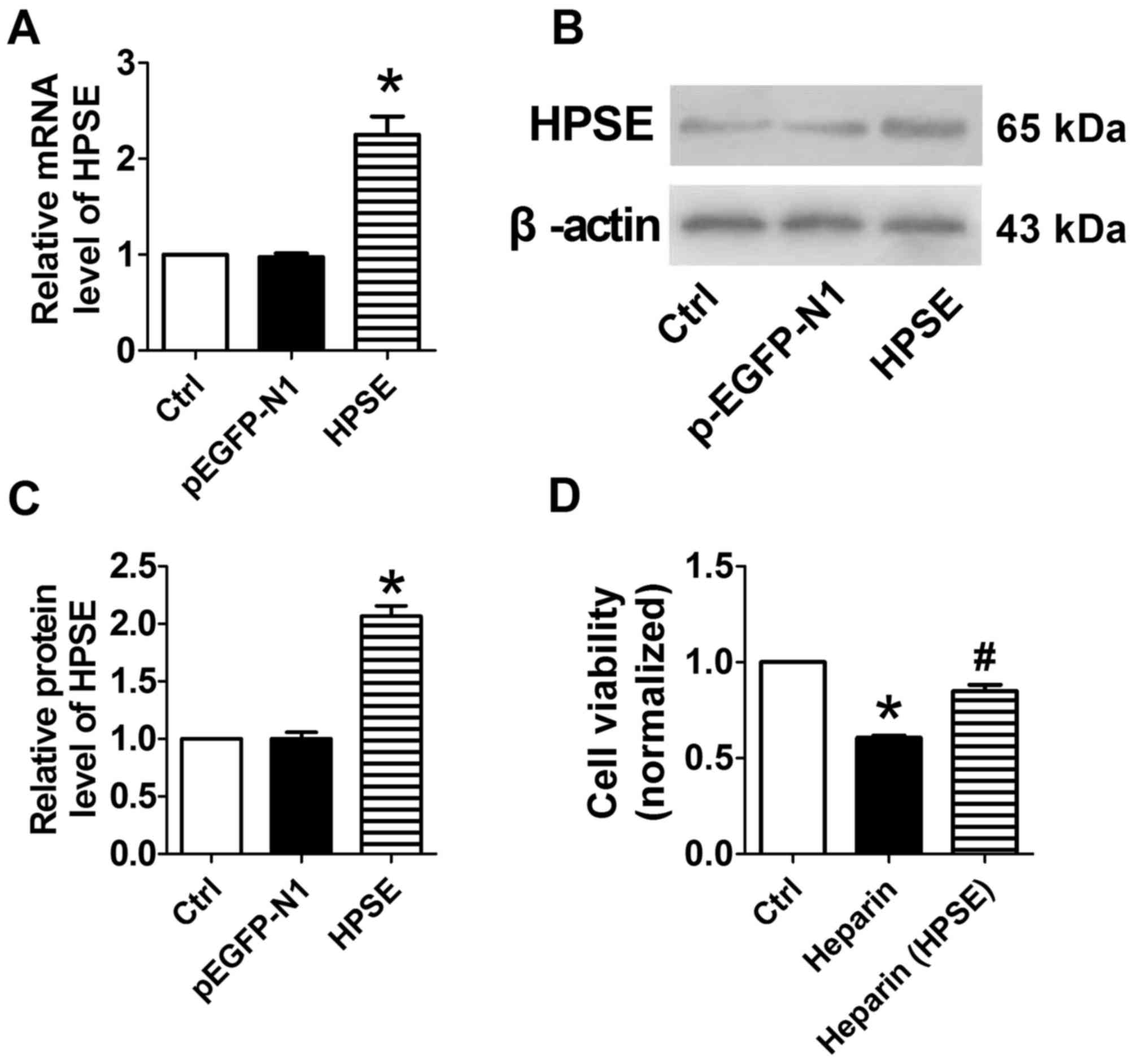
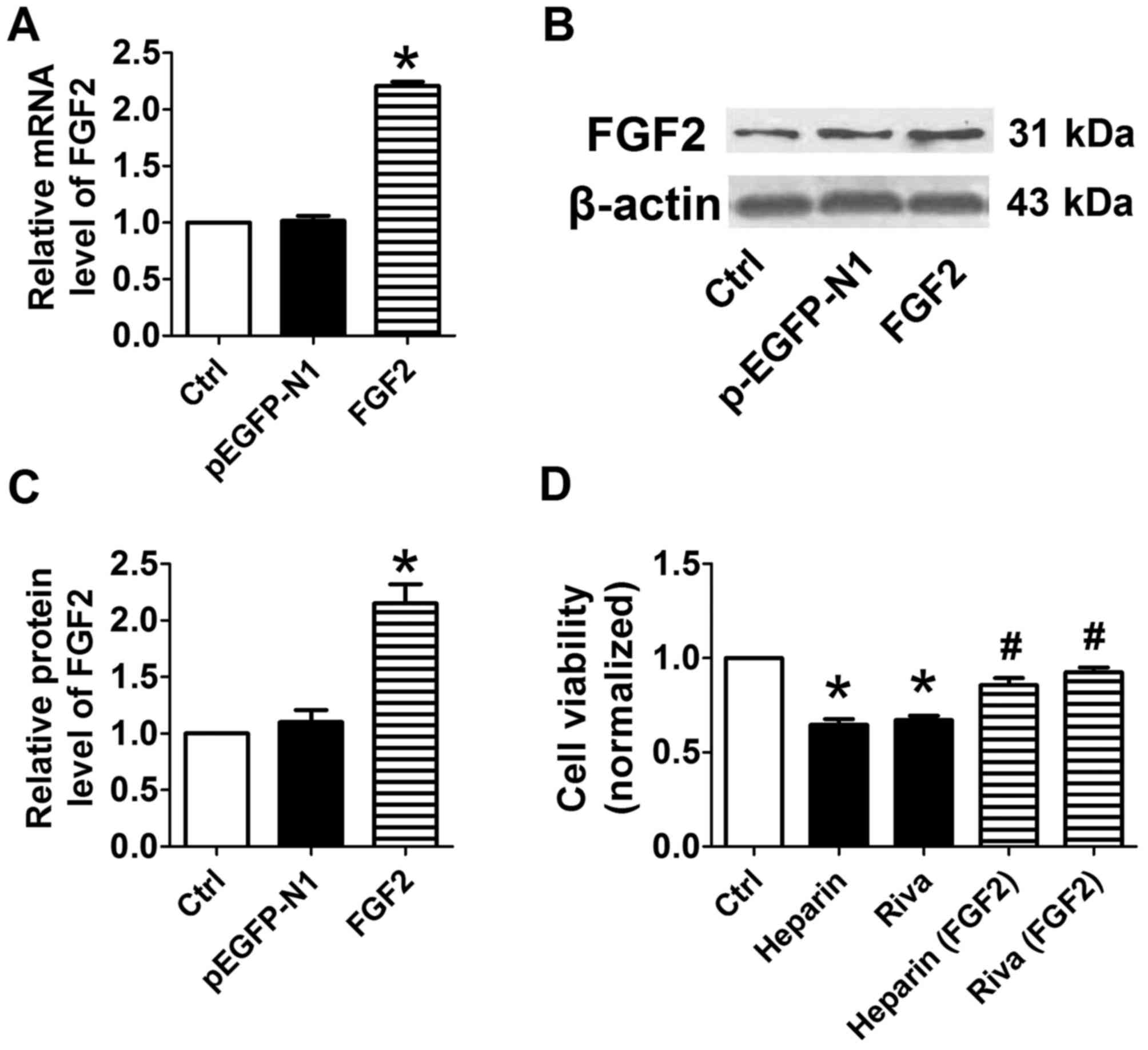
Overexpression of HPSE or FGF2
increases osteoblast viability
The transfection of cells with plasmids encoding
HPSE (Fig. 3A-C) markedly enhanced
the expression mRNA and protein levels of FGF2, compared with the
control and p-EGFP-N1-transfected cells. The assays of cell
viability showed that the inhibitory effect of unfractionated
heparin was overcome in HPSE-overexpressing cells (Fig. 3D). The transfection of cells with
plasmids encoding FGF2 (Fig. 4A-C)
markedly enhanced the expression levels of their respective mRNA
and protein, and the inhibitory effects of unfractionated heparin
or rivaroxaban were overcome in FGF2-overexpressing cells (Fig. 4D). These findings indicated that
the overexpression of HPSE or FGF2 markedly reversed the adverse
effects of unfractionated heparin or rivaroxaban on
osteoblasts.
Discussion
The results of the present study demonstrated that
the incubation of human osteoblasts with unfractionated heparin or
rivaroxaban for 3 days inhibited the growth of the osteoblasts by
downregulating the expression of HPSE and/or FGF2. The
overexpression of HPSE and FGF2 overcame the adverse effects of
unfractionated heparin and rivaroxaban. In our previous study, it
was found that treatment with unfractionated heparin and
rivaroxaban for 4 weeks had negative effects on bone microstructure
and function in adult rats (9).
Therefore, the results of the present in vitro study
suggested that the negative effects of long-term treatment with
unfractionated heparin or rivaroxaban on bone health were due to
their ability to inhibit the growth of osteoblasts.
The expression of HPSE and HSPG affect chondrogenic
and osteogenic processes during endochondral bone formation
(20,21). The present study showed that
unfractionated heparin significantly reduced the expression of HPSE
and increased the expression of HSPG, however, these effects were
not observed with rivaroxaban. FGF signals are important in bone
formation and metabolism (22,23).
A previous study showed that bone mass and bone formation were
markedly reduced in basic FGF2-knockout mice (24), whereas the overexpression of FGF2
by the osteoblastic lineage of transgenic mice resulted in
increased bone mass (25). In the
present study, unfractionated heparin and rivaroxaban did not
affect the expression of FGF2 or other members of the FGF family,
including FGF1 and FGF18 (data not shown), however, the
contribution of other FGF members cannot be excluded.
Rivaroxaban treatment reduces the expression of the
bone marker, osteocalcin, the major osteoblast factor, runt-related
transcription factor-2, and osteogenic bone morphogenic protein-2,
resulting in a negative effect on osteoblast function (17). Although the previous study found
that treatment with 1.3 and 13 µg/ml rivaroxaban for 3 days did not
have an adverse effect on osteoblast viability, it also showed that
treatment with lower concentrations of rivaroxaban (0.013 and 0.13
µg/ml) increased osteoblast viability by 15 and 10%, respectively.
By contrast, the present study found that treatment with 13 µg/ml
rivaroxaban for 3 days markedly reduced cell viability. Therefore,
further investigations are required to determine the reasons for
this discrepancy, and the reasons why treatment with 1.3 and 13
µg/ml rivaroxaban for 5 days reduced osteoblast viability.
In our previous study, it was found that
unfractionated heparin had a more marked adverse effect on bone
microstructure and function, compared with rivaroxaban (9). The present study showed that
rivaroxaban inhibited the expression of FGF2, whereas
unfractionated heparin repressed the expression of HPSE and FGF2.
These findings may partly explain previous in vivo
differences observed between these two drugs.
The present study had two limitations, one of which
was its in vitro design. In vivo investigations in
animals are required to confirm that unfractionated heparin and
rivaroxaban regulate the expression of HPSE and FGF2 in
osteoblasts. In addition, further investigations are required to
examine the potential association between HPSE and FGF2.
In conclusion, the present study showed that
treatment of osteoblasts with anticoagulants altered the expression
of HPSE and FGF2. These findings may provide novel information
regarding the side effects of unfractionated heparin and
rivaroxaban in clinical practice.
Acknowledgements
This study was supported by a Research Grant from
the Education Department of Heilongjiang Province of China (grant
no. 12541443).
References
|
1
|
Mazziotti G, Canalis E and Giustina A:
Drug-induced osteoporosis: Mechanisms and clinical implications. Am
J Med. 123:877–884. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Panday K, Gona A and Humphrey MB:
Medication-induced osteoporosis: Screening and treatment
strategies. Ther Adv Musculoskelet Dis. 6:185–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bilen O and Teruya J: Complications of
anticoagulation. Dis Mon. 58:440–447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rajgopal R, Bear M, Butcher MK and
Shaughnessy SG: The effects of heparin and low molecular weight
heparins on bone. Thromb Res. 122:293–298. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duggan ST, Scott LJ and Plosker GL:
Rivaroxaban: A review of its use for the prevention of venous
thromboembolism after total hip or knee replacement surgery. Drugs.
69:1829–1851. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klüter T, Weuster M, Brüggemann S,
Menzdorf L, Fitschen-Oestern S, Steubesand N, Acil Y, Pufe T,
Varoga D, Seekamp A and Lippross S: Rivaroxaban does not impair
fracture healing in a rat femur fracture model: An experimental
study. BMC Musculoskelet Disord. 16:792015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Somjen D, Katzburg S, Gigi R, Dolkart O,
Sharon O, Salai M and Stern N: Rivaroxaban, a direct inhibitor of
the coagulation factor Xa interferes with hormonal-induced
physiological modulations in human female osteoblastic cell line
SaSO2. J Steroid Biochem Mol Biol. 135:67–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gigi R, Salai M, Dolkart O, Chechik O,
Katzburg S, Stern N and Somjen D: The effects of direct factor Xa
inhibitor (Rivaroxaban) on the human osteoblastic cell line SaOS2.
Connect Tissue Res. 53:446–450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xia J, Zhang Z, Wang J, Zu J, Wang N and
Wang D: Comparison of the effects of heparin and the direct factor
Xa inhibitor, rivaroxaban, on bone microstructure and metabolism in
adult rats. Connect Tissue Res. 56:477–482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saijo M, Kitazawa R, Nakajima M, Kurosaka
M, Maeda S and Kitazawa S: Heparanase mRNA expression during
fracture repair in mice. Histochem Cell Biol. 120:493–503. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kram V, Zcharia E, Yacoby-Zeevi O, Metzger
S, Chajek-Shaul T, Gabet Y, Müller R, Vlodavsky I and Bab I:
Heparanase is expressed in osteoblastic cells and stimulates bone
formation and bone mass. J Cell Physiol. 207:784–792. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smith PN, Freeman C, Yu D, Chen M, Gatenby
PA, Parish CR and Li RW: Heparanase in primary human osteoblasts. J
Orthop Res. 28:1315–1322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ornitz DM and Marie PJ: Fibroblast growth
factor signaling in skeletal development and disease. Genes Dev.
29:1463–1486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takei Y, Minamizaki T and Yoshiko Y:
Functional diversity of fibroblast growth factors in bone
formation. Int J Endocrinol. 2015:7293522015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hurley MM, Marie PJ and Florkiewics RZ:
Fibroblast Growth Factor and Fibroblast Growth Factor Receptor
Families. Principles of Bone Biology. Bilezikian JP, Raisz LG and
Rodan G: San Diego, CA: Academic Press; pp. 627–645. 2002
|
|
16
|
Debiais F, Hott M, Graulet AM and Marie
PJ: The effects of fibroblast growth factor-2 on human neonatal
calvaria osteoblastic cells are differentiation stage specific. J
Bone Miner Res. 13:645–654. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Solayar GN, Walsh PM and Mulhall KJ: The
effect of a new direct factor Xa inhibitor on human osteoblasts: An
in-vitro study comparing the effect of rivaroxaban with enoxaparin.
BMC Musculoskelet Disord. 12:2472011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ramani VC, Yang Y, Ren Y, Nan L and
Sanderson RD: Heparanase plays a dual role in driving hepatocyte
growth factor (HGF) signaling by enhancing HGF expression and
activity. J Biol Chem. 286:6490–6499. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brown AJ, Alicknavitch M, D'Souza SS,
Daikoku T, Kirn-Safran CB, Marchetti D, Carson DD and Farach-Carson
MC: Heparanase expression and activity influences chondrogenic and
osteogenic processes during endochondral bone formation. Bone.
43:689–699. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han Q, Liu F and Zhou Y: Increased
expression of heparanase in osteogenic differentiation of rat
marrow stromal cells. Exp Ther Med. 5:1697–1700. 2013.PubMed/NCBI
|
|
22
|
Fei Y, Gronowicz G and Hurley MM:
Fibroblast growth factor-2, bone homeostasis and fracture repair.
Curr Pharm Des. 19:3354–3363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marie PJ, Miraoui H and Sévère N: FGF/FGFR
signaling in bone formation: Progress and perspectives. Growth
Factors. 30:117–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Montero A, Okada Y, Tomita M, Ito M,
Tsurukami H, Nakamura T, Doetschman T, Coffin JD and Hurley MM:
Disruption of the fibroblast growth factor-2 gene results in
decreased bone mass and bone formation. J Clin Invest.
105:1085–1093. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao L, Ueno D, Catros S, Homer-Bouthiette
C, Charles L, Kuhn L and Hurley MM: Fibroblast growth factor-2
isoform (low molecular weight/18 kDa) overexpression in
preosteoblast cells promotes bone regeneration in critical size
calvarial defects in male mice. Endocrinology. 155:965–974. 2014.
View Article : Google Scholar : PubMed/NCBI
|